Latest Evidence Regarding the Effects of Photosensitive Drugs on the Skin: Pathogenetic Mechanisms and Clinical Manifestations
Abstract
:1. Introduction
2. Pathogenesis
2.1. Phototoxicity
2.2. Photoallergy
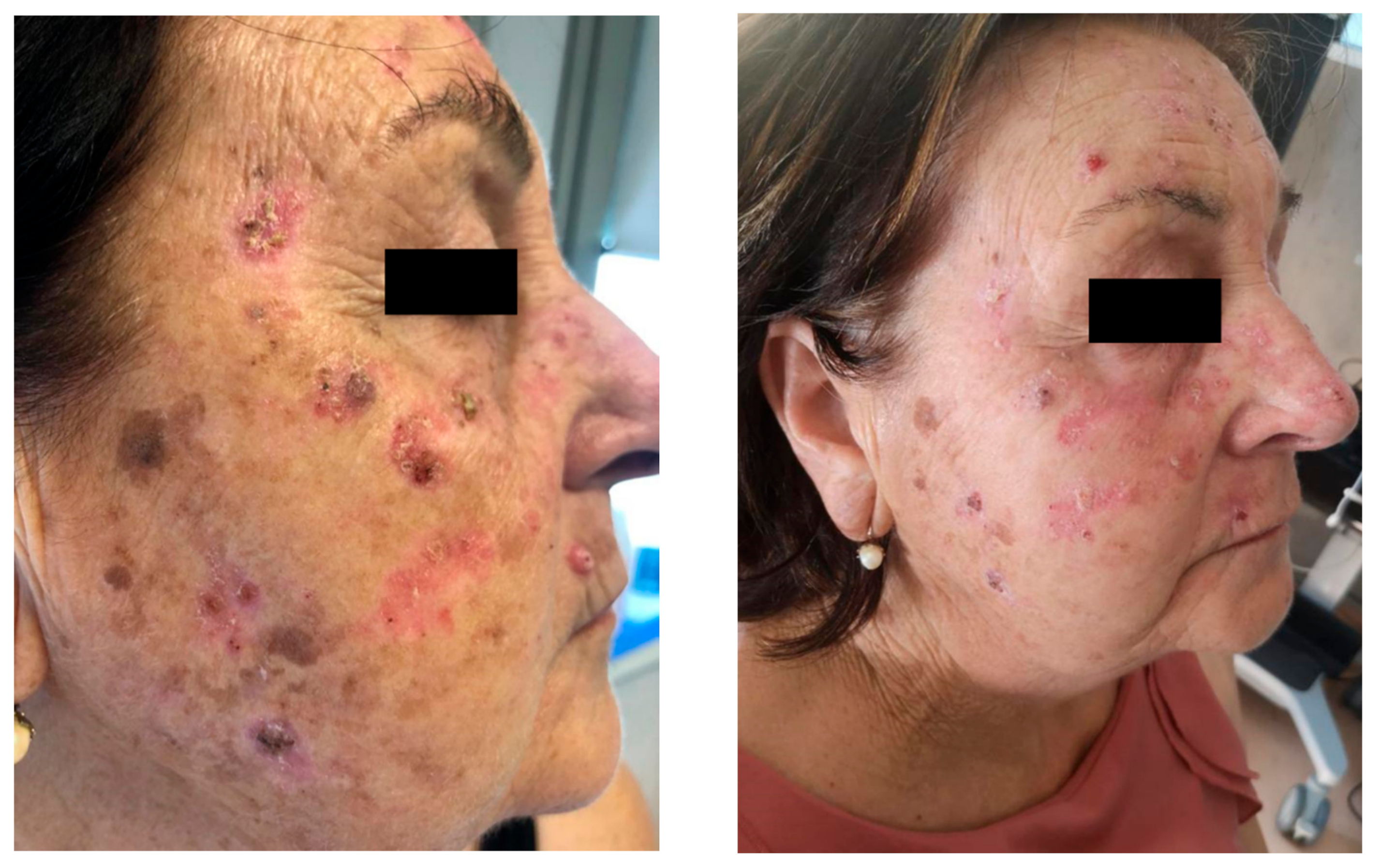
3. Clinical Manifestations and Diagnostic Approach
4. Prediction and Evaluation of Drug-Induced Photosensitivity
5. Photosensitive Drugs
5.1. Systemic Drugs
5.1.1. Antimicrobials
5.1.2. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
5.1.3. Anti-Hypertensives
5.1.4. Anti-Arrhythmics
5.1.5. Chemotherapeutics
5.1.6. Psychotropic Drugs
5.1.7. Others
5.2. Topical Drugs and Cosmetics
6. Discussion
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Khandpur, S.; Porter, R.M.; Boulton, S.J.; Anstey, A. Drug-induced photosensitivity: New insights into pathomechanisms and clinical variation through basic and applied science. Br. J. Dermatol. 2017, 176, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Drucker, A.M.; Rosen, C.F. Drug-induced photosensitivity: Culprit drugs, management and prevention. Drug Saf. 2011, 34, 821–837. [Google Scholar] [CrossRef] [PubMed]
- Blakely, K.M.; Drucker, A.M.; Rosen, C.F. Drug-induced photosensitivity—An update: Culprit drugs, prevention and management. Drug Saf. 2019, 42, 827–847. [Google Scholar] [CrossRef] [PubMed]
- Hinton, A.N.; Goldminz, A.M. Feeling the burn: Phototoxicity and photoallergy. Dermatol. Clin. 2020, 38, 165–175. [Google Scholar] [CrossRef]
- Kutlubay, Z.; Sevim, A.; Engin, B.; Tuzun, Y. Photodermatoses, including phototoxic and photoallergic reactions (internal and external). Clin. Dermatol. 2014, 32, 73–79. [Google Scholar] [CrossRef]
- Wilm, A.; Berneburg, M. Photoallergy. J. Dtsch. Dermatol. Ges. 2015, 13, 7–12. [Google Scholar] [CrossRef]
- Addo, H.A.; Ferguson, J.; Frain-Bell, W. Thiazide-induced photosensitivity: A study of 33 subjects. Br. J. Dermatol. 1987, 116, 749–760. [Google Scholar] [CrossRef]
- Jensen, A.O.; Thomsen, H.F.; Engebjerg, M.C.; Olesen, A.B.; Sorensen, H.T.; Karagas, M.R. Use of photosensitising diuretics and risk of skin cancer: A population-based case-control study. Br. J. Cancer 2008, 99, 1522–1528. [Google Scholar] [CrossRef] [Green Version]
- Williams, K.; Mansh, M.; Chin-Hong, P.; Singer, J.; Arron, S.T. Voriconazole-associated cutaneous malignancy: A literature review on photocarcinogenesis in organ transplant recipients. Clin. Infect. Dis. 2014, 58, 997–1002. [Google Scholar] [CrossRef] [Green Version]
- Food and Drug Administration; HHS. International Conference on Harmonisation; S10 Photosafety Evaluation of Pharmaceuticals; guidance for industry; availability. Notice. Fed. Regist. 2015, 80, 4282–4283. [Google Scholar]
- Monteiro, A.F.; Rato, M.; Martins, C. Drug-induced photosensitivity: Photoallergic and phototoxic reactions. Clin. Dermatol. 2016, 34, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Andreu, I.; Mayorga, C.; Miranda, M.A. Generation of reactive intermediates in photoallergic dermatitis. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, P. Diagnostic approach to photodermatoses. J. Dtsch. Dermatol. Ges. 2006, 4, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Onoue, S.; Seto, Y.; Sato, H.; Nishida, H.; Hirota, M.; Ashikaga, T.; Api, A.M.; Basketter, D.; Tokura, Y. Chemical photoallergy: Photobiochemical mechanisms, classification, and risk assessments. J. Dermatol. Sci. 2017, 85, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Cimino, G.D.; Gamper, H.B.; Isaacs, S.T.; Hearst, J.E. Psoralens as photoactive probes of nucleic acid structure and function: Organic chemistry, photochemistry, and biochemistry. Ann. Rev. Biochem. 1985, 54, 1151–1193. [Google Scholar] [CrossRef]
- Vassileva, S.G.; Mateev, G.; Parish, L.C. Antimicrobial photosensitive reactions. Arch. Intern. Med. 1998, 158, 1993–2000. [Google Scholar] [CrossRef]
- Storck, H. Photoallergy and photosensitivity due to systemically administered drugs. Arch. Dermatol. 1965, 91, 469–482. [Google Scholar] [CrossRef]
- Moore, D.E. Drug-induced cutaneous photosensitivity: Incidence, mechanism, prevention and management. Drug Saf. 2002, 25, 345–372. [Google Scholar] [CrossRef]
- Inoue, K.; Hosoi, J.; Ideta, R.; Ohta, N.; Ifuku, O.; Tsuchiya, T. Stress augmented ultraviolet-irradiation-induced pigmentation. J. Investig. Dermatol. 2003, 121, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Deleo, V.A. Photocontact dermatitis. Dermatol. Ther. 2004, 17, 279–288. [Google Scholar] [CrossRef]
- Lane, P.R.; Massey, K.L.; Worobetz, L.J.; Jutras, M.N.; Hull, P.R. Acute hereditary coproporphyria induced by the androgenic/anabolic steroid methandrostenolone (Dianabol). J. Am. Acad. Dermatol. 1994, 30, 308–312. [Google Scholar] [CrossRef]
- Benavente, C.A.; Schnell, S.A.; Jacobson, E.L. Effects of niacin restriction on sirtuin and PARP responses to photodamage in human skin. PLoS ONE 2012, 7, e42276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, P.; Ferguson, J. Photodistributed nifedipine-induced facial telangiectasia. Br. J. Dermatol. 1993, 129, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Kerr, A.; Shareef, M.; Dawe, R.; Ferguson, J. Photopatch testing negative in systemic quinine phototoxicity. Photodermatol. Photoimmunol. Photomed. 2010, 26, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Onoue, S.; Yamauchi, Y.; Kojima, T.; Igarashi, N.; Tsuda, Y. Analytical studies on photochemical behavior of phototoxic substances; effect of detergent additives on singlet oxygen generation. Pharm. Res. 2008, 25, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Beani, J.C.; Gautron, R.; Amblard, P.; Bastrenta, F.; Harrouch, L.; Jardon, P.; Reymond, J.L. Screening for drug photosensitization activity by measuring the variations in oxygen consumption of Bacillus subtilis. Photo-Dermatol. 1985, 2, 101–106. [Google Scholar]
- Selvaag, E. Evaluation of phototoxic properties of oral antidiabetics and diuretics. Photohemolysis model as a screening method for recognizing potential photosensitizing drugs. Arzneimittelforschung 1997, 47, 1031–1034. [Google Scholar]
- Przybilla, B.; Schwab-Przybilla, U.; Ruzicka, T.; Ring, J. Phototoxicity of non-steroidal anti-inflammatory drugs demonstrated in vitro by a photo-basophil-histamine-release test. Photo-Dermatol. 1987, 4, 73–78. [Google Scholar]
- Lim, D.S.; Triscott, J. O’Brien’s actinic granuloma in association with prolonged doxycycline phototoxicity. Australas. J. Dermatol. 2003, 44, 67–70. [Google Scholar] [CrossRef]
- Susong, J.; Carrizales, S. Lichenoid photosensitivity: An unusual reaction to doxycycline and an unusual response. Cutis 2014, 93, E1–E2. [Google Scholar]
- Gventer, M.; Brunetti, V.A. Photo-onycholysis secondary to tetracycline. A case report. J. Am. Podiatr. Med. Assoc. 1985, 75, 658–660. [Google Scholar] [CrossRef] [PubMed]
- Badri, T.; Ben Tekaya, N.; Cherif, F.; Ben Osman Dhahri, A. Photo-onycholysis: Two cases induced by doxycycline. Acta Dermatovenerol. Alp Pannonica Adriat 2004, 13, 135–136. [Google Scholar] [PubMed]
- Rabar, D.; Combemale, P.; Peyron, F. Doxycycline-induced photo-onycholysis. J. Travel Med. 2004, 11, 386–387. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Q.; Drucker, A.M.; Cho, E.; Laden, F.; VoPham, T.; Li, S.; Weinstock, M.A.; Qureshi, A.A. Tetracycline use and risk of incident skin cancer: A prospective study. Br. J. Cancer 2018, 118, 294–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, J.P.; Gogliotti, R.D.; Domagala, J.M.; Gracheck, S.J.; Huband, M.D.; Sesnie, J.A.; Cohen, M.A.; Shapiro, M.A. The synthesis, structure-activity, and structure-side effect relationships of a series of 8-alkoxy- and 5-amino-8-alkoxyquinolone antibacterial agents. J. Med. Chem. 1995, 38, 4478–4487. [Google Scholar] [CrossRef]
- Hayashi, N.; Nakata, Y.; Yazaki, A. New findings on the structure-phototoxicity relationship and photostability of fluoroquinolones with various substituents at position 1. Antimicrob. Agents Chemother. 2004, 48, 799–803. [Google Scholar] [CrossRef] [Green Version]
- Borgia, F.; Vaccaro, M.; Guarneri, F.; Cannavo, S.P. Photodistributed telangiectasia following use of cefotaxime. Br. J. Dermatol. 2000, 143, 674–675. [Google Scholar] [CrossRef]
- Vinks, S.A.; Heijerman, H.G.; de Jonge, P.; Bakker, W. Photosensitivity due to ambulatory intravenous ceftazidime in cystic fibrosis patient. Lancet 1993, 341, 1221–1222. [Google Scholar] [CrossRef]
- De, D.; Dogra, S.; Kaur, I. Dapsone induced acute photosensitivity dermatitis; a case report and review of literature. Lepr. Rev. 2007, 78, 401–404. [Google Scholar] [CrossRef]
- Chandler, M.J. Recurrence of phototoxic skin eruption due to trimethoprim. J. Infect. Dis. 1986, 153, 1001. [Google Scholar] [CrossRef]
- Lee, A.Y.; Jung, S.Y. Two patients with isoniazid-induced photosensitive lichenoid eruptions confirmed by photopatch test. Photodermatol. Photoimmunol. Photomed. 1998, 14, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Bihari, S.; Prakash, S. Pyrazinamide-induced phototoxicity: A case report and review of literature. Indian J. Dermatol. 2010, 55, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.B.; Shelley, A.J.; Novice, K.; Joo, J.; Lim, H.W.; Glassman, S.J. Drug-induced phototoxicity: A systematic review. J. Am. Acad. Dermatol. 2018, 79, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Kolaitis, N.A.; Duffy, E.; Zhang, A.; Lo, M.; Barba, D.T.; Chen, M.; Soriano, T.; Hu, J.; Nabili, V.; Saggar, R.; et al. Voriconazole increases the risk for cutaneous squamous cell carcinoma after lung transplantation. Transpl. Int. 2017, 30, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.D.; Cowen, E.W.; Nguyen, J.C.; McCalmont, T.H.; Fox, L.P. Melanoma associated with long-term voriconazole therapy: A new manifestation of chronic photosensitivity. Arch. Dermatol. 2010, 146, 300–304. [Google Scholar] [CrossRef] [Green Version]
- Haylett, A.K.; Felton, S.; Denning, D.W.; Rhodes, L.E. Voriconazole-induced photosensitivity: Photobiological assessment of a case series of 12 patients. Br. J. Dermatol. 2013, 168, 179–185. [Google Scholar] [CrossRef]
- Gould, J.W.; Mercurio, M.G.; Elmets, C.A. Cutaneous photosensitivity diseases induced by exogenous agents. J. Am. Acad. Dermatol. 1995, 33, 551–573; quiz 574–556. [Google Scholar] [CrossRef]
- Ferguson, J.; Addo, H.A.; Johnson, B.E.; Frain-Bell, W. Quinine induced photosensitivity: Clinical and experimental studies. Br. J. Dermatol. 1987, 117, 631–640. [Google Scholar] [CrossRef]
- Ljunggren, B.; Hindsen, M.; Isaksson, M. Systemic quinine photosensitivity with photoepicutaneous cross-reactivity to quinidine. Contact Dermat. 1992, 26, 1–4. [Google Scholar] [CrossRef]
- van Weelden, H.; Bolling, H.H.; Baart de la Faille, H.; van der Leun, J.C. Photosensitivity caused by chloroquine. Arch. Dermatol. 1982, 118, 290. [Google Scholar] [CrossRef]
- Lisi, P.; Assalve, D.; Hansel, K. Phototoxic and photoallergic dermatitis caused by hydroxychloroquine. Contact Dermat. 2004, 50, 255–256. [Google Scholar] [CrossRef] [PubMed]
- Amelot, A.; Dupouy-Camet, J.; Jeanmougin, M. Phototoxic reaction associated with Malarone (atovaquone/proguanil) antimalarial prophylaxis. J. Dermatol. 2014, 41, 346–348. [Google Scholar] [CrossRef] [PubMed]
- Furue, M. Photosensitive drug eruption induced by efavirenz in a patient with HIV infection. Intern. Med. 2004, 43, 533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, R.; Vasudevan, B.; Shankar, S.; Pragasam, V.; Suwal, B.; Venugopal, R. First reported case of tenofovir-induced photoallergic reaction. Indian J. Pharmacol. 2012, 44, 651–653. [Google Scholar] [CrossRef]
- Hoosen, K.; Mosam, A.; Dlova, N.C.; Grayson, W. An update on adverse cutaneous drug reactions in HIV/AIDS. Dermatopathology 2019, 6, 111–125. [Google Scholar] [CrossRef]
- Koch, K. Photosensitive disorders in HIV. South Afr. J. HIV Med. 2017, 18, 676. [Google Scholar] [CrossRef]
- Bosca, F.; Miranda, M.A.; Vano, L.; Vargas, F. New photodegradation pathways for naproxen, a phototoxic nonsteroidal antiinflammatory drug. J. Photoch. Photobio. A 1990, 54, 131–134. [Google Scholar] [CrossRef]
- Costanzo, L.L.; De Guidi, G.; Condorelli, G.; Cambria, A.; Fama, M. Molecular mechanism of drug photosensitization—II. Photohemolysis sensitized by ketoprofen. Photochem. Photobiol. 1989, 50, 359–365. [Google Scholar] [CrossRef]
- LaDuca, J.R.; Bouman, P.H.; Gaspari, A.A. Nonsteroidal antiinflammatory drug-induced pseudoporphyria: A case series. J. Cutan. Med. Surg. 2002, 6, 320–326. [Google Scholar] [CrossRef]
- Al-Khenaizan, S.; Schechter, J.F.; Sasseville, D. Pseudoporphyria induced by propionic acid derivatives. J. Cutan. Med. Surg. 1999, 3, 162–166. [Google Scholar] [CrossRef] [Green Version]
- Yazici, A.C.; Baz, K.; Ikizoglu, G.; Kokturk, A.; Uzumlu, H.; Tataroglu, C. Celecoxib-induced photoallergic drug eruption. Int. J. Dermatol. 2004, 43, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Bernal, S.; Alvarez-Perez, A.; Rodriguez-Pazos, L.; Gutierrez-Gonzalez, E.; Rodriguez-Granados, M.T.; Toribio, J. Photosensitivity due to thiazides. Actas Dermosifiliogr. 2014, 105, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Johnston, G.A. Thiazide-induced lichenoid photosensitivity. Clin. Exp. Dermatol. 2002, 27, 670–672. [Google Scholar] [CrossRef] [PubMed]
- Robinson, H.N.; Morison, W.L.; Hood, A.F. Thiazide diuretic therapy and chronic photosensitivity. Arch. Dermatol. 1985, 121, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, A.; Herrmann, J. Hydrochlorothiazide-induced photosensitivity in a psoriasis patient following exposure to narrow-band ultraviolet B excimer therapy. Photodermatol. Photoimmunol. Photomed. 2019, 35, 369–371. [Google Scholar] [CrossRef]
- Burry, J.N.; Lawrence, J.R. Phototoxic blisters from high frusemide dosage. Br. J. Dermatol. 1976, 94, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Takeichi, S.; Kubo, Y.; Arase, S.; Hashimoto, T.; Ansai, S. Brunsting-Perry type localized bullous pemphigoid, possibly induced by furosemide administration and sun exposure. Eur. J. Dermatol. 2009, 19, 500–503. [Google Scholar] [CrossRef]
- Rodriguez Granados, M.T.; Abalde, T.; Garcia Doval, I.; De la Torre, C. Systemic photosensitivity to quinapril. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 389–390. [Google Scholar] [CrossRef]
- Wagner, S.N.; Welke, F.; Goos, M. Occupational UVA-induced allergic photodermatitis in a welder due to hydrochlorothiazide and ramipril. Contact Dermat. 2000, 43, 245–246. [Google Scholar]
- Viola, E.; Coggiola Pittoni, A.; Drahos, A.; Moretti, U.; Conforti, A. Photosensitivity with angiotensin II receptor blockers: A retrospective study using data from VigiBase®. Drug Saf. 2015, 38, 889–894. [Google Scholar] [CrossRef]
- Bakkour, W.; Haylett, A.K.; Gibbs, N.K.; Chalmers, R.J.; Rhodes, L.E. Photodistributed telangiectasia induced by calcium channel blockers: Case report and review of the literature. Photodermatol. Photoimmunol. Photomed. 2013, 29, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Boyer, M.; Katta, R.; Markus, R. Diltiazem-induced photodistributed hyperpigmentation. Dermatol. Online J. 2003, 9, 10. [Google Scholar] [PubMed]
- Scherschun, L.; Lee, M.W.; Lim, H.W. Diltiazem-associated photodistributed hyperpigmentation: A review of 4 cases. Arch. Dermatol. 2001, 137, 179–182. [Google Scholar] [PubMed]
- Garrido, P.M.; Borges-Costa, J. Hydrochlorothiazide treatment and risk of non-melanoma skin cancer: Review of the literature. Rev. Port. Cardiol. 2020, 39, 163–170. [Google Scholar] [CrossRef] [PubMed]
- MHRA drug safety update: Hydrochlorothiazide and non-melanoma skin cancer. Drug Ther. Bull. 2019, 57, 70. [CrossRef]
- Mazzilli, S.; Garofalo, V.; Ventura, A.; Diluvio, L.; Milani, M.; Bianchi, L.; Campione, E. Effects of topical 0.8% piroxicam and 50+ sunscreen filters on actinic keratosis in hypertensive patients treated with or without photosensitizing diuretic drugs: An observational cohort study. Clin. Cosmet. Investig. Dermatol. 2018, 11, 485–490. [Google Scholar] [CrossRef] [Green Version]
- Campione, E.; Di Prete, M.; Diluvio, L.; Bianchi, L.; Orlandi, A. Efficacy of ingenol mebutate gel for actinic keratosis in patients treated by thiazide diuretics. Clin. Cosmet. Investig. Dermatol. 2016, 9, 405–409. [Google Scholar] [CrossRef] [Green Version]
- Gandini, S.; Palli, D.; Spadola, G.; Bendinelli, B.; Cocorocchio, E.; Stanganelli, I.; Miligi, L.; Masala, G.; Caini, S. Anti-hypertensive drugs and skin cancer risk: A review of the literature and meta-analysis. Crit. Rev. Oncol. Hematol. 2018, 122, 1–9. [Google Scholar] [CrossRef]
- Tatu, A.L.; Elisei, A.M.; Chioncel, V.; Miulescu, M.; Nwabudike, L.C. Immunologic adverse reactions of beta-blockers and the skin. Exp. Ther. Med. 2019, 18, 955–959. [Google Scholar] [CrossRef]
- Alrashidi, A.; Rhodes, L.E.; Sharif, J.C.H.; Kreeshan, F.C.; Farrar, M.D.; Ahad, T. Systemic drug photosensitivity-Culprits, impact and investigation in 122 patients. Photodermatol. Photoimmunol. Photomed. 2020. [Google Scholar] [CrossRef]
- Bongard, V.; Marc, D.; Philippe, V.; Jean-Louis, M.; Maryse, L.M. Incidence rate of adverse drug reactions during long-term follow-up of patients newly treated with amiodarone. Am. J. Ther. 2006, 13, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.; McKenna, W.J.; Rowland, E.; Holt, D.W.; Storey, G.C.; Krikler, D.M. Side effects of long-term amiodarone therapy. Circulation 1983, 67, 45–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rappersberger, K.; Honigsmann, H.; Ortel, B.; Tanew, A.; Konrad, K.; Wolff, K. Photosensitivity and hyperpigmentation in amiodarone-treated patients: Incidence, time course, and recovery. J. Investig. Dermatol. 1989, 93, 201–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zachary, C.B.; Slater, D.N.; Holt, D.W.; Storey, G.C.; MacDonald, D.M. The pathogenesis of amiodarone-induced pigmentation and photosensitivity. Br. J. Dermatol. 1984, 110, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Ladizinski, B.; Elpern, D.J. Dronaderone-induced phototoxicity. J. Drugs Dermatol. 2013, 12, 946–947. [Google Scholar] [PubMed]
- Falkson, G.; Schulz, E.J. Skin changes in patients treated with 5-fluorouracil. Br. J. Dermatol. 1962, 74, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.R. Photodistributed erythema multiforme: Paclitaxel-related, photosensitive conditions in patients with cancer. J. Drugs Dermatol. 2009, 8, 61–64. [Google Scholar] [PubMed]
- Hussain, S.; Anderson, D.N.; Salvatti, M.E.; Adamson, B.; McManus, M.; Braverman, A.S. Onycholysis as a complication of systemic chemotherapy: Report of five cases associated with prolonged weekly paclitaxel therapy and review of the literature. Cancer 2000, 88, 2367–2371. [Google Scholar] [CrossRef]
- Beutler, B.D.; Cohen, P.R. Nab-paclitaxel-associated photosensitivity: Report in a woman with non-small cell lung cancer and review of taxane-related photodermatoses. Dermatol. Pract. Concept. 2015, 5, 121–124. [Google Scholar] [CrossRef]
- Lacouture, M.E.; Duvic, M.; Hauschild, A.; Prieto, V.G.; Robert, C.; Schadendorf, D.; Kim, C.C.; McCormack, C.J.; Myskowski, P.L.; Spleiss, O.; et al. Analysis of dermatologic events in vemurafenib-treated patients with melanoma. Oncologist 2013, 18, 314–322. [Google Scholar] [CrossRef] [Green Version]
- Gelot, P.; Dutartre, H.; Khammari, A.; Boisrobert, A.; Schmitt, C.; Deybach, J.C.; Nguyen, J.M.; Seite, S.; Dreno, B. Vemurafenib: An unusual UVA-induced photosensitivity. Exp. Dermatol. 2013, 22, 297–298. [Google Scholar] [CrossRef] [PubMed]
- Khanna, U.; Richardson, V.; Hexner, E.; Nguyen, C.V.; Elenitsas, R.; Rosenbach, M. A photo-distributed papulopustular eruption and multiple squamous cell carcinomas in a patient on ruxolitinib. JAAD Case Rep. 2019, 5, 895–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanamandra, U.; Sahu, K.K.; Malhotra, P.; Varma, S. Photodermatosis secondary to hydroxyurea. BMJ Case Rep. 2014, 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leon-Mateos, A.; Zulaica, A.; Caeiro, J.L.; Fabeiro, J.M.; Calvino, S.; Peteiro, C.; Toribio, J. Photo-induced granulomatous eruption by hydroxyurea. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 1428–1429. [Google Scholar] [CrossRef] [PubMed]
- Satanove, A.; McIntosh, J.S. Phototoxic reactions induced by high doses of chlorpromazine and thioridazine. JAMA 1967, 200, 209–212. [Google Scholar] [CrossRef]
- Rodriguez-Pazos, L.; Sanchez-Aguilar, D.; Rodriguez-Granados, M.T.; Pereiro-Ferreiros, M.M.; Toribio, J. Erythema multiforme photoinduced by statins. Photodermatol. Photoimmunol. Photomed. 2010, 26, 216–218. [Google Scholar] [CrossRef]
- Montanaro, S.; Lhiaubet-Vallet, V.; Iesce, M.I.; Previtera, L.; Miranda, M.A. A mechanistic study on the phototoxicity of atorvastatin: Singlet oxygen generation by a phenanthrene-like photoproduct. Chem. Res. Toxicol. 2009, 22, 173–178. [Google Scholar] [CrossRef]
- Tsai, K.C.; Yang, J.H.; Hung, S.J. Fenofibrate-induced photosensitivity—A case series and literature review. Photodermatol. Photoimmunol. Photomed. 2017, 33, 213–219. [Google Scholar] [CrossRef]
- Kastalli, S.; El Aidli, S.; Chaabane, A.; Amrani, R.; Daghfous, R.; Belkahia, C. Photosensitivity induced by metformin: A report of 3 cases. Tunis. Med. 2009, 87, 703–705. [Google Scholar]
- Dogra, S.; Kanwar, A.J. Clopidogrel bisulphate-induced photosensitive lichenoid eruption: First report. Br. J. Dermatol. 2003, 148, 609–610. [Google Scholar] [CrossRef]
- Bourezane, Y.; Girardin, P.; Aubin, F.; Vigan, M.; Adessi, B.; Humbert, P.; Laurent, R. Allergic contact dermatitis to Zovirax cream. Allergy 1996, 51, 755–756. [Google Scholar] [CrossRef] [PubMed]
- Horio, T. Photosensitivity reaction to dibucaine. Case report and experimental induction. Arch. Dermatol. 1979, 115, 986–987. [Google Scholar] [CrossRef] [PubMed]
- Rietschel, R.L. Photocontact dermatitis to hydrocortisone. Contact Dermat. 1978, 4, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Horio, T. Chlorpromazine photoallergy. Coexistence of immediate and delayed type. Arch. Dermatol. 1975, 111, 1469–1471. [Google Scholar] [CrossRef] [PubMed]
- Kaidbey, K.H.; Kligman, A.M. Clinical and histological study of coal tar phototoxicity in humans. Arch. Dermatol. 1977, 113, 592–595. [Google Scholar] [CrossRef]
- Bagheri, H.; Lhiaubet, V.; Montastruc, J.L.; Chouini-Lalanne, N. Photosensitivity to ketoprofen: Mechanisms and pharmacoepidemiological data. Drug Saf. 2000, 22, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Loh, T.Y.; Cohen, P.R. Ketoprofen-induced photoallergic dermatitis. Indian J. Med. Res. 2016, 144, 803–806. [Google Scholar] [CrossRef]
- Szczurko, C.; Dompmartin, A.; Michel, M.; Moreau, A.; Leroy, D. Photocontact allergy to oxybenzone: Ten years of experience. Photodermatol. Photoimmunol. Photomed. 1994, 10, 144–147. [Google Scholar]
- Kowalzick, L.; Ziegler, H. Photoallergic contact dermatitis from topical diclofenac in Solaraze gel. Contact Dermat. 2006, 54, 348–349. [Google Scholar] [CrossRef]
- Fernandez-Jorge, B.; Goday-Bujan, J.J.; Murga, M.; Molina, F.P.; Perez-Varela, L.; Fonseca, E. Photoallergic contact dermatitis due to diclofenac with cross-reaction to aceclofenac: Two case reports. Contact Dermat. 2009, 61, 236–237. [Google Scholar] [CrossRef]
- Canelas, M.M.; Cardoso, J.C.; Goncalo, M.; Figueiredo, A. Photoallergic contact dermatitis from benzydamine presenting mainly as lip dermatitis. Contact Dermat. 2010, 63, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Elgezua, O.L.; Gorrotxategi, P.E.; Garcia, J.G.; Nieto, J.A.R.; Perez, J.L.D. Photoallergic hand eczema due to benzydamine. Eur. J. Dermatol. 2004, 14, 69–70. [Google Scholar]
- Youn, J.I.; Lee, H.G.; Yeo, U.C.; Lee, Y.S. Piroxicam photosensitivity associated with vesicular hand dermatitis. Clin. Exp. Dermatol. 1993, 18, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Varela, P.; Amorim, I.; Massa, A.; Sanches, M.; Silva, E. Piroxicam-beta-cyclodextrin and photosensitivity reactions. Contact Dermat. 1998, 38, 229. [Google Scholar] [CrossRef] [PubMed]
- Dosik, J.; Ellman, H.; Stuart, I. Topical minocycline foam 4%: Results of four phase 1 studies evaluating the potential for phototoxicity, photoallergy, sensitization, and cumulative irritation. J. Immunotoxicol. 2019, 16, 133–139. [Google Scholar] [CrossRef]
- Onoue, S.; Suzuki, G.; Kato, M.; Hirota, M.; Nishida, H.; Kitagaki, M.; Kouzuki, H.; Yamada, S. Non-animal photosafety assessment approaches for cosmetics based on the photochemical and photobiochemical properties. Toxicol. In Vitro 2013, 27, 2316–2324. [Google Scholar] [CrossRef]
- Scheuer, E.; Warshaw, E. Sunscreen allergy: A review of epidemiology, clinical characteristics, and responsible allergens. Dermatitis 2006, 17, 3–11. [Google Scholar] [CrossRef]
- Honari, G. Photoallergy. Rev. Environ. Health 2014, 29, 233–242. [Google Scholar] [CrossRef]
- Schauder, S.; Ippen, H. Contact and photocontact sensitivity to sunscreens. Review of a 15-year experience and of the literature. Contact Dermat. 1997, 37, 221–232. [Google Scholar] [CrossRef]
- Santoro, F.A.; Lim, H.W. Update on photodermatoses. Semin. Cutan. Med. Surg. 2011, 30, 229–238. [Google Scholar] [CrossRef]
- Gordon-Thomson, C.; Gupta, R.; Tongkao-on, W.; Ryan, A.; Halliday, G.M.; Mason, R.S. 1alpha,25 dihydroxyvitamin D3 enhances cellular defences against UV-induced oxidative and other forms of DNA damage in skin. Photochem. Photobiol. Sci. 2012, 11, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Song, E.J.; Gordon-Thomson, C.; Cole, L.; Stern, H.; Halliday, G.M.; Damian, D.L.; Reeve, V.E.; Mason, R.S. 1alpha,25-Dihydroxyvitamin D3 reduces several types of UV-induced DNA damage and contributes to photoprotection. J. Steroid Biochem. Mol. Biol. 2013, 136, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Ann. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, F.F.; Zhang, F.F.; Lai, X.D.; Wang, L.J.; Yang, L.; Wang, X.; Singh, G.; Zhong, J.L. Nrf2-mediated protection against UVA radiation in human skin keratinocytes. Biosci. Trends 2011, 5, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Kalra, S.; Knatko, E.V.; Zhang, Y.; Honda, T.; Yamamoto, M.; Dinkova-Kostova, A.T. Highly potent activation of Nrf2 by topical tricyclic bis(cyano enone): Implications for protection against UV radiation during thiopurine therapy. Cancer Prev. Res. (Phila) 2012, 5, 973–981. [Google Scholar] [CrossRef] [Green Version]
- Johansson, E.K.; Krynitz, B.; Holmsten, M.; Klockhoff, K.; Isaksson Friman, E.; Xie, H. Severe photo toxicity recalled by docetaxel. Case Rep. Oncol. 2018, 11, 751–755. [Google Scholar] [CrossRef]
- Droitcourt, C.; Le Ho, H.; Adamski, H.; Le Gall, F.; Dupuy, A. Docetaxel-induced photo-recall phenomenon. Photodermatol. Photoimmunol. Photomed. 2012, 28, 222–223. [Google Scholar] [CrossRef]
- Camidge, D.R. Methotrexate-induced radiation recall. Am. J. Clin. Oncol. 2001, 24, 211–213. [Google Scholar] [CrossRef]
- Jibbe, A.; Tefft, K.; Weir, G.; Aires, D.; Liu, D. Photo recall reaction following the use of vancomycin. Dermatol. Online J. 2013, 19, 20396. [Google Scholar]
- Damian, D.L. Photoprotective effects of nicotinamide. Photochem. Photobiol. Sci. 2010, 9, 578–585. [Google Scholar] [CrossRef]
- Snaidr, V.A.; Damian, D.L.; Halliday, G.M. Nicotinamide for photoprotection and skin cancer chemoprevention: A review of efficacy and safety. Exp. Dermatol. 2019, 28 (Suppl. S1), 15–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damian, D.L. Nicotinamide for skin cancer chemoprevention. Australas. J. Dermatol. 2017, 58, 174–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristics | Phototoxicity | Photoallergy |
|---|---|---|
| Incidence | high | Low |
| Sensitization | + | − |
| Time of onset | Few min-h | >24 h |
| Most common clinical presentation | Exaggerate sunburn | Dermatitis |
| Dose dependency | + | +/− |
| Histologic features | cell necrosis and, neutrophilic and lymphocytic infiltration of derma | Epidermal spongiosis, vesiculation, exocytosis of lymphocytes into the epidermis, perivascular inflammatory infiltrates |
| PSD Group | Molecule |
|---|---|
| Antimicrobials | Tetracycline Doxycycline Minocycline Lymecycline Nalidix acid and fluorochinolones Cefotaxime Ceftazidime Dapsone Trimethoprim Isoniazid Pyrazinamide Voriconazole Quinine Quinidine Chloroquine Hydroxychloroquine Atovaquone/proguanil Efavirenz Tenofovir Tipranavir |
| NSAIDs | Naproxen Ibuprofen Ketoprofen Suprofen Benoxaprofen Tiaprofenic acid |
| Antihypertensives | Hydrochlorothiazide Furosemide Ramipril Quinapril Enalapril |
| Antihypertensives | Losartan Irbesartan Valsartan Amlodipine Nifedipine Diltiazem Amiloride Indapamide Tilisolol Bisoprolol Atenolol |
| Antiarrhythmics | Amiodarone Dronedarone |
| Chemotherapeutics | Fluorouracil Tegafur Capecitabine Dacarbazine Paclitaxel and nab-paclitaxel Vemurafenib Ruxolitinib Hydroxyurea |
| Psychotropic drugs | Chlorpromazine Thioridazine Tricyclic antidepressant |
| Others | Simvastatin, atorvastatin, fenofibrate, metformin, clopidogrel |
| Topical Drugs | Cosmetics |
|---|---|
| Acyclovir Dibucaine Hydrocortisone Chlorpromazine gel Furocoumarins (bergapten, 5- and 8-methoxypsoralen) Coal tar Ketoprofen Tiaprofenic acid Suprofen Oxybenzone Fenofibrate Diclofenac Aceclofenac Benzydamine Piroxicam | Furocoumarins (bergapten, 5- and 8-methoxypsoralen) 6-methylcoumarin Musk ambrette Hexachlorophene PABA derivatives Benzophenones Salicylates Dibenzoylmethane derivatives Anthranilates Mexoryl SX Triclosan Fenticlor |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozzi, F.; Di Raimondo, C.; Lanna, C.; Diluvio, L.; Mazzilli, S.; Garofalo, V.; Dika, E.; Dellambra, E.; Coniglione, F.; Bianchi, L.; et al. Latest Evidence Regarding the Effects of Photosensitive Drugs on the Skin: Pathogenetic Mechanisms and Clinical Manifestations. Pharmaceutics 2020, 12, 1104. https://doi.org/10.3390/pharmaceutics12111104
Lozzi F, Di Raimondo C, Lanna C, Diluvio L, Mazzilli S, Garofalo V, Dika E, Dellambra E, Coniglione F, Bianchi L, et al. Latest Evidence Regarding the Effects of Photosensitive Drugs on the Skin: Pathogenetic Mechanisms and Clinical Manifestations. Pharmaceutics. 2020; 12(11):1104. https://doi.org/10.3390/pharmaceutics12111104
Chicago/Turabian StyleLozzi, Flavia, Cosimo Di Raimondo, Caterina Lanna, Laura Diluvio, Sara Mazzilli, Virginia Garofalo, Emi Dika, Elena Dellambra, Filadelfo Coniglione, Luca Bianchi, and et al. 2020. "Latest Evidence Regarding the Effects of Photosensitive Drugs on the Skin: Pathogenetic Mechanisms and Clinical Manifestations" Pharmaceutics 12, no. 11: 1104. https://doi.org/10.3390/pharmaceutics12111104
APA StyleLozzi, F., Di Raimondo, C., Lanna, C., Diluvio, L., Mazzilli, S., Garofalo, V., Dika, E., Dellambra, E., Coniglione, F., Bianchi, L., & Campione, E. (2020). Latest Evidence Regarding the Effects of Photosensitive Drugs on the Skin: Pathogenetic Mechanisms and Clinical Manifestations. Pharmaceutics, 12(11), 1104. https://doi.org/10.3390/pharmaceutics12111104






