Electro-Hydrodynamic Drop-on-Demand Printing of Aqueous Suspensions of Drug Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
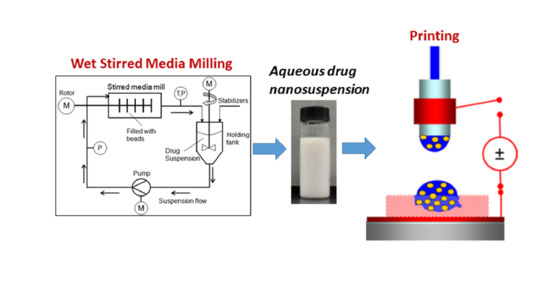
2.2. Preparation of Suspensions
2.3. Characterization of Suspensions
2.4. Preparation of Porous HPMC Films
2.5. EHD DOD Printing
2.6. Characterization of HPMC Film with Printed Suspension Droplets
2.6.1. Differential Scanning Calorimetry (DSC)
2.6.2. X-Ray Diffractometry (XRD)
2.6.3. Measurement of Griseofulvin Content
2.6.4. Thermo-Gravimetric Analysis (TGA)
2.6.5. Dissolution Tests
3. Results and Discussion
3.1. Processing Characteristics
3.2. Drug Release Profile
3.3. Drug Content and Its Uniformity
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Katsanis, S.H.; Javitt, G.; Hudson, K. A case study of personalized medicine. Science 2008, 320, 53–54. [Google Scholar] [CrossRef] [PubMed]
- Govender, R.; Abrahmsén-Alami, S.; Larsson, A.; Folestad, S. Therapy for the individual: Towards patient integration into the manufacturing and provision of pharmaceuticals. Eur. J. Pharm. Biopharm. 2020, 149, 58–76. [Google Scholar] [CrossRef] [PubMed]
- Personalized Medicine Coalition. Personalized Medicine at FDA: The Scope & Significance of Progress in 2019. Available online: http://www.personalizedmedicinecoalition.org/Resources/Personalized_Medicine_at_FDA_An_Annual_Research_Report (accessed on 6 September 2020).
- Norman, J.; Madurawe, R.D.; Moor, C.M.V.; Khan, M.A.; Khairuzzaman, A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv. Drug Deliv. Rev. 2017, 108, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Trenfield, S.J.; Awad, A.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D printing pharmaceuticals: Drug development to frontline care. Trends Pharmacol. Sci. 2018, 39, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Vo, A.Q.; Feng, X.; Bandari, S.; Repka, M.A. Pharmaceutical additive manufacturing: A novel tool for complex and personalized drug delivery systems. AAPS PharmSciTech 2018, 19, 3388–3402. [Google Scholar] [CrossRef] [PubMed]
- Lind, J.; Kälvemark Sporrong, S.; Kaae, S.; Rantanen, J.; Genina, N. Social aspects in additive manufacturing of pharmaceutical products. Expert Opin. Drug Deliv. 2017, 14, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Siiskonen, M.; Malmqvist, J.; Folestad, S. Integrated product and manufacturing system platforms supporting the design of personalized medicines. J. Manuf. Syst. 2020, 56, 281–295. [Google Scholar] [CrossRef]
- Aprecia Pharmaceuticals, LLC. ZipDose® Technology. Available online: https://www.aprecia.com/technology/zipdose (accessed on 6 September 2020).
- Boyd, B.J.; Bergström, C.A.S.; Vinarov, Z.; Kuentz, M.; Brouwers, J.; Augustijns, P.; Brandl, M.; Bernkop-Schnürch, A.; Shrestha, N.; Préat, V.; et al. Successful oral delivery of poorly water-soluble drugs both depends on the intraluminal behavior of drugs and of appropriate advanced drug delivery systems. Eur. J. Pharm. Sci. 2019, 137, 104967. [Google Scholar] [CrossRef]
- Sandler, N.; Määttänen, A.; Ihalainen, P.; Kronberg, L.; Meierjohann, A.; Viitala, T.; Peltonen, J. Inkjet printing of drug substances and use of porous substrates-towards individualized dosing. J. Pharm. Sci. 2011, 100, 3386–3395. [Google Scholar] [CrossRef]
- Iftimi, L.-D.; Edinger, M.; Bar-Shalom, D.; Rantanen, J.; Genina, N. Edible solid foams as porous substrates for inkjet-printable pharmaceuticals. Eur. J. Pharm. Biopharm. 2019, 136, 38–47. [Google Scholar] [CrossRef]
- Fastø, M.M.; Genina, N.; Susanne Kaae, S.; Sporrong, S.K. Perceptions, preferences and acceptability of patient designed 3D printed medicine by polypharmacy patients: A pilot study. Int. J. Clin. Pharm. 2019, 41, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Wen-Kai Hsiao, W.-K.; Lorber, B.; Reitsamer, H.; Khinast, J. 3D printing of oral drugs: A new reality or hype? Expert Opin. Drug Deliv. 2018, 15, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.H.; Kathuria, H.; Justin Jia Yao Tan, J.J.Y.; Kang, L. 3D printed drug delivery and testing systems—A passing fad or the future? Adv. Drug Deliv. Rev. 2018, 132, 139–168. [Google Scholar] [CrossRef]
- Kipphan, H. Handbook of Print Media: Technologies and Production Methods; Springer: Berlin/Heidelberg, Germany, 2001; pp. 1–202. [Google Scholar]
- Hutchings, I.M.; Martin, G.D. Fundamentals of inkjet technology. In Book Inkjet Technology for Digital Fabrication; Hutchings, I.M., Martin, G.D., Eds.; Joh Wiley & Sons: Chichester, UK, 2013; pp. 21–44. [Google Scholar]
- Elele, E.; Shen, Y.; Khusid, B. Electrodeless electro-hydrodynamic printing of personalized medicines. Appl. Phys. Lett. 2010, 97, 233501. [Google Scholar] [CrossRef]
- Elele, E.; Shen, Y.; Susarla, R.; Khusid, B.; Keyvan, G.; Michniak-Kohn, B. Electrodeless electro-hydrodynamic drop-on-demand encapsulation of drugs into porous polymer films for fabrication of personalized dosage units. J. Pharm. Sci. 2012, 101, 2523–2533. [Google Scholar] [CrossRef] [PubMed]
- Anand, O.; Yu, L.X.; Conner, D.P.; Davit, B.M. Dissolution testing for generic drugs: An FDA perspective. Am. Assoc. Pharm. Sci. J. 2011, 13, 328–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesisoglou, F.; Panmai, S.; Wu, Y. Nanosizing—Oral formulation development and biopharmaceutical evaluation. Adv. Drug Deliv. Rev. 2007, 59, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Junghanns, J.-U.A.; Müller, R.H. Nanocrystal technology, drug delivery and clinical applications. Int. J. Nanomed. 2008, 3, 295–309. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Azad, M.; Davé, R.; Bilgili, E. Nanomilling of drugs for bioavailability enhancement: A holistic formulation–process perspective. Pharmaceutics 2016, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Bhakay, A.; Rahman, M.; Davé, R.N.; Bilgili, E. Bioavailability enhancement of poorly-water soluble drugs via nanocomposites: Formulation–processing aspects and challenges. Pharmaceutics 2018, 10, 86. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Shao, J.; Fu, Q.; Li, J.; Sun, J.; He, Z. Spray-dried nanocrystals for a highly hydrophobic drug: Increased drug loading, enhanced redispersity, and improved oral bioavailability. Int. J. Pharm. 2017, 516, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Bhakay, A.; Azad, M.; Bilgili, E.; Dave, R. Redispersible fast dissolving nanocomposite microparticles of poorly water-soluble drugs. Int. J. Pharm. 2014, 461, 367–379. [Google Scholar] [CrossRef]
- Li, M.; Lopez, N.; Bilgili, E. A study of the impact of polymer–surfactant in drug nanoparticle coated pharmatose composites on dissolution performance. Adv. Powder Technol. 2016, 27, 1625–1636. [Google Scholar] [CrossRef] [Green Version]
- Möschwitzer, J.; Müller, R.H. Spray coated pellets as carrier system for mucoadhesive drug nanocrystals. Eur. J. Pharm. Biopharm. 2006, 62, 282–287. [Google Scholar] [CrossRef]
- Chung, N.-O.; Lee, M.K.; Lee, J. Mechanism of freeze-drying drug nanosuspensions. Int. J. Pharm. 2012, 437, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Beirowski, J.; Inghelbrecht, S.; Arien, A.; Gieseler, H. Freeze-drying of nanosuspensions, part 3: Investigation of factors compromising storage stability of highly concentrated drug nanosuspensions. J. Pharm. Sci. 2012, 101, 354–362. [Google Scholar] [CrossRef]
- Rao, V.M.; Lin, M.; Larive, C.K.; Southard, M.Z. A mechanistic study of griseofulvin dissolution into surfactant solutions under laminar flow conditions. J. Pharm. Sci. 1997, 86, 1132–1137. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Bhagwant, D.; Rege, B.D.; Amidon, G.L.; Poll, J.E. Surfactant-mediated dissolution: Contributions of solubility enhancement and relatively low micelle diffusivity. J. Pharm. Sci. 2004, 93, 2064–2075. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.; Liversidge, G.G.; Cooper, E.R. Nanosizing: A formulation approach for poorly-water soluble compounds. Eur. J. Pharm. Sci. 2003, 18, 113–120. [Google Scholar] [CrossRef]
- Shegokar, R.; Muller, R.H. Nanocrystals: Industrially feasible multifunctional formulation technology for poorly soluble actives. Int. J. Pharm. 2010, 399, 129–139. [Google Scholar] [CrossRef]
- Bhakay, A.; Merwade, M.; Bilgili, E.; Dave, R.N. Novel aspects of wet milling for the production of microsuspensions and nanosuspensions of poorly water-soluble drugs. Drug Dev. Ind. Pharm. 2011, 37, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Bilgili, E.; Afolabi, A. A combined microhydrodynamics–polymer adsorption analysis for elucidation of the roles of stabilizers in wet stirred media milling. Int. J. Pharm. 2012, 439, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.; Afolabi, A.; Bilgili, E. Continuous production of drug nanoparticle suspensions via wet stirred media milling: A fresh look at the Rehbinder effect. Drug Dev. Ind. Pharm. 2013, 39, 266–283. [Google Scholar] [CrossRef]
- Afolabi, A.; Akinlabi, O.; Bilgili, E. Impact of process parameters on the breakage kinetics of poorly water-soluble drugs during wet stirred media milling: A microhydrodynamic view. Eur. J. Pharm. Sci. 2014, 51, 75–86. [Google Scholar] [CrossRef]
- Dow Chemical Company. METHOCEL Cellulose Ethers. Technical Handbook; Dow Chemical Company: Midland, MI, USA, 2002; pp. 1–30. Available online: https://cdn.chempoint.com/ic/getattachment/2d8a4ff1-a232-46b7-b47d-956f2753cb3b/attachment.aspx (accessed on 6 September 2020).
- Heng, D.; Cutler, D.; Chan, H.; Yun, J.; Raper, J. What is a suitable dissolution method for drug nanoparticles? Pharm. Res. 2008, 25, 1696–1701. [Google Scholar] [CrossRef]
- Bhardwaj, U.; Burgess, D.J. A novel USP apparatus 4 based release testing method for dispersed systems. Int. J. Pharm. 2010, 388, 287–294. [Google Scholar] [CrossRef]
- Sievens-Figueroa, L.; Pandya, N.; Bhakay, A.; Keyvan, G.; Michniak-Kohn, B.; Bilgili, E.; Davé, R. Using USP I and USP IV for discriminating dissolution rates of nano- and microparticle-loaded pharmaceutical strip-films. AAPS PharmSciTech 2012, 13, 1473–1482. [Google Scholar] [CrossRef] [Green Version]
- Moore, J.W.; Flanner, H.H. Mathematical comparison of curves with an emphasis on in vitro dissolution profiles. Pharm. Tech. 1996, 20, 64–74. Available online: https://www.worldcat.org/title/mathematical-comparison-of-dissolution-profiles/oclc/92120469 (accessed on 20 October 2020).
- Costa, P. An alternative method to the evaluation of similarity factor in dissolution testing. Int. J. Pharm. 2001, 220, 77–83. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2001, 48, 139–157. [Google Scholar] [CrossRef]
- Lu, Z.; Alfano, J.; Race, D.; Dave, R.N. Zero-order release of poorly water-soluble drug from polymeric films made via aqueous slurry casting. Eur. J. Pharm. Sci. 2018, 117, 245–254. [Google Scholar] [CrossRef]









| Property | 20% Griseofulvin, 1.5% HPMC, 0.5% SDS | 20% Griseofulvin, 2.5% HPMC, 0.5% SDS |
|---|---|---|
| Density (g/cm3) | 1.11 ± 0.02 | 1.12 ± 0.01 |
| Shear viscosity (mPa·s) | 32.0 ± 2.0 | 60.0 ± 4.0 |
| Surface tension (mN/m) | 37.0 ± 2.0 | 39.0 ± 1.0 |
| Particle size, D50 (nm) | 162.0 ± 3.0 | 155.0 ± 5.0 |
| Electrical conductivity (µS/cm) | 700.0 ± 5.0 | 690.0 ± 5.0 |
| Dielectric constant | 83.0 ± 2.0 | 82.0 ± 3.0 |
| Capillary time ** (ms) | 1.3 | 1.3 |
| Ohnesorge number ** | 0.3 | 0.5 |
| Bond number ** | 0.5 | 0.5 |
| Pulse impulse ** | 2.8 | 2.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elele, E.; Shen, Y.; Boppana, R.; Afolabi, A.; Bilgili, E.; Khusid, B. Electro-Hydrodynamic Drop-on-Demand Printing of Aqueous Suspensions of Drug Nanoparticles. Pharmaceutics 2020, 12, 1034. https://doi.org/10.3390/pharmaceutics12111034
Elele E, Shen Y, Boppana R, Afolabi A, Bilgili E, Khusid B. Electro-Hydrodynamic Drop-on-Demand Printing of Aqueous Suspensions of Drug Nanoparticles. Pharmaceutics. 2020; 12(11):1034. https://doi.org/10.3390/pharmaceutics12111034
Chicago/Turabian StyleElele, Ezinwa, Yueyang Shen, Rajyalakshmi Boppana, Afolawemi Afolabi, Ecevit Bilgili, and Boris Khusid. 2020. "Electro-Hydrodynamic Drop-on-Demand Printing of Aqueous Suspensions of Drug Nanoparticles" Pharmaceutics 12, no. 11: 1034. https://doi.org/10.3390/pharmaceutics12111034
APA StyleElele, E., Shen, Y., Boppana, R., Afolabi, A., Bilgili, E., & Khusid, B. (2020). Electro-Hydrodynamic Drop-on-Demand Printing of Aqueous Suspensions of Drug Nanoparticles. Pharmaceutics, 12(11), 1034. https://doi.org/10.3390/pharmaceutics12111034