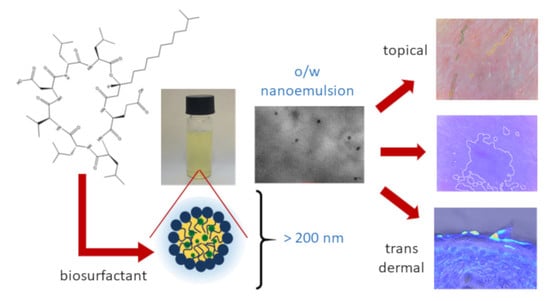
Nanoemulsion Stabilized by Safe Surfactin from Bacillus subtilis as a Multifunctional, Custom-Designed Smart Delivery System
Abstract
1. Introduction
2. Materials and Methods
2.1. Surfactin Preparation
2.2. Analytical Identity—HPLC and ESI Analysis
2.3. SEDDS—Key Components
2.3.1. Co-Solvents
2.3.2. Oils and Active Substances
2.3.3. Other Reagents
2.4. Construction of Diagrams
2.5. Characterization of SEDDS System Stabilized by Biosurfactant
2.5.1. DLS Analysis
2.5.2. TEM Microscopy
2.5.3. Stability Studies
2.5.4. Scavenging Free Radicals
2.6. Biological Evaluation
2.6.1. Cytotoxicity Assay
2.6.2. Ex Vivo Skin Transdermal Studies
2.6.3. Visualization of Penetration
2.6.4. In Vivo Evaluation of Nanoemulsions—Skin Interaction Study
2.7. Statistical Analysis
3. Results and Discussion
3.1. Optimization, Characterization and Stability of Smart SEDDS Systems Obtained through Nanoemulsion Structural Design
3.2. Biological Evaluation
3.2.1. Cytotoxicity Assay
3.2.2. Scavenging of Free Radicals
3.2.3. Ex Vivo—Transdermal Studies
3.2.4. In Vivo Evaluation of Nanoemulsions—Skin Interaction Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lewińska, A.; Jaromin, A.; Jezierska, J. Role of architecture of N-oxide surfactants in the design of nanoemulsions for Candida skin infection. Colloids Surf. B Biointerf. 2020, 187, 110639. [Google Scholar] [CrossRef] [PubMed]
- Bazylinska, U.; Kulbacka, J.; Chodaczek, G. Nanoemulsion Structural Design in Co-Encapsulation of Hybrid Multifunctional Agents: Influence of the Smart PLGA Polymers on the Nanosystem-Enhanced Delivery and Electro-Photodynamic Treatment. Pharmaceutics 2019, 11, 405. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Bandyopadhyay, S.; Kapil, R.; Singh, R.; Katare, O.P. Self-emulsifying drug delivery systems (SEDDS): Formulation development, characterization, and applications. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 427–451. [Google Scholar] [CrossRef]
- Khames, A. Formulation and Characterization of Eplerenone Nanoemulsion Liquisolids, An Oral Delivery System with Higher Release Rate and Improved Bioavailability. Pharmaceutics 2019, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Trommer, H.; Neubert, R. Overcoming the Stratum Corneum: The Modulation of Skin Penetration. Skin Pharmacol. Physiol. 2006, 19, 106–121. [Google Scholar] [CrossRef]
- Abd, E.; Namjoshi, S.; Mohammed, Y.; Roberts, M.; Grice, J. Synergistic Skin Penetration Enhancer and Nanoemulsion Formulations Promote the Human Epidermal Permeation of Caffeine and Naproxen. J. Pharm. Sci. 2016, 105, 212–220. [Google Scholar] [CrossRef]
- Lindman, B.; Shinoda, K.; Olsson, U.; Anderson, D.; Karlström, G.; Wennerström, H. On the demonstration of bicontinuous structures in microemulsions. Coll. Surf. 1989, 38, 205–224. [Google Scholar] [CrossRef]
- Hadgraft, J. Modulation of the barrier function of the skin. Skin Pharmacol. Appl. Skin Physiol. 2001, 14, 72–81. [Google Scholar] [CrossRef]
- Biniarz, P.; Łukaszewicz, M.; Janek, T. Screening concepts, characterization and structural analysis of microbial-derived bioactive lipopeptides: A review. Crit. Rev. Biotechnol. 2016, 37, 393–410. [Google Scholar] [CrossRef]
- Desai, J.D.; Banat, I.M. Microbial production of surfactants and their commercial potential. Microbiol. Mol. Biol. Rev. 1997, 61, 47–64. [Google Scholar] [CrossRef]
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in Food Science: Applications, Recent Trends, and Future Perspectives. Nano-Micro Lett. 2020, 12, 1–34. [Google Scholar] [CrossRef]
- Chow, C.K. Vitamin E and oxidative stress. Free. Radic. Biol. Med. 1991, 11, 215–232. [Google Scholar] [CrossRef]
- Hayden, R.E.; Paniello, R.C.; Yeung, C.S.T.; Bello, S.L.; Dawson, S.M. The effect of glutathione and vitamins A, C, and E on acute skin flap survival. Laryngoscope 1987, 97, 1176–1179. [Google Scholar] [CrossRef]
- Thiele, J.J.; Ekanayake-Mudiyanselage, S. Vitamin E in human skin: Organ-specific physiology and considerations for its use in dermatology. Mol. Asp. Med. 2007, 28, 646–667. [Google Scholar] [CrossRef]
- Yang, Y.; McClements, D.J. Vitamin E bioaccessibility: Influence of carrier oil type on digestion and release of emulsified α-tocopherol acetate. Food Chem. 2013, 141, 473–481. [Google Scholar] [CrossRef]
- Barbosa, E.; Faintuch, J.; Moreira, E.A.M.; Da Silva, V.R.G.; Pereima, M.J.L.; Fagundes, R.L.M.; Filho, D.W. Supplementation of Vitamin E, Vitamin C, and Zinc Attenuates Oxidative Stress in Burned Children: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. J. Burn. Care Res. 2009, 30, 859–866. [Google Scholar] [CrossRef]
- Kojo, S. Vitamin C: Basic Metabolism and Its Function as an Index of Oxidative Stress. Curr. Med. Chem. 2004, 11, 1041–1064. [Google Scholar] [CrossRef]
- Niedziółka, K.; Szymula, M.; Lewinska, A.; Wilk, K.A.; Narkiewicz-Michałek, J. Studies of vitamin C antioxidative activity in the N-oxide surfactant solutions. Coll. Surf. A Physicochem. Eng. Asp. 2012, 413, 33–37. [Google Scholar] [CrossRef]
- Kligman, A.M. COSMETICS. Dermatol. Clin. 2000, 18, 699–709. [Google Scholar] [CrossRef]
- Ramos-E-Silva, M.; Celem, L.R.; Ramos-E-Silva, S.; Fucci-Da-Costa, A.P. Anti-aging cosmetics: Facts and controversies. Clin. Dermatol. 2013, 31, 750–758. [Google Scholar] [CrossRef]
- Farris, P.K. Topical Vitamin C: A Useful Agent for Treating Photoaging and Other Dermatologic Conditions. Dermatol. Surg. 2006, 31, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Chung, W.S.; Lee, H.; Jung, S.W. Whitening Effect of Cosmetics Containing Magnesium L-Ascorbyl-2-Phosphate (VC-PMG, Vitamin C Derivatives) Assessed by Colorimeter. Ann. Dermatol. 2002, 14, 63. [Google Scholar] [CrossRef][Green Version]
- Yoo, S.-H.; Song, Y.-B.; Chang, P.-S.; Lee, H.G. Microencapsulation of α-tocopherol using sodium alginate and its controlled release properties. Int. J. Biol. Macromol. 2006, 38, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Di Cola, E.; Cantu’, L.; Brocca, P.; Rondelli, V.; Fadda, G.C.; Canelli, E.; Martelli, P.; Clementino, A.; Sonvico, F.; Bettini, R.; et al. Novel O/W nanoemulsions for nasal administration: Structural hints in the selection of performing vehicles with enhanced mucopenetration. Coll. Surf. Biointerf. 2019, 183, 110439. [Google Scholar] [CrossRef] [PubMed]
- De Matos, S.P.; Teixeira, H.F.; Lima, E.S.; Júnior, V.F.D.V.; Koester, L.S. Essential Oils and Isolated Terpenes in Nanosystems Designed for Topical Administration: A Review. Biomolecules 2019, 9, 138. [Google Scholar] [CrossRef]
- Sita, V.G.; Vavia, P.R. Bromocriptine Nanoemulsion-Loaded Transdermal Gel: Optimization Using Factorial Design, In Vitro and In Vivo Evaluation. AAPS Pharm. Sci. Tech. 2020, 21, 80. [Google Scholar] [CrossRef]
- Jajor, P.; Piłakowska-Pietras, D.; Krasowska, A.; Łukaszewicz, M. Surfactin analogues produced by Bacillus subtilis strains grown on rapeseed cake. J. Mol. Struct. 2016, 1126, 141–146. [Google Scholar] [CrossRef]
- Craig, D. An investigation into the mechanisms of self-emulsification using particle size analysis and low frequency dielectric spectroscopy. Int. J. Pharm. 1995, 114, 103–110. [Google Scholar] [CrossRef]
- Jaromin, A.; Zarnowski, R.; Pietka-Ottlik, M.; Andes, D.; Gubernator, J. Topical delivery of ebselen encapsulated in biopolymeric nanocapsules: Drug repurposing enhanced antifungal activity. Nanomedicine 2018, 13, 1139–1155. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Chem. Biol. 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Filon, F.L.; Mauro, M.; Adami, G.; Bovenzi, M.; Crosera, M. Nanoparticles skin absorption: New aspects for a safety profile evaluation. Regul. Toxicol. Pharmacol. 2015, 72, 310–322. [Google Scholar] [CrossRef]
- Shakeel, F.; Baboota, S.; Ahuja, A.; Ali, J.; Shafiq, S. Skin permeation mechanism and bioavailability enhancement of celecoxib from transdermally applied nanoemulsion. J. Nanobiotech. 2008, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Edible nanoemulsions: Fabrication, properties, and functional performance. Soft Mat. 2011, 7, 2297–2316. [Google Scholar] [CrossRef]
- Karwal, R.; Garg, T.; Rath, G.; Markandeywar, T.S. Current Trends in Self-Emulsifying Drug Delivery Systems (SEDDSs) to Enhance the Bioavailability of Poorly Water-Soluble Drugs. Crit. Rev. Ther. Drug Carr. Syst. 2016, 33, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Morozowich, W.; Gao, P. Improving the Oral Absorption of Poorly Soluble Drugs Using SEDDS and S-SEDDS Formulations. Develop. Sol. Or. Dos. For. 2009, 443–468. [Google Scholar] [CrossRef]
- Yousef, S.A.; Mohammed, Y.; Namjoshi, S.; Grice, J.; Benson, H.; Sakran, W.; Roberts, M. Mechanistic Evaluation of Enhanced Curcumin Delivery through Human Skin In Vitro from Optimised Nanoemulsion Formulations Fabricated with Different Penetration Enhancers. Pharmaceutics 2019, 11, 639. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Mandal, A. Surfactant Stabilized Oil-in-Water Nanoemulsion: Stability, Interfacial Tension, and Rheology Study for Enhanced Oil Recovery Application. Energy Fuels 2018, 32, 6452–6466. [Google Scholar] [CrossRef]
- Rodrigues, F.; Diniz, L.; Sousa, R.; Honorato, T.; Simão, D.; Araújo, C.; Gonçalves, T.; Rolim, L.; Goto, P.; Tedesco, A.; et al. Preparation and characterization of nanoemulsion containing a natural naphthoquinone. Quím. Nov. 2018, 41, 756–761. [Google Scholar] [CrossRef]
- Lowry, G.V.; Hill, R.J.; Harper, S.; Rawle, A.F.; Hendren, C.O.; Klaessig, F.; Nobbmann, U.; Sayre, P.; Rumble, J. Guidance to improve the scientific value of zeta-potential measurements in nanoEHS. Environ. Sci. Nano 2016, 3, 953–965. [Google Scholar] [CrossRef]
- Nozhat, Z.; Asadi, A.; Zahri, S. Properties of Surfactin C-15 Nanopeptide and Its Cytotoxic Effect on Human Cervix Cancer (HeLa) Cell Line. J. Nanomater. 2012, 2012, 526580. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, J.Y.; Kim, S.-H.; Bae, H.J.; Yi, H.; Yoon, S.H.; Koo, B.S.; Kwon, M.; Cho, J.Y.; Lee, C.-E.; et al. Surfactin from Bacillus subtilis displays anti-proliferative effect via apoptosis induction, cell cycle arrest and survival signaling suppression. FEBS Lett. 2007, 581, 865–871. [Google Scholar] [CrossRef]
- Huang, X.; Lu, Z.; Zhao, H.; Bie, X.; Lü, F.; Yang, S. Antiviral Activity of Antimicrobial Lipopeptide from Bacillus subtilis fmbj Against Pseudorabies Virus, Porcine Parvovirus, Newcastle Disease Virus and Infectious Bursal Disease Virus in Vitro. Int. J. Pept. Res. Ther. 2006, 12, 373–377. [Google Scholar] [CrossRef]
- Liu, X.; Tao, X.; Zou, A.; Yang, S.; Zhang, L.; Mu, B. Effect of themicrobial lipopeptide on tumor cell lines: Apoptosis induced by disturbing the fatty acid composition of cell membrane. Prot. Cell 2010, 1, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, O.; Poulson, R.; Thakkar, D.; Yapp, C.; Carr, A. Ascorbic acid is essential for significant collagen deposition by human tenocytes in vitro. Oxid. Antioxid. Med Sci. 2014, 3, 119. [Google Scholar] [CrossRef]
- Dulińska-Molak, I.; Pasikowska-Piwko, M.; Dębowska, R.; Święszkowski, W.; Rogiewicz, K.; Eris, I. Determining the effectiveness of vitamin C in skin care by atomic force microscope. Microsc. Res. Tech. 2019, 82, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, G.; Anuszewska, E.L. Influence of vitamins C and E on cytotoxic activity of adriamycin in chosen cell cultures. Acta Pol. Pharm. Drug Res. 2002, 59, 31–35. [Google Scholar]
- Wei, C.-W.; Yu, Y.; Chen, Y.; Hung, Y.; Yiang, G.-T. Anticancer effects of methotrexate in combination with α-tocopherol and α-tocopherol succinate on triple-negative breast cancer. Oncol. Rep. 2019, 41, 2060–2066. [Google Scholar] [CrossRef]
- Levi-Schaffer, F.; Dayan, N.; Touitou, E. Diethylene Glycol Monoethyl Ether (Transcutol®) Displays Antiproliferative Properties Alone and in Combination with Xanthines. Skin Pharmacol. Physiol. 1996, 9, 53–59. [Google Scholar] [CrossRef]
- Alvi, M.M.; Chatterjee, P. A Prospective Analysis of Co-Processed Non-Ionic Surfactants in Enhancing Permeability of a Model Hydrophilic Drug. AAPS Pharm. Sci. Tech. 2013, 15, 339–353. [Google Scholar] [CrossRef]
- Landeros, J.M.; Belmont-Bernal, F.; Pérez-González, A.T.; Pérez-Padrón, M.I.; Guevara-Salazar, P.; González-Herrera, I.G.; Guadarrama, P. A two-step synthetic strategy to obtain a water-soluble derivative of curcumin with improved antioxidant capacity and in vitro cytotoxicity in C6 glioma cells. Mater. Sci. Eng. C 2017, 71, 351–362. [Google Scholar] [CrossRef]
- Semsri, S.; Krig, S.R.; Kotelawala, L.; Sweeney, C.A.; Anuchapreeda, S. Inhibitory mechanism of pure curcumin onWilms’ tumor 1(WT1) gene expression through the PKCα signaling pathway in leukemic K562 cells. FEBS Lett. 2011, 585, 2235–2242. [Google Scholar] [CrossRef] [PubMed]
- Pund, S.; Pawar, S.; Gangurde, S.; Divate, D. Transcutaneous delivery of leflunomide nanoemulgel: Mechanistic investigation into physicomechanical characteristics, in vitro anti-psoriatic and anti-melanoma activity. Int. J. Pharm. 2015, 487, 148–156. [Google Scholar] [CrossRef]
- Hosmer, J.; Reed, R.; Bentley, M.V.L.; Nornoo, A.; Lopes, L.B. Microemulsions Containing Medium-Chain Glycerides as Transdermal Delivery Systems for Hydrophilic and Hydrophobic Drugs. AAPS Pharm. Sci. Tech. 2009, 10, 589–596. [Google Scholar] [CrossRef]
- Keemink, J.; Bergström, C.A. Caco-2 Cell Conditions Enabling Studies of Drug Absorption from Digestible Lipid-Based Formulations. Pharm. Res. 2018, 35, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Roohinejad, S.; Oey, I.; Wen, J.; Lee, S.J.; Everett, D.W.; Burritt, D.J. Formulation of oil-in-water β-carotene microemulsions: Effect of oil type and fatty acid chain length. Food Chem. 2015, 174, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Ganapathi, M.R.; Hermann, R.; Naumov, S.; Brede, O. Free electron transfer from several phenols to radical cations of non-polar solvents. Phys. Chem. Chem. Phys. 2000, 2, 4947–4955. [Google Scholar] [CrossRef]
- Menon, G.K.; Cleary, G.W.; Lane, M.E. The structure and function of the stratum corneum. Int. J. Pharm. 2012, 435, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Gorzelanny, C.; Mess, C.; Schneider, S.W.; Huck, V.; Brandner, J.M. Skin Barriers in Dermal Drug Delivery: Which Barriers Have to Be Overcome and How Can We Measure Them? Pharmaceutics 2020, 12, 684. [Google Scholar] [CrossRef] [PubMed]
- Sarheed, O.; Shouqair, D.; Ramesh, K.; Khaleel, T.; Amin, M.; Boateng, J.; Drechsler, M. Formation of stable nanoemulsions by ultrasound-assisted two-step emulsification process for topical drug delivery: Effect of oil phase composition and surfactant concentration and loratadine as ripening inhibitor. Int. J. Pharm. 2020, 576, 118952. [Google Scholar] [CrossRef]
- Nastiti, C.M.R.R.; Ponto, T.; Mohammed, Y.; Roberts, M.; Benson, H. Novel Nanocarriers for Targeted Topical Skin Delivery of the Antioxidant Resveratrol. Pharmaceutics 2020, 12, 108. [Google Scholar] [CrossRef]
- Nowicka, D. Diagnostyka kosmetologiczna–studium przypadków. Dermatologia 2012, 38–42. [Google Scholar]
- Hwang, S.-W.; Oh, D.-J.; Lee, D.; Kim, J.-W.; Park, S.-W. Clinical Efficacy of 25% l-Ascorbic Acid (C’ensil) in the Treatment of Melasma. J. Cutan. Med. Surg. 2009, 13, 74–81. [Google Scholar] [CrossRef] [PubMed]
- May, J.M.; Harrison, F.E. Role of Vitamin C in the Function of the Vascular Endothelium. Antioxid. Redox. Sign. 2013, 19, 2068–2083. [Google Scholar] [CrossRef]
- Sardana, K.; Sarkar, R.; Sehgal, V.N. Pigmented purpuric dermatoses: An overview. Int. J. Dermatol. 2004, 43, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Jaros, A.; Zasada, M.; Budzisz, E.; Debowska, R.; Gębczyńska-Rzepka, M.; Rotsztejn, H. Evaluation of selected skin parameters following the application of 5% vitamin C concentrate. J. Cosmet. Dermatol. 2018, 18, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.J.; Hsieh, S.N.; Ekanayake-Mudiyanselage, S. Vitamin E: Critical Review of Its Current Use in Cosmetic and Clinical Dermatology. Dermatol. Surg. 2006, 31, 805–813. [Google Scholar] [CrossRef]
- Ekanayake-Mudiyanselage, S.; Tavakkol, A.; Polefka, T.; Nabi, Z.; Elsner, P.; Thiele, J. Vitamin E Delivery to Human Skin by a Rinse-Off Product: Penetration of?–Tocopherol versus Wash-Out Effects of Skin Surface Lipids. Skin Pharmacol. Physiol. 2004, 18, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Rona, C.; Vailati, F.; Berardesca, E. The cosmetic treatment of wrinkles. J. Cosmet. Dermatol. 2004, 3, 26–34. [Google Scholar] [CrossRef]
- Hahn, H.J.; Jung, H.J.; Schrammek-Drusios, M.C.; Lee, S.N.; Kim, J.-H.; Bin Kwon, S.; An, I.-S.; An, S.; Ahn, K.J. Instrumental evaluation of anti-aging effects of cosmetic formulations containing palmitoyl peptides, Silybum marianum seed oil, vitamin E and other functional ingredients on aged human skin. Exp. Ther. Med. 2016, 12, 1171–1176. [Google Scholar] [CrossRef]

 , 30:20:50
, 30:20:50  , 50:30:20
, 50:30:20  ).
).
 , 30:20:50
, 30:20:50  , 50:30:20
, 50:30:20  ).
).

 vitamin E,
vitamin E,  CA,
CA,  CAC; **** p < 0.0001, *** p > 0.0001, ** p < 0.001, * p < 0.05.
CAC; **** p < 0.0001, *** p > 0.0001, ** p < 0.001, * p < 0.05.
 vitamin E,
vitamin E,  CA,
CA,  CAC; **** p < 0.0001, *** p > 0.0001, ** p < 0.001, * p < 0.05.
CAC; **** p < 0.0001, *** p > 0.0001, ** p < 0.001, * p < 0.05.




| Nanoemulsion System | DH [nm] | PdI | ξ [mV] |
|---|---|---|---|
| SF:TR:CA | 69.3 ± 1.4 | 0.084 ± 0.019 | −77.36 ± 1.61 |
| SF:TR:Vitamin C | 176.46 ± 0.50 | 0.108 ± 0.014 | −82.7 ± 1.9 |
| SF:TR:Vitamin E | 183.9 ± 7.64 | 0.328 ± 0.01 | −95.03 ± 5.11 |
| SF:TR:CAC | 89.18 ± 1.35 | 0.371 ± 0.02 | −43.57 ± 7.10 |
| Samples | Nanoemulsion with Vit C | Nanoemulsion with Vit E | ||||
|---|---|---|---|---|---|---|
| 0 day | 14 day | 28 day | 0 day | 14 day | 28 day | |
| Wrinkle size (mm) | ||||||
| 1 | 0.089 | 0.081 | 0.070 | 0.101 | 0.081 | 0.080 |
| 2 | 0.107 | 0.079 | 0.076 | 0.100 | 0.078 | 0.073 |
| 3 | 0.106 | 0.087 | 0.070 | 0.120 | 0.087 | 0.079 |
| Vascular lesions (mm2) | ||||||
| 1 | 1.40 | 0.97 | 0.21 | 3.80 | 2.21 | 2.18 |
| 2 | 1.53 | 0.55 | 0.41 | 2.10 | 0.65 | 0.60 |
| 3 | 1.72 | 0.66 | 0.31 | 3.42 | 2.69 | 1.67 |
| Discoloration (%) | ||||||
| 1 | 14.10 | 11.31 | 10.10 | 11.6 | 10.1 | normal |
| 2 | 12.89 | 10.70 | normal | 10.47 | 10.00 | normal |
| 3 | 11.72 | 10.15 | 10.42 | 12.16 | 11.25 | 10.20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewińska, A.; Domżał-Kędzia, M.; Jaromin, A.; Łukaszewicz, M. Nanoemulsion Stabilized by Safe Surfactin from Bacillus subtilis as a Multifunctional, Custom-Designed Smart Delivery System. Pharmaceutics 2020, 12, 953. https://doi.org/10.3390/pharmaceutics12100953
Lewińska A, Domżał-Kędzia M, Jaromin A, Łukaszewicz M. Nanoemulsion Stabilized by Safe Surfactin from Bacillus subtilis as a Multifunctional, Custom-Designed Smart Delivery System. Pharmaceutics. 2020; 12(10):953. https://doi.org/10.3390/pharmaceutics12100953
Chicago/Turabian StyleLewińska, Agnieszka, Marta Domżał-Kędzia, Anna Jaromin, and Marcin Łukaszewicz. 2020. "Nanoemulsion Stabilized by Safe Surfactin from Bacillus subtilis as a Multifunctional, Custom-Designed Smart Delivery System" Pharmaceutics 12, no. 10: 953. https://doi.org/10.3390/pharmaceutics12100953
APA StyleLewińska, A., Domżał-Kędzia, M., Jaromin, A., & Łukaszewicz, M. (2020). Nanoemulsion Stabilized by Safe Surfactin from Bacillus subtilis as a Multifunctional, Custom-Designed Smart Delivery System. Pharmaceutics, 12(10), 953. https://doi.org/10.3390/pharmaceutics12100953