Stability Study of Isoniazid and Rifampicin Oral Solutions Using Hydroxypropyl-Β-Cyclodextrin to Treat Tuberculosis in Paediatrics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ultra Performance Liquid Chromatography (UPLC) Method
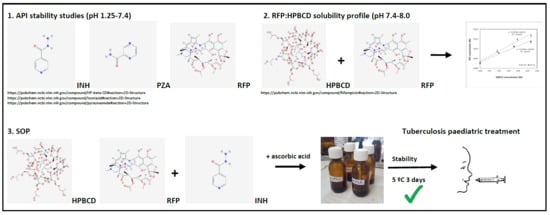
2.2. API Stability Studies
2.3. RFP:HPBCD Solubility Profiles
2.4. RFP:HPBCD Characterization
2.5. General Standard Operating Procedure (SOP)
- The buffer phosphate vehicle at pH 8.0 is prepared [24]. For 1 L: 55 mL solution A (908 mg KH2PO4 in 100 mL purified water) is added to 945 mL solution B (11.9 g Na2HPO4·2H2O in 100 mL purified water).
- HPβCD solution is prepared (5.6 % p/v).
- RFP dose (1.0 g) is weighted and transferred to a 100 mL glass amber bottle.
- 100 mL of HPβCD solution is transferred to glass amber bottle, a magnetic stirrer is introduced, and the bottle is closed.
- Preparation is kept in constant shaking during 24 h at 25 °C.
- Only for F2 and F3, 10 mg, or 100 mg of ascorbic acid, respectively, is weighted and transferred to the 100 mL glass amber bottle. Preparation is kept in constant shaking during 15 min at 25 °C.
- INH dose (0.7 g) is weighted and transferred to the 100 mL glass amber bottle.
- Preparation is kept in constant shaking during 30 min at 25 °C.
- The magnetic stirrer is removed, and the bottle is closed with a dispenser closed.
2.6. Formulation Stability Study
3. Results
3.1. API Stability Studies
3.2. RFP:HPBCD Solubility Profile
3.3. RFP:HPBCD Characterization
3.4. Formulation Stability Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. New Fixed-Dose Combinations for the Treatment of TB in Children 2016. Available online: https://www.who.int/tb/FDC_Factsheet.pdf (accessed on 24 January 2020).
- Dailymed. Isoniazid. 2018. Bethesda, USA: NIH 2018. Available online: https://dailymed.nlm.nih.gov/dailymed/search.cfm?labeltype=all&query=ISONIAZID&pagesize=20&page=3&vfile= (accessed on 24 January 2020).
- Piñeiro, R.; Santiago, B.; Rodríguez, B.; Baquero-Artigao, F.; Fernández-Llamazares, C.M.; López-Ramos, M.G.; Vinent, J.; Gómez-Pastrana, D.; Mellado, M.J. Recomendaciones para la elaboración y administración de fármacos antituberculosos en niños. Segunda fase del proyecto magistral de la red española de estudio de la tuberculosis pediátrica (pTBred). In Anales de Pediatría; Elsevier: Amsterdam, The Netherlands, 2016; Volume 85, p. 323-e1. [Google Scholar]
- World Health Organization. Medicines/Finished Pharmaceutical Products TB309 2017. Available online: https://extranet.who.int/prequal/content/prequalified-lists/medicines?label=&field_medicine_applicant=&field_medicine_fpp_site_value=&search_api_aggregation_1=&field_medicine_pq_date&field_medicine_pq_date_1&field_therapeutic_area=All&field_medicine_status=&field_basis_of_listing=All&field_combi_excl_copack_num_op=%3D&field_combi_excl_copack_num=&field_combi_with_copack_num_op=%3D&field_combi_with_copack_num=&page=10 (accessed on 24 January 2020).
- UNITAID. Euro Health Group End of Project Evaluation Team: Bernadette Bourdin Trunz and Miranda Brouwer Global Alliance for TB Drug Development (TB Alliance) Speeding Treatments to End Paediatric Tuberculosis (STEP–TB) End of Project Evaluation 2017. Available online: https://unitaid.eu/assets/UNITAID-STEPTB-Final-Evaluation-04July2017.pdf (accessed on 24 January 2019).
- World Health Organization. Rapid Advice Treatment of Tuberculosis in Children 2010. Available online: http://apps.who.int/iris/bitstream/handle/10665/44444/9789241500449_eng.pdf?sequence=1&isAllowed=y2010 (accessed on 24 January 2020).
- Bhutani, H.; Mariappan, T.T.; Singh, S. The physical and chemical stability on anti-tuberculosis fixed-dose combination products under accelerated climatic conditions. Int. J. Tuberc. Lung Dis. 2004, 8, 1073–1080. [Google Scholar] [PubMed]
- Battini, S.; Mannava, M.K.C.; Nangia, A. Improved stability of TB drug fixed dose combination using isoniazid-caffeic acid and vanillic acid cocrystal. J. Pharm. Sci. 2018, 107, 1667–1679. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Bhutani, H.; Mariappan, T.T. Quality problems of anti-tuberculosis fixed-dose combinations (FDCs): A way forward. Indian J. Tuberc. 2006, 53, 201–205. [Google Scholar]
- Panchagnula, R.; Agrawal, S. Biopharmaceutic and pharmacokinetic aspects of variable bioavailability of rifampicin. Int. J. Pharm. 2004, 71, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Mariappan, T.T.; Singh, S. Positioning of rifampicin in the biopharmaceutics classification system (BCS). Clin. Res. Regul. Aff. 2006, 23, 1–10. [Google Scholar] [CrossRef]
- Alves, D.; Gilberto, A.; Vizzotto, L.; Federman, A.; Gomes, A. Analysis of the molecular association of rifampicin with hydroxypropyl-β-cyclodextrin. Braz. J. Pharm. Sci. 2004, 40, 43–51. [Google Scholar]
- Tewes, F.; Brillault, J.; Couet, W.; Olivier, J.-C. Formulation of rifampicin-cyclodextrin complexes for lung nebulization. J. Control Release 2008, 129, 93–99. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Deng, P.; Yang, L.; Tan, Q.; Liu, J.; Yang, M.; Zhang, J. Molecular encapsulation of rifampicin as an inclusión complex of hydroxypropyl-β-cyclodextrin: Design; characterization and in vitro dissolution. Colloids Surf. B Biointerfaces 2013, 203, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Rajewski, R.A.; Stella, J.V. Pharmaceutical applications of cyclodextrins 2. In vivo drug delivery. J. Pharm. Sci. 1996, 85, 1142–1168. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, N.O. Improvement of dissolution properties of furosemide by complexation with HP-b-Cyclo-dextrin. Drug Dev. Ind. Pharm. 1998, 24, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.-T.N.; Guillarme, D.; Rudaz, S.; Veuthey, J.-L. Validation of an ultra-fast UPLC-UV method for the separation of antituberculosis tablets. J. Sep. Sci. 2008, 31, 1050–1056. [Google Scholar]
- Moreno-Exebio, L.; Grande-Ortiz, M. Validación de un método de cromatografía líquida para la determinación de rifampicina en plasma humano. Rev. Peru. Med. Exp. Salud Pública 2014, 31, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Suárez-González, J.; Santoveña-Estévez, A.; Soriano, M.; Fariña, J.B. Design and optimization of a child-friendly dispersible tablet containing Isoniazid, Pyrazinamide and Rifampicin for treating Tuberculosis in pediatrics. Drug Dev. Ind. Pharm. 2020. accepted. [Google Scholar] [CrossRef] [PubMed]
- Real Farmacopea Española. Recomendaciones Relativas al Ensayo de Disolución Real Farmacopea Española, 5th ed.; Ministerio de Sanidad y Consumo, Agencia Española de Medicamentos y Productos Sanitarios: Madrid, Spain, 2010; p. 51701.
- World Health Organization, TB alliance, Unicef. New fixed-dose combinations for the treatment of TB in children. 2016. Available online: http://www.who.int/tb/FDC_Factsheet.pdf (accessed on 24 February 2020).
- World Health Organization. World Health Organization Guidance for National Tuberculosis Programmes on the Management of Tuberculosis in Children 2014. Available online: http://apps.who.int/medicinedocs/documents/s21535en/s21535en.pdf (accessed on 24 January 2020).
- delMoral-Sanchez, J.-M.; Gonzalez-Alvarez, I.; Gonzalez-Alvarez, M.; Navarro, A.; Bermejo, M. Classification of WHO eseential oral medicines for children applying a provisional pediatric bipharmaceutics classification system. Pharmaceutics 2019, 11, 567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geigy, J.R. Documenta Geigy Tablas Cientificas, 5th ed.; Sociedad Alianza de Artes Gráficas: Barcelona, Spain, 1965. [Google Scholar]
- OMS. Child Growth Standards. Weight for Age. Available online: https://www.who.int/childgrowth/standards/weight_for_age/en/ (accessed on 24 January 2020).
- Agencia Europea de Medicamentos. EMEA. Reflection Paper: Formulations of Choice for the Paediatric Population 2006. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-formulations-choice-paediatric-population_en.pdf (accessed on 24 January 2020).
- EMA. EMA/CHMP/333892/2013. Cyclodextrins Used as Excipients. 9 October 2017. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/questions-answers-cyclodextrins-used-excipients-medicinal-products-human-use_en.pdf (accessed on 24 January 2020).
- Rajaram, S.; Vemuri, V.D.; Natham, R. Ascorbic acid improves stability and pharmacokinetics of rifampicin in the presence of isoniazid. J. Pharm. Biomed. Anal. 2014, 100, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. (Eds.) Handbook of Pharmaceutical Excipients, 6th ed.; Libros Digitales-Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- NIH Office of Dietary Supplements. Vitamin C. Available online: https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/ (accessed on 24 January 2020).
- International Conference of Armonization. Stability Testing of New Drug Substances and Products Q2(R1) 1994. Available online: https://database.ich.org/sites/default/files/Q2_R1__Guideline.pdf (accessed on 24 January 2020).
- Bonner, J.J.; Vajjah, P.; Abduljalil, K.; Rostami-Hodjegan, A.; Tucker, G.T.; Johnson, T. Does age affect gastric empyting time? A model-based meta-analysis of data from premature neonates through to adults. Biopharm. Drug Doispos. 2015, 36, 245–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loftsson, T.; Hreinsdóttir, D.; Másson, M. Evaluation of cyclodextrin solubilization of drugs. Int. J. Pharm. 2005, 302, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Mariappan, T.T.; Singh, S. Regional gastrointestinal permeability of rifampicin and isoniazid (alone and their combination) in the rat. Int. J. Tuberc. Lung Dis. 2003, 7, 797–803. [Google Scholar] [PubMed]
- Shawahna, R. Pediatric Biopharmaceutical Classification System: Using Age-appropriate Initial Gastric Volume. AAPS J. 2016, 18, 728–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliabadi, H.M.; Romanick, M.; Desai, S.; Lavasanifar, A. Effect of buffer and antioxidant on stability of a mercaptopurine suspensión. Am. J. Health Syst. Pharm. 2008, 65, 441–447. [Google Scholar] [CrossRef] [PubMed]





| Products | F1 | F2 | F3 |
|---|---|---|---|
| INH (g) | 0.7 | 0.7 | 0.7 |
| RFP (g) | 1.0 | 1.0 | 1.0 |
| HPBCD (% w/v) | 5.6 | 5.6 | 5.6 |
| Ascorbic acid (% w/v) | - | 0.01 | 0.1 |
| Phosphate buffer pH 8.0 | 100 mL | 100 mL | 100 mL |
| INH | INDIVIDUAL | COMBINED | ||
| pH | R (%) | SD | R (%) | SD |
| 1.25 | 100.1 | 2.84 | 97.2 | 1.08 |
| 3.0 | 95.7 | 1.10 | 99.8 | 0.00 |
| 6.3 | 98.3 | 5.25 | 103.2 | 5.22 |
| 7.4 | 104.4 | 4.00 | 99.8 | 2.0 |
| PZA | INDIVIDUAL | COMBINED | ||
| pH | R (%) | SD | R (%) | SD |
| 1.25 | 112.6 | 3.03 | 99.8 | 0.20 |
| 3.0 | 99.2 | 1.76 | 99.8 | 0.00 |
| 6.3 | 95.0 | 2.14 | 96.2 | 1.99 |
| 7.4 | 97.4 | 1.20 | 99.7 | 4.10 |
| RFP | INDIVIDUAL | COMBINED | ||
| pH | R (%) | SD | R (%) | SD |
| 1.25 | 94.7 | 0.18 | 89.4 | 2.28 |
| 3.0 | 89.0 | 0.82 | 88.7 | 3.90 |
| 6.3 | 96.6 | 6.25 | 95.05 | 12.8 |
| 7.4 | 103.4 | 1.40 | 100.8 | 3.50 |
| INH | INDIVIDUAL | COMBINED | ||||
| pH | Order | t5% (h) | r | Order | t5% (h) | r |
| 1.25 | 2 | 6.6 | 0.988 | 0 | 7.6 | 0.990 |
| 3.0 | 1 | 6.2 | 0.900 | 2 | 6.6 | 0.691 |
| 6.3 | 2 | 3.9 | 0.913 | 2 | 11.2 | 0.922 |
| 7.4 | 0 | 18.0 | 0.995 | 2 | 9.4 | 0.97 |
| PZA | INDIVIDUAL | COMBINED | ||||
| pH | Order | t5% (h) | r | Order | t5% (h) | r |
| 1.25 | 2 | 96.1 | 0.970 | 0 | 44.6 | 0.922 |
| 3.0 | 2 | 8.2 | 0.880 | - | > 24 | - |
| 6.3 | 2 | 173.9 | 0.941 | - | > 24 | - |
| 7.4 | 2 | 78.7 | 0.843 | 1 | 27.8 | 0.999 |
| RFP | INDIVIDUAL | COMBINED | ||||
| pH | Order | t5% (h) | r | Order | t5% (h) | R |
| 1.25 | 1 | 1.2 | 0.995 | 1 | 0.5 | 0.975 |
| 3.0 | 1 | 2.1 | 0.991 | 1 | 0.7 | 0.994 |
| 6.3 | 1 | 2.5 | 0.952 | 2 | 1.8 | 0.940 |
| 7.4 | 1 | 7.8 | 0.978 | 1 | 5.8 | 0.995 |
| INH | t (days) | |||||||||
| Initial Dose (mg/mL) | SD | 1 | 3 | 7 | 14 | |||||
| R (%) | SD | R (%) | SD | R (%) | SD | R (%) | SD | |||
| F1 | 6.5 | 0.1 | 100 | 0.00 | 96.6 | 2.37 | 92.6 | 6.27 | 92.3 | 10.9 |
| F2 | 7.3 | 0.0 | 100 | 0.00 | 86.4 | 1.00 | 83.7 | 3.29 | 76.8 | 1.25 |
| F3 | 7.0 | 0.3 | 100 | 0.00 | 95.0 | 0.03 | 89.1 | 0.68 | 83.4 | 0.26 |
| RFP | Initial Dose (mg/mL) | SD | t (days) | |||||||
| 1 | 3 | 7 | 14 | |||||||
| R (%) | SD | R (%) | SD | R (%) | SD | R (%) | SD | |||
| F1 | 10.5 | 0.4 | 100 | 0.00 | 75.5 | 1.87 | 68.3 | 3.92 | 45.3 | 4.76 |
| F2 | 10.3 | 0.4 | 100 | 0.00 | 88.1 | 0.21 | 61.0 | 3.80 | 41.3 | 0.82 |
| F3 | 9.03 | 0.2 | 100 | 0.00 | 91.6 | 7.86 | 64.2 | 4.92 | 48.0 | 2.56 |
| pH | t (days) | |||||||||
| 1 | 3 | 7 | 14 | |||||||
| pH | SD | pH | SD | pH | SD | pH | SD | |||
| F1 | 7.47 | 0.03 | 7.45 | 0.07 | 7.53 | 0.01 | 7.47 | 0.06 | ||
| F2 | 7.35 | 0.01 | 7.53 | 0.01 | 7.25 | 0.04 | 7.11 | 0.05 | ||
| F3 | 7.24 | 0.00 | 7.28 | 0.00 | 7.20 | 0.01 | 7.19 | 0.01 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoveña-Estévez, A.; Suárez-González, J.; Cáceres-Pérez, A.R.; Ruiz-Noda, Z.; Machado-Rodríguez, S.; Echezarreta, M.; Soriano, M.; Fariña, J.B. Stability Study of Isoniazid and Rifampicin Oral Solutions Using Hydroxypropyl-Β-Cyclodextrin to Treat Tuberculosis in Paediatrics. Pharmaceutics 2020, 12, 195. https://doi.org/10.3390/pharmaceutics12020195
Santoveña-Estévez A, Suárez-González J, Cáceres-Pérez AR, Ruiz-Noda Z, Machado-Rodríguez S, Echezarreta M, Soriano M, Fariña JB. Stability Study of Isoniazid and Rifampicin Oral Solutions Using Hydroxypropyl-Β-Cyclodextrin to Treat Tuberculosis in Paediatrics. Pharmaceutics. 2020; 12(2):195. https://doi.org/10.3390/pharmaceutics12020195
Chicago/Turabian StyleSantoveña-Estévez, Ana, Javier Suárez-González, Amor R. Cáceres-Pérez, Zuleima Ruiz-Noda, Sara Machado-Rodríguez, Magdalena Echezarreta, Mabel Soriano, and José B. Fariña. 2020. "Stability Study of Isoniazid and Rifampicin Oral Solutions Using Hydroxypropyl-Β-Cyclodextrin to Treat Tuberculosis in Paediatrics" Pharmaceutics 12, no. 2: 195. https://doi.org/10.3390/pharmaceutics12020195
APA StyleSantoveña-Estévez, A., Suárez-González, J., Cáceres-Pérez, A. R., Ruiz-Noda, Z., Machado-Rodríguez, S., Echezarreta, M., Soriano, M., & Fariña, J. B. (2020). Stability Study of Isoniazid and Rifampicin Oral Solutions Using Hydroxypropyl-Β-Cyclodextrin to Treat Tuberculosis in Paediatrics. Pharmaceutics, 12(2), 195. https://doi.org/10.3390/pharmaceutics12020195