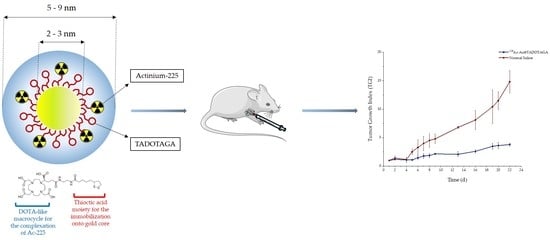
A Proof-of-Concept Study on the Therapeutic Potential of Au Nanoparticles Radiolabeled with the Alpha-Emitter Actinium-225
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Macrocycle-Coated Gold Nanoparticles
2.1.1. UV–Visible Spectroscopy
2.1.2. Hydrodynamic Diameter and Zeta Potential Measurements
2.1.3. Transmission Electron Microscopy (TEM)
2.2. Radiolabeling of Au@TADOTAGA Gold Nanoparticles with Actinium-225
2.3. In Vitro Stability Studies of [225Ac]225Ac-Au@TADOTAGA
2.4. Cell Cultures
2.5. MTT Toxicity Assay
2.6. Ex Vivo Biodistribution Studies of [225Ac]225Ac-Au@TADOTAGA
2.7. Therapeutic Efficacy Studies
2.8. Histopathology Studies
2.9. Statistical Analysis
3. Results
3.1. Synthesis and Characterization of Gold Nanoparticles
3.2. Radiolabeling of Au@TADOTAGA Gold Nanoparticles with Actinium-225
3.3. In Vitro Stability Studies
3.4. In Vitro Cytotoxicity Study of Au@TADOTAGA/[225Ac]225Ac-Au@TADOTAGA
3.5. Ex Vivo Biodistribution Studies: Intravenous vs. Intratumoral Injection of [225Ac]225Ac-Au@TADOTAGA
3.6. Therapeutic Efficacy Study
3.7. Histopathology Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Older, R.A.; Synder, B.; Krupski, T.L.; Glembocki, D.J.; Gillenwater, J.Y. Radioactive implant migration in patients treated for localized prostate cancer with interstitial brachytherapy. J. Urol. 2001, 165, 1590–1592. [Google Scholar] [CrossRef]
- Biswal, B.M.; Yusoff, Z. Application of nanotechnology in cancer treatment. In Engineering Applications of Nanotechnology; Korada, V.S., Hamid, N.H., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 269–311. [Google Scholar] [CrossRef]
- Laprise-Pelletier, M.; Simão, T.; Fortin, M.A. Gold nanoparticles in radiotherapy and recent progress in nanobrachytherapy. Adv. Healthc. Mater. 2018, 7. [Google Scholar] [CrossRef]
- Ehlerding, E.B.; Cai, W. Smaller agents for larger therapeutic indices: Nanoscale brachytherapy with 177Lu-labeled gold nanoparticles. J. Nucl. Med. 2016, 57, 834–835. [Google Scholar] [CrossRef] [Green Version]
- Laprise-Pelletier, M.; Lagueux, J.; Côté, M.F.; LaGrange, T.; Fortin, M.A. Low-dose prostate cancer brachytherapy with radioactive palladium–gold nanoparticles. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef]
- Thiele, N.A.; Wilson, J.J. Actinium-225 for targeted α therapy: Coordination chemistry and current chelation approaches. Cancer Biother. Radiopharm. 2018, 33, 336–348. [Google Scholar] [CrossRef]
- Kampf, G. Induction of DNA double-strand breaks by ionizing radiation of different quality and their relevance for cell inactivation. Radiobiol. Radiother. 1988, 29, 631–658. [Google Scholar]
- Hall, E.J.; Giaccia, A.J.; Technologies, I.O. Radiobiology for the Radiologist, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006. [Google Scholar]
- Gadbois, D.M.; Crissman, H.A.; Nastasi, A.; Habbersett, R.; Wang, S.K.; Chen, D.; Lehnert, B.E. Alterations in the progression of cells through the cell cycle after exposure to alpha particles or gamma rays. Radiat. Res. 1996, 146, 414–424. [Google Scholar] [CrossRef]
- Mulford, D.A.; Scheinberg, D.A.; Jurcic, J.G. The promise of targeted α-particle therapy. J. Nucl. Med. 2005, 46, 199S–204S. [Google Scholar]
- Brust, M.; Fink, J.; Bethell, D.; Schiffrin, D.J.; Kiely, C. Synthesis and reactions of functionalised gold nanoparticles. J. Chem. Soc. Chem. Commun. 1995, 1655–1656. [Google Scholar] [CrossRef]
- Yook, S.; Cai, Z.; Lu, Y.; Winnik, M.A.; Pignol, J.P.; Reilly, R.M. Intratumorally injected 177Lu-labeled gold nanoparticles: Gold nanoseed brachytherapy with application for neoadjuvant treatment of locally advanced breast cancer. J. Nucl. Med. 2016, 57, 936–942. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Jacobson, O.; Tian, R.; Mease, R.C.; Kiesewetter, D.O.; Niu, G.; Pomper, M.G.; Chen, X. Radioligand therapy of prostate cancer with a long-lasting prostate-specific membrane antigen targeting agent 90Y-DOTA-EB-MCG. Bioconjug. Chem. 2018, 29, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Thiele, N.A.; Brown, V.; Kelly, J.M.; Amor-Coarasa, A.; Jermilova, U.; MacMillan, S.N.; Nikolopoulou, A.; Ponnala, S.; Ramogida, C.F.; Robertson, A.K.H.; et al. An eighteen-membered macrocyclic ligand for actinium-225 targeted alpha therapy. Angew. Chem. Int. Ed. 2017, 56, 14712–14717. [Google Scholar] [CrossRef] [PubMed]
- Pruszynski, M.; D’Huyvetter, M.; Bruchertseifer, F.; Morgenstern, A.; Lahoutte, T. Evaluation of an Anti-HER2 nanobody labeled with 225Ac for targeted α-particle therapy of cancer. Mol. Pharm. 2018, 15, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Graf, F.; Fahrer, J.; Maus, S.; Morgenstern, A.; Bruchertseifer, F.; Venkatachalam, S.; Fottner, C.; Weber, M.M.; Huelsenbeck, J.; Schreckenberger, M.; et al. DNA double strand breaks as predictor of efficacy of the alpha-particle emitter Ac-225 and the electron emitter Lu-177 for somatostatin receptor targeted radiotherapy. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [Green Version]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [Green Version]
- Ballal, S.; Yadav, M.P.; Bal, C.; Sahoo, R.K.; Tripathi, M. Broadening horizons with 225Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to 177Lu-DOTATATE PRRT: First clinical experience on the efficacy and safety. Eur. J. Nucl. Med. Mol. Imaging 2019. [Google Scholar] [CrossRef]
- Salvanou, E.A.; Bouziotis, P.; Tsoukalas, C. Radiolabeled nanoparticles in nuclear oncology. ANR 2018, 1, 38–55. [Google Scholar] [CrossRef] [Green Version]
- Vilchis-Juárez, A.; Ferro-Flores, G.; Santos-Cuevas, C.; Morales-Avila, E.; Ocampo-García, B. Molecular targeting radiotherapy with Cyclo-RGDfK (C) peptides conjugated to 177Lu-labeled gold nanoparticles in tumor-bearing mice. J. Biomed. Nanotechnol. 2014, 10, 393–404. [Google Scholar] [CrossRef]
- Jiménez-mancilla, N.; Ferro-flores, G.; Santos-Cuevas, C.; Ocampo-garcía, B.; Luna-gutiérrez, M.; Azorín-vega, E.; Isaac-olivé, K.; Camacho-lópez, M.; Torres-garcía, E. Multifunctional targeted therapy system based on 99mTc/177Lu-labeled gold nanoparticles-Tat(49-57)-Lys3-bombesin internalized in nuclei of prostate cancer cells. J. Label. Compd. Radiopharm. 2013, 56, 663–671. [Google Scholar] [CrossRef]
- Cai, Z.; Yook, S.; Lu, Y.; Bergstrom, D.; Winnik, M.A.; Pignol, J.P.; Reilly, R.M. Local radiation treatment of HER2-positive breast cancer using trastuzumab-modified gold nanoparticles labeled with 177Lu. Pharm. Res. 2017, 34, 579–590. [Google Scholar] [CrossRef]
- Yook, S.; Cai, Z.; Lu, Y.; Winnik, M.A.; Pignol, J.; Reilly, R.M. Radiation nanomedicine for EGFR-Positive Breast Cancer:Panitumumab Modified Gold Nanoparticles Complexed to the β-Particle-Emitter, 177Lu. Mol. Pharm. 2015. [Google Scholar] [CrossRef]
- Ferro-Flores, G.; Ocampo-García, B.; Santos-Cuevas, C.; María Ramírez, F.; Azorín-Vega, E.; Meléndez-Alafort, L. Theranostic radiopharmaceuticals based on gold nanoparticles labeled with 177lu and conjugated to peptides. Curr. Radiopharm. 2015, 8, 150–159. [Google Scholar] [CrossRef]
- Cędrowska, E.; Pruszynski, M.; Majkowska-Pilip, A.; Męczyńska-Wielgosz, S.; Bruchertseifer, F.; Morgenstern, A.; Bilewicz, A. Functionalized TiO2 nanoparticles labelled with 225Ac for targeted alpha radionuclide therapy. J. Nanoparticle Res. 2018, 20. [Google Scholar] [CrossRef] [Green Version]
- Sempkowski, M.; Zhu, C.; Menzenski, M.Z.; Kevrekidis, I.G.; Bruchertseifer, F.; Morgenstern, A.; Sofou, S. Sticky patches on lipid nanoparticles enable the selective targeting and killing of untargetable cancer cells. Langmuir 2016, 32, 8329–8338. [Google Scholar] [CrossRef]
- Mclaughlin, M.F.; Robertson, D.; Pevsner, P.H.; Wall, J.S. LnPO4 nanoparticles doped with Ac-225 and sequestered daughters for targeted alpha therapy. Cancer Biotherary Radiopharm. 2013, 29, 34–41. [Google Scholar] [CrossRef]
- Laurent, G.; Bernhard, C.; Dufort, S.; Jiménez Sánchez, G.; Bazzi, R.; Boschetti, F.; Moreau, M.; Vu, T.H.; Collin, B.; Oudot, A.; et al. Minor changes in the macrocyclic ligands but major consequences on the efficiency of gold nanoparticles designed for radiosensitization. Nanoscale 2016, 8, 12054–12065. [Google Scholar] [CrossRef]
- Alric, C.; Miladi, I.; Kryza, D.; Taleb, J.; Lux, F.; Bazzi, R.; Billotey, C.; Janier, M.; Perriat, P.; Roux, S.; et al. The biodistribution of gold nanoparticles designed for renal clearance. Nanoscale 2013, 5, 5930–5939. [Google Scholar] [CrossRef]
- Miladi, I.; Alric, C.; Dufort, S.; Mowat, P.; Dutour, A.; Mandon, C.; Laurent, G.; Bräuer-Krisch, E.; Herath, N.; Coll, J.; et al. The in vivo radiosensitizing effect of gold nanoparticles based MRI contrast agents. Small 2014, 10, 1116–1124. [Google Scholar] [CrossRef]
- Lee, J.; Chatterjee, D.K.; Lee, M.H.; Krishnan, S. Gold nanoparticles in breast cancer treatment: Promise and potential pitfalls. Cancer Lett. 2014, 347, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Davis, I.A.; Glowienka, K.A.; Boll, R.A.; Deal, K.A.; Brechbiel, M.W.; Stabin, M.; Bochsler, P.N.; Mirzadeh, S.; Kennel, S.J. Comparison of 225actinium chelates: Tissue distribution and radiotoxicity. Nucl. Med. Biol. 1999, 26, 581–589. [Google Scholar] [CrossRef]
- Vonarbourg, A.; Passirani, C.; Saulnier, P.; Benoit, J.P. Parameters influencing the stealthiness of colloidal drug delivery systems. Biomaterials 2006, 27, 4356–4373. [Google Scholar] [CrossRef]
- Bertrand, N.; Leroux, J.C. The journey of a drug-carrier in the body: An anatomo-physiological perspective. J. Control. Release 2012, 161, 152–163. [Google Scholar] [CrossRef]
- Bouziotis, P.; Stellas, D.; Thomas, E.; Truillet, C.; Tsoukalas, C.; Lux, F.; Tsotakos, T.; Xanthopoulos, S.; Paravatou-Petsotas, M.; Gaitanis, A.; et al. 68Ga-radiolabeled AGuIX nanoparticles as dual-modality imaging agents for PET/MRI-guided radiation therapy. Nanomedicine 2017, 12, 1561–1574. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Jim, Z.; Bao, A.; Goins, B.; Phillips, W.T. In vivo PET imaging and biodistribution of radiolabeled gold nanoshells in rats with tumor xenografts. Int. J. Pharm. 2010, 395, 324–330. [Google Scholar] [CrossRef]
- Chattopadhyay, N.; Fonge, H.; Cai, Z.; Scollard, D.; Lechtman, E.; Done, S.J.; Pignol, J.-P.; Reilly, R.M. Role of antibody-mediated tumor targeting and route of administration in nanoparticle tumor accumulation in vivo. Mol. Pharm. 2012, 9, 2168–2179. [Google Scholar] [CrossRef]
- Huang, X.; Peng, X.; Wang, Y.; Wang, Y.; Shin, D.M.; El-Sayed, M.A.; Nie, S. A reexamination of active and passive tumor targeting by using rod-shaped gold nanocrystals and covalently conjugated peptide ligands. ACS Nano 2010, 4, 5887–5896. [Google Scholar] [CrossRef] [Green Version]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvanou, E.-A.; Stellas, D.; Tsoukalas, C.; Mavroidi, B.; Paravatou-Petsotas, M.; Kalogeropoulos, N.; Xanthopoulos, S.; Denat, F.; Laurent, G.; Bazzi, R.; et al. A Proof-of-Concept Study on the Therapeutic Potential of Au Nanoparticles Radiolabeled with the Alpha-Emitter Actinium-225. Pharmaceutics 2020, 12, 188. https://doi.org/10.3390/pharmaceutics12020188
Salvanou E-A, Stellas D, Tsoukalas C, Mavroidi B, Paravatou-Petsotas M, Kalogeropoulos N, Xanthopoulos S, Denat F, Laurent G, Bazzi R, et al. A Proof-of-Concept Study on the Therapeutic Potential of Au Nanoparticles Radiolabeled with the Alpha-Emitter Actinium-225. Pharmaceutics. 2020; 12(2):188. https://doi.org/10.3390/pharmaceutics12020188
Chicago/Turabian StyleSalvanou, Evangelia-Alexandra, Dimitris Stellas, Charalampos Tsoukalas, Barbara Mavroidi, Maria Paravatou-Petsotas, Nikolaos Kalogeropoulos, Stavros Xanthopoulos, Franck Denat, Gautier Laurent, Rana Bazzi, and et al. 2020. "A Proof-of-Concept Study on the Therapeutic Potential of Au Nanoparticles Radiolabeled with the Alpha-Emitter Actinium-225" Pharmaceutics 12, no. 2: 188. https://doi.org/10.3390/pharmaceutics12020188
APA StyleSalvanou, E.-A., Stellas, D., Tsoukalas, C., Mavroidi, B., Paravatou-Petsotas, M., Kalogeropoulos, N., Xanthopoulos, S., Denat, F., Laurent, G., Bazzi, R., Roux, S., & Bouziotis, P. (2020). A Proof-of-Concept Study on the Therapeutic Potential of Au Nanoparticles Radiolabeled with the Alpha-Emitter Actinium-225. Pharmaceutics, 12(2), 188. https://doi.org/10.3390/pharmaceutics12020188