Characteristic of K3 (CpG-ODN) as a Transcutaneous Vaccine Formulation Adjuvant
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
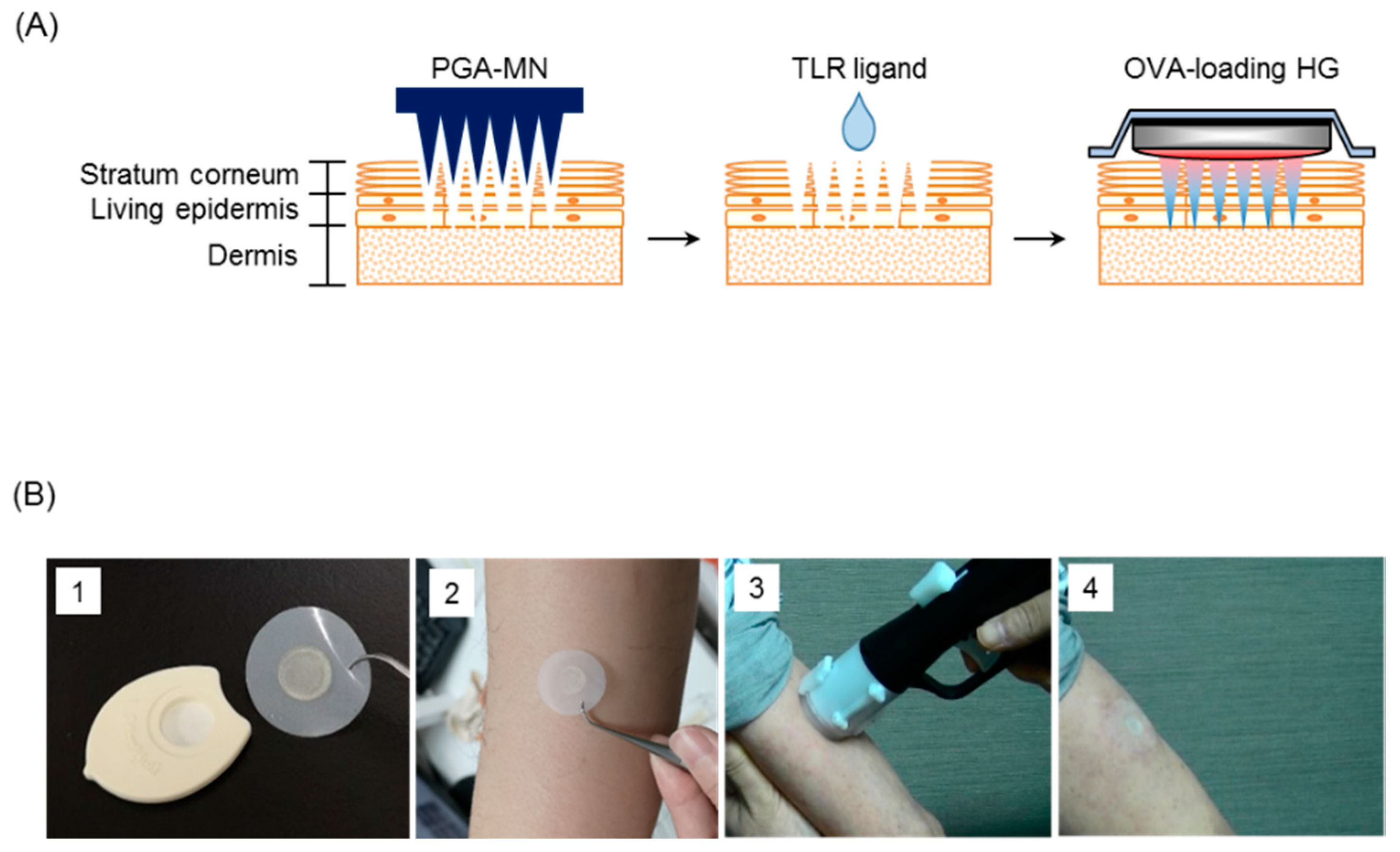
2.2. Preparation of Ovalbumin (OVA)-Loaded Hydrophilic Gel Patch (HG)
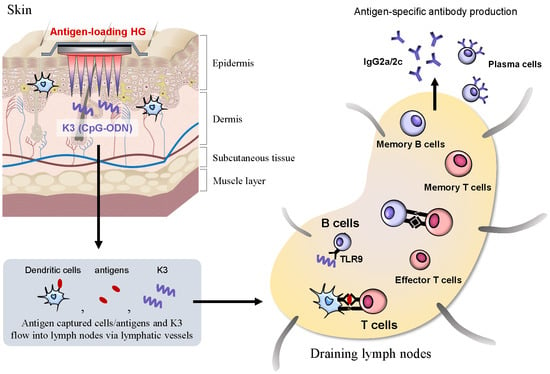
2.3. Immunization
2.4. Epidermal and Dermal Sheets
2.5. Flow Cytometry Analysis
2.6. Measurement of OVA-Specific Antibody Titer
2.7. Immunofluorescence Staining
2.8. Cytokine Assay
2.9. In Vivo Proliferation Assay for OVA-Specific T Cells
2.10. In Vitro Stimulation of B Cells
2.11. Safety Evaluation for TCI of K3: Clinical Trial (Phase I)
2.12. Statistical Analysis
3. Results
3.1. Screening for Effective Adjuvants for TCI Using sdMN
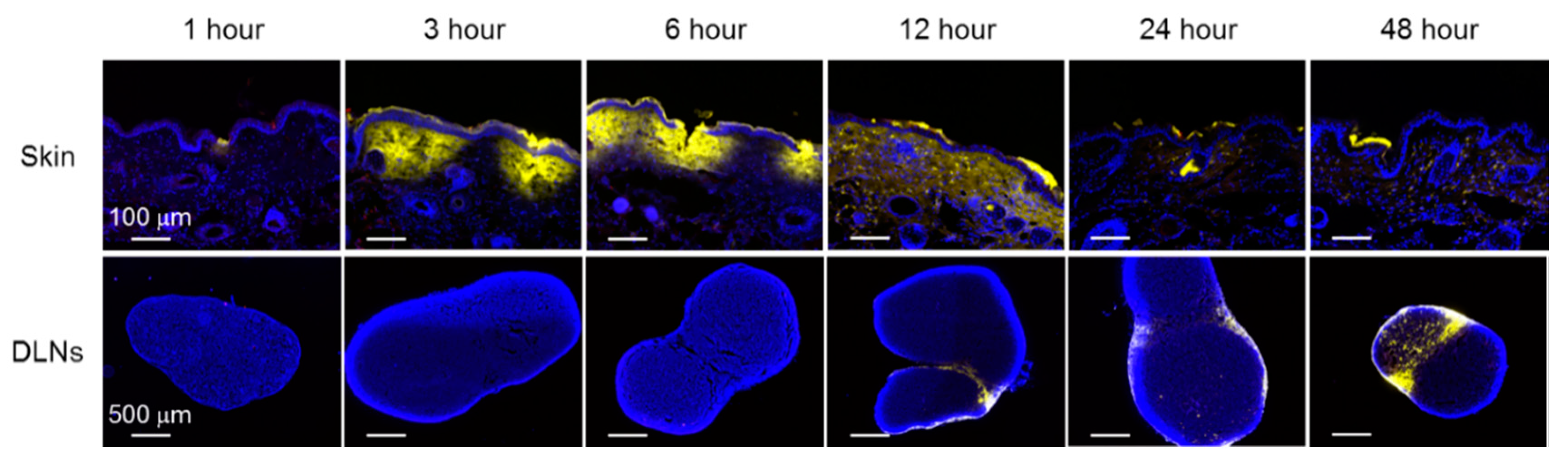
3.2. Localization of OVA and K3 in Skin and DLNs
3.3. Activation and Differentiation of T cells and B cells after Combination Treatment with K3
3.4. Characteristics of the K3-Induced Primary Immune Response after Transcutaneous Vaccination
3.5. Human Safety Evaluation for Transcutaneous Administration of K3
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Dawood, F.S.; Jain, S.; Finelli, L.; Shaw, M.W.; Lindstrom, S.; Garten, R.J.; Gubareva, L.V.; Xu, X.; Bridges, C.B.; Uyeki, T.M.; et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 2009, 360, 2605–2615. [Google Scholar] [PubMed]
- Baize, S.; Pannetier, D.; Oestereich, L.; Rieger, T.; Koivogui, L.; Magassouba, N.; Soropogui, B.; Sow, M.S.; Keïta, S.; De Clerck, H.; et al. Emergence of Zaire Ebola virus disease in Guinea. N. Engl. J. Med. 2014, 371, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Yokota, Y.; Zhai, Y.; Quan, Y.-S.; Kamiyama, F.; Mukai, Y.; Okada, N.; Nakagawa, S. A low-invasive and effective transcutaneous immunization system using a novel dissolving microneedle array for soluble and particulate antigens. J. Control. Release 2012, 161, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Ono, A.; Azukizawa, H.; Ito, S.; Nakamura, Y.; Asada, H.; Quan, Y.-S.; Kamiyama, F.; Katayama, I.; Hirobe, S.; Okada, N. Development of novel double-decker microneedle patches for transcutaneous vaccine delivery. Int. J. Pharm. 2017, 532, 374–383. [Google Scholar] [CrossRef]
- Matsuo, K.; Hirobe, S.; Yokota, Y.; Ayabe, Y.; Seto, M.; Quan, Y.-S.; Kamiyama, F.; Tougan, T.; Horii, T.; Mukai, Y.; et al. Transcutaneous immunization using a dissolving microneedle array protects against tetanus, diphtheria, malaria, and influenza. J. Control. Release 2012, 160, 495–501. [Google Scholar] [CrossRef]
- Ono, A.; Ito, S.; Sakagami, S.; Asada, H.; Saito, M.; Quan, Y.-S.; Kamiyama, F.; Hirobe, S.; Okada, N. Development of novel faster-dissolving microneedle patches for transcutaneous vaccine delivery. Pharmaceutics 2017, 9, 27. [Google Scholar] [CrossRef]
- Hirobe, S.; Azukizawa, H.; Matsuo, K.; Zhai, Y.; Quan, Y.-S.; Kamiyama, F.; Suzuki, H.; Katayama, I.; Okada, N.; Nakagawa, S. Development and clinical study of a self-dissolving microneedle patch for transcutaneous immunization device. Pharm. Res. 2013, 30, 2664–2674. [Google Scholar] [CrossRef]
- Hirobe, S.; Azukizawa, H.; Hanafusa, T.; Matsuo, K.; Quan, Y.-S.; Kamiyama, F.; Katayama, I.; Okada, N.; Nakagawa, S. Clinical study and stability assessment of a novel transcutaneous influenza vaccination using a dissolving microneedle patch. Biomaterials 2015, 57, 50–58. [Google Scholar] [CrossRef]
- Kuroda, E.; Coban, C.; Ishii, K.J. Particulate adjuvant and innate immunity: Past achievements, present findings, and future prospects. Int. Rev. Immunol. 2013, 32, 209–220. [Google Scholar] [CrossRef]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. TLR signaling. Semin. Immunol. 2007, 19, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, K.; Takeuchi, O.; Kawai, T.; Sanjo, H.; Ogawa, T.; Takeda, Y.; Takeda, K.; Akira, S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J. Immunol. 1999, 162, 3749–3752. [Google Scholar] [PubMed]
- Suzuki, O.; Koura, M.; Noguchi, Y.; Uchio-Yamada, K.; Matsuda, J. Zygosity determination in hairless mice by PCR based on Hr(hr) gene analysis. Exp. Amin. 2013, 62, 267–273. [Google Scholar]
- Hemmi, H.; Takeuchi, O.; Kawai, T.; Kaisho, T.; Sato, S.; Sanjo, H.; Matsumoto, M.; Hoshino, K.; Wagner, H.; Takeda, K.; et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000, 408, 740–745. [Google Scholar] [CrossRef]
- Ishii, Y.; Nakae, T.; Sakamoto, F.; Matsuo, K.; Matsuo, K.; Quan, Y.-S.; Kamiyama, F.; Fujita, T.; Yamamoto, A.; Nakagawa, S.; et al. A transcutaneous vaccination system using a hydrogel patch for viral and bacterial infection. J. Control. Release 2008, 131, 113–120. [Google Scholar] [CrossRef]
- Matsuo, K.; Ishii, Y.; Quan, Y.-S.; Kamiyama, F.; Mukai, Y.; Okada, N.; Nakagawa, S. Characterization of transcutaneous protein delivery by a hydrogel patch in animal, human, and tissue-engineered skin models. Biol. Pharm. Bull. 2011, 34, 586–589. [Google Scholar] [CrossRef][Green Version]
- Verthelyi, D.; Ishii, K.J.; Gursel, M.; Takeshita, F.; Klinman, D.M. Human peripheral blood cells differentially recognize and respond to two distinct CPG motifs. J. Immunol. 2001, 166, 2372–2377. [Google Scholar] [CrossRef]
- Van der Maaden, K.; Jiskoot, W.; Bouwstra, J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J. Control. Release 2012, 161, 645–655. [Google Scholar] [CrossRef]
- Chen, Q.; Davidson, T.S.; Huter, E.N.; Shevach, E.M. Engagement of TLR2 does not reverse the suppressor function of mouse regulatory T cells, but promotes their survival. J. Immunol. 2009, 183, 4458–4466. [Google Scholar] [CrossRef]
- Leemans, J.C.; Vervoordeldonk, M.J.; Florquin, S.; van Kessel, K.P.; van der Poll, T. Differential role of interleukin-6 in lung inflammation induced by lipoteichoic acid and peptidoglycan from Staphylococcus aureus. Am. J. Respir. Crit. Care Med. 2002, 165, 1445–1450. [Google Scholar] [CrossRef]
- Kanaya, K.; Kondo, K.; Suzukawa, K.; Sakamoto, T.; Kikuta, S.; Okada, K.; Yamasoba, T. Innate immune responses and neuroepithelial degeneration and regeneration in the mouse olfactory mucosa induced by intranasal administration of Poly(I:C). Cell Tissue Res. 2014, 357, 279–299. [Google Scholar] [CrossRef] [PubMed]
- Hickling, J.K.; Jones, K.R.; Friede, M.; Zehrung, D.; Chen, D.; Kristensen, D. Intradermal delivery of vaccines: Potential benefits and current challenges. Bull. World Health Organ. 2011, 89, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, W.; Wang, S. Effect of vaccine administration modality on immunogenicity and efficacy. Expert Rev. Vaccines 2015, 14, 1509–1523. [Google Scholar] [CrossRef] [PubMed]
- Ciabattini, A.; Nardini, C.; Santoro, F.; Garagnani, P.; Franceschi, C.; Medaglini, D. Vaccination in the elderly: The challenge of immune changes with aging. Semin. Immunol. 2018, 40, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Nagao, M.; Fujisawa, T.; Ihara, T.; Kino, Y. Highly increased levels of IgE antibodies to vaccine components in children with influenza vaccine-associated anaphylaxis. J. Allergy Clin. Immunol. 2016, 137, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Skountzou, I.; Quan, F.S.; Jacob, J.; Compans, R.W.; Kang, S.M. Transcutaneous immunization with inactivated influenza virus induces protective immune responses. Vaccine 2006, 24, 6110–6119. [Google Scholar] [CrossRef]
- Krieg, A.M. Therapeutic potential of Toll-like receptor 9 activation. Nat. Rev. Drug Discov. 2006, 5, 471–484. [Google Scholar] [CrossRef]
- Sivori, S.; Carlomagno, S.; Moretta, L.; Moretta, A. Comparison of different CpG oligodeoxynucleotide classes for their capability to stimulate human NK cells. Eur. J. Immunol. 2006, 36, 961–967. [Google Scholar] [CrossRef]
- Gürsel, M.; Verthelyi, D.; Gürsel, I.; Ishii, K.J.; Klinman, D.M. Differential and competitive activation of human immune cells by distinct classes of CpG oligodeoxynucleotide. J. Leukoc. Biol. 2002, 71, 813–820. [Google Scholar]
- Marshall, J.D.; Fearon, K.; Abbate, C.; Subramanian, S.; Yee, P.; Gregorio, J.; Coffman, R.L.; Van Nest, G. Identification of a novel CpG DNA class and motif that optimally stimulate B cell and plasmacytoid dendritic cell functions. J. Leukoc. Biol. 2003, 73, 781–792. [Google Scholar] [CrossRef]
- Tougan, T.; Aoshi, T.; Coban, C.; Katakai, Y.; Kai, C.; Yasutomi, Y.; Ishii, K.J.; Horii, T. TLR9 adjuvants enhance immunogenicity and protective efficacy of the SE36/AHG malaria vaccine in nonhuman primate models. Hum. Vaccin. Immunother. 2013, 9, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Clinical Development of BK-SE36CpG Malaria Vaccine Safety Evaluation of BK-SE36CpG in the Malaria Endemic Population. Available online: https://www.ghitfund.org/investment/portfoliodetail/detail/99 (accessed on 20 January 2020).




| TLR Ligand | Description | Molecular Weight | Dose | Target TLR |
|---|---|---|---|---|
| Pam3CSK4 | Synthetic mimic of bacterial lipoprotein | 1500 Da | 20 μg | TLR1/2 |
| LTA-SA | Lipoteichoic acid from Staphylococcus aureus | 4000–8000 Da | 100 μg | TLR2 |
| Poly I:C | Synthetic analogue of double stranded RNA | 1500–8000 Da | 50 μg | TLR3 |
| Imiquimod | Small molecule agonist | 240 Da | 100 μg | TLR7 |
| K3 | CpG-oligodeoxyribonucleotide (ODN) | 6300 Da | 20 μg | TLR9 |
| Day | 0 | 1 | 2–7 | 8–20 | 21 | 22 | 23–28 | 29–41 | 42 |
|---|---|---|---|---|---|---|---|---|---|
| Vaccination | ● | ||||||||
| Medical examination | ● | ● | ● | ● | ● | ||||
| Vital sign measurement | ● | ● | ● | ● | ● | ||||
| Blood count and biochemistry | ● | ● | ● | ● | ● | ||||
| Urinalysis | ● | ● | ● | ● | ● | ||||
| Cytokine production | ● | ● | ● | ● | ● | ||||
| Diary by subjects | ● | ● | ● | ▲ | ● | ● | ● | ▲ | ▲ |
| Confirmation of adverse events | ● | ● |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, S.; Hirobe, S.; Kawakita, T.; Saito, M.; Quan, Y.-S.; Kamiyama, F.; Ishii, K.J.; Nagao, M.; Fujisawa, T.; Tachibana, M.; et al. Characteristic of K3 (CpG-ODN) as a Transcutaneous Vaccine Formulation Adjuvant. Pharmaceutics 2020, 12, 267. https://doi.org/10.3390/pharmaceutics12030267
Ito S, Hirobe S, Kawakita T, Saito M, Quan Y-S, Kamiyama F, Ishii KJ, Nagao M, Fujisawa T, Tachibana M, et al. Characteristic of K3 (CpG-ODN) as a Transcutaneous Vaccine Formulation Adjuvant. Pharmaceutics. 2020; 12(3):267. https://doi.org/10.3390/pharmaceutics12030267
Chicago/Turabian StyleIto, Sayami, Sachiko Hirobe, Takuto Kawakita, Mio Saito, Ying-Shu Quan, Fumio Kamiyama, Ken J. Ishii, Mizuho Nagao, Takao Fujisawa, Masashi Tachibana, and et al. 2020. "Characteristic of K3 (CpG-ODN) as a Transcutaneous Vaccine Formulation Adjuvant" Pharmaceutics 12, no. 3: 267. https://doi.org/10.3390/pharmaceutics12030267
APA StyleIto, S., Hirobe, S., Kawakita, T., Saito, M., Quan, Y.-S., Kamiyama, F., Ishii, K. J., Nagao, M., Fujisawa, T., Tachibana, M., & Okada, N. (2020). Characteristic of K3 (CpG-ODN) as a Transcutaneous Vaccine Formulation Adjuvant. Pharmaceutics, 12(3), 267. https://doi.org/10.3390/pharmaceutics12030267