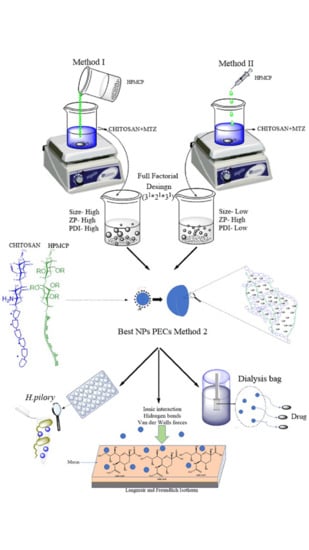
3.1. Evaluation of the Polymeric Ratio and Method on the Nano PECs Obtention
The samples prepared by method I, HP 12.5 and HP 15, in which the mass of HP was higher than the CS mass (
Table 1,
Section 2.2.1), the formation of large aggregates and phase separation was observed. Possibly, the higher proportion of HP results in the reduction of particles zeta potential, due to the charges annulment from the protonated amine groups (
NH3+) of CS and the carboxylate group (
COO–) of HP. This reduction in the zeta potential, near to zero, should result in a reduction in the electrostatic repulsion of the particles and, consequently, in their attraction and aggregation [
37].
Samples HP 1, HP 5, and HP 10 showed an opalescent visual aspect [
19]. This opalescent aspect is the result of the Tyndall effect, due to the interference in the light passage through the system. The observation of the Tyndall effect in the systems indicates the nanostructured particles formation [
38]. Samples HP 1, HP 5, and HP 10 were selected for characterization according
Section 2.2.2 and the results are shown in
Table 2.
The nano PECs diameter ranged from 734 to 810.8 nm. The diameter is one characteristic responsible for the cellular uptake, smaller particles tend to be more easily internalized by cells [
39].
Nano PECs showed positive surface charge between +17.5 and +25.7 mV (
Table 2), it means that the zeta potential of nano PECs is more strongly influenced by CS. Modular zeta potential values around 25–30 mV are desirable for the physical stability of the system, due the interparticle electrostatic repulsion [
31,
32]. The positive charge is also interesting to favor the interaction with the anionic group of lipids of the cell membrane or to the mucous, formed by monosaccharide sialic acid [
40].
By
Table 2, it was also possible to observe that with the increase in the HP ratio from 0.1 to 1, the zeta potential of the nano PECs decreases from +25.7 to +17.5 mV.
This reduction in zeta potential may occur because the HP molecules preferably associate more closely in the external region of the particle, due to their more rigid and bulk chain. The HP chains must have difficulty interpenetrating the formed precomplex, remaining more in the external region, giving a less positive character to nano PECs [
41,
42].
The analysis of PDI shows values in the range of 0.60–0.88. PDI represents the deviance of the particles size distribution, PDI < 0.4 indicates that the particles present low defiance in size [
43].
According
Table 2, nano PECs obtained by higher proportions of HP presented higher PDI values and consequently, the size distribution is less homogeneous.
This may be related to the polymeric dilution regime in the medium. With more polymer chains competing for solvent in the medium (concentrated regime), the probability of thermodynamically oriented arrangements are less, the kinetically oriented arrangements prevailing due to the reduction mobility in the medium, as well as the conformational limitations of the chains in unfolding [
44].
Nano PECs were formed in regions with high polymer concentrations (center of the container) to have larger polymer clusters and regions with lower concentrations of these polymer chains (periphery of the container). In these regions with lower polymeric density there is greater configurational and conformational freedom, for more uniform and thermodynamically oriented arrangements of the polymer chains.
Following the studies, the load of MT and the complexes obtainment was performed according to the two methodologies mentioned in
Section 2.2.1, and the characteristics of these nano PECs as presented in
Table 3.
According to
Table 3, the association of MT resulted in an increase in the average diameter of nano PECs, compared to the empty nano PECs, with some samples reaching the micrometric size (>1000 nm). Probably, when MT was added, it interacted with the polymers in order to decrease the probability of CS-HP interactions, which is an organized association, forming a more disordered matrix, with higher dimension.
Results in
Table 3 also showed that for HP 1 and HP 5 the drug association resulted in a reduction in the PDI value from 0.60 and 0.78 to values between 0.47 and 0.52. This effect was not observed for sample HP 10, with a 1:1 ratio of CS: HP, which even after the drug association the higher PDI values were maintained (>0.80). For samples composed by higher polymeric proportions (1:1) the polymer–polymer interactions are favored due to the high probability of the polymer chains being found, becoming less sensitive to the influence of MT addition. In conditions where the likelihood of encounters between polymer chains is low, interactions between MT–polymer are favored, in order to alter the formation of nano PECs [
45].
As the HP10 sample showed a high PDI value and decreased zeta potential, which may be indicative of low physical stability, it was discarded from the test of obtainment by method II (dripp).
Seeking better results in diameter and PDI, the nano PECs were obtained by dripping (method II).
The results of nano PECs obtained by method II are shown in
Table 4. The gradual and slow addition of the polymer allows the conditions of thermodynamic equilibrium to be established to a greater extent, as in this case the system has greater conformational and configurational freedom, which should result in more intense interactions between CS and HP, resulting in smaller and homogeneous particles [
44].
For all the samples analyzed, practically all the mass of MT added in the system was incorporated into the structure of the nano PECs and the association efficiencies were greater than 95%. Through the analysis of the content of drug associated, it was possible to infer the efficiency of the developed system. From the results obtained, nano PECs are classified as systems with high capacity for drug incorporation, showing itself as a promising system in the MT delivery. Regardless of the HP and MT amount, the selected nano PECs had a high association capacity.
From the data obtained in the experiments, method I proved not to be suitable for obtaining particles on a nanometric scale with homogeneous size distribution.
Among the nano PECs obtained by method II, the HP 1 and HP 5 systems presented the most suitable characteristics for the application, such as reduced diameter, low PDI, and high ZP values.
Then the values of polymeric ratios and drug were used together with the mixing method to compose a full factorial experiment (2
1 × 3
1 × 2
1) as shown in
Table 5, with the aim to assess the influence and effect of the interaction of these factors on the size, PDI, and ZP of the nano PECs.
The analysis of each factor’s influence and their interactions with the nano PECs characteristics are shown in
Figure 2,
Figure 3 and
Figure 4. They contain a bar for each effect, sorted from most significant to least significant. The length of each bar is proportional to the standardized effect. A vertical line is drawn at the location of the 0.05 critical value for the statistical test.
The results observed in
Table 4 together with the data in
Figure 2A, reinforce the fact that the average diameter of the nano PECs is mainly dependent on the method and the interaction between the method and the HP proportion. In
Figure 2B it is possible to verify the strong influence of the obtainment method on the average diameter of the nano PECs. While method I formed particles with average sizes varying between 871 and 1292 nm, method II (drip) formed particles with diameters between 553 and 664 nm, concluding that quantitatively method II is the most suitable for the formation of smaller nano PECs.
In
Figure 3, is possible to observe the influence of method, HP ratio, and MT in the PDI of nano PECs. A similar result to that of diameter, the method strongly influenced the PDI of nano PECs, in which the average values obtained by method I were ±0.49 while the average observed by method II was ±0.26, showing a homogeneity of particle diameter distribution, when it was obtained by dripping. They indicate that the method II was more efficient in reducing the average particle diameter, as well as, the PDI.
Finally, the influence of the factors tested on the zeta potential of the nano PECs was evaluated and the results showed in the
Figure 4, once again, a significant influence of the method and HP proportion were observed. As was discussed previously, the HP contributes to the lower positive charge density of nano PECs, due to it anionic nature and its conformation during the nano PECs formation.
A possible explanation for the differences in zeta potential, observed between the nano PECs obtained by the different methods, may be the fact that in method I, large amounts of HP were added to the reaction medium, containing QS-MT, this favored the formation of larger particles, as there was no sufficient time for the complete dispersion of HP in this medium and the electrostatic interactions, Van der Waals forces, and hydrogen bonds between the nearby chains, of both polymers, were quickly established, forming particles containing polymeric tangles, with discontinuous and interstice regions containing MT. Thus, it can be considered that the formation process of these nano PECs by method I was governed kinetically. As it is a dynamic and kinetically controlled process, the lack of control when adding the HP dispersion, would promote the formation of nano PECs with varying diameters, impacting in the PDI [
44].
In the systems obtained by drip method II, in which the addition of HP was carried out in a controlled and slow manner, the process would be governed thermodynamically, since smaller amounts of HP were introduced in the medium, allowing this material to disperse and interact with the nearby CS chains, slowly and initially due to strong, electrostatic interactions. As these interactions occurred, the loads of these complexes would become smaller and CS chains with higher loads would interact with the new available HP chains, dripped in the medium, so that the formation of these complexes would be associated with the reduction of the system’s overall energy by reducing polymer loads. These complexes would present smaller PDI values and smaller particle diameters as well, since in these cases the interactions would be much more governed by electrostatic interactions than other types of interactions, a fact evidenced by the low values of zeta potential (
Table 3 and
Table 4). Based on all the results and discussion, method II was selected to obtain the nano PECs. Thus, all samples analyzed in the subsequent tests were obtained using this method.
3.2. Fourier-Transform Infrared Spectroscopy (FTIR)
Figure 5A shows the absorption spectra of CS, HP, and MT in the infrared region. In the MT spectrum, bands in the regions from 1300 to 1600 cm
−1 are assigned to the
NO2 group. The bands at 2900 and 3100 cm
−1 represent stretching of sp
3 and sp
2 carbons, respectively. The bands in the regions of 3200–3600 cm
−1 are attributed to the stretch of the
O–H group [
46].
In the CS absorption spectrum, bands were observed in the regions from 1650 to 1665 cm
−1, which can be attributed to the
C=O stretch from the secondary amide group. The bands from 1560 to 1610 cm
−1 are attributed to the axial deformation of the
NH2 group in the plane, the bands from 1200 to 1000 cm
−1 are attributed to the
CO stretching, and the bands from 3350 to 3180 cm
−1 to the stretching of the NH
2 group [
47]. In the HP absorption spectrum, bands from 3500 to 3200 cm
−1 were noted, attributed to the stretching of the
O–H group. The band observed at 2800 cm
−1 is attributed to the methoxy group (
C–CH3) and at 1725 cm
−1 it is attributed to the stretching of the
C=O group of the ester group; from 1600 to 1550 cm
−1 attributed to the aromatic ring and 740 cm
−1 attributed to the monosubstituted aromatic ring [
48].
Figure 5B shows the absorption spectra of empty and drug-containing nano PECs. It is possible to notice in the loaded nano PECs the presence of the characteristic band of the MT between 1200 and 1400 cm
−1, which is not noticed in the empty samples. Between 3500 and 3100 cm
−1 referring to
NH2 and
OH, it can be noted that there was a displacement of the bands in all samples, when compared to CS, HP, and MT, which may indicate the interaction of protonated amino groups of CS with the carboxylic groups HP, as well as the MT NO and
OH groups of CS and the
C=O, C–O, and
OH groups in HP through dipole–dipole interactions and hydrogen bonds. In addition, the differences found in the 500–1700 cm
−1 bands of the samples, in relation to the free polymers, can also suggest an interaction between them.
It is possible to see two bands, one at 1200 and the other at 1250, only in systems with MT. These bands are related to stretching of the C-N bonds, abundant in MT. One observation that confirms this statement is the fact that both bands are becoming less intense as the concentration of MT in the systems is reduced [
49]. At 1650 cm it is possible to identify an intense band in loaded nano PECs spectra, which is low in the empty nano PECs spectrum. This increase in the intensity of the C=C carbonyl band, already present due to the double bonds of the aromatic rings of the HP, may be related to the presence of a C=C bond in the imidazolidinic ring [
49,
50]. At 1550 cm
−1, related to NH
2 stretch, abundant in CS, it is possible to observe the reduction in the intensity of this band, which is quite intense in the empty nano PECs, and becomes progressively less intense, as the incorporations of MT occur. This observation may indicate that there are interactions between the NH
2→NH
3+ groups of chitosan with the NO
− groups present in metronidazole [
51]. In
Table 3, it is possible to see that the addition of MT in the nano PECs leads to a reduction in the system’s PDI. The reduction in the PDI value can be associated with the fact that MT may be interacting with CS, forming more monodispersed systems. The effect of adding MT on the systems stems from the fact that MT can interact more with polymers, especially with CS, as evidenced in the FTIR spectrum, forming larger particles, however, despite the particles having a larger diameter, with a greater probability of interaction, we will have more uniformly distributed particles [
52].
3.3. In Vitro Mucin Interaction Assay
Figure 6 shows the results of mucin adsorption on nano PECs. It was observed that for all samples the amount of adsorbed mucin increased with the increase in the amount of added mucin, highlighting the high mucoadhesiveness of the nano PECs.
The nano PECs mucoadhesion derives from the fact that CS, predominant in nano PECs composition, in acidic media, such as the stomach, present protonated amino groups. Mucus is mainly composed by mucin (pKa-2,6), a glycoprotein and its sialic acid residues, in acid pH values, are ionized presenting negative charge. Electrostatic interactions and Van der Waals forces must be established between the system surface and the mucin, favoring the adsorption [
52,
53].
To the mucin adsorption data, Freundlich and Langmuir models were applied and the results are shown in
Table 6.
The Freundlich model describes the adsorption of a single solute, on the adsorbent, in multiple layers and in a reversible way, assuming that the solid surface is irregular and heterogeneous [
54]. Langmuir’s model considers the adsorption on a homogeneous surface, in monolayer, over the entire surface of the adsorbent, in which the adjacent molecules of the solute do not interact with each other, remaining in solution [
20].
For HP1MT5 the adsorption was better adjusted to the Langmuir model, this particle presented a greater positive zeta potential. Therefore, the adsorption must be governed by electrostatic interactions, between its surface and mucin, which adsorb quickly due to electrostatic attraction. These interactions are strong and are established at shorter distances, must be able to form a homogeneous mucin layer, and closely adhered to the particle surface [
55]. In this case, the system has better adhesivity and more force of adhesion to the negative surfaces, like cells and mucous layers. We believe that mucin adsorbed in the surface of positive nano PECss it actuate as a repulsive layer, not leaving other nano PECs to approximate the negative mucin negative and adsorb to that, forming multiple absorption layers (particle-mucin) [
56].
The other systems listed in
Table 6 are more fitted to the Freundlich model. A possible explanation for this is the fact that these systems also have positive zeta potential, although, relatively lower <+20 mV. Possibly, the interactions that occur between mucin and these system surfaces also are majority electrostatic type, however, some weaker interactions such as hydrogen bonds and Van der Waals forces, are established at a greater distance than when compared to the HP1MT5 system, making this type of adsorption less intense [
56].
In addition, the lower zeta potential allows interactions between nano PECs, making it possible to establish adsorption in multilayers, that is, nano PECs already with mucin adsorbed on its surface can interact with other nano PECs, increasing the number of layers on the adsorbent surface [
57].
Regarding the
n coefficient, it indicates the intensity of mucin adsorption with the surface of the particles,
n values greater than 1.0 represent favorable conditions for adsorption [
58,
59]. Thus, all particles, even with higher concentrations of MT, showed strong adsorption interaction. The
k coefficient is related to the adsorption capacity. For HP1MT5, with a lower ratio of HP and HP5MT5 with a higher concentration of MT, the values found were lower, which may indicate that the addition of drug reduces the adsorption capacity, perhaps due to the formation of a more compact matrix and less positive residual load, which makes it difficult to interact with mucin molecules [
60].
3.4. In Vitro Dissolution Test
Based on the data found for the adsorption isotherms and zeta potential values, samples HP1MT5 and HP5MT3 were chosen to perform the in vitro dissolution test. These systems were also chosen because they have higher and lower zeta potential values, respectively, and variations on the release profile can be justified by some of these physical-chemical characteristics. Free MT was also tested, to evaluate the influence of nano-compartmentalization on the drug dissolution rates.
The results in
Figure 7 suggest that both the free MT and the MT incorporated in the HP1MT5 system present statistically similar dissolution profile and release rates.
Around 100% of the MT was released at 120 min (
Figure 7). This may be associated with the fact that in strongly acidic media (stomach pH = 1.5–3.5), CS quickly becomes protonated. The extensively protonated CS chains electrostatically repel, consequently, the matrix swelling allowed the diffusion of medium to the particle structure, dissolving the drug and favoring the diffusion to the dissolution medium. In addition, the proton-rich solvent accesses the HP chains by neutralizing their charges, this event can culminate in the disruption of the particle matrix, also favoring the rapid release of the drug.
The best model that represented the dissolution kinetics of MT was the Korsmeyer–Peppas (Equation (5)).
where
F is the total drug concentration in the medium,
Mt is amount of released drug in at time “
t”,
M is amount total drug in the system
k is the constant of incorporation of structural modifications and geometrical characteristics of the system (also considered the release velocity constant), and
n is the exponent of release (related to the drug release mechanism) as a function of time t [
61,
62].
In all systems, a value of
n greater than 0.5 and lower then 1.0 (
Table 7) was observed, such experimental data indicate that the observed release profile can be classified as anomalous, in which the drug release was controlled by a combination of many processes, including the swelling and dissolution of the polymeric matrix, diffusion and drug dissolution, as discussed above [
63].
The results indicate that the system did not allow a prolonged release of the drug. However, considering the high mucoadhesive capacity of the developed system, in the physiological environment a large number of nano PECs can adhere to the mucous layer, resisting gastric emptying, since due to the small diameter the nano PECs behave similarly to liquids and may remain in the stomach for less time, resulting also in a close contact with
H. pylori [
64]. In addition, with the nano PECs matrix erosion, the polysaccharides can interact with the bacteria membrane, resulting in destabilization and, consequently, in the greatest internalization of the drug [
64] and/or acting in the inhibition of membrane efflux pumps.