Rheological and Drug Delivery Characteristics of Poloxamer-Based Diclofenac Sodium Formulations for Chronic Wound Site Analgesia
Abstract
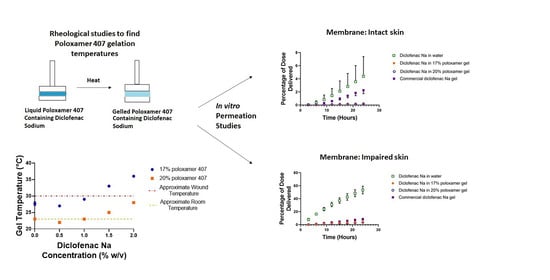
:1. Introduction
2. Materials and Methods
2.1. Preparation of Poloxamer Gels
2.2. Rheologic Characterization of Poloxamer Gels
2.3. In vitro Diclofenac Sodium Release Studies
2.4. In vitro Permeation Studies with Intact and Impaired Skin
2.5. HPLC Quantification of Diclofenac Sodium
2.6. Data and Statistical Analysis
3. Results
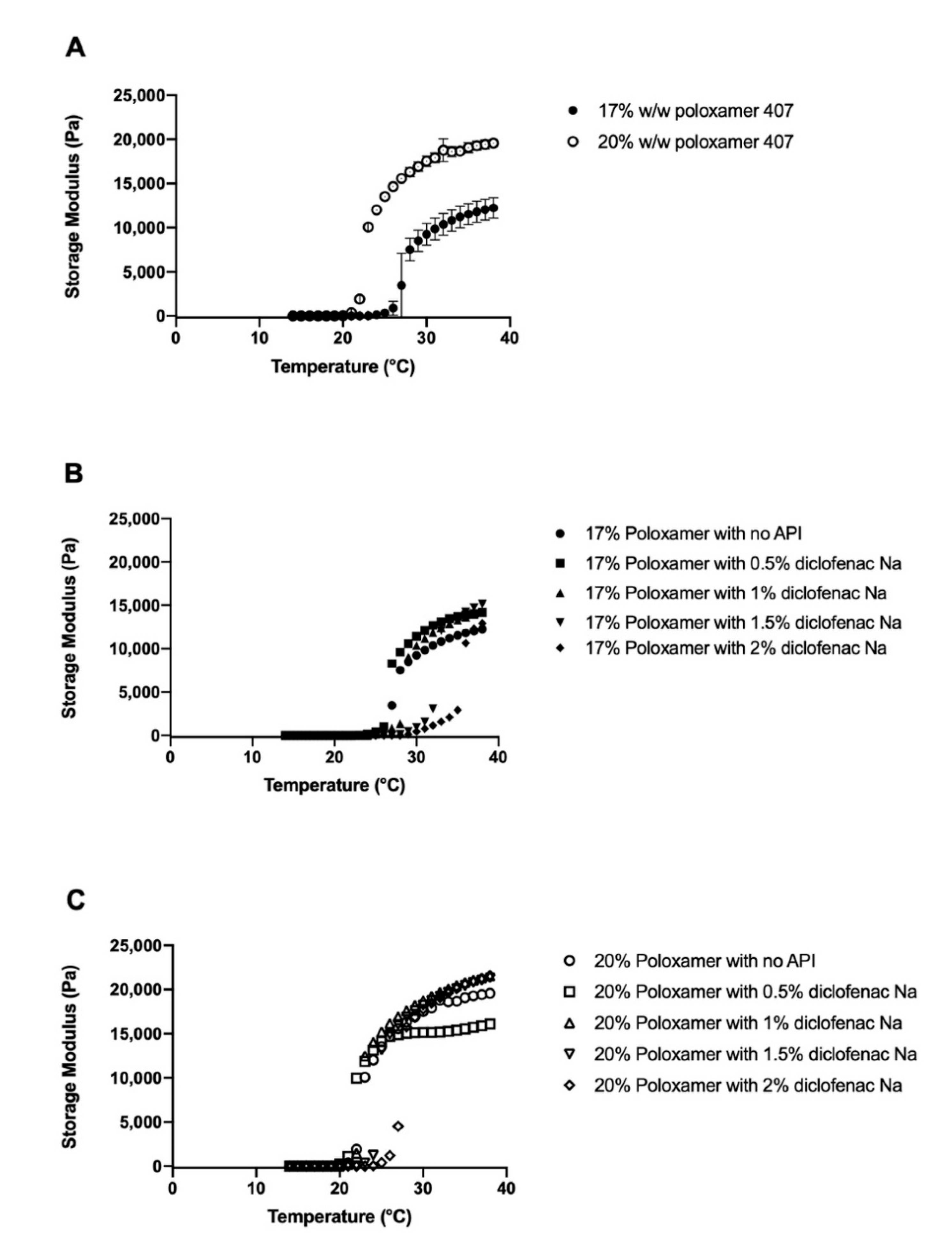
3.1. Determination of Poloxamer Gel Linear Viscoelastic Regions
3.2. Effects of Diclofenac Sodium on Gelation
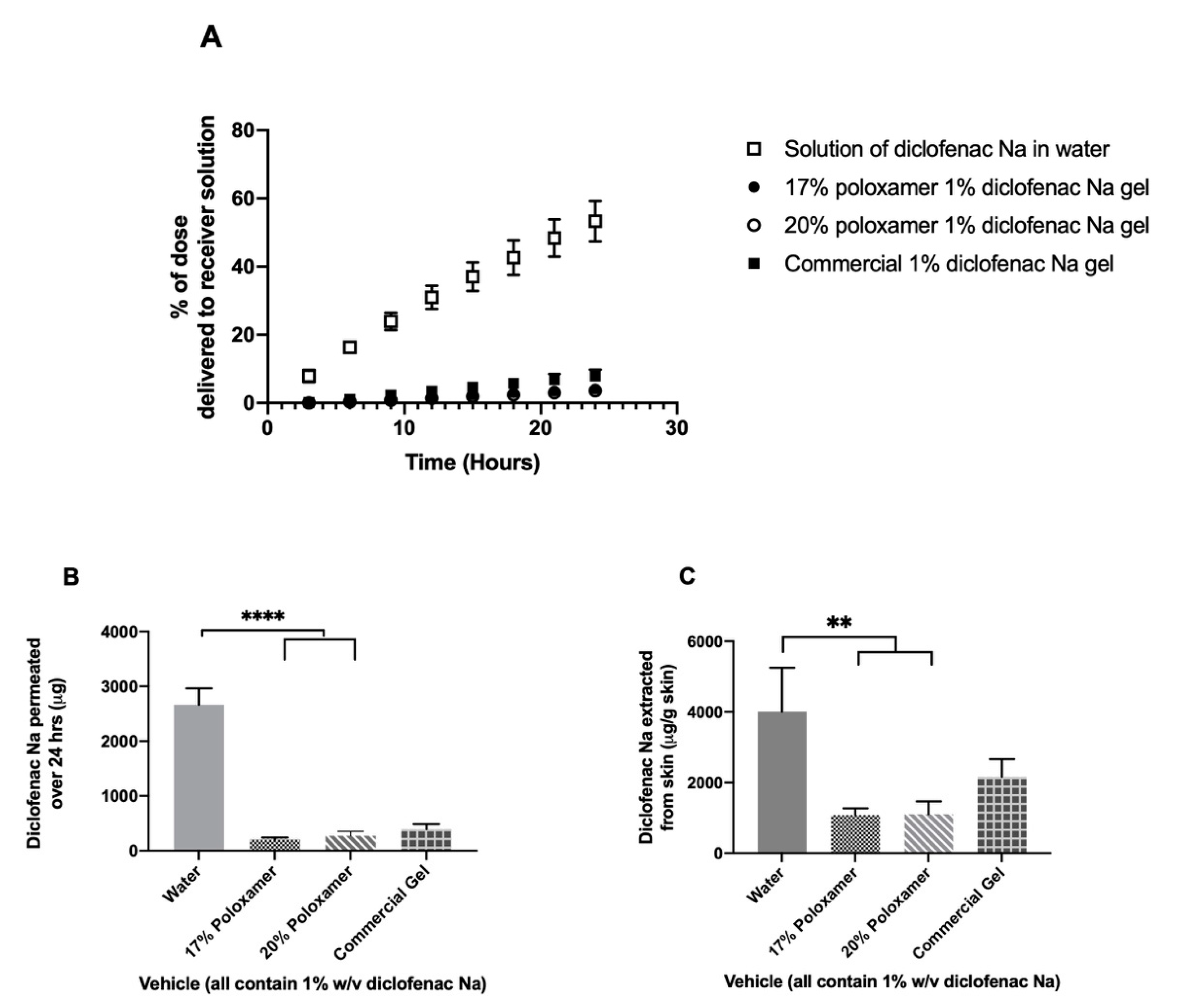
3.3. In vitro Diclofenac Sodium Release and Permeation Studies
3.4. Permeation through Tape-Stripped Skin
4. Discussion
4.1. Effect of Diclofenac on Gelation Temperature
4.2. Storage Modulus and Gel Strength
4.3. Diclofenac Release from Poloxamer Gels
4.4. Diclofenac Sodium Permeation through Intact Skin
4.5. Effect of Skin Impairment on Diclofenac Permeation
5. Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Harder, J.; Schroder, J.M.; Glaser, R. The skin surface as antimicrobial barrier: Present concepts and future outlooks. Exp. Dermatol. 2013, 22, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Russo, J.; Fiegel, J.; Brogden, N. Antibiotic Delivery Strategies to Treat Skin Infections When Innate Antimicrobial Defense Fails. Antibiotics 2020, 9, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawton, S. Skin 1: The structure and functions of the skin. Nurs. Times 2019, 115, 30–33. [Google Scholar]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walburn, J.; Vedhara, K.; Hankins, M.; Rixon, L.; Weinman, J. Psychological stress and wound healing in humans: A systematic review and meta-analysis. J. Psychosom. Res. 2009, 67, 253–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padgett, D.A.; Marucha, P.T.; Sheridan, J.F. Restraint stress slows cutaneous wound healing in mice. Brain Behav. Immun. 1998, 12, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Cole-King, A.; Harding, K.G. Psychological factors and delayed healing in chronic wounds. Psychosom. Med. 2001, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Gouin, J.P.; Kiecolt-Glaser, J.K. The impact of psychological stress on wound healing: Methods and mechanisms. Immunol. Allergy Clin. N. Am. 2011, 31, 81–93. [Google Scholar] [CrossRef] [Green Version]
- Kolodny, A.; Courtwright, D.T.; Hwang, C.S.; Kreiner, P.; Eadie, J.L.; Clark, T.W.; Alexander, G.C. The prescription opioid and heroin crisis: A public health approach to an epidemic of addiction. Annu. Rev. Public Health 2015, 36, 559–574. [Google Scholar] [CrossRef]
- Benyamin, R.; Trescot, A.M.; Datta, S.; Buenaventura, R.; Adlaka, R.; Sehgal, N.; Glaser, S.E.; Vallejo, R. Opioid complications and side effects. Pain Physician 2008, 11, S105–S120. [Google Scholar]
- Langemo, D.K.; Black, J.; Panel, N.P.U.A. Pressure ulcers in individuals receiving palliative care: A National Pressure Ulcer Advisory Panel white paper. Adv. Skin Wound Care 2010, 23, 59–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peppin, J.F.; Albrecht, P.J.; Argoff, C.; Gustorff, B.; Pappagallo, M.; Rice, F.L.; Wallace, M.S. Skin Matters: A Review of Topical Treatments for Chronic Pain. Part Two: Treatments and Applications. Pain Ther. 2015, 4, 33–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalia, Y.N.; Guy, R.H. Modeling transdermal drug release. Adv. Drug Deliv. Rev. 2001, 48, 159–172. [Google Scholar] [CrossRef]
- Cohn, D.; Sosnik, A.; Levy, A. Improved reverse thermo-responsive polymeric systems. Biomaterials 2003, 24, 3707–3714. [Google Scholar] [CrossRef]
- Escobar-Chavez, J.J.; Lopez-Cervantes, M.; Naik, A.; Kalia, Y.N.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. Applications of thermoreversible pluronic F-127 gels in pharmaceutical formulations. J. Pharm. Pharm. Sci. 2006, 9, 339–358. [Google Scholar]
- Akash, M.S.; Rehman, K.; Sun, H.; Chen, S. Assessment of release kinetics, stability and polymer interaction of poloxamer 407-based thermosensitive gel of interleukin-1 receptor antagonist. Pharm. Dev. Technol. 2014, 19, 278–284. [Google Scholar] [CrossRef]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef] [Green Version]
- Yong, C.S.; Choi, Y.-K.; Kim, Y.-I.; Park, B.-J.; Quan, Q.-Z.; Rhee, J.-D.; Kim, C.-K.; Choi, H.-G. Physicochemical characterization and in vivo evaluation of thermosensitive diclofenac liquid suppository. Arch. Pharm. Res. 2003, 26, 162–167. [Google Scholar] [CrossRef]
- Ricci, E.J.; Bentley, M.V.L.B.; Farah, M.; Bretas, R.E.S.; Marchetti, J.M. Rheological Characterization of Poloxamer 407 Lidocaine Hydrochloride Gels. Eur. J. Pharm. Sci. 2002, 2002, 161–167. [Google Scholar] [CrossRef]
- Heilmann, S.; Kuchler, S.; Wischke, C.; Lendlein, A.; Stein, C.; Schafer-Korting, M. A thermosensitive morphine-containing hydrogel for the treatment of large-scale skin wounds. Int. J. Pharm. 2013, 444, 96–102. [Google Scholar] [CrossRef]
- Price, P.; Fogh, K.; Glynn, C.; Krasner, D.L.; Osterbrink, J.; Sibbald, R.G. Managing Painful Chronic Wounds: The Wound Pain Management Model. Int. Wound J. 2007, 4, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Hamm, R.L. Factors That Impair Wound Healing. J. Am. Coll. Clin. Wound Spec. 2012, 4, 84–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broughton, G., 2nd; Janis, J.E.; Attinger, C.E. The basic science of wound healing. Plast. Reconstr. Surg. 2006, 117, 12–34. [Google Scholar] [CrossRef] [PubMed]
- Geesala, R.; Dhoke, N.R.; Das, A. Cox-2 inhibition potentiates mouse bone marrow stem cell engraftment and differentiation-mediated wound repair. Cytotherapy 2017, 19, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Duchesne, E.; Dufresne, S.S.; Dumont, N.A. Impact of inflammation and anti-inflammatory modalities on skeletal muscle healing: From fundamental research to the clinic. Phys. Ther. 2017, 97, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Rosique, R.G.; Rosique, M.J.; Farina Junior, J.A. Curbing Inflammation in Skin Wound Healing: A Review. Int. J. Inflamm. 2015, 2015, 316235. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava, S.; Das, A. Interaction between ethoxylated emulsifiers and propylene glycol based solvents: Gelation and rheology study. Colloid Surf. A 2019, 582. [Google Scholar] [CrossRef]
- Edsman, K.; Carlfors, J.; Petersson, R. Rheological evaluation of poloxamer as an in situ gel for ophthalmic use. Eur. J. Pharm. Sci. 1998, 6, 105–112. [Google Scholar] [CrossRef]
- Sekkat, N.; Kalia, Y.N.; Guy, R.H. Biophysical study of porcine ear skin in vitro and its comparison to human skin in vivo. J. Pharm. Sci. 2002, 91, 2376–2381. [Google Scholar] [CrossRef]
- Ricci, E.J.; Lunardi, L.O.; Nanclares, D.M.; Marchetti, J.M. Sustained release of lidocaine from Poloxamer 407 gels. Int. J. Pharm. 2005, 288, 235–244. [Google Scholar] [CrossRef]
- Djekic, L.; Čalija, B.; Medarević, Đ. Gelation behavior, drug solubilization capacity and release kinetics of poloxamer 407 aqueous solutions: The combined effect of copolymer, cosolvent and hydrophobic drug. J. Mol. Liq. 2020, 303. [Google Scholar] [CrossRef]
- Zhang, L.; Parsons, D.L.; Navarre, C.; Kompella, U.B. Development and in-vitro evaluation of sustained release poloxamer 407 (P407) gel formulations of ceftiofur. J. Control Release 2002, 85, 73–81. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Bruschi, M.L., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 63–86. [Google Scholar] [CrossRef]
- Gethin, G.; O’Connor, G.M.; Abedin, J.; Newell, J.; Flynn, L.; Watterson, D.; O’Loughlin, A. Monitoring of pH and temperature of neuropathic diabetic and nondiabetic foot ulcers for 12 weeks: An observational study. Wound Repair Regen. 2018, 26, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, K. Phase behaviour of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock-coplymer dissolved in water. EPL 1992, 19, 599–604. [Google Scholar] [CrossRef]
- Mortensen, K.; Brown, W.; Norden, B. Inverse melting transition and evidence of three-dimensional cubatic structure in a block-copolymer micellar system. Phys. Rev. Lett. 1992, 68, 2340–2343. [Google Scholar] [CrossRef]
- Mortensen, K. Block copolymer in aqueous solution: Micelle formation and hard-sphere crystallization. Colloid Polym. Sci. 1993, 93, 72–75. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Ramadan, E.M.; Borg, T.M.; Elkayal, M.O. Formulation of site-specific mucoadhesive liquid suppositories as a method to decrease hepatotoxicity in rabbits. Bull. Pharm. Sci. Assiut 2008. [Google Scholar] [CrossRef]
- Sharma, P.K.; Bhatia, S.R. Effect of anti-inflammatories on Pluronic F127: Micellar assembly, gelation and partitioning. Int. J. Pharm. 2004, 278, 361–377. [Google Scholar] [CrossRef]
- Janmey, P.A.; Schliwa, M. Rheology. Curr. Biol. 2008, 18, 639–641. [Google Scholar] [CrossRef] [Green Version]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Shelke, S.; Shahi, S.; Jalalpure, S.; Dhamecha, D.; Shengule, S. Formulation and evaluation of thermoreversible mucoadhesive in-situ gel for intranasal delivery of naratriptan hydrochloride. J. Drug Deliv. Sci. Technol. 2015, 29, 238–244. [Google Scholar] [CrossRef]
- Soni, G.; Yadav, K.S. High encapsulation efficiency of poloxamer-based injectable thermoresponsive hydrogels of etoposide. Pharm. Dev. Technol. 2014, 19, 651–661. [Google Scholar] [CrossRef]
- Gou, M.; Li, X.; Dai, M.; Gong, C.; Wang, X.; Xie, Y.; Deng, H.; Chen, L.; Zhao, X.; Qian, Z.; et al. A novel injectable local hydrophobic drug delivery system: Biodegradable nanoparticles in thermo-sensitive hydrogel. Int. J. Pharm. 2008, 359, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Sulimai, N.H.; Ko, J.C.; Jones-Hall, Y.L.; Weng, H.-Y.; Deng, M.; Breur, G.J.; Knipp, G.T. Evaluation of 25% Poloxamer as a Slow Release Carrier for Morphine in a Rat Model. Front. Vet. Sci. 2018, 5, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayol, L.; Quaglia, F.; Borzacchiello, A.; Ambrosio, L.; La Rotonda, M.I. A novel poloxamers/hyaluronic acid in situ forming hydrogel for drug delivery: Rheological, mucoadhesive and in vitro release properties. Eur. J. Pharm. Biopharm. 2008, 70, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, L.; Temchenko, M.; Alakhov, V.; Hatton, T.A. Bioadhesive properties and rheology of polyether-modified poly(acrylic acid) hydrogels. Int. J. Pharm. 2004, 282, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Koffi, A.A.; Agnely, F.; Ponchel, G.; Grossiord, J.L. Modulation of the rheological and mucoadhesive properties of thermosensitive poloxamer-based hydrogels intended for the rectal administration of quinine. Eur. J. Pharm. Sci. 2006, 27, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Mukhija, A.; Kishore, N. Partitioning of drugs in micelles and effect on micellization: Physicochemical insights with tryptophan and diclofenac sodium. Colloids Surf. A Physicochem. Eng. Asp. 2017, 513, 204–214. [Google Scholar] [CrossRef]
- Herkenne, C.; Alberti, I.; Naik, A.; Kalia, Y.N.; Mathy, F.X.; Preat, V.; Guy, R.H. In vivo methods for the assessment of topical drug bioavailability. Pharm. Res. 2008, 25, 87–103. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Alikhan, A.; Maibach, H.I. Biology of Stratum Corneum: Tape Stripping and Protein Quantification. In Textbook of Aging Skin, 2nd ed.; Farage, M.A., Miller, K.W., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 387–396. [Google Scholar] [CrossRef]
- Bashir, S.J.; Chew, A.; Anigbogu, A.; Dreher, F.; Maibach, H.I. Physical and physiological effects of stratum corneum tape stripping. Skin Res. Technol. 2001, 2001, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Murawsky, M.; LaCount, T.; Kasting, G.B.; Li, S.K. Transepidermal water loss and skin conductance as barrier integrity tests. Toxicol. Vitr. 2018, 51, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Sotoodian, B.; Maibach, H.I. Noninvasive test methods for epidermal barrier function. Clin. Dermatol. 2012, 30, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.J.; Herranz, P.; Cruz, S.B.; Parodi, A. Treatment of actinic keratosis through inhibition of cyclooxygenase-2: Potential mechanism of action of diclofenac sodium 3% in hyaluronic acid 2.5. Dermatol. Ther. 2019, 32, e12800. [Google Scholar] [CrossRef]






| Model Parameters | 17% Poloxamer | 20% Poloxamer | ||||
|---|---|---|---|---|---|---|
| Zero-Order | Higuchi | Korsmeyer–Peppas | Zero-Order | Higuchi | Korsmeyer–Peppas | |
| r2 | 0.992 | 0.833 | 0.995 | 0.998 | 0.799 | 0.996 |
| k | 89.9 µg/h | 365.0 µg/h | 80.7 µg/h | 84.7 µg/h | 341.5 µg/h | 62.0 µg/h |
| n (Korsmeyer–Peppas) | 1.044 | 1.113 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, J.; Fiegel, J.; Brogden, N.K. Rheological and Drug Delivery Characteristics of Poloxamer-Based Diclofenac Sodium Formulations for Chronic Wound Site Analgesia. Pharmaceutics 2020, 12, 1214. https://doi.org/10.3390/pharmaceutics12121214
Russo J, Fiegel J, Brogden NK. Rheological and Drug Delivery Characteristics of Poloxamer-Based Diclofenac Sodium Formulations for Chronic Wound Site Analgesia. Pharmaceutics. 2020; 12(12):1214. https://doi.org/10.3390/pharmaceutics12121214
Chicago/Turabian StyleRusso, Jackson, Jennifer Fiegel, and Nicole K. Brogden. 2020. "Rheological and Drug Delivery Characteristics of Poloxamer-Based Diclofenac Sodium Formulations for Chronic Wound Site Analgesia" Pharmaceutics 12, no. 12: 1214. https://doi.org/10.3390/pharmaceutics12121214
APA StyleRusso, J., Fiegel, J., & Brogden, N. K. (2020). Rheological and Drug Delivery Characteristics of Poloxamer-Based Diclofenac Sodium Formulations for Chronic Wound Site Analgesia. Pharmaceutics, 12(12), 1214. https://doi.org/10.3390/pharmaceutics12121214