Self-Assembled Polyester Dendrimer/Cellulose Nanofibril Hydrogels with Extraordinary Antibacterial Activity
Abstract
:1. Introduction
2. Methods
2.1. Chemicals
2.2. Instruments
2.3. Dendrimer Synthesis
2.4. Preparation of TEMPO Oxidized Fibrils and Preparation of Colloidally Stable Dispersions
2.5. Preparation of the Hybrid Hydrogels
2.6. Characterization of Dendrimers and CNFs
2.6.1. NMR of the Dendrimers
2.6.2. MALDI-TOF of the Dendrimers
2.6.3. Total Charge Determination of the Fibers
2.6.4. FTIR of the Dendrimers and Hybrid Hydrogels
2.6.5. Polyelectrolyte Titration of Dendrimers and CNFs
2.6.6. Quartz Crystal Microbalance with Dissipation (QCM-D)
2.6.7. Nitrogen Analysis of the Hybrid Hydrogels
2.6.8. Leaching Analysis of the Hybrid Hydrogels
2.6.9. Rheology of the Hybrid Hydrogels
2.7. Antibacterial Study of the Cationic Dendrimers and the Hybrid Hydrogels
2.7.1. Minimum Inhibitory Concentration (MIC) and Minimum Microbicidal Concentration (MMC) Assays
2.7.2. Antibacterial Assays of the Hybrid Hydrogels
2.8. Cytotoxicity Assay
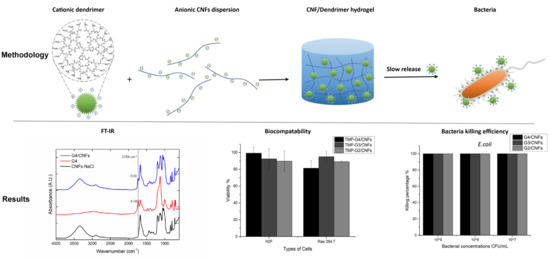
3. Results and Discussion
3.1. Synthesis and Characterization of Cationic Dendrimers and Carboxylated CNFs
3.2. Hybrid Hydrogel Formation and Characterization
3.3. Antibacterial Testing
3.3.1. MIC and MMC Assays of the Cationic Dendrimers
3.3.2. Antibacterial Property of the Hybrid Hydrogels
3.3.3. Biocompatibility of the Cationic Dendrimers and Hybrid Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hultman, J.; Rahkila, R.; Ali, J.; Rousu, J.; Bjorkroth, K.J. Meat Processing Plant Microbiome and Contamination Patterns of Cold-Tolerant Bacteria Causing Food Safety and Spoilage Risks in the Manufacture of Vacuum-Packaged Cooked Sausages. Appl. Environ. Microbiol. 2015, 81, 7088–7097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mugoyela, V.; Mwambete, K.D. Microbial contamination of nonsterile pharmaceuticals in public hospital settings. Ther. Clin. Risk Manag. 2010, 6, 443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fijan, S.; Šostar-Turk, S.; Cencič, A. Implementing hygiene monitoring systems in hospital laundries in order to reduce microbial contamination of hospital textiles. J. Hosp. Infect. 2005, 61, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Schierholz, J.M.; Beuth, J. Implant infections: A haven for opportunistic bacteria. J. Hosp. Infect. 2001, 49, 87–93. [Google Scholar] [CrossRef]
- Ashbolt, N.J. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology 2004, 198, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.; Carmeli, Y.; Falagas, M.; Giske, C.; Harbarth, S.; Hindler, J.; Kahlmeter, G.; Olsson-Liljequist, B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Arroll, B. Antibiotics for upper respiratory tract infections: An overview of Cochrane reviews. Respir. Med. 2005, 99, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Banin, E.; Hughes, D.; Kuipers, O.P. Editorial: Bacterial pathogens, antibiotics and antibiotic resistance. FEMS Microbiol. Rev. 2017, 41, 450–452. [Google Scholar] [CrossRef] [Green Version]
- Aminov, R.I. A brief history of the antibiotic era: Lessons learned and challenges for the future. Front. Microbiol. 2010, 1, 134. [Google Scholar] [CrossRef] [Green Version]
- Larsson, D.J. Antibiotics in the environment. Ups. J. Med. Sci. 2014, 119, 108–112. [Google Scholar] [CrossRef]
- Du, H.; Zha, G.; Gao, L.; Wang, H.; Li, X.; Shen, Z.; Zhu, W. Fully biodegradable antibacterial hydrogels via thiol–ene “click” chemistry. Polym. Chem. 2014, 5, 4002–4008. [Google Scholar] [CrossRef]
- Lichter, J.A.; Van Vliet, K.J.; Rubner, M.F. Design of antibacterial surfaces and interfaces: Polyelectrolyte multilayers as a multifunctional platform. Macromolecules 2009, 42, 8573–8586. [Google Scholar] [CrossRef]
- Bosman, D.A.; Janssen, H.; Meijer, E. About dendrimers: Structure, physical properties, and applications. Chem. Rev. 1999, 99, 1665–1688. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.V.; Malkoch, M. Simplifying the synthesis of dendrimers: Accelerated approaches. Chem. Soc. Rev. 2012, 41, 4593–4609. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Dufes, C.; Uchegbu, I.F.; Schätzlein, A.G. Dendrimers in gene delivery. Adv. Drug Deliv. Rev. 2005, 57, 2177–2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorain, B.; Tekade, M.; Kesharwani, P.; Iyer, A.K.; Kalia, K.; Tekade, R.K. The use of nanoscaffolds and dendrimers in tissue engineering. Drug Discov. Today 2017, 22, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; MacKay, J.A.; Frechet, J.M.; Szoka, F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005, 23, 1517–1526. [Google Scholar] [CrossRef]
- Soler, M.; Mesa-Antunez, P.; Estevez, M.-C.; Ruiz-Sanchez, A.J.; Otte, M.A.; Sepulveda, B.; Collado, D.; Mayorga, C.; Torres, M.J.; Perez-Inestrosa, E. Highly sensitive dendrimer-based nanoplasmonic biosensor for drug allergy diagnosis. Biosens. Bioelectron. 2015, 66, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Feng, G.; Djordjevic, I.; Mogal, V.; O’Rorke, R.; Pokholenko, O.; Steele, T.W. Elastic light tunable tissue adhesive dendrimers. Macromol. Biosci. 2016, 16, 1072–1082. [Google Scholar] [CrossRef]
- Esfand, R.; Tomalia, D.A. Poly (amidoamine)(PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar] [CrossRef]
- Araújo, R.V.D.; Santos, S.D.S.; Igne Ferreira, E.; Giarolla, J. New advances in general biomedical applications of PAMAM dendrimers. Molecules 2018, 23, 2849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, A.I.; Reins, R.Y.; McDermott, A.M.; Trautner, B.W.; Cai, C. Antibacterial activity and cytotoxicity of PEGylated poly (amidoamine) dendrimers. Mol. Biosyst. 2009, 5, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Klaykruayat, B.; Siralertmukul, K.; Srikulkit, K. Chemical modification of chitosan with cationic hyperbranched dendritic polyamidoamine and its antimicrobial activity on cotton fabric. Carbohydr. Polym. 2010, 80, 197–207. [Google Scholar] [CrossRef]
- Holmes, A.M.; Heylings, J.R.; Wan, K.-W.; Moss, G.P. Antimicrobial efficacy and mechanism of action of poly (amidoamine)(PAMAM) dendrimers against opportunistic pathogens. Int. J. Antimicrob. Agents. 2019, 53, 500–507. [Google Scholar] [CrossRef]
- Serri, A.; Mahboubi, A.; Zarghi, A.; Moghimi, H.R. PAMAM-dendrimer enhanced antibacterial effect of vancomycin hydrochloride against gram-negative bacteria. J. Pharm. Pharm. 2019, 22, 10–21. [Google Scholar] [CrossRef]
- Kolhatkar, R.B.; Kitchens, K.M.; Swaan, P.W.; Ghandehari, H. Surface acetylation of polyamidoamine (PAMAM) dendrimers decreases cytotoxicity while maintaining membrane permeability. Bioconjug. Chem. 2007, 18, 2054–2060. [Google Scholar] [CrossRef]
- Nam, H.Y.; Nam, K.; Hahn, H.J.; Kim, B.H.; Lim, H.J.; Kim, H.J.; Choi, J.S.; Park, J.-S. Biodegradable PAMAM ester for enhanced transfection efficiency with low cytotoxicity. Biomaterials 2009, 30, 665–673. [Google Scholar] [CrossRef]
- Hoque, J.; Akkapeddi, P.; Yadav, V.; Manjunath, G.B.; Uppu, D.S.; Konai, M.M.; Yarlagadda, V.; Sanyal, K.; Haldar, J. Broad spectrum antibacterial and antifungal polymeric paint materials: Synthesis, structure–activity relationship, and membrane-active mode of action. ACS Appl. Mater. Interfaces. 2015, 7, 1804–1815. [Google Scholar] [CrossRef]
- Murata, H.; Koepsel, R.R.; Matyjaszewski, K.; Russell, A.J. Permanent, non-leaching antibacterial surfaces—2: How high density cationic surfaces kill bacterial cells. Biomaterials 2007, 28, 4870–4879. [Google Scholar] [CrossRef]
- Milović, N.M.; Wang, J.; Lewis, K.; Klibanov, A.M. Immobilized N-alkylated polyethylenimine avidly kills bacteria by rupturing cell membranes with no resistance developed. Biotechnol. Bioeng. 2005, 90, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Gillies, E.R.; Dy, E.; Fréchet, J.M.; Szoka, F.C. Biological evaluation of polyester dendrimer: Poly (ethylene oxide)“bow-tie” hybrids with tunable molecular weight and architecture. Mol. Pharm. 2005, 2, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Feliu, N.; Walter, M.V.; Montañez, M.I.; Kunzmann, A.; Hult, A.; Nyström, A.; Malkoch, M.; Fadeel, B. Stability and biocompatibility of a library of polyester dendrimers in comparison to polyamidoamine dendrimers. Biomaterials 2012, 33, 1970–1981. [Google Scholar] [CrossRef]
- García-Gallego, S.; Hult, D.; Olsson, J.V.; Malkoch, M. Fluoride-Promoted Esterification with Imidazolide-Activated Compounds: A Modular and Sustainable Approach to Dendrimers. Angew. Chem. Int. Ed. 2015, 54, 2416–2419. [Google Scholar] [CrossRef] [PubMed]
- Stenstrom, P.; Hjorth, E.; Zhang, Y.; Andren, O.C.J.; Guette-Marquet, S.; Schultzberg, M.; Malkoch, M. Synthesis and in Vitro Evaluation of Monodisperse Amino-Functional Polyester Dendrimers with Rapid Degradability and Antibacterial Properties. Biomacromolecules 2017, 18, 4323–4330. [Google Scholar] [CrossRef] [PubMed]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef] [Green Version]
- Ciolkowski, M.; Petersen, J.F.; Ficker, M.; Janaszewska, A.; Christensen, J.B.; Klajnert, B.; Bryszewska, M. Surface modification of PAMAM dendrimer improves its biocompatibility. Nanomedicine 2012, 8, 815–817. [Google Scholar] [CrossRef]
- Jevprasesphant, R.; Penny, J.; Jalal, R.; Attwood, D.; McKeown, N.; D’emanuele, A. The influence of surface modification on the cytotoxicity of PAMAM dendrimers. Int. J. Pharm. 2003, 252, 263–266. [Google Scholar] [CrossRef]
- Alexandrescu, L.; Syverud, K.; Gatti, A.; Chinga-Carrasco, G. Cytotoxicity tests of cellulose nanofibril-based structures. Cellulose 2013, 20, 1765–1775. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef]
- Henriksson, M.; Henriksson, G.; Berglund, L.; Lindström, T. An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers. Eur. Polym. J. 2007, 43, 3434–3441. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Henschen, J.; Illergård, J.; Larsson, P.A.; Ek, M.; Wågberg, L. Contact-active antibacterial aerogels from cellulose nanofibrils. Colloids Surf. B 2016, 146, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Henschen, J.; Larsson, P.A.; Illergård, J.; Ek, M.; Wågberg, L. Bacterial adhesion to polyvinylamine-modified nanocellulose films. Colloids Surf. B 2017, 151, 224–231. [Google Scholar] [CrossRef]
- Chen, C.; Petterson, T.; Illergård, J.; Ek, M.; Wågberg, L. Influence of cellulose charge on bacteria adhesion and viability to PVAm/CNF/PVAm-modified cellulose model surfaces. Biomacromolecules 2019, 20, 2075–2083. [Google Scholar] [CrossRef]
- Fall, A.B.; Lindström, S.B.; Sprakel, J.; Wågberg, L. A physical cross-linking process of cellulose nanofibril gels with shear-controlled fibril orientation. Soft Matter. 2013, 9, 1852–1863. [Google Scholar] [CrossRef]
- Dong, H.; Snyder, J.F.; Williams, K.S.; Andzelm, J.W. Cation-induced hydrogels of cellulose nanofibrils with tunable moduli. Biomacromolecules 2013, 14, 3338–3345. [Google Scholar] [CrossRef]
- Ingverud, T.; Erlandsson, J.; Wågberg, L.; Malkoch, M. Dendritic polyampholyte assisted formation of functional cellulose nanofibril materials. Biomacromolecules 2020. [Google Scholar] [CrossRef]
- Azhar, F.F.; Shahbazpour, E.; Olad, A. pH sensitive and controlled release system based on cellulose nanofibers-poly vinyl alcohol hydrogels for cisplatin delivery. Fibers Polym. 2017, 18, 416–423. [Google Scholar] [CrossRef]
- Kaldéus, T.; Nordenström, M.; Carlmark, A.; Wågberg, L.; Malmström, E. Insights into the EDC-mediated PEGylation of cellulose nanofibrils and their colloidal stability. Carbohydr. Polym. 2018, 181, 871–878. [Google Scholar] [CrossRef]
- Saito, T.; Hirota, M.; Tamura, N.; Kimura, S.; Fukuzumi, H.; Heux, L.; Isogai, A. Individualization of nano-sized plant cellulose fibrils by direct surface carboxylation using TEMPO catalyst under neutral conditions. Biomacromolecules 2009, 10, 1992–1996. [Google Scholar] [CrossRef] [PubMed]
- Fall, A.B.; Burman, A.; Wågberg, L. Cellulosic nanofibrils from eucalyptus, acacia and pine fibers. Nord. Pulp Paper Res. J. 2014, 29, 176–184. [Google Scholar] [CrossRef]
- Katz, S.; Beatson, R.; Scallan, A.M. The determineation of strong and weak acidic groups in sulfite pulps. Sven. Papp. Nord. Cellul. 1984, 87, R48–R53. [Google Scholar]
- Kharlampieva, E.; Sukhishvili, S.A. Ionization and pH stability of multilayers formed by self-assembly of weak polyelectrolytes. Langmuir 2003, 19, 1235–1243. [Google Scholar] [CrossRef]
- Horn, D. Optisches Zweistrahlverfahren zur Bestimmung von Polyeketrolyten in Wasser und zur Messung der Polymeradsorption an Grenzflächen. In Progress in Colloid & Polymer Science; Springer: Berlin/Heidelberg, Germany, 1978; pp. 251–264. [Google Scholar]
- Nordenström, M.; Fall, A.; Nyström, G.; Wågberg, L. Formation of colloidal nanocellulose glasses and gels. Langmuir 2017, 33, 9772–9780. [Google Scholar] [CrossRef] [PubMed]
- Rizo-Gorrita, M.; Herráez-Galindo, C.; Torres-Lagares, D.; Serrera-Figallo, M.-Á.; Gutiérre-Pérez, J.-L. Biocompatibility of polymer and ceramic CAD/CAM materials with human gingival fibroblasts (HGFs). Polymers 2019, 11, 1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Generation | Amount Dendrimer | Charge Density (Dendrimers) | Amount CNFs | Charge Density (CNFs) | Carboxy to Amine Ratio | |
|---|---|---|---|---|---|---|
| Unit | mg | µe/g | mg | µe/g | COO- | NH3 + |
| TMP-G2-NH3+ | 8.5 | 2416.0 | 1.7 | 550.0 | 1.0 | 20.7 |
| TMP-G3-NH3+ | 8.5 | 1775.0 | 1.7 | 550.0 | 1.0 | 15.2 |
| TMP-G4-NH3+ | 8.5 | 1765.0 | 1.7 | 550.0 | 1.0 | 15.1 |
| Measurement | Bacterial Strains | E. coli | S. aureus | P. aeruginosa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unit | μg/mL | μM | NH3+ | μg/mL | μM | NH3+ | μg/mL | μM | NH3+ | |
| (μM) | (μM) | (μM) | ||||||||
| MIC value | TMP-G2-NH3+ | 125 | 36.8 | 441 | 1000 | 294.1 | 3529 | 500 | 147.1 | 1765 |
| TMP-G3-NH3+ | 62.5 | 8.9 | 214 | 500 | 71.3 | 1710 | 125 | 17.8 | 428 | |
| TMP-G4-NH3+ | 31.25 | 2.2 | 105 | 62.5 | 4.4 | 211 | 31.25 | 2.2 | 105 | |
| CNFs | >2000 | >2000 | >2000 | |||||||
| Unit | μg/mL | μM | NH3+ | μg/mL | μM | NH3+ | μg/mL | μM | NH3+ | |
| (μM) | (μM) | (μM) | ||||||||
| MMC value | TMP-G2-NH3+ | 125 | 36.8 | 441 | 4000 | 1176 | 14116 | 500 | 147.1 | 1765 |
| TMP-G3-NH3+ | 62.5 | 8.9 | 214 | 1000 | 142.6 | 3420 | 125 | 17.8 | 428 | |
| TMP-G4-NH3+ | 31.25 | 2.2 | 105 | 125 | 8.8 | 422 | 31.25 | 2.2 | 105 | |
| CNFs | >2000 | >2000 | >2000 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Namata, F.; Erlandsson, J.; Zhang, Y.; Wågberg, L.; Malkoch, M. Self-Assembled Polyester Dendrimer/Cellulose Nanofibril Hydrogels with Extraordinary Antibacterial Activity. Pharmaceutics 2020, 12, 1139. https://doi.org/10.3390/pharmaceutics12121139
Fan Y, Namata F, Erlandsson J, Zhang Y, Wågberg L, Malkoch M. Self-Assembled Polyester Dendrimer/Cellulose Nanofibril Hydrogels with Extraordinary Antibacterial Activity. Pharmaceutics. 2020; 12(12):1139. https://doi.org/10.3390/pharmaceutics12121139
Chicago/Turabian StyleFan, Yanmiao, Faridah Namata, Johan Erlandsson, Yuning Zhang, Lars Wågberg, and Michael Malkoch. 2020. "Self-Assembled Polyester Dendrimer/Cellulose Nanofibril Hydrogels with Extraordinary Antibacterial Activity" Pharmaceutics 12, no. 12: 1139. https://doi.org/10.3390/pharmaceutics12121139
APA StyleFan, Y., Namata, F., Erlandsson, J., Zhang, Y., Wågberg, L., & Malkoch, M. (2020). Self-Assembled Polyester Dendrimer/Cellulose Nanofibril Hydrogels with Extraordinary Antibacterial Activity. Pharmaceutics, 12(12), 1139. https://doi.org/10.3390/pharmaceutics12121139