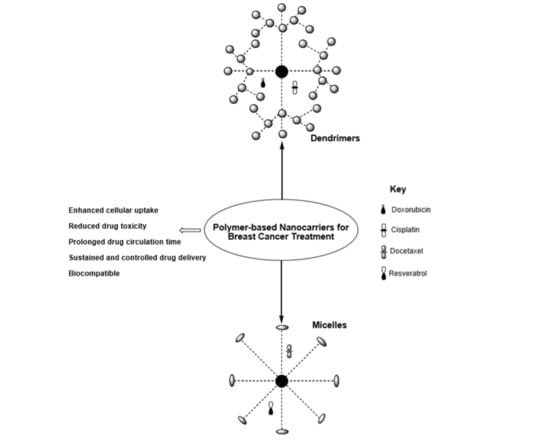
The Therapeutic Efficacy of Dendrimer and Micelle Formulations for Breast Cancer Treatment
Abstract
:1. Introduction
2. Breast Cancer and Its Chemotherapy
3. Micelles and Dendrimers Nanoformulations That Are Currently in the Clinical Trials
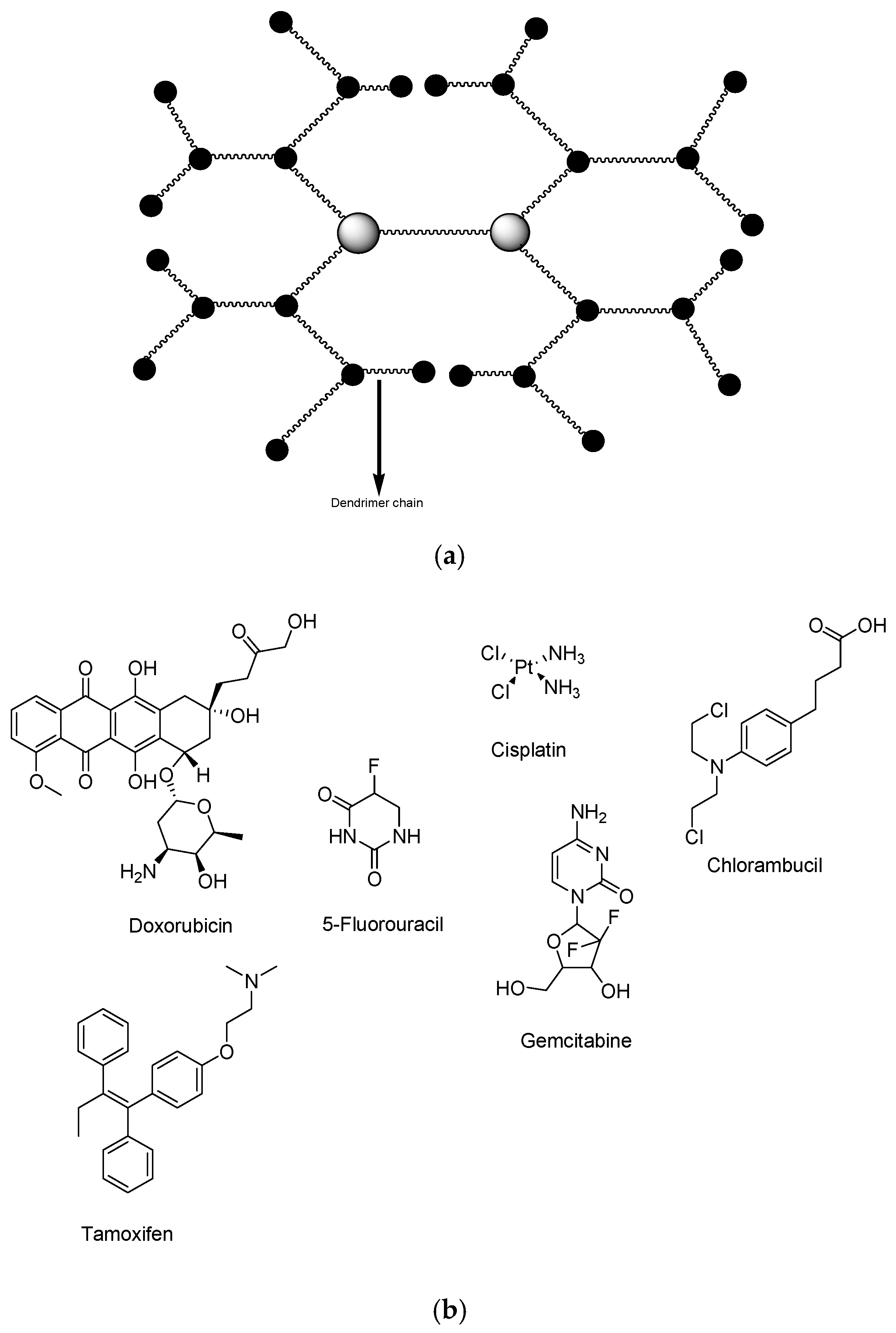
4. Dendrimers for Breast Cancer Therapy
4.1. Dendrimers Loaded with Doxorubicin
4.2. Dendrimers Loaded with Oligodeoxynucleotides
4.3. Dendrimers Loaded with Trastuzumab
4.4. Dendrimers Loaded with Other Anticancer Drugs
4.5. Limitations of Dendrimers
5. Polymeric Micelles
5.1. Polymeric Micelles Loaded with Docetaxel
5.2. Polymeric Micelles Loaded with Doxorubicin
5.3. Polymeric Micelles Loaded with Paclitaxel
5.4. Polymeric Micelles Loaded with Curcumin
5.5. Polymeric Micelles Loaded with Platinum Drugs
5.6. Polymeric Micelles Loaded with Other Anticancer Drugs
5.7. Limitations of Micelles
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Singh, S.; Sharma, B.; Kanwar, S.S.; Kumar, A. Lead Phytochemicals for Anticancer Drug Development. Front. Plant Sci. 2016, 7, 1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, P.; Kunnumakara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef] [PubMed]
- Okuhara, T.; Ishikawa, H.; Urakubo, A.; Hayakama, M.; Yamaki, T.; Tarayama, T.; Kuchi, T. Cancer information needs according to cancer type: A content analysis of data from Japan’s largest cancer information website. Prev. Med. Rep. 2018, 12, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.; Aderibigbe, B.A. Ferrocene-Based Compounds with Antimalaria/Anticancer Activity. Molecules 2019, 24, 3604. [Google Scholar] [CrossRef] [Green Version]
- Feng, S.; Chien, S. Chemotherapeutic engineering: Application and further development of chemical engineering principles for chemotherapy of cancer and other diseases. Chem. Eng. Sci. 2003, 58, 4087–4114. [Google Scholar] [CrossRef]
- International Agency for Research. Latest Global Cancer Data: Cancer Burden Rises to 18.1 Million New Cases and 9.6 Million Cancer Deaths in 2018; International Agency for Research on Cancer: Lyon, France, 2018. [Google Scholar]
- Siegel, R.; Ward, E.; Brawley, O.; Jemal, A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J. Clin. 2011, 61, 212–236. [Google Scholar] [CrossRef]
- Tinoco, G.; Warsch, S.; Glück, S.; Avancha, K.; Montero, A.J. Treating Breast Cancer in the 21st Century: Emerging Biological Therapies. J. Cancer 2013, 4, 117–132. [Google Scholar] [CrossRef] [Green Version]
- American Cancer Society. Cancer Facts and Figures; American Cancer Society: Atlanta, GA, USA, 2011. [Google Scholar]
- Carlson, R.W.; Allred, D.C.; Anderson, B.O.; Burstein, H.J.; Carter, W.B.; Edge, S.B.; Erban, J.K.; Farrar, W.B.; Goldstein, L.J.; Gradishar, W.J.; et al. Breast cancer. Clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2009, 7, 122–192. [Google Scholar] [CrossRef]
- Peng, J.; Chen, J.; Xie, F.; Bao, W.; Xu, H.; Wang, H.; Xu, Y.; Du, Z. Herceptin-conjugated paclitaxel loaded PCL-PEG worm-like nanocrystal micelles for the combinatorial treatment of HER2-positive breast cancer. Biomaterials 2019, 222, 119420. [Google Scholar] [CrossRef]
- Chang, L.; Weiner, L.S.; Hartman, S.J.; Horvath, S.; Jeste, D.; Mischel, P.S.; Kado, D.M. Breast cancer treatment and its effects on aging. J. Geriatr. Oncol. 2019, 10, 346–355. [Google Scholar] [CrossRef]
- Alven, S.; Nqoro, X.; Buyana, B.; Aderibigbe, B.A. Polymer-Drug Conjugate, a Potential Therapeutic to Combat Breast and Lung Cancer. Pharmaceutics 2020, 12, 406. [Google Scholar] [CrossRef] [PubMed]
- Rania, M.H.; Abdelkader, A.M.; Sherweit, H.E.; Eman, S.M.; Noha, A.G.; Salma, A.; Tarek, E.; Rosaline, A.; Maha, F.; Abdullah, I.E.; et al. Dual stimuli-responsive polypyrrole nanoparticles for anticancer therapy. J. Drug Deliv. Sci. Technol. 2018, 47, 176–180. [Google Scholar]
- Ma, W.; Xu, A.; Ying, J.; Li, B.; Jin, Y. Biodegradable core-shell copolymer-phospholipid nanoparticles for combination chemotherapy: An in vitro study. J. Biomed. Nanotechnol. 2015, 11, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, J.; Wang, Y.; Zhou, M.; Li, D.; Zheng, S.; Luo, C.; Zhang, H.; Zhong, L.; Li, W.; et al. In situ apolipoprotein E-enriched corona guides dihydroartemisinin-decorating nanoparticles towards LDLr-mediated tumor-homing chemotherapy. Asian J. Pharm. Sci. 2019, 2. [Google Scholar] [CrossRef]
- Liu, L.; Wei, Y.; Zhai, S.; Chen, Q.; Xing, D. Dihydroartemisinin and transferrin dual-dressed nano-graphene oxide for a pH-triggered chemotherapy. Biomaterials 2015, 62, 35–46. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Tran, T.H.; Kim, J.O.; Yong, C.S.; Nguyen, C.N. Enhancing the in vitro anti-cancer efficacy of artesunate by loading into poly-d,l-lactide-co-glycolide (PLGA) nanoparticles. Arch. Pharm. Res. 2015, 38, 716–724. [Google Scholar] [CrossRef]
- Jieqing, M.; Rongfa, G.; Haitao, S.; Fei, L.; Chaogeng, X.; Mingqi, L.; Tianshu, K. Comparison of anticancer activity between lactoferrin nanoliposome and lactoferrin in Caco-2 cells in vitro. Food Chem. Toxicol. 2013, 59, 72–77. [Google Scholar]
- Zucker, D.; Andriyanov, A.V.; Steiner, A.; Raviv, U.; Barenholz, Y. Characterization of PEGylated nanoliposomes co-remotely loaded with topotecan and vincristine: Relating structure and pharmacokinetics to therapeutic efficacy. J. Control. Release 2012, 160, 281–289. [Google Scholar] [CrossRef]
- Nancy, D.; Samantha, M.; Jason, T.; Cynthia, L.; Vasilios, P.; Elena, G.; Christopher, W.E.; Lia, L.; Walid, S.K.; Omid, G.; et al. Nanoliposome targeting in breast cancer is influenced by the tumor microenvironment. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 71–81. [Google Scholar]
- Perillo, E.; Allard-vannier, E.; Falanga, A.; Stiuso, P.; Teresa, M.; Galdiero, M.; Galdiero, S.; Chourpa, I. Quantitative and qualitative effect of gH625 on the nanoliposome-mediated delivery of mitoxantrone anticancer drug to HeLa cells. Int. J. Pharm. 2015, 488, 59–66. [Google Scholar] [CrossRef]
- Liang, X.-J.; Chen, C.; Zhao, Y.; Wang, P.C. Circumventing Tumor Resistance to Chemotherapy by Nanotechnology. Methods Mol. Biol. 2010, 596, 467–488. [Google Scholar]
- Larson, L.; Ghandehari, H. Polymeric Conjugates Drug Delivery. Chem. Mater. 2012, 24, 840–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alven, S.; Aderibigbe, B.A.; Balogun, M.O.; Matshe, W.M.R.; Ray, S.S. Polymer-drug conjugates containing antimalarial drugs and antibiotics. J. Drug Deliv. Sci. Technol. 2019, 53, 101171. [Google Scholar] [CrossRef]
- Cai, H.; Wang, X.; Zhang, H.; Sun, L.; Pan, D.; Gong, Q.; Gu, Z.; Luo, K. Enzyme-sensitive biodegradable and multifunctional polymeric conjugate as theranostic nanomedicine. Appl. Mater. Today 2018, 11, 207–218. [Google Scholar] [CrossRef]
- Vogus, D.R.; Evans, M.E.; Pusuluri, A.; Barajas, A.; Zhang, M.; Krishnan, V.; Nowak, M.; Menegatti, S.; Helgeson, M.E.; Squires, T.M.; et al. A hyaluronic acid conjugate engineered to synergistically and sequentially deliver gemcitabine and doxorubicin to treat triple negative breast cancer. J. Control. Release 2017, 267, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Armiñán, A.; Palomino-Schätzlein, M.; Deladriere, C.; Arroyo-Crespo, J.J.; Vicente-Ruiz, S.; Vicent, M.J.; Pineda-Lucena, A. Metabolomics facilitates the discrimination of the specific anti-cancer effects of free- and polymer-conjugated doxorubicin in breast cancer models. Biomaterials 2018, 162, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lv, S.; Zhang, D.; Deng, M.; Zhang, X.; Tang, Z. A polypeptide based podophyllotoxin conjugate for the treatment of multi drug resistant breast cancer with enhanced efficiency and minimal toxicity. Acta Biomater. 2018, 73, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Sultana, F.; Imran-Ul-Haque, M.M.; Arafat, M.; Sharmin, S. An overview of nanogel drug delivery system. J. Appl. Pharm. Sci. 2013, 3, S95–S105. [Google Scholar]
- Martin, L.; Wilson, C.G.; Koosha, F.; Tetley, L.; Gray, A.; Senel, S.; Uchegbu, I.F. The release of model macromolecules may be controlled by the hydrophobicity of palmitoyl glycol chitosan hydrogels. J. Control. Release 2002, 80, 87–100. [Google Scholar] [CrossRef]
- Namazi, H.; Adeli, M. Dendrimers of citric acid and poly (ethylene glycol) as the new drug-delivery agents. Biomaterials 2005, 26, 1175–1183. [Google Scholar] [CrossRef]
- Saluja, V.; Mankoo, A.; Saraogi, G.K.; Tambuwala, M.M.; Mishra, V. Smart dendrimers: Synergizing the targeting of anticancer bioactives. J. Drug Deliv. Sci. Technol. 2019, 52, 15–26. [Google Scholar] [CrossRef]
- Manjili, H.K.; Malvandi, H.; Mousavi, M.S.; Attari, E.; Danafar, H. In vitro and in vivo delivery of artemisinin loaded PCL–PEG–PCL micelles and its pharmacokinetic study. Artif. Cells Nanomed. Biotechnol. 2018, 46, 926–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramazani, A.; Keramati, M.; Malvandi, H.; Danafar, H.; Kheiri Manjili, H. Preparation and in vivo evaluation of anti-plasmodial properties of artemisinin-loaded PCL-PEG-PCL nanoparticles. Pharm. Dev. Technol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Liu, Y.; Kou, L.; Tu, Y.; Tang, X.; Zhu, L. Tumor-targeted drug delivery and sensitization by MMP2-responsive polymeric micelles. Nanomed. Nanotechnol. Biol. Med. 2019, 19, 71–80. [Google Scholar] [CrossRef]
- Movellan, J.; Urban, P.; Moles, E.; Fuente, J.M.; Sierra, T.; Serrano, J.L.; Fernandez-busquetts, X. Amphiphilic dendritic derivatives as nanocarriers for the targeted delivery of antimalarial drugs. Biomaterials 2014, 13, 7940–7950. [Google Scholar] [CrossRef]
- Bhadra, D.; Bhadra, S.; Jain, N.K. Pegylated Lysine Based Copolymeric Dendritic Micelles For Solubilization And Delivery Of Artemether. J. Pharm. Pharm. Sci. 2005, 8, 467–482. [Google Scholar]
- Anders, C.; Carey, L. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin. Breast Cancer 2009, 9, S73–S81. [Google Scholar] [CrossRef]
- Ikhuoria, E.B.; Bach, C. Introduction to Breast Carcinogenesis Symptoms, Risks Factors, Treatment and Management. Eur. J. Eng. Res. Sci. 2018, 3, 58–66. [Google Scholar] [CrossRef]
- Humberto, P.J.; Marilie, D.G.; Hope, L.E.; Jia, C.; Antonia, M.C.; Alfred, I.N.; Regina, M.S.; Mary, S.W.; Susan, L.T. Urinary concentrations of environmental phenols and their associations with breast cancer incidence and mortality following breast cancer. Environ. Int. 2019, 130, 104890. [Google Scholar]
- Lee, S. Human serum albumin: A nanomedicine platform targeting breast cancer cells. J. Drug Deliv. Sci. Technol. 2019, 52, 652–659. [Google Scholar] [CrossRef]
- Smoot, B.; Wampler, M.; Topp, K.S. Breast Cancer Treatments and Complications: Implications for Rehabilitation. Rehabil. Oncol. 2009, 27. [Google Scholar] [CrossRef]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Bedell, C. A changing paradigm for cancer treatment: The advent of new oral chemotherapy agents. Clin. J. Oncol. Nurs. 2003, 7, 5–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birner, A. Pharmacology of oral chemotherapy agents. Clin. J. Oncol. Nurs. 2003, 7, 11–19. [Google Scholar] [CrossRef]
- Shapiro, D.E.; Boggs, S.R.; Rodrigue, J.R.; Urry, H.L.; Algina, J.J.; Hellman, R.; Ewen, F. Stage II breast cancer: Differences between four coping patterns in side effects during adjuvant chemotherapy. J. Psychosom. Res. 1997, 43, 143–157. [Google Scholar] [CrossRef]
- Cabral, H.; Kataoka, K. Progress of drug-loaded polymeric micelles into clinical studies. J. Control. Release 2014, 19, 465–476. [Google Scholar] [CrossRef] [Green Version]
- Lua, Y.; Park, K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int. J. Pharm. 2013, 453, 198–214. [Google Scholar] [CrossRef] [Green Version]
- Kato, K.; Chin, K.; Yoshikawa, T.; Yamaguchi, K.; Tsuji, Y.; Esaki, T. Phase II study of NK105, a paclitaxel incorporating micellar nanoparticle, for previously treated advanced or recurrent gastric cancer. Investig. New Drugs 2012, 30, 1621–1627. [Google Scholar] [CrossRef]
- Fields, J.S.; Burris, H.A.; Infante, J.R.; Greco, F.A.; Spigel, D.R.; Kawamura, S.; Ishioka, T.; Yamazaki, H.; Bendell, J.C. A phase I study of NK012 in combination with 5 fluorouracil with or without leucovorin in patients (pts) with advanced solid tumors. J. Clin. Oncol. 2012, 30. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Chauhan, P.N.; Noolvi, M.N.; Chaturvedi, K.; Ganguly, K.; Shukla, S.S.; Nadagouda, M.N.; Aminabhavi, T.M. Polymeric micelles: Basic research to clinical practice. Int. J. Pharm. 2017, 532, 249–268. [Google Scholar] [CrossRef]
- Takahashi, A.; Yamamoto, Y.; Yasunaga, M.; Koga, Y.; Kuroda, J.; Takigahira, M.; Harada, M.; Saito, H.; Hayashi, T.; Kato, Y.; et al. NC-6300, an epirubicin-incorporating micelle, extends the antitumor effect and reduces the cardiotoxicity of epirubicin. Cancer Sci. 2013, 104, 920–925. [Google Scholar] [CrossRef]
- Gong, J.; Chen, M.; Zheng, Y.; Wang, S.; Wang, Y. Polymeric micelles drug delivery system in oncology. J. Control. Release 2012, 159, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Plummer, R.; Wilson, R.H.; Calvert, H.; Boddy, A.V.; Griffin, M.; Sludden, J.; Tilby, M.J.; Eatock, M.; Pearson, D.G.; Ottley, C.J.; et al. A Phase I clinical study of cisplatin-incorporated polymeric micelles (NC-6004) in patients with solid tumours. Br. J. Cancer 2011, 104, 593–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valle, J.W.; Armstrong, A.; Newman, C.; Alakhov, V.; Pietrzynski, G.; Brewer, J. A phase 2 study of SP1049C, doxorubicin in P-glycoprotein- targeting pluronics in patients with advanced adeno-carcinoma of the esophagus and gastro esophageal junction. Investig. New Drugs 2011, 29, 1029–1037. [Google Scholar] [CrossRef]
- Mendes, L.P.; Pan, J.; Torchilin, V.P. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- StarPharma DEP™ Docetaxel. Available online: https://starpharma.com/drug_delivery/dep_docetaxel (accessed on 5 May 2020).
- Dias, A.P.; Santos, S.S.; da Silva, J.V.; Parise-Filhoa, R.; Ferreira, E.I.; Seoud, O.E.; Giaroll, J. Dendrimers in the context of nanomedicine. Int. J. Pharm. 2020, 573, 118814. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B.A. Combination Therapy Strategies for the Treatment. Molecules 2019, 24, 3601. [Google Scholar] [CrossRef] [Green Version]
- Narmani, A.; Mohammadnejad, J.; Yavari, K. Synthesis and evaluation of polyethylene glycol- and folic acid-conjugated polyamidoamine G4 dendrimer as nanocarrier. J. Drug Deliv. Sci. Technol. 2019, 50, 278–286. [Google Scholar] [CrossRef]
- Uram, Ł.; Filipowicz, A.; Misiorek, M.; Pienkowska, N.; Markowicz, J.; Walajtys-Rode, E.; Wolowiec, S. Biotinylated PAMAM G3 dendrimer conjugated with celecoxib and/or Fmoc-L-Leucine and its cytotoxicity for normal and cancer human cell lines. Eur. J. Pharm. Sci. 2018, 124, 1–9. [Google Scholar] [CrossRef]
- Qiu, L.Y.; Bae, Y.H. Polymer architecture and drug delivery. Pharm. Res. 2006, 23. [Google Scholar] [CrossRef]
- Li, J.; Liang, H.; Liu, J.; Wang, Z. Poly (amidoamine)(PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy. Int. J. Pharm. 2018, 546, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska1, J.; Posadas, I.; Játiva, P.; Bugaj-Zarebska, M.; Urbanczyk-Lipkowska, Z.; Ceña, V. Second Generation Amphiphilic Poly-Lysine Dendrons Inhibit Glioblastoma Cell Proliferation without Toxicity for Neurons or Astrocytes. PLoS ONE 2016, 11, e0165704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorzkiewicz, M.; Konopka, M.; Janaszewska, A.; Tarasenko, I.I.; Nadezhda, N.; Sheveleva, N.N.; Gajeke, A.; Neelov, I.M.; Klajnert-Maculewicz, B. Application of new lysine-based peptide dendrimers D3K2 and D3G2 for gene delivery: Specifi cytotoxicity to cancer cells and transfection in vitro. Bioinorg. Chem. 2020, 95, 103504. [Google Scholar] [CrossRef]
- Caminade, A.-M. Phosphorus Dendrimers as Nanotools against Cancers. Molecules 2020, 25, 3333. [Google Scholar] [CrossRef]
- Liu, M.; Frechet, J. Designing dendrimers for drug delivery. Pharm. Sci. Technol. Today 1999, 2, 393–401. [Google Scholar] [CrossRef]
- Shrivastava, P.K.; Singh, R.; Shrivastava, S.K. Polyamidoamine dendrimer and dextran conjugates: Preparation, characterization, and in vitro and in vivo evaluation. Chem. Pap. 2010, 64, 592–601. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M.V. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013, 334, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Ghalandarlaki, N.; Alizadeh, A.M.; Ashkani-esfahani, S. Nanotechnology-Applied Curcumin for Different Diseases Therapy. BioMed Res. Int. 2014, 2014, 394264. [Google Scholar] [CrossRef] [Green Version]
- Gha, M.; Dehghan, G.; Baradaran, B.; Zarebkohan, A.; Mansoori, B.; Soleymani, J.; Dolatabadi, J.; Ezzati, N.; Hamblin, M.R. Co-delivery of curcumin and Bcl-2 siRNA by PAMAM dendrimers for enhancement of the therapeutic e ffi cacy in HeLa cancer cells. Colloids Surf. B Biointerfaces 2020, 188, 110762. [Google Scholar]
- Guo, X.; Kang, X.; Wang, Y.; Zhang, X.; Li, C.; Liu, Y.; Du, L. Co-delivery of cisplatin and doxorubicin by covalently conjugating with polyamidoamine dendrimer for enhanced synergistic cancer therapy. Acta Biomaterilia 2019, 84, 367–377. [Google Scholar] [CrossRef]
- Kitchens, K.M.; Kolhatkar, R.B.; Swaan, P.W.; Ghandehari, H. Endocytosis inhibitors prevent poly(amidoamine) dendrimer internalization and permeability across Caco-2 cells. Mol. Pharm. 2008, 5, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Chittasupho, C.; Anuchapreeda, S.; Sarisuta, N. CXCR4 targeted dendrimer for anti-cancer drug delivery and breast cancer cell migration inhibition. Eur. J. Pharm. Biopharm. 2017, 119, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Y.; HuangFu, M.; Xiao, Y.; Zhang, T.; Han, M.; Xu, D.; Li, F.; Ling, D.; Jin, Y.; et al. Pluronic-attached polyamidoamine dendrimer conjugates overcome drug resistance in breast cancer. Nanomedicine 2016, 11, 2917–2934. [Google Scholar] [CrossRef]
- Kojima, C.; Suehiro, T.; Watanabe, K.; Ogawa, M.; Fukuhara, A.; Nishisaka, E.; Harada, A.; Kono, K.; Inui, T.; Magata, Y. Doxorubicin-conjugated dendrimer/collagen hybrid gels for metastasis-associated drug delivery systems. Acta Biomater. 2013, 9, 5673–5680. [Google Scholar] [CrossRef]
- Kaminskas, L.M.; Kelly, B.D.; McLeod, V.M.; Sberna, G.; Owen, D.J.; Boyd, B.J.; Porter, C.J. Characterisation and tumour targeting of PEGylated polylysine dendrimers bearing doxorubicin via a pH labile linker. J. Control. Release 2011, 152, 241–248. [Google Scholar] [CrossRef]
- Mehta, D.; Leong, N.; McLeod, V.M.; Kelly, B.D.; Pathak, R.; Owen, D.J.; Porter, C.J.; Kaminskas, L.M. Reducing dendrimer generation and PEG chain length increases drug release and promotes anticancer activity of PEGylated polylysine dendrimers conjugated with doxorubicin via a cathepsin-cleavable peptide linker. Mol. Pharm. 2018, 15, 4568–4576. [Google Scholar] [CrossRef] [PubMed]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, X.; Wang, Z.; Meng, M.; Li, X.; Ning, Q. Generation 4 polyamidoamine dendrimers is a novel candidate of nano-carrier for gene delivery agents in breast cancer treatment. Cancer Lett. 2010, 298, 34–49. [Google Scholar] [CrossRef]
- Chen, A.M.; Santhakumaran, L.M.; Nair, S.K.; Amenta, P.S.; Thomas, T.; He, H.; Thomas, T.J. Oligodeoxynucleotide nanostructure formation in the presence of polypropyleneimine dendrimers and their uptake in breast cancer cells. Nanotechnology 2006, 17, 5449–5460. [Google Scholar] [CrossRef]
- Pourianazar, N.T.; Gunduz, U. CpG oligodeoxynucleotide-loaded PAMAM dendrimer-coated magnetic nanoparticles promote apoptosis in breast cancer cells. Biomed. Pharmacother. 2016, 78, 81–91. [Google Scholar] [CrossRef]
- Xin, G.; Zhao, X.; Duan, X.; Ning, Q.; Meng, M.; Meng, D.; Liu, L. Antitumor Effect of a Generation 4 Polyamidoamine Dendrimer/Cyclooxygenase-2 Antisense Oligodeoxynucleotide Complex on Breast Cancer In Vitro and In Vivo. Cancer Biother. Radiopharm. 2012, 27, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Kulhari, H.; Pooja, D.; Shrivastava, S.; Kuncha, M.; Naidu, V.G.; Bansal, V.; Sistla, R.; Adams, D.J. Trastuzumab-grafted PAMAM dendrimers for the selective delivery of anticancer drugs to HER2-positive breast cancer. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Miyano, T.; Wijagkanalan, W.; Kawakami, S.; Yamashita, F.; Hashida, M. Anionic Amino Acid Dendrimer−Trastuzumab Conjugates for Specific Internalization in HER2-Positive Cancer Cells. Mol. Pharm. 2010, 7, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowska, M.; Stanczyk, M.; Janaszewska, A.; Sobierajska, E.; Chworos, A.; Klajnert-Maculewicz, B. Multicomponent conjugates of anticancer drugs and monoclonal antibody with PAMAM dendrimers to increase efficacy of HER-2 positive breast cancer therapy. Pharm. Res. 2019, 36, 154. [Google Scholar] [CrossRef] [Green Version]
- Aleanizy, F.S.; Alqahtani, F.Y.; Seto, S.; Al Khalil, N.; Aleshaiwi, L.; Alghamdi, M.; Alquadeib, B.; Alkahtani, H.; Aldarwesh, A.; Alqahtani, Q.H.; et al. Trastuzumab Targeted Neratinib Loaded Poly-Amidoamine Dendrimer Nanocapsules for Breast Cancer Therapy. Int. J. Nanomed. 2020, 15, 5433. [Google Scholar] [CrossRef]
- Chan, C.; Cai, Z.; Reilly, R.M. Trastuzumab Labeled to High Specific Activity with 111 In by Conjugation to G4 PAMAM Dendrimers Derivatized with Multiple DTPA Chelators Exhibits Increased Cytotoxic Potency on HER2-Positive Breast Cancer Cells. Pharm. Res. 2013, 30, 1999–2009. [Google Scholar] [CrossRef]
- Oddone, N.; Lecot, N.; Fernández, M.; Rodriguez-Haralambides, A.; Cabral, P.; Cerecetto, H.; Benech, J.C. In vitro and in vivo uptake studies of PAMAM G4.5 dendrimers in breast cancer. J. Nanobiotechnol. 2016, 14, 45. [Google Scholar] [CrossRef] [Green Version]
- Bielawski, K.; Bielawska, A.; Muszy, A.; Czarnomysy, R. Cytotoxic activity of G3 PAMAM-NH 2 dendrimer-chlorambucil conjugate in human breast cancer cells. Environ. Toxicol. Pharmacol. 2011, 32, 364–372. [Google Scholar] [CrossRef]
- Kociecka, B.; Surazynski, A.; Miltyk, W.; Palka, J. The effect of telmisartan on collagen biosynthesis depends on the status of estrogen activation in breast cancer cells. Eur. J. Pharmacol. 2010, 628, 51–56. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Al-Abd, A.M. Thermoresponsive dendrimers based on oligoethylene glycols: Design, synthesis and cytotoxic activity against MCF-7 breast cancer cells. Eur. J. Med. Chem. 2013, 69, 848–854. [Google Scholar] [CrossRef]
- Li, K.; Wen, S.; Larson, A.C.; Shen, M.; Zhang, Z.; Chen, Q.; Shi, X.; Zhang, G. Multifunctional dendrimer-based nanoparticles for in vivo MR/CT dual-modal molecular imaging of breast cancer. Int. J. Nanomed. 2013, 8, 2589–2600. [Google Scholar] [CrossRef] [Green Version]
- Finlay, J.; Roberts, C.M.; Lowe, G.; Loeza, J.; Rossi, J.J.; Glackin, C.A. RNA-Based TWIST1 Inhibition via Dendrimer Complex to Reduce Breast Cancer Cell Metastasis. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Wallerand, H.; Robert, G.; Pasticier, G.; Ravaud, A.; Ballanger, P.; Reiter, R.E.; Ferrière, J.M. September. The epithelial-mesenchymal transition-inducing factor TWIST is an attractive target in advanced and/or metastatic bladder and prostate cancers. Urol. Oncol. Semin. Orig. Investig. 2010, 28, 473–479. [Google Scholar]
- Winnicka, K.; Bielawski, K.; Rusak, M.; Bielawska, A. The Effect of Generation 2 and 3 Poly (amidoamine) Dendrimers on Viability of Human Breast Cancer Cells. J. Health Sci. 2009, 55, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Debnath, S.; Saloum, D.; Dolai, S.; Sun, C.; Averick, S.; Raja, K.; Fata, J. Dendrimer-curcumin conjugate: A water soluble and effective cytotoxic agent against breast cancer cell lines. Anti-Cancer Agents Med. Chem. 2013, 13, 1531–1539. [Google Scholar] [CrossRef]
- Yao, H.; Veine, D.M.; Fay, K.S.; Staszewski, E.D.; Zeng, Z.Z.; Livant, D.L. The PHSCN dendrimer as a more potent inhibitor of human breast cancer cell invasion, extravasation, and lung colony formation. Breast Cancer Res. Treat. 2011, 125, 363–375. [Google Scholar] [CrossRef]
- Lozano-Cruz, T.; Gómez, R.; de la Mata, F.J.; Ortega, P. New bow-tie cationic carbosilane dendritic system with a curcumin core as an anti-breast cancer agent. New J. Chem. 2018, 42, 11732–11738. [Google Scholar] [CrossRef]
- Winnicka, K.; Bielawski, K.; Bielawska, A. Synthesis and cytotoxic activity of G3 PAMAM-NH 2 dendrimer-modified digoxin and proscillaridin A conjugates in breast cancer cells. Pharmacol. Rep. 2010, 62, 414–423. [Google Scholar] [CrossRef]
- Mei, M.; Ren, Y.; Zhou, X.; Yuan, X.B.; Li, F.; Jiang, L.H.; Kang, C.S.; Yao, Z. Suppression of breast cancer cells in vitro by polyamidoamine-dendrimer-mediated 5-fluorouracil chemotherapy combined with antisense micro-RNA 21 gene therapy. J. Appl. Polym. Sci. 2011, 114, 3760–3766. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, D.; Zhang, M.; Sun, Y.; Zhang, X.; Guan, G.; Zhao, X.; Qiao, M.; Chen, D.; Hu, H. The cellular uptake mechanism, intracellular transportation, and exocytosis of polyamidoamine dendrimers in multidrug-resistant breast cancer cells. Int. J. Nanomed. 2016, 11, 3677–3690. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Pan, D.; Li, J.; Hu, J.; Bains, A.; Guys, N.; Zhu, H.; Li, X.; Luo, K.; Gong, Q.; et al. Enzyme-responsive peptide dendrimer-gemcitabine conjugate as a controlled-release drug delivery vehicle with enhanced antitumor efficacy. Acta Biomater. 2017, 55, 153–162. [Google Scholar] [CrossRef]
- Matai, L.; Gopinath, P. Hydrophobic myristic acid modified PAMAM dendrimers augment the delivery of tamoxifen to breast cancer cells. RSC Adv. 2016, 6, 24808–24819. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, Q.; Wang, C.; Xu, J.; Wu, J.P.; Kirk, T.B.; Ma, D.; Xue, W. Star-shaped amphiphilic hyperbranched polyglycerol conjugated with dendritic poly (L-lysine) for the codelivery of docetaxel and MMP-9 siRNA in cancer therapy. ACS Appl. Mater. Interfaces 2016, 8, 12609–12619. [Google Scholar] [CrossRef]
- Kaminskas, L.M.; McLeod, V.M.; Porter, C.J.; Boyd, B.J. Association of chemotherapeutic drugs with dendrimer nanocarriers: An assessment of the merits of covalent conjugation compared to noncovalent encapsulation. Mol. Pharm. 2012, 9, 355–373. [Google Scholar] [CrossRef]
- Bugno, J.; Hsu, H.J.; Hong, S. Tweaking dendrimers and dendritic nanoparticles for controlled nano-bio interactions: Potential nanocarriers for improved cancer targeting. J. Drug Target. 2015, 23, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.M.; Turrin, C.O. Dendrimers for drug delivery. J. Mater. Chem. B 2014, 2, 4055–4066. [Google Scholar] [CrossRef]
- Labieniec-Watala, M.; Watala, C. PAMAM dendrimers: Destined for success or doomed to fail? Plain and modified PAMAM dendrimers in the context of biomedical applications. J. Pharm. Sci. 2015, 104, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Jain, K.; Mehra, N.K.; Jain, N.K. Dendrimers in anticancer drug delivery: Mechanism of interaction of drug and dendrimers. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1626–1634. [Google Scholar] [CrossRef]
- Jones, C.F.; Campbell, R.A.; Franks, Z.; Gibson, C.C.; Thiagarajan, G.; Vieira-de-Abreu, A.; Sukavaneshvar, S.; Mohammad, S.F.; Li, D.Y.; Ghandehari, H.; et al. Cationic PAMAM dendrimers disrupt key platelet functions. Mol. Pharm. 2012, 9, 1599–1611. [Google Scholar] [CrossRef] [Green Version]
- Lesniak, W.G.; Mishra, M.K.; Jyoti, A.; Balakrishnan, B.; Zhang, F.; Nance, E.; Romero, R.; Kannan, S.; Kannan, R.M. Biodistribution of fluorescently labeled PAMAM dendrimers in neonatal rabbits: Effect of neuroinflammation. Mol. Pharm. 2013, 10, 4560–4571. [Google Scholar] [CrossRef]
- Valenzuela-Oses, J.K.; García, M.C.; Feitosa, V.A.; Pachioni-Vasconcelos, J.A.; Gomes-Filho, S.M.; Lourenço, F.R.; Cerize, N.N.P.; Bassères, D.S.; Rangel-Yagui, C.O. Development and characterization of miltefosine-loaded polymeric micelles for cancer treatment. Mater. Sci. Eng. C 2017, 81, 327–333. [Google Scholar] [CrossRef]
- Mahmud, A.; Lavasanifar, A. The effect of block copolymer structure on the internalization of polymeric micelles by human breast cancer cells. Colloids Surf. B Biointerfaces 2005, 45, 82–89. [Google Scholar] [CrossRef]
- Suroshe, S.; Nerkar, P.; Patil, K.; Chalikwar, S. Breast cancer: Recent review on micelles as nano-carriers for treatment. Indo Am. J. Pharm. Res. 2019, 9, 2231–6876. [Google Scholar]
- Zhong, G.; Yang, C.; Liu, S.; Zheng, Y.; Lou, W.; Yng, J.; Bao, C.; Cheng, W.; Tan, J.P.K.; Gao, S.; et al. Polymers with distinctive anticancer mechanism that kills MDR cancer cells and inhibits tumor metastasis. Biomaterials 2019, 199, 76–87. [Google Scholar] [CrossRef]
- Chen, T.; Tu, L.; Wang, G.; Qi, N.; Wu, W.; Zhang, W.; Feng, J. Multi-functional chitosan polymeric micelles as oral paclitaxel delivery systems for enhanced bioavailability and anti-tumor e fficacy. Intern. J. Pharm. 2020, 578, 119105. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, B.; Meng, X.; She, P.; Zhang, P.; Cao, Y.; Zhang, X. Preparation of dual-drug conjugated polymeric micelles with synergistic anti-cancer efficacy in vitro. J. Drug Deliv. Sci. Technol. 2018, 43, 388–396. [Google Scholar] [CrossRef]
- Wei, X.; Liu, L.; Li, X.; Wang, Y.; Guo, X.; Zhao, J.; Zhou, S. Selectively targeting tumor-associated macrophages and tumor cells with polymeric micelles for enhanced cancer chemo-immunotherapy. J. Control. Release 2019, 313, 42–53. [Google Scholar] [CrossRef]
- Senevirathne, S.A.; Washington, K.E.; Biewer, M.C.; Stefan, M.C. PEG based anti-cancer drug conjugated prodrug micelles for the delivery of anti-cancer agents. J. Mater. Chem. B. 2016, 4, 360–370. [Google Scholar] [CrossRef]
- Guo, X.; Zhao, Z.; Chen, D.; Qiao, M.; Wan, F.; Cun, D.; Sun, Y.; Yang, M. Co-delivery of resveratrol and docetaxel via polymeric micelles to improve the treatment of drug-resistant tumors. Asian J. Pharm. Sci. 2019, 14, 78–85. [Google Scholar] [CrossRef]
- Lang, T.; Dong, X.; Zheng, Z.; Liu, Y.; Wang, G.; Yin, Q.; Li, Y. Tumor microenvironment-responsive docetaxel-loaded micelle combats metastatic breast cancer. Sci. Bull. 2019, 64, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Logie, J.; Ganesh, A.N.; Aman, A.M.; Al-awar, R.S.; Shoichet, M.S. Preclinical evaluation of taxane-binding peptide-modified polymeric micelles loaded with docetaxel in an orthotopic breast cancer mouse model. Biomaterials 2017, 123, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Rijcken, C.J.; Bansal, R.; Hennink, W.E.; Storm, G.; Prakash, J. Complete regression of breast tumour with a single dose of docetaxel-entrapped core-cross-linked polymeric micelles. Biomaterials 2015, 53, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Peng, J.; Tan, L.; Wu, J.; Shi, K.; Qu, Y.; Wei, X.; Qian, Z. Mild photothermal therapy/photodynamic therapy/chemotherapy of breast cancer by Lyp-1 modi fi ed Docetaxel/IR820 Co-loaded micelles. Biomaterials 2016, 106, 119–133. [Google Scholar] [CrossRef]
- Kutty, R.V.; Feng, S. Cetuximab conjugated vitamin E TPGS micelles for targeted delivery of docetaxel for treatment of triple negative breast cancers. Biomaterials 2013, 34, 10160–10171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Chen, L.; Tan, L.; Zhao, Q.; Luo, F.; Wei, Y.; Qian, Z. PEG-PCL based micelle hydrogels as oral docetaxel delivery systems for breast cancer therapy. Biomaterials 2014, 35, 6972–6985. [Google Scholar] [CrossRef]
- Tan, L.; Ma, B.; Chen, L.; Peng, J.; Qian, Z. Toxicity evaluation and anti-tumor study of docetaxel loaded mPEG-polyester micelles for breast cancer therapy. J. Biomed. Nanotechnol. 2017, 13, 393–408. [Google Scholar] [CrossRef]
- Li, Y.; Jin, M.; Shao, S.; Huang, W.; Yang, F.; Chen, W.; Zhang, S.; Xia, G.; Gao, Z. Small-sized polymeric micelles incorporating docetaxel suppress distant metastases in the clinically-relevant 4T1 mouse breast cancer model. BMC Cancer 2014, 14, 329. [Google Scholar] [CrossRef] [Green Version]
- Raza, K.; Kumar, N.; Misra, C.; Kaushik, L.; Guru, S.K.; Kumar, P.; Malik, R.; Bhushan, S.; Katare, O.P. Dextran-PLGA-loaded docetaxel micelles with enhanced cytotoxicity and, better pharmacokinetic profile. Int. J. Biol. Macromol. 2016, 88, 206–212. [Google Scholar] [CrossRef]
- Kutty, R.V.; Chia, S.L.; Setyawati, M.I.; Muthu, M.S.; Feng, S.; Leong, T.D. In vivo and ex vivo proofs of concept that cetuximab conjugated vitamin E TPGS micelles increases ef fi cacy of delivered docetaxel against triple negative breast cancer. Biomaterials 2015, 63, 58–69. [Google Scholar] [CrossRef] [Green Version]
- Koo, A.N.; Min, K.H.; Lee, H.J.; Lee, S.; Kim, K.; Kwon, I.C.; Cho, S.H.; Jeong, S.Y.; Lee, S.C. Tumor accumulation and antitumor efficacy of docetaxel-loaded core-shell-corona micelles with shell-specific redox-responsive cross-links. Biomaterials 2012, 33, 1489–1499. [Google Scholar] [CrossRef]
- Muthu, M.S.; Kutty, R.V.; Luo, Z.; Xie, J.; Feng, S. Theranostic vitamin E TPGS micelles of transferrin conjugation for targeted co-delivery of docetaxel and ultra bright gold nanoclusters. Biomaterials 2015, 39, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Peng, J.; Zhao, Q.; Zhang, L.; Tang, X.; Chen, L.; Lei, M.; Qian, Z. A Novel MPEG-PDLLA-PLL Copolymer for Docetaxel Delivery in Breast Cancer Therapy. Theranostics 2017, 7, 2652–2672. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zeng, X.; Liang, X.; Yang, Y.; Li, X.; Chen, H.; Huang, L.; Mei, L.; Feng, S.-S. The chemotherapeutic potential of PEG-b-PLGA copolymer micelles that combine chloroquine as autophagy inhibitor and docetaxel as an anti-cancer drug. Biomaterials 2014, 35, 9144–9154. [Google Scholar] [CrossRef]
- Jun, Y.J.; Jadhav, V.B.; Min, J.H.; Cui, J.X.; Chae, S.W.; Cjoi, J.M.; Kim, I.; Choi, S.; Lee, H.J.; Sohn, Y.S. Stable and efficient delivery of docetaxel by micelle-encapsulation using a tripodal cyclotriphosphazene amphiphile. Int. J. Pharm. 2012, 422, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Enteshari, S.; Varshosaz, J.; Minayian, M.; Hassanzadeh, F. Antitumor activity of raloxifene-targeted poly (styrene maleic loaded with docetaxel in breast cancer-bearing mice. Investig. New Drugs 2018, 32, 206–216. [Google Scholar] [CrossRef]
- Varshosaz, J.; Dehkordi, A.J.; Setayesh, S. Magnetic polyvinyl caprolactam–polyvinyl acetate–polyethylene glycol micelles for docetaxel delivery in breast cancer: An in vitro study on two cell lines of breast cancer. Pharm. Dev. Technol. 2017, 22, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zheng, M.; Gong, P.; Deng, J.; Yi, H.; Zhang, P.; Zhang, Y.; Liu, P.; Ma, Y.; Cai, L. Polypeptide cationic micelles mediated co-delivery of docetaxel and siRNA for synergistic tumor therapy. Biomaterials 2013, 34, 3431–3438. [Google Scholar] [CrossRef]
- Tong, S.; Xiang, B.; Dong, D.; Qi, X. Enhanced antitumor efficacy and decreased toxicity by self-associated docetaxel in phospholipid-based micelles. Int. J. Pharm. 2012, 434, 413–419. [Google Scholar] [CrossRef]
- Ho, M.Y.; Mackey, J.R. Presentation and management of docetaxel-related adverse effects in patients with breast cancer. Cancer Manag. Res. 2014, 6, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Esmaeli, B.; Valero, V. Epiphora and canalicular stenosis associated with adjuvant docetaxel in early breast cancer: Is excessive tearing clinically important? J. Clin. Oncol. 2013, 31, 2076–2077. [Google Scholar] [CrossRef] [PubMed]
- Wakharde, A.A.; Awad, A.H.; Bhagat, A.; Karuppayil, S.M. Synergistic activation of doxorubicin against cancer: A review. Am. J. Clin. Microbiol. Antimicrob. 2018, 1, 1–6. [Google Scholar]
- Zhao, N.; Woodle, M.C.; Mixson, A.J. Advances in delivery systems for doxorubicin. J. Nanomed. Nanotechnol. 2018, 9, 519. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhang, C.; Zhang, E.; Chen, H.; Zhen, Y.; Zhang, S.; Zhang, S. Zwitterionic pH-responsive hyaluronic acid polymer micelles for delivery of doxorubicin. Colloids Surf. B Biointerfaces 2019, 178, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Tian, L.; Hu, J.; Park, I.; Bae, Y.H. Prevention of metastasis in a 4T1 murine breast cancer model by doxorubicin carried by folate conjugated pH sensitive polymeric micelles. J. Control. Release 2011, 152, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Alakhova, D.Y.; Zhao, X.; Band, V.; Batrakova, E.V.; Kabanov, A.V. Eradication of cancer stem cells in triple negative breast cancer using doxorubicin/pluronic polymeric micelles. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102124. [Google Scholar] [CrossRef]
- Bae, Y.; Diezi, T.A.; Zhao, A.; Kwon, G.S. Mixed polymeric micelles for combination cancer chemotherapy through the concurrent delivery of multiple chemotherapeutic agents. J. Control. Release 2007, 122, 324–330. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Li, C.; Zheng, H.; Song, H.; Xiong, F.; Qiu, T.; Yang, J. Cisplatin-crosslinked glutathione-sensitive micelles loaded with doxorubicin for combination and targeted therapy of tumors. Carbohydr. Polym. 2017, 155, 407–415. [Google Scholar] [CrossRef]
- Varshosaz, J.; Sadeghi-Aliabadi, H.; Ghasemi, S.; Behdadfar, B. Use of Magnetic Folate-Dextran-Retinoic Acid Micelles for Dual Targeting of Doxorubicin in Breast Cancer. BioMed Res. Int. 2013, 2013, 680712. [Google Scholar] [CrossRef]
- Lv, L.; Qiu, K.; Yu, X.; Chen, C.; Qin, F.; Shi, Y.; Ou, J.; Zhang, T.; Zhu, H.; Wu, J.; et al. Amphiphilic copolymeric micelles for doxorubicin and curcumin co-delivery to reverse multidrug resistance in breast cancer. J. Biomed. Nanotechnol. 2016, 12, 973–985. [Google Scholar] [CrossRef]
- Cuong, N.; Jiang, J.; Li, Y.; Chen, J.; Jwo, S.; Hsieh, M. Doxorubicin-Loaded PEG-PCL-PEG Micelle Using Xenograft Model of Nude Mice: Effect of Multiple Administration of Micelle on the Suppression of Human Breast Cancer. Cancers 2011, 3, 61–78. [Google Scholar] [CrossRef]
- Yu, H.; Cui, Z.; Yu, P.; Guo, C.; Feng, B.; Jiang, T.; Wang, S.; Yin, Q.; Zhong, D.; Yang, X.; et al. pH and NIR light-responsive micelles with hyperthermia-triggered tumor penetration and cytoplasm drug release to reverse doxorubicin resistance in breast cancer. Adv. Funct. Mater. 2015, 25, 2489–2500. [Google Scholar] [CrossRef]
- Lee, E.S.; Na, K.; Bae, Y.H. Doxorubicin loaded pH-sensitive polymeric micelles for reversal of resistant MCF-7 tumor. J. Control. Release 2005, 103, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Hassanzadeh, F.; Sadeghi-Aliabadi, H.; Larian, Z.; Rostami, M. Synthesis of Pluronic Ò F127-poly (methyl vinyl ether-alt-maleic acid ) copolymer and production of its micelles for doxorubicin delivery in breast cancer. Chem. Eng. J. 2014, 240, 133–146. [Google Scholar] [CrossRef]
- Sun, Y.; Zou, W.; Bian, S.; Huang, Y.; Tan, Y.; Liang, J.; Fan, Y.; Zhang, X. Bioreducible PAA-g-PEG graft micelles with high doxorubicin loading for targeted antitumor effect against mouse breast carcinoma. Biomaterials 2013, 34, 6818–6828. [Google Scholar] [CrossRef]
- Shuai, X.; Ai, H.; Nasongkla, N.; Kim, S.; Gao, J. Micellar carriers based on block copolymers of poly (q-caprolactone) and poly (ethylene glycol) for doxorubicin delivery. J. Control. Release 2004, 98, 415–426. [Google Scholar] [CrossRef]
- Cuong, N.V.; Li, Y.L.; Hsieh, M.F. Targeted delivery of doxorubicin to human breast cancers by folate-decorated. J. Mater. Chem. 2012, 22, 1006. [Google Scholar] [CrossRef]
- Cuong, N.V.; Hsieh, M.F.; Chen, Y.T.; Liau, I. Doxorubicin-Loaded Nanosized Micelles of a Star-Shaped Poly (ε-Caprolactone)-Polyphosphoester Block Co-polymer for Treatment of Human Breast Cancer for Treatment of Human Breast Cancer. J. Biomater. Sci. Polym. Ed. 2012, 22, 1409–1426. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Wang, S.; Ying, X.; Wang, Y.; Geng, P.; Deng, A.; Yu, Z. Doxorubicin-loaded redox-responsive micelles based on dextran and indomethacin for resistant breast cancer. Int. J. Nanomed. 2017, 12, 6153–6168. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.; Sun, Q.; Liang, B.; Shen, J.; Shuai, X. Targeting EGFR-overexpressing tumor cells using Cetuximab-immunomicelles loaded with doxorubicin and superparamagnetic iron oxide. Eur. J. Radiol. 2011, 80, 699–705. [Google Scholar] [CrossRef]
- Chen, C.; Cuong, N.Y.; Chen, Y.; So, R.C.; Liau, I.; Hsieh, M.F. Overcoming Multidrug Resistance of Breast Cancer Cells by the Micellar Doxorubicin Nanoparticles of mPEG-PCL-Graft-Cellulose. J. Nanosci. Nanotechnol. 2010, 10, 53–60. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, Y.; Wu, Z.; Lundberg, P.; Malkoch, M.; Nystrom, A.M. Hyperbranched Copolymer Micelles as Delivery Vehicles of Doxorubicin in Breast Cancer Cells. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 280–288. [Google Scholar] [CrossRef]
- Cheng, Y.; Hao, J.; Lee, L.A.; Biewer, M.C.; Wang, Q.; Stefan, M.C. Thermally Controlled Release of Anticancer Drug from Self-Assembled Î3-Substituted Amphiphilic Poly (ε-caprolactone) Micellar Nanoparticles. Biomacromolecules 2012, 13, 2163–2173. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Kouhé, T.T.; Duhem, N.; Ucakar, B.; Staub, A.; Draoui, N.; Feron, O.; Préat, V. Vitamin E-based micelles enhance the anticancer activity of doxorubicin. Int. J. Pharm. 2014, 476, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, Y.; Feng, X.; Deng, M.; Xie, G.; Wang, J.; Zhang, L.; Liu, Q.; Yuan, P. Micellar nanoparticles loaded with gemcitabine and doxorubicin showed synergistic effect. Colloids Surf. B Biointerfaces 2014, 113, 158–168. [Google Scholar] [CrossRef]
- Cagel, M.; Bernabeu, E.; Gonzalez, L.; Lagomarsino, E.; Zubillaga, M.; Moretton, M.A.; Chiappetta, D.A. Mixed micelles for encapsulation of doxorubicin with enhanced in vitro cytotoxicity on breast and ovarian cancer cell lines versus Doxil®. Biomed. Pharmacother. 2017, 95, 894–903. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Huang, Y.; Gao, F.; Sha, X.; Fang, X. Pluronic-based functional polymeric mixed micelles for co-delivery of doxorubicin and paclitaxel to multidrug resistant tumor. Int. J. Pharm. 2014, 488, 44–58. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, S.; Xie, Z.; Zhang, H. A synergistic combination therapy with paclitaxel and doxorubicin loaded micellar nanoparticles. Colloids Surf. B Biointerfaces 2014, 116, 41–48. [Google Scholar] [CrossRef]
- Abu Samaan, T.M.; Samec, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Paclitaxel’s Mechanistic and Clinical Effects on Breast Cancer. Biomolecules 2019, 9, 789. [Google Scholar] [CrossRef] [Green Version]
- Barbuti, A.M.; Chen, Z.S. Paclitaxel through the ages of anticancer therapy: Exploring its role in chemoresistance and radiation therapy. Cancers 2015, 7, 2360–2371. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Qiu, L.; Wang, X.; Li, T.; Han, L. Preparation and evaluation of carboxymethyl chitosan-rhein polymeric micelles with synergistic antitumor effect for oral delivery of paclitaxel. Carbohydr. Polym. 2019, 206, 121–131. [Google Scholar] [CrossRef]
- Zajdel, A.; Wilczok, A.; Jelonek, K.; Musiał-Kulik, M.; Foryś, A.; Li, S.; Kasperczyk, J. Cytotoxic effect of paclitaxel and lapatinib Co-delivered in polylactide-co-Poly (ethylene glycol) micelles on HER-2-Negative breast Cancer cells. Pharmaceutics 2019, 11, 169. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Zheng, Y.; Hu, X.; Fan, Z.; Jing, X. Anti-tumor activity of folate targeted biodegradable polymer—Paclitaxel conjugate micelles on EMT-6 breast cancer model. Mater. Sci. Eng. C 2015, 53, 68–75. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Wang, D.; Zou, Y.; Qu, X.; He, C.; Deng, Y.; Jin, Y.; Zhou, Y.; Zhou, Y.; et al. Concurrently suppressing multidrug resistance and metastasis of breast cancer by co-delivery of paclitaxel and honokiol with pH-sensitive polymeric micelles. Acta Biomater. 2017, 62, 144–156. [Google Scholar] [CrossRef]
- Oda, C.M.R.; Barros, A.L.B.; Farnandes, R.S.; Miranda, S.E.M.; Teixeira, X.M.; Cardoso, N.V.; Oliveira, C.M.; Leite, A.E. Freeze-dried diethylenetriaminepentaacetic acid-functionalized polymeric micelles containing paclitaxel: A kit formulation for theranostic application in cancer. J. Drug Deliv. Sci. Technol. 2018, 46, 182–187. [Google Scholar] [CrossRef]
- Zhang, L.; He, Y.; Ma, G.; Song, C.; Sun, H. Paclitaxel-loaded polymeric micelles based on poly (ɛ-caprolactone)-poly (ethylene glycol)-poly (ɛ-caprolactone) triblock copolymers: In vitro and in vivo evaluation. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 925–934. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. The eradication of breast cancer and cancer stem cells using octreotide modified paclitaxel active targeting micelles and salinomycin passive targeting micelles. Biomaterials 2012, 33, 679–691. [Google Scholar] [CrossRef]
- Yin, T.; Wang, L.; Yin, L.; Zhou, J.; Huo, M. Co-delivery of hydrophobic paclitaxel and hydrophilic AURKA speci fi c siRNA by redox-sensitive micelles for effective treatment of breast cancer. Biomaterials 2015, 61, 10–25. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H.; Peng, J.; Chen, L.; Tan, L.; Huang, Y.; Qian, Z. Targeting therapy of neuropilin-1 receptors overexpressed breast cancer by paclitaxel-loaded CK3-conjugated polymeric micelles. J. Biomed. Nanotechnol. 2016, 12, 2097–2111. [Google Scholar] [CrossRef]
- Kelishady, P.D.; Saadat, E.; Ravar, F.; Akbari, H.; Dorkoosh, F. Pluronic F127 polymeric micelles for co-delivery of paclitaxel and lapatinib against metastatic breast cancer: Preparation, optimization and in vitro evaluation. Pharm. Dev. Technol. 2014, 7450, 1009–1017. [Google Scholar]
- Hou, J.; Sun, E.; Sun, C.; Wang, J.; Yang, L.; Jia, X.B.; Zhang, Z.H. Improved oral bioavailability and anticancer ef fi cacy on breast cancer of paclitaxel via Novel Soluplus—Solutol 1 HS15 binary mixed micelles system. Int. J. Pharm. 2016, 512, 186–193. [Google Scholar] [CrossRef]
- Zhong, Y.; Goltsche, K.; Cheng, L.; Xie, F.; Meng, F.; Deng, C.; Zhong, Z.; Haag, R. Hyaluronic acid-shelled acid-activatable paclitaxel prodrug micelles effectively target and treat CD44-overexpressing human breast tumor xenografts in vivo. Biomaterials 2016, 84, 250–261. [Google Scholar] [CrossRef]
- Hasenstein, J.R.; Shin, H.; Kasmerchak, K.; Buehler, D.; Kwon, G.S.; Kozak, K.R. Antitumor Activity of Triolimus: A Novel Multidrug-Loaded Micelle Containing Paclitaxel, Rapamycin, and 17-AAG. Mol. Cancer Ther. 2012, 11, 2233–2243. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.S.; Chung, H.C.; Im, S.A.; Park, Y.H.; Kim, C.S.; Kim, S.B.; Rha, S.Y.; Lee, M.Y.; Ro, J. Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res. Treat. 2008, 108, 241–250. [Google Scholar] [CrossRef]
- Mei, D.; Lin, Z.; Fu, J.; He, B.; Gao, W.; Ma, L.; Dai, W.; Zhang, H.; Wang, X.; Wang, J.; et al. The use of α-conotoxin ImI to actualize the targeted delivery of paclitaxel micelles to a 7 nAChR-overexpressing breast cancer. Biomaterials 2015, 42, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Situ, J.Q.; Li, W.S.; Shan, C.L.; You, J.; Yuan, H.; Hu, F.Q.; Du, Y.Z. High tolerated paclitaxel nano-formulation delivered by poly (lactic-co-glycolic acid)-g-dextran micelles to efficient cancer therapy. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 855–866. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, N.; Cheng, R.; Zhao, C.; Liu, Z.; Li, X.; Liu, J.; Tian, Z. pH multistage responsive micellar system with charge-switch and PEG layer detachment for co-delivery of paclitaxel and curcumin to synergistically eliminate breast cancer stem cells. Biomaterials 2017, 147, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Dong, X.; Li, J.; Wang, M.; Luo, L.; Li, Z.; Lu, X.; He, R.; Xu, R.; Gong, M. Free paclitaxel-loaded E-selectin binding peptide modified micelle self-assembled from hyaluronic acid-paclitaxel conjugate inhibit breast cancer metastasis in a murine model. Int. J. Pharm. 2017, 258, 33–46. [Google Scholar] [CrossRef]
- Wang, T.; Petrenko, V.A.; Torchilin, V.P. Paclitaxel-loaded polymeric micelles modified with MCF-7 cell-specific phage protein: Enhanced binding to target cancer cells and increased cytotoxicity. Mol. Pharm. 2010, 7, 1007–1014. [Google Scholar] [CrossRef] [Green Version]
- Bernabeu, E.; Gonzalez, L.; Cagel, M.; Gergic, E.P.; Moretton, M.A.; Chiappetta, D.A. Novel Soluplus ®—TPGS mixed micelles for encapsulation of paclitaxel with enhanced in vitro cytotoxicity on breast and ovarian cancer cell lines. Colloids Surf. B Biointerfaces 2016, 140, 403–411. [Google Scholar] [CrossRef]
- Chen, Y.; Yue, Q.; De, G.; Wang, J.; Li, Z.; Xiao, S.; Yu, H.; Ma, H.; Sui, F.; Zhao, Q. Inhibition of breast cancer metastasis by paclitaxel-loaded pH responsive poly (β-amino ester) copolymer micelles. Nanomedicine 2017, 12, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, S.; Mei, L.A.; Parmar, C.K.; Gillespie, J.W.; Praveen, K.P.; Petrenko, V.A.; Torchilin, V.P. Paclitaxel-Loaded PEG-PE—Based Micellar Nanopreparations Targeted with Tumor-Speci fi c Landscape Phage Fusion Protein Enhance Apoptosis and efficiently Reduce Tumors. Mol. Cancer Ther. 2014, 13, 2864–2875. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Wang, Z.; Nie, S.; Song, L.; He, T.; Yang, S.; Yang, X.; Yi, C.; Wu, Q.; Gong, C. Biodegradable polymeric micelles coencapsulating paclitaxel and honokiol: A strategy for breast cancer therapy in vitro and in vivo. Int. J. Nanomed. 2017, 12, 1499–1514. [Google Scholar] [CrossRef] [Green Version]
- Lang, T.; Dong, X.; Huang, Y.; Ran, W.; Yin, Q.; Zhang, P.; Zhang, Z.; Yu, H.; Li, Y. Ly6Chi Monocytes Delivering pH-Sensitive Micelle Loading Paclitaxel Improve Targeting Therapy of Metastatic Breast Cancer. Adv. Funct. Mater. 2017, 27, 3. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, L.; Han, L.; Sha, X.; Fang, X. Difunctional Pluronic copolymer micelles for paclitaxel delivery: Synergistic effect of folate-mediated targeting and Pluronic-mediated overcoming multidrug resistance in tumor cell lines. Int. J. Pharm. 2007, 337, 63–73. [Google Scholar] [CrossRef]
- Lu, J.; Huang, Y.; Zhao, W.; Marquez, R.T.; Meng, X.; Li, J.; Gao, X.; Venkataramanan, R.; Wang, Z.; Li, S. PEG-derivatized embelin as a nanomicellar carrier for delivery of paclitaxel to breast and prostate cancers. Biomaterials 2013, 34, 1591–1600. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.J.; Yang, C.X.S.; Zhu, Q.L.; Chen, W.L.; Li, F.; Yuan, Z.Q.; Liu, Y.; You, B.G.; Zhang, X.N. Low-density lipoprotein-coupled micelles with reduction and pH dual sensitivity for intelligent co-delivery of paclitaxel and siRNA to breast tumor. Int. J. Nanomed. 2017, 12, 3375–3393. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Li, L.; Zhang, X.; Liang, Y.; Pu, Z.; Wang, L.; Mo, J. Curcumin: A calixarene derivative micelle potentiates anti-breast cancer stem cells effects in xenografted, triple-negative breast cancer mouse models. Drug Deliv. 2017, 24, 1470–1481. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Liu, J.; Zhu, H.; Hussain, A.; Liu, Q.; Li, J.; Shen, Y.; Cheng, J.; Guo, S. PEGylated Doxorubicin Micelles Loaded with Curcumin Exerting Synergic Effects on Multidrug Resistant Tumor Cells. J. Nanosci. Nanotechnol. 2016, 16, 2873–2880. [Google Scholar] [CrossRef]
- Jung, K.H.; Lee, J.H.; Park, J.W.; Kim, D.H.; Moon, S.H.; Cho, Y.S.; Lee, K.H. Targeted therapy of triple negative MDA-MB-468 breast cancer with curcumin delivered by epidermal growth factor—Conjugated phospholipid nanoparticles. Oncol. Lett. 2018, 15, 9093–9100. [Google Scholar] [CrossRef] [Green Version]
- Medel, S.; Syrova, Z.; Kovacik, L.; Hrdy, J.; Hornacek, M.; Jager, E.; Hruby, M.; Lund, R.; Cmarko, D.; Stepanek, P.; et al. Curcumin-bortezomib loaded polymeric nanoparticles for synergistic cancer therapy. Eur. Polym. J. 2017, 93, 116–131. [Google Scholar] [CrossRef]
- Liu, L.; Sun, L.; Wu, Q.; Guo, W.; Li, L.; Chen, Y.; Li, Y.; Gong, C.; Qian, Z.; Wei, Y. Curcumin loaded polymeric micelles inhibit breast tumor growth and spontaneous pulmonary metastasis. Int. J. Pharm. 2013, 443, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Beaudoin, J.J.; Vinod, N.; Min, Y.; Makita, N.; Bludau, H.; Jordan, R.; Wang, A.; Sokolsky, M.; Kabanov, A.V. Co-delivery of paclitaxel and cisplatin in poly (2-oxazoline) polymeric micelles: Implications for drug loading, release, pharmacokinetics and outcome of ovarian and breast cancer treatments. Biomaterials 2019, 192, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Tang, Z.; Shah, A.; Lv, S.; Zhang, D.; Zhang, Y.; Chen, X. Cisplatin Loaded Methoxy Poly (ethylene glycol)-block-Poly (l-glutamic acid-co-l-Phenylalanine) Nanoparticles against Human Breast Cancer Cell. Macromol. Biosci. 2014, 14, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hu, X.; Wu, S.; Xiao, H.; Cai, H.; Xie, Z.; Huang, Y.; Jing, X. Biological Characterization of Folate-Decorated Biodegradable Polymer—Platinum(II) Complex Micelles. Mol. Pharm. 2012, 9, 3200–3208. [Google Scholar] [CrossRef]
- He, S.; Yang, H.; Zhang, R.; Li, Y.; Duan, L. Preparation and in vitro–in vivo evaluation of teniposide nanosuspensions. Int. J. Pharm. 2015, 478, 131–137. [Google Scholar] [CrossRef]
- Kubisz, P.; Seghier, F.; Dobrotora, M.; Stasko, J.; Cronberg, S. Influence of teniposide on platelet functions in vitro. Thromb. Res. 1995, 77, 145–148. [Google Scholar] [CrossRef]
- Chu, B.; Shi, S.; Li, X.; Hu, L.; Shi, L.; Zhang, H.; Xu, Q.; Ye, L.; Lin, G.; Zhang, N.; et al. Preparation and evaluation of teniposide-loaded polymeric micelles for breast cancer therapy. Int. J. Pharm. 2016, 513, 118–129. [Google Scholar] [CrossRef]
- Zhang, J.; Kinoh, H.; Hespel, L.; Liu, X.; Quader, S.; Martin, J.; Chida, T.; Cabral, H.; Kataoka, K. Effective treatment of drug resistant recurrent breast tumors harboring cancer stem-like cells by staurosporine/epirubicin co-loaded polymeric micelles. J. Control. Release 2017, 264, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Min, H.K.; Kim, J.; Bae, S.M.; Shin, H.; Kim, M.S.; Park, S.; Lee, H.; Park, R.; Kim, I.; Kim, K.; et al. Tumoral acidic pH-responsive MPEG-poly (β-amino ester) polymeric micelles for cancer targeting therapy. J. Control. Release 2010, 144, 259–266. [Google Scholar] [CrossRef]
- Lee, E.S.; Gao, Z.; Kim, D.; Park, K.; Kwon, I.C.; Bae, Y.H. Super pH-sensitive multifunctional polymeric micelle for tumor pH(e) specific TAT exposure and multidrug resistance. J. Control. Release 2008, 129, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Sethuraman, V.A.; Na, K.; Bae, Y.H. pH-responsive sulfonamide/PEI system for tumor specific gene delivery: An in vitro study. Biomacromolecules 2006, 7, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Chida, T.; Miura, Y.; Cabral, H.; Nomoto, T.; Kataoka, K.; Nishiyama, N. Epirubicin-loaded polymeric micelles effectively treat axillary lymph nodes metastasis of breast cancer through selective accumulation and pH-triggered drug release. J. Control. Release 2018, 292, 130–140. [Google Scholar] [CrossRef]
- Gener, P.; Montero, S.; Xandri-Monje, H.; Díaz-Riascos, Z.V.; Rafael, D.; Andrade, F.; Martínez-Trucharte, F.; González, P.; Seras-Franzoso, J.; Manzano, A.; et al. Zileuton TM loaded in polymer micelles effectively reduce breast cancer circulating tumor cells and intratumoral cancer stem cells. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102106. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Hall, R.R.; Ahmed, A.U. Cancer stem cells: Cellular plasticity, niche and its Clinical Relevance. J. Stem Cell Res. Ther. 2016, 6, 363. [Google Scholar] [CrossRef] [PubMed]
- Lamch, L.; Bazyli, U.; Kulbacka, J.; Pietkiewicz, J.; Biezunska-Kusiak, K.; Wilk, K.A. Polymeric micelles for enhanced Photofrin II ® delivery, cytotoxicity and pro-apoptotic activity in human breast and ovarian cancer cells. Photodiagnosis Photodyn. Ther. 2014, 11, 570–585. [Google Scholar] [CrossRef]
- Marcos, X.; Padilla-beltrán, C.; Bernad-bernad, M.J.; Rosales-hernández, M.C.; Pérez-casas, S.; Correa-basurto, J. Controlled release of N-(2-hydroxyphenyl)-2-propylpentanamide nanoencapsulated in polymeric micelles of P123 and F127 tested as anti- proliferative agents in MDA-MB-231 cells. J. Drug Deliv. Sci. Technol. 2018, 48, 403–413. [Google Scholar] [CrossRef]
- Lu, H.; Blunden, B.M.; Scarano, W.; Lu, M.; Stenzel, M.H. Anti-metastatic effects of RAPTA-C conjugated polymeric micelles on multicellular tumor spheroids. Acta Biomater. 2016, 32, 68–76. [Google Scholar] [CrossRef]
- Wu, B.; Ong, M.S.; Groessl, M.; Adhireksan, Z.; Hartinger, C.G.; Dyson, P.J.; Davey, C.A. A ruthenium antimetastasis agent forms specific histone protein adducts in the nucleosome core. Chem. Eur. J. 2011, 17, 3562–3566. [Google Scholar] [CrossRef]
- Brinkman, A.M.; Chen, G.; Wang, Y.; Hedman, C.J.; Sherer, N.M.; Havighurst, T.C.; Gong, S.; Xu, W. Aminoflavone-loaded EGFR-targeted unimolecular micelle nanoparticles exhibit anti-cancer effects in triple negative breast cancer. Biomaterials 2016, 101, 20–31. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Pearson, R.M.; Lee, O.; Lee, C.W.; Chatterton, J.R.T.; Khan, S.A.; Hong, S. Dendron-Based Micelles for Topical Delivery of Endoxifen: A Potential Chemo-Preventive Medicine for Breast Cancer. Adv. Funct. Mater. 2014, 24, 2442–2449. [Google Scholar] [CrossRef]
- Zhong, P.; Gu, X.; Cheng, R.; Deng, C.; Meng, F.; Zong, Z. αvβ3 integrin-targeted micellar mertansine prodrug effectively inhibits triple-negative breast cancer in vivo. Int. J. Nanomed. 2017, 12, 7913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Zhang, E.; Yang, J.; Cao, Z. Strategies to improve micelle stability for drug delivery. Nano Res. 2018, 11, 4985–4998. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Yang, X.J.; Yin, Q.; Cai, K.M.; Wang, H.; Chaudhury, I.; Yao, C.; Zhou, Q.; Kwon, M.; Hartman, J.A. Investigating the optimal size of anticancer nanomedicine. Proc. Natl. Acad. Sci. USA 2014, 111, 15344–15349. [Google Scholar] [CrossRef] [Green Version]
- Stirland, D.L.; Nichols, J.W.; Miura, S.; Bae, Y.H. Mind the gap: A survey of how cancer drug carriers are susceptible to the gap between research and practice. J. Control. Release 2013, 172, 1045–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Shi, Y.; Kim, J.Y.; Park, K.; Cheng, J.X. Overcoming the barriers in micellar drug delivery: Loading efficiency, in vivo stability, and micelle-cell interaction. Expert Opin. Drug Deliv. 2010, 7, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Wakaskar, R. Polymeric Micelles and their Properties. J. Nanomed. Nanotechnol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, M. Clinical Applications of Polymeric Micelle Carrier Systems in Chemotherapy and Image Diagnosis of Solid Tumors. J. Exp. Clin. Med. 2011, 3, 151–158. [Google Scholar] [CrossRef]
- van Nostrum, C.F. Covalently cross-linked amphiphilic block copolymer micelles. Soft Matter 2011, 7, 3246–3259. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.F.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Talelli, M.; Barz, M.; Rijcken, C.J.; Kiessling, F.; Hennink, W.E.; Lammers, T. Core-Crosslinked Polymeric Micelles: Principles, Preparation, Biomedical Applications and Clinical Translation. Nano Today 2015, 10, 93–117. [Google Scholar] [CrossRef] [Green Version]
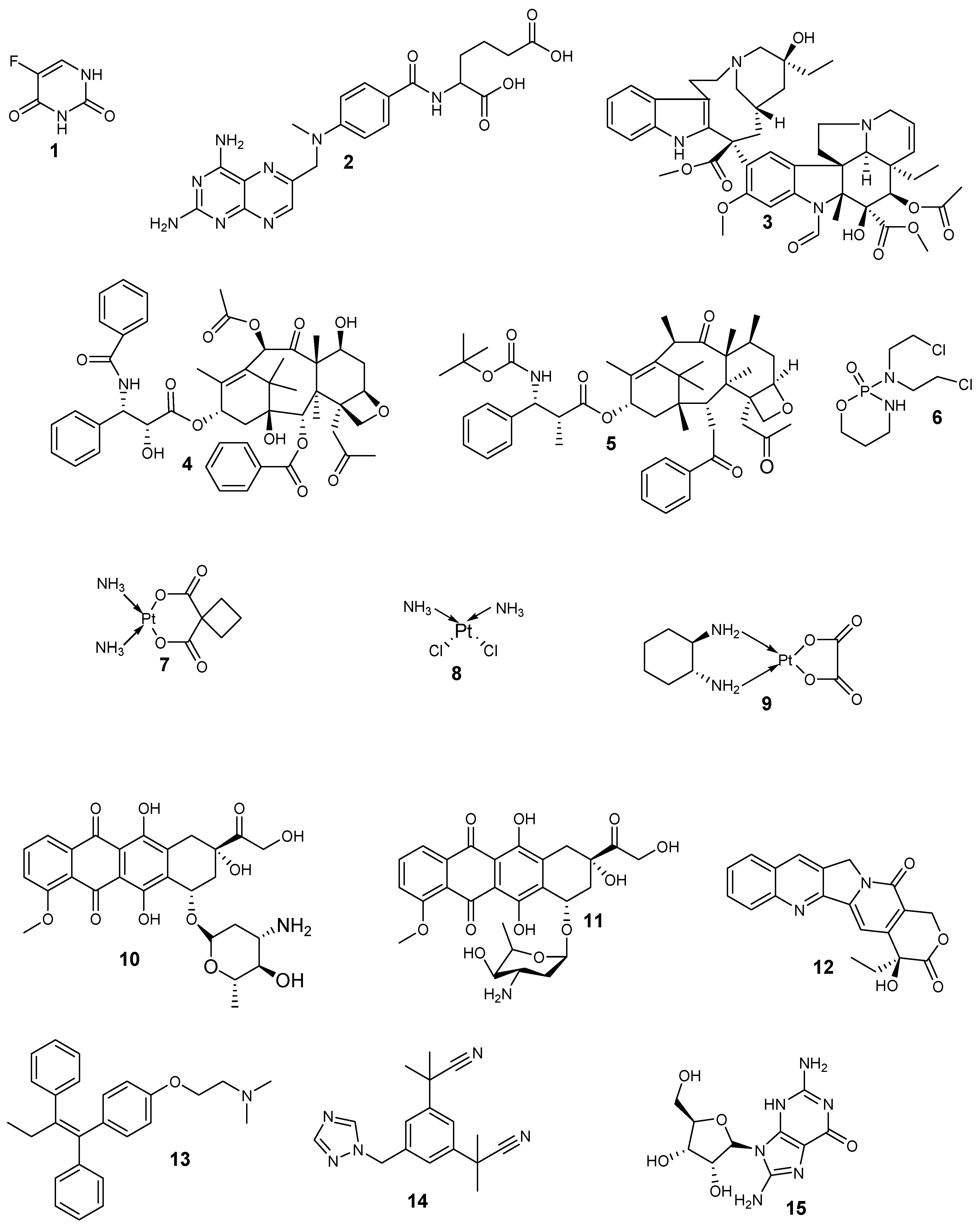


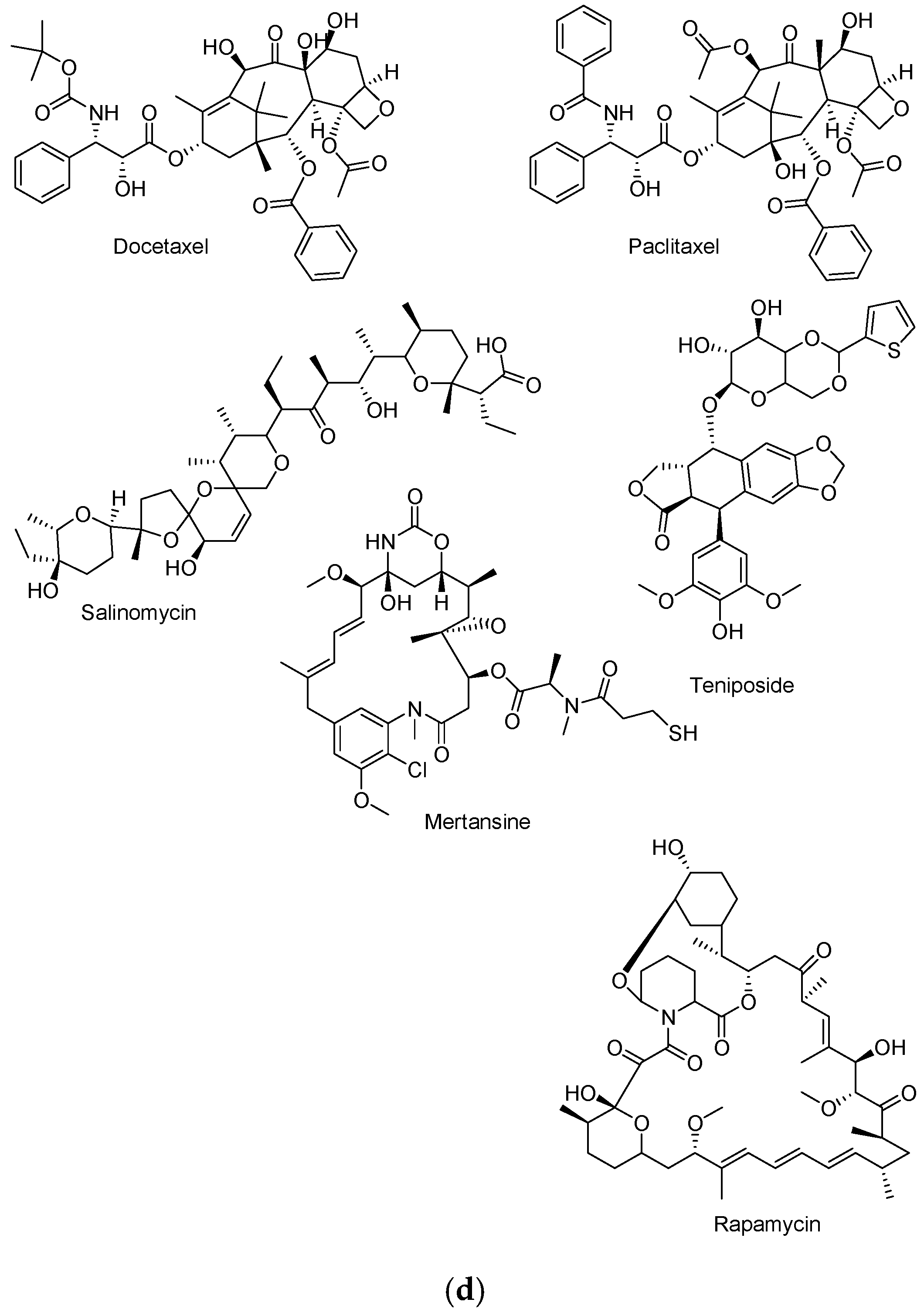
| Anticancer Drugs | Drug Class | Mode of Action | Side Effects |
|---|---|---|---|
| Doxorubicin Epirubicin Camptothecin | Antitumor antibiotics | Binds to DNA and prevent DNA synthesis. They cause changes in the chromatin structure via inhibition of topoisomerase II. | Vomiting/nausea, weight loss, alopecia, thrombocytopenia, neutropenia, anorexia, and impaired immunity. |
| Cyclophosphamide (Cytoxan) | Alkylating agent Anti-neoplastic drug | Result in cross-linkage in DNA strands. Inhibit DNA biosynthesis and cell division. | Severe vomiting/nausea, neurotoxicity, pulmonary fibrosis, immune suppression, and alopecia. |
| Paclitaxel (taxol) Docetaxel (Taxotere) | Plant alkaloids Taxane class | Hinders microtubule disassembly and increase microtubule assembly. Terminate cell division in metaphase. | Leutropenia, hypersensitivity, anaphylaxis, thrombocytopenia, myalgias, fatigue, neutropenia, arthralgias, stomatitis, and Peripheral neuropathy |
| Vincristine | Vinca alkaloids Mitotic inhibitor | Binds to mitotic tubules and prevent the formation of microtubule in the mitotic spindle, inhibits mitosis I metaphase. | Mucositis, leukopenis, weight change, neurotoxicity, constipation, fatigue, secondary neoplasm, and thrombocytopenia |
| 5-Fluorouracil | Pyrimidine antimetabolite | Inhibits enzyme formation needed for the synthesis of DNA. | Thrombocytopenia, peripheral neuropathy, mucositis, anaemia, neutropenia, neurotoxicity, cerebellar ataxia, and skin changes |
| Cisplatin Carboplatin | Platinum drugs | Inhibits the synthesis of DNA and prevents cell replication. | Ototoxicity, neurotoxicity, cardiotoxicity, nephrotoxicity, myelosuppression, neutropenia, and delayed hypersensitivity |
| Arimidex | aromatase inhibitor | Hinders aromatase, which intermediates transformation of androstenedione to estrone, which is then converted to estradiol. | Pain, mild nausea, vaginal dryness, osteoporosis, chest pain, increased risk for fractures, oedema, weakness, mild diarrhea, headache, and arthralgias |
| Tamoxifen | Selective estrogen receptor modulator | Strives with estrogen for receptor binding | Vaginal discharge, altered menses, amenorrhea, cough oedema, bone pain, musculoskeletal pain, dizziness, and endometrial hyperplasia. |
| Bevacizumab | Monoclonal antibody biological modifier | Binds to HER2 positive cancer cells and bring them up to be destroyed by the immune system. Cell proliferation inhibition. | Weakness, pain, flu-like symptoms, chills, diarrhea, abdominal pain, back pain, anorexia, congestive heart failure, and left ventricular cardiac dysfunction |
| Nanocarriers | Product/Trade Name | Copolymer Composition | Entrapped Drug | Cancer Therapy | Clinical Trial Phase | Ref |
|---|---|---|---|---|---|---|
| Micelles | Genexol®-PM/Cynviloq™ | mPEG-PDLLA | Paclitaxel | Lung and Breast Cancer | Phase IV | [48,49] |
| Micelles | NK105 | PEG-poly(aspartic acid) copolymer | Paclitaxel | Breast, colon, and gastric cancer | Phase III | [50] |
| Micelles | NK012 | poly(l-glutamic acid) | Irinotecan | TNBC and small lung cancer | Phase II | [51] |
| Micelles | NC-6300 | PEG-b-poly(aspartate-hydrazone) | Epirubicin | Breast and liver cancer | Phase I | [53] |
| Micelles | NK911 | PEG-P (Asp)-DOX | Doxorubicin | Solid tumors | Phase II | [53] |
| Micelles | NC-4016 | PEG-b-poly(β-glutamic acid) | Oxaliplatin | Solid Tumor | Phase I | [54] |
| Micelles | NC-6004 | PEG-b-poly(l-glutamic acid) | Cisplatin | Pancreatic cancer | Phase III | [55] |
| Micelles | siRNA micelles | siRNA | siRNA | Lung Cancer | Phase I | [55] |
| Micelles | SP1049C | Pluronic L61 and F127 | Doxorubicin | Adenocarcinoma | Phase III | [56] |
| Dendrimers | DEP® docetaxel | PEGylated PLL | Docetaxel | breast, Lung, Prostate, and ovarian cancer | Phase I | [58] |
| Dendrimers | DEP®cabazitaxel | Polylysine | Cabazitaxel | Testicular, ovarian, breast, bladder, and head and neck | Phase I/11 | [59] |
| Dendrimers | ImDendrim | Polylysine | 188Rerhenium complex | Liver cancer | Phase I | [59] |
| Dendrimers | MAG-Tn3 | Carbohydrate peptide lysine | Vaccine composed of tri Tn glycotope | Breast cancer | Phase I | [59] |
| Polymers | Anticancer Drugs | Breast Cancer Models | Therapeutic Outcomes | References |
|---|---|---|---|---|
| PAMAM | Doxorubicin and cisplatin | MDA-MB-231 and MCF-7 | The HA modified polymeric dendrimers showed good anticancer efficacy of when compared to the unmodified dendrimers | [73] |
| PAMAM | Doxorubicin | T47D and BT-549-Luc | High cellular uptake and binding. | [75] |
| pluronic F68- PAMAM | Doxorubicin | MCF-7/ADR | Improved antitumor activity | [76] |
| Collagen | Doxorubicin | MCF-7 and MDA-MB-231 | Potential anticancer efficacy | [77] |
| PAMAM | Antisense oligodeoxynucleotides | MDA-MB-231 | High cellular accumulation of the loaded drug. | [81] |
| polypropyleneimine | Oligodeoxynucleotide nanoparticles | MDA-MB-231 | High cellular uptake | [82] |
| PAMAM | CpG oligodeoxynucleotide | SKBR3 and MDA-MB231 | Decreased cell viability. | [83] |
| PAMAM | Antisense oligodeoxynucleotide | - | Growth tumor inhibition | [84] |
| PAMAM | Trastuzumab | MDA-MB-231and MDA-MB-453 | Sustained drug release profile and reduced breast cancer cell viability. | [85] |
| polylysine | Trastuzumab | SKBR3 and MCF-7 | High cellular internalization | [86] |
| PAMAM | Trastuzumab | MDA-MB-231 and SK-Br-3 | Increased anticancer efficacy | [89] |
| PAMAM | Florescein isothiocyanated | 4T1 | Good cellular uptake | [90] |
| oligoethylene glycol | Tetrabromohydroquinone | MCF-7 | Potent cytotoxicity efficacy against breast tumor. | [93] |
| PEG | Au nanoparticles | MCF-7 | Excellent antitumor efficacy. | [94] |
| PAMAM | siRNA | SUM1315 | High cellular uptake. | [95] |
| PAMAM | - | MDA-MB-231 and MCF-7 | Good cytotoxicity | [97] |
| - | Curcumin | BT549 and SKBr3 | Good anticancer activity | [98] |
| polylysine | PHSCN peptide | MDA-MB-231 and SUM-149 | High cytotoxicity | [99] |
| PAMAM | Proscillaridin A and digoxin | MDA-MB-231 and MCF-7 | High cell apoptosis | [101] |
| PAMAM | 5-fluorouracil | MCF-7 | Improved anticancer efficacy | [102] |
| PAMAM-NH2 | - | MCF-7/ADR | Concentration-dependent cytotoxicity | [103] |
| PEG | Gemcitabine | 4T1 | Suppressed tumor volume | [104] |
| PAMAM | Tamoxifen | MCF-7 | High cancer cell inhibitory effect | [105] |
| Polymers | Drugs | Cancer Cell Lines | Therapeutic Outcomes | References |
|---|---|---|---|---|
| mPEG-PDLA | Docetaxel and resveratrol | MCF-7 | Improved pharmacokinetic parameters, good biocompatibility, and safety. | [122] |
| BD-Peptide-PEG and BD-PEI | Docetaxel | 4T1 | High cellular uptake | [123] |
| Poly(d,l-lactide-co-2-methyl-2-carboxy-trimethylene carbonate) | Docetaxel | MDA-MB-231/H2N | High tumor growth inhibition and improvement in pharmacokinetic parameters | [124] |
| Monomethoxy PEG | Docetaxel | MDA-MB-231 | Sustained in vitro drug release profile and potent in vivo antitumor efficacy. | [125] |
| PCL-g-PEI | Docetaxel and NIR dye | 4T1 | High tumor growth inhibition | [126] |
| TPGS | Docetaxel | MDA-MB-231 and MDA-MB-468 | Greatly enhanced anticancer activity | [127] |
| PEG–PCL | Docetaxel | 4T1 | High tumor growth inhibition and decreased systemic toxicity | [128] |
| mPEG-polyester | Docetaxel | - | High tumor growth inhibition | [129] |
| mPEG2000-b-PDLLA1300 | Docetaxel | 4T1 | greater anticancer activity | [130] |
| PLGA | Docetaxel | MDA-MB-231 and MCF-7 | Improved antitumor activity | [131] |
| TPGS | Docetaxel | MDA-MB-231 | Hindering of EGFR-overexpressing tumor cell lines | [132] |
| PLys-PPhe | Docetaxel | - | Improved tumor specificity | [133] |
| TPGS | Docetaxel | MDA-MB-231 | High antitumor efficacy | [134] |
| MPEG-PDLLA-PLL | Docetaxel | 4T1 and MCF-7 | High tumor growth inhibition | [135] |
| PEG-b-PLGA | Docetaxel and chloroquine | MCF-7 | Good anticancer activity | [136] |
| [NP(PEG750)(GlyPheLeu)2Et]3 | Docetaxel | MDA-MB-231 | Excellent anticancer efficacy | [137] |
| Poly(styrene maleic acid)-poly (amide-ether-ester-imide) | Docetaxel | MC4-L2 | High tumor inhibition and increased survival in vivo | [138] |
| Polyvinyl caprolactam–polyvinyl acetate–PEG | Docetaxel and Fe3O4 | MDA-MB-231 and MCF-7 | Good anticancer activity | [139] |
| PEG-PLL-PLLeu | Docetaxel and siRNA-Bcl-2 | MCF-7 | Improved antitumor efficacy | [140] |
| mPEG2000- DSPE | Docetaxel | MCF-7 | Better antitumor activity | [141] |
| HA | Doxorubicin | MCF-7 | High tumor growth inhibition | [146] |
| Phis-PEG and PLLA-PEG | Doxorubicin | 4T1 | Moderate anticancer activity | [147] |
| Pluronic block copolymers | Doxorubicin | MDA-MB-231 and MDA-MB-468 | Potent tumor growth inhibition | [148] |
| PEG-poly(aspartate hydrazide) block copolymers | Doxorubicin and wortmannin | MCF-7 | Small particle size which is suitable for tumor-specific drug delivery | [149] |
| Carboxymethyl chitosan | Doxorubicin and cisplatin | HeLa | Synergistic anticancer effect | [150] |
| Dextran-retinoic acid | Doxorubicin | MDA-MB-468 and MCF-7 | Good antitumor activity | [151] |
| PEG2k-PLA5k | Doxorubicin and curcumin | MCF-7/ADR | Higher tumor accumulation and tumor growth inhibitory effect | [152] |
| PEG-PCL-PEG | Doxorubicin | MCF-7 | Suppressed tumor cells | [153] |
| - | Doxorubicin | MCF-7/ADR | Potential tumor growth inhibition | [154] |
| PLLA/PEG | Doxorubicin | MCF-7/DOXR | High cytotoxicity | [155] |
| Pluronic® F127-poly (methyl-vinyl ether-alt-maleic acid) copolymer | Doxorubicin | MCF-7 | Sustained drug release kinetics with good anticancer activity | [156] |
| PAA-g-PEG | Doxorubicin | 4T1 | High tumor accumulation | [157] |
| Poly (ε-caprolactone)-PEG | Doxorubicin | MCF-7 | Time-delayed anticancer activity | [158] |
| PEG–PCL | Doxorubicin | MCF-7 | High drug cellular uptake | [159] |
| Poly(ε-caprolactone)-polyphospho-ester | Doxorubicin | MCF-7 | Improved antitumor activity | [160] |
| Dextran and indomethacin | Doxorubicin | MCF-7 | Potential tumor growth inhibition | [161] |
| PEG–PCL | Doxorubicin and iron oxide | A431and MDA-MB-453 | Significant tumor inhibitory effect | [162] |
| mPEG-PCL-g-cellulose | Doxorubicin | MCF7/ADR | Good cellular internalization | [163] |
| Poly 2,2-bis (methylol) propionic acid (bis-MPA)-PEG | Doxorubicin | MCF-7 and MDA-MB-468 | Concentration-dependent anticancer activity | [164] |
| Poly (ε-caprolactone) | Doxorubicin | MCF-7 | Higher antitumor activity | [165] |
| TPGS | Doxorubicin | MCF-7 | 100% long-term survival of the treated mice | [166] |
| mPEG-PLA | Doxorubicin and gemcitabine | MCF-7 | Synergistic anticancer efficacy | [167] |
| Tetronic T1107, Pluronic F127, and TPGS | Doxorubicin | MDA-MB- 231 | High anticancer efficacy | [168] |
| Pluronic | Doxorubicin and paclitaxel | MCF-7 | Decreased cancer cell viability | [169] |
| MPEG-PCL-4-FBA | Doxorubicin and paclitaxel | MCF-7 | Synergistic antitumor efficacy | [170] |
| Carboxymethyl chitosan | Paclitaxel | MCF-7 | Improved oral drug bioavailability and synergistic anticancer activity. | [173] |
| Poly(2-oxazoline)- | Paclitaxel and cisplatin | LCC-6-MDR | Extended of the average lifespan of animals models in vivo | [174] |
| MPEG-b-P(LA-co-DHC/FA) | Paclitaxel | EMT-6 | Reduced tumor mass and high tumor growth inhibition | [175] |
| Poly(2-ethyl-2-oxazoline)-poly(d,l-lactide)- | Paclitaxel and honokiol | MCF-7/ADR | Decreased cancer cell viability and small particle size which is beneficial for tumor-targeted drug delivery. | [176] |
| 1,2-distearoyl-sn-glycero-3-phosphoethanol- amine-N-[methoxy(PEGl)-2000] | Paclitaxel | 4T1 | Great potential for theranostic application in the breast cancer therapy | [177] |
| Poly(ɛ-caprolactone)-PEG-poly(ɛ-caprolactone) triblock copolymers | Paclitaxel | EMT6 | Low cell viability | [178] |
| PEG-b-PCL | Paclitaxel and salinomycin | MCF-7 | High tumor growth inhibition | [179] |
| HA | paclitaxel and hydrophilic AURKA | MDA-MB- 231 | Decreased tumor volume | [180] |
| PEG-PDLLA | Paclitaxel | MDA-MB-231 | Significant tumor growth inhibition and cell apoptosis | [181] |
| Pluronic F127 | Paclitaxel and lapatinib | T-47D | Suppressed proliferation of breast cancer cell | [182] |
| Soluplus®—Solutol® HS15 | Paclitaxel | MDA-MB-231 | Good anticancer efficacy | [183] |
| HA | Paclitaxel | MCF-7 | Good anticancer activity with reduced drug toxicity | [184] |
| PEG-PLA | Paclitaxel, 17-AAG, and Rapamycin | MDA-MB-231 and 549 | High tumor growth inhibition | [185] |
| mPEG-b-poly(d,l-lactide) | Paclitaxel | - | Good clinical response rate | [186] |
| PEG-DSPE | Paclitaxel | MCF-7 | Greater anticancer activity | [187] |
| PLGA-g-dextran | Paclitaxel | MCF-7 | High anticancer efficacy | [188] |
| PPBV | Paclitaxel and curcumin | MCF-7 | Small tumor volume | [189] |
| HA | Paclitaxel | 4T1 | Good cellular uptake | [190] |
| PEG-phosphatidyl-ethanolamine | Paclitaxel | MCF-7 | High antitumor efficacy | [191] |
| Caprolactam–polyvinyl acetate–PEG and TPGS | Paclitaxel | MCF-7 and MDA-MB-231 | Superior anticancer efficacy | [192] |
| Poly(β-amino ester) | Paclitaxel | MDA-MB-231 | Good cellular uptake and suppression of the tumor metastasis | [193] |
| PEG-PE | Paclitaxel | MCF-7 | Enhanced anticancer activity | [194] |
| mPEG-poly(capro-lactone | Paclitaxel and honokiol | 4T1 | High cellular uptake and increased anticancer efficacy | [195] |
| - | Paclitaxel | 4T1 | High growth inhibition on tumor metastasis | [196] |
| Pluronic | Paclitaxel | MCF-7 | High anticancer activity | [197] |
| PEG | Paclitaxel | 4T1.2 | High anticancer activity | [198] |
| lipoprotein–N-succinyl chitosan–cystamine–urocanic acid | Paclitaxel and siRNA | MCF-7 | Superior anticancer activity | [199] |
| phosphorylated calixarene | Curcumin | BT-549 | Concentration-dependent cytotoxicity | [200] |
| PEG | Curcumin and doxorubicin | MCF-7/ADR | High synergistic anticancer activity | [201] |
| DSPE-PEG | Curcumin | MDA-MB-468 | Decreased cancer cell viability | [202] |
| mPEG-b-PLA- | Curcumin and bortezomib | MDA-MB-231 and MCF-7 | Maximum cellular uptake | [203] |
| MPEG-PCL | Curcumin | 4T1 | High anticancer activity | [204] |
| poly (2-oxazoline) | Cisplatin and paclitaxel | LCC-6-MDR | Prolonged plasma half-life and superior antitumor activity. | [205] |
| MPEG-block-poly (l-glutamic acid-co-l-phenylalanine) | Cisplatin | ZR-75-30 | Inhibition of cancer cell proliferation | [206] |
| mPEG-b-poly(l-lactide-co-2-methyl-2-carboxyl-propylene carbonate | Platinum (II) drug | MCF-7 | Dose-dependent cytotoxicity | [207] |
| MPEG-PCLA copolymer | Teniposide | MCF-7 | Significant cell growth inhibition and reduced tumor volumes in vivo | [210] |
| PEG-b-poly(aspartate-hydrazide-epirubicin) copolymer | Epirubicin and staurosporine | orthotopic 4T1-luc | Potent anticancer efficacy and prolonged animal survival. | [211] |
| MPEG-poly(β-amino ester) copolymer | Camptothecin | MDA-MB231 | Non-toxicity of the plain micelles and low cell viability for drug-loaded micelles | [212] |
| PEG-b-PBLA | Epirubicin | MDA-MB-231 | Good tumor growth inhibition and suppression of ALNM | [215] |
| Pluronic® F127 | Zileuton™ | MDA-MB-231 and MCF-7 | Significant tumor growth inhibition and inhibition of metastatic spread and cancer cell blood circulation. | [216] |
| Pluronic | Photofrin II® | MCF-7 | Improved in vitro pro-apoptotic and cytotoxic activity | [218] |
| Poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymers | N-(2-Hydroxy-phenyl)-2-propyl-pentanamide | MDA-MB-231 | Sustained drug release mechanism and anti-proliferative properties | [219] |
| PLA-P(HEA-CEMA-F) | Ruthenium complexes | MDA-MB-231 and MCF-7 | Improved anti-metastatic effect and tumor growth inhibition | [220] |
| PAMAM-PLA | Aminoflavone | MDA-MB-468and BT474 | Decreased cancer cell viability | [222] |
| Dendron | Endoxifen | - | Sustained drug release and improved permeation through skin | [223] |
| PEG | Mertansine | MDA-MB-231 | Suppressed tumor growth | [224] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alven, S.; Aderibigbe, B.A. The Therapeutic Efficacy of Dendrimer and Micelle Formulations for Breast Cancer Treatment. Pharmaceutics 2020, 12, 1212. https://doi.org/10.3390/pharmaceutics12121212
Alven S, Aderibigbe BA. The Therapeutic Efficacy of Dendrimer and Micelle Formulations for Breast Cancer Treatment. Pharmaceutics. 2020; 12(12):1212. https://doi.org/10.3390/pharmaceutics12121212
Chicago/Turabian StyleAlven, Sibusiso, and Blessing Atim Aderibigbe. 2020. "The Therapeutic Efficacy of Dendrimer and Micelle Formulations for Breast Cancer Treatment" Pharmaceutics 12, no. 12: 1212. https://doi.org/10.3390/pharmaceutics12121212
APA StyleAlven, S., & Aderibigbe, B. A. (2020). The Therapeutic Efficacy of Dendrimer and Micelle Formulations for Breast Cancer Treatment. Pharmaceutics, 12(12), 1212. https://doi.org/10.3390/pharmaceutics12121212