Influence of Formulation Parameters on Redispersibility of Naproxen Nanoparticles from Granules Produced in a Fluidized Bed Process
Abstract
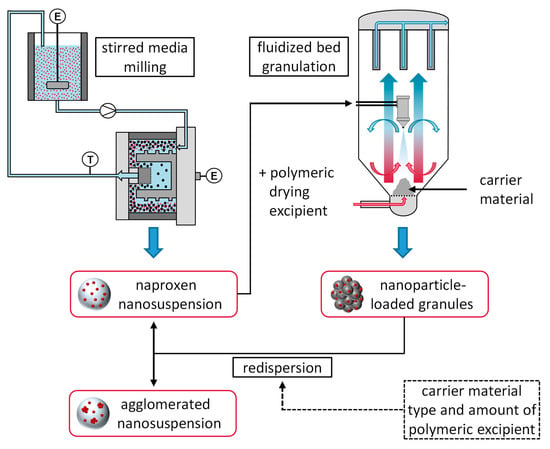
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Naproxen Nanosuspensions
2.3. Fluidized Bed Granulation
2.4. Characterization of Materials and Granules
2.4.1. Particle Size Measurement
2.4.2. Redispersibility of Granules
2.4.3. Gas Pycnometry
2.4.4. Apparent Intrinsic Dissolution Rate of Carrier Materials
2.4.5. Scanning Electron Microscopy
2.4.6. Confocal Raman Microscopy
3. Results and Discussion
3.1. Production of Naproxen Nanosuspensions
3.2. Fluidized Bed Granulation of Naproxen Nanosuspensions
3.2.1. Influence of Carrier Material
3.2.2. Influence of Polymer Concentration in the Nanosuspension
3.2.3. Influence of Polymer Type in the Nanosuspension
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lipinski, C.A. Drug-like properties and the cause of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Lipinski, C.A. Poor aqueous solubility—An industry wide problem in drug discovery. Am. Pharm. Rev. 2002, 5, 82–85. [Google Scholar]
- van Hoogevest, P.; Liu, X.; Fahr, A. Drug delivery strategies for poorly water-soluble drugs: The industrial perspective. Expert Opin. Drug Deliv. 2011, 8, 1481–1500. [Google Scholar] [CrossRef] [PubMed]
- Noyes, A.A.; Whitney, W.R. The rate of solution on solid substances in their own solutions. J. Am. Chem. Soc. 1897, 19, 930–934. [Google Scholar] [CrossRef] [Green Version]
- Nernst, W. Theorie der Reaktionsgeschwindigkeit in heterogenen Systemen. Z. Phys. Chem. 1904, 47U, 52–55. [Google Scholar] [CrossRef]
- Brunner, E. Reaktionsgeschwindigkeit in heterogenen Systemen. Z. Phys. Chem. 1904, 47U, 56–102. [Google Scholar] [CrossRef]
- Melzig, S.; Niedbalka, D.; Schilde, C.; Kwade, A. Spray drying of amorphous ibuprofen nanoparticles for the production of granules with enhanced drug release. Colloids Surf. A 2018, 536, 133–141. [Google Scholar] [CrossRef]
- Melzig, S.; Finke, J.H.; Schilde, C.; Kwade, A. Formation of long-term stable amorphous ibuprofen nanoparticles via antisolvent melt precipitation (AMP). Eur. J. Pharm. Biopharm. 2018, 131, 224–231. [Google Scholar] [CrossRef]
- Möschwitzer, J.P. Drug nanocrystals in the commercial pharmaceutical development process. Int. J. Pharm. 2013, 453, 142–156. [Google Scholar] [CrossRef]
- van Eerdenbrugh, B.; Martens, J.A.; Froyen, L.; van Humbeeck, J.; Augustijns, P.; van den Mooter, G. A screening study of surface stabilization during the production of drug nanocrystals. J. Pharm. Sci. 2009, 98, 2091–2103. [Google Scholar] [CrossRef]
- Cerdeira, A.M.; Mazzotti, M.; Gander, B. Miconazole nanosuspensions: Influence of formulation variables on particle size reduction and physical stability. Int. J. Pharm. 2010, 396, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Rong, X.; Laru, J.; van Veen, B.; Kiesvaara, J.; Hirvonen, J.; Laaksonen, T.; Peltonen, L. Nanosuspensions of poorly soluble drugs: Preparation and development by wet milling. Int. J. Pharm. 2011, 411, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Bilgili, E.; Afolabi, A. A combined microhydrodynamics-polymer adsorption analysis for elucidation of the roles of stabilizers in wet stirred media milling. Int. J. Pharm. 2012, 439, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Bitterlich, A.; Laabs, C.; Busmann, E.; Grandeury, A.; Juhnke, M.; Bunjes, H.; Kwade, A. Challenges in nanogrinding of active pharmaceutical ingredients. Chem. Eng. Technol. 2014, 37, 840–846. [Google Scholar] [CrossRef]
- Bitterlich, A.; Laabs, C.; Krautstrunk, I.; Dengler, M.; Juhnke, M.; Grandeury, A.; Bunjes, H.; Kwade, A. Process parameter dependent growth phenomena of naproxen nanosuspension manufactured by wet media milling. Eur. J. Pharm. Biopharm. 2015, 92, 171–179. [Google Scholar] [CrossRef]
- Flach, F.; Breitung-Faes, S.; Kwade, A. Scaling wet fine grinding processes of organic particles using stirred media mills. Chem. Ing. Tech. 2017, 89, 1051–1059. [Google Scholar] [CrossRef]
- Flach, F.; Breitung-Faes, S.; Kwade, A. Tailoring product formulation properties to reduce grinding media wear. Chem. Eng. Sci. 2019, 207, 69–78. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J.; Watanabe, W. Physical and chemical stability of drug nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 456–469. [Google Scholar] [CrossRef]
- Kesisoglou, F.; Panmai, S.; Wu, Y. Nanosizing—Oral formulation development and biopharmaceutical evaluation. Adv. Drug Deliv. Rev. 2007, 59, 631–644. [Google Scholar] [CrossRef]
- Kumar, S.; Gokhale, R.; Burgess, D.J. Sugars as bulking agents to prevent nano-crystal aggregation during spray or freeze-drying. Int. J. Pharm. 2014, 471, 303–311. [Google Scholar] [CrossRef]
- van Eerdenbrugh, B.; Froyen, L.; van Humbeeck, J.; Martens, J.A.; Augustijns, P.; van den Mooter, G. Alternative matrix formers for nanosuspension solidification: Dissolution performance and X-ray microanalysis as an evaluation tool for powder dispersion. Eur. J. Pharm. Sci. 2008, 35, 344–353. [Google Scholar] [CrossRef]
- van Eerdenbrugh, B.; Froyen, L.; van Humbeeck, J.; Martens, J.A.; Augustijns, P.; van den Mooter, G. Drying of crystalline drug nanosuspensions—The importance of surface hydrophobicity on dissolution behavior upon redispersion. Eur. J. Pharm. Sci. 2008, 35, 127–135. [Google Scholar] [CrossRef]
- van Eerdenbrugh, B.; van den Mooter, G.; Augustijns, P. Top-down production of drug nanocrystals: Nanosuspension stabilization, miniaturization and transformation into solid products. Int. J. Pharm. 2008, 364, 64–75. [Google Scholar] [CrossRef]
- Nair, A.; Khunt, D.; Misra, M. Application of quality by design for optimization of spray drying process used in drying of Risperidone nanosuspension. Powder Technol. 2019, 342, 156–165. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, J.; Ni, R.; Li, L.C.; Mao, S. Preparation and solidification of redispersible nanosuspensions. J. Pharm. Sci. 2014, 103, 2166–2176. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.-F.; Li, Y.; Wan, J.; Yang, M.; Zhu, W.-F.; Wang, C.-H. Study on formability of solid nanosuspensions during nanodispersion and solidification: I. Novel role of stabilizer/drug property. Int. J. Pharm. 2013, 454, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Kim, M.Y.; Kim, S.; Lee, J. Cryoprotectants for freeze drying of drug nano-suspensions: Effect of freezing rate. J. Pharm. Sci. 2009, 98, 4808–4817. [Google Scholar] [CrossRef] [PubMed]
- Šantl, M.; Ilić, I.; Vrečer, F.; Baumgartner, S. A compressibility and compactibility study of real tableting mixtures: The impact of wet and dry granulation versus a direct tableting mixture. Int. J. Pharm. 2011, 414, 131–139. [Google Scholar] [CrossRef]
- Wang, P.; Luo, Q.; Miao, Y.; Ying, L.; He, H.; Cai, C.; Tang, X. Improved dissolution rate and bioavailability of fenofibrate pellets prepared by wet-milled-drug layering. Drug Dev. Ind. Pharm. 2012, 38, 1344–1353. [Google Scholar] [CrossRef]
- Kayaert, P.; Anné, M.; van den Mooter, G. Bead layering as a process to stabilize nanosuspensions: Influence of drug hydrophobicity on nanocrystal reagglomeration following in-vitro release from sugar beads. J. Pharm. Pharmacol. 2011, 63, 1446–1453. [Google Scholar] [CrossRef]
- Möschwitzer, J.P.; Müller, R.H. Spray coated pellets as carrier system for mucoadhesive drug nanocrystals. Eur. J. Pharm. Biopharm. 2006, 62, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.; Moreno, J.; Bilgili, E.; Davé, R. Fast dissolution of poorly water soluble drugs from fluidized bed coated nanocomposites: Impact of carrier size. Int. J. Pharm. 2016, 513, 319–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhakay, A.; Azad, M.; Vizzotti, E.; Dave, R.N.; Bilgili, E. Enhanced recovery and dissolution of griseofulvin nanoparticles from surfactant-free nanocomposite microparticles incorporating wet-milled swellable dispersants. Drug Dev. Ind. Pharm. 2014, 40, 1509–1522. [Google Scholar] [CrossRef]
- Figueroa, C.E.; Bose, S. Spray granulation: Importance of process parameters on in vitro and in vivo behavior of dried nanosuspensions. Eur. J. Pharm. Biopharm. 2013, 85, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Schenck, D.; Ghosh, I.; Hollywood, A.; Maulit, E.; Ruegger, C. Application of spray granulation for conversion of a nanosuspension into a dry powder form. Eur. J. Pharm. Sci. 2012, 47, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Basa, S.; Muniyappan, T.; Karatgi, P.; Prabhu, R.; Pillai, R. Production and in vitro characterization of solid dosage form incorporating drug nanoparticles. Drug Dev. Ind. Pharm. 2008, 34, 1209–1218. [Google Scholar] [CrossRef]
- Saveyn, H.; Mermuys, D.; Thas, O.; van der Meeren, P. Determination of the refractive index of water-dispersible granules for use in laser diffraction experiments. Part. Part. Syst. Charact. 2002, 19, 426–432. [Google Scholar] [CrossRef]
- Europäisches Arzneibuch. Amtliche deutsche Ausgabe, 9 th ed.; Deutscher Apotheker Verlag: Stuttgart, Germany, 2017. [Google Scholar]
- Jaiyeoba, K.T.; Spring, M.S. The granulation of ternary mixtures: The effect of the solubility of the excipients. J. Pharm. Pharmacol. 1980, 32, 1–5. [Google Scholar] [CrossRef]
- Steiner, D.; Finke, J.H.; Kwade, A. Redispersion of nanoparticle-loaded orodispersible films: Preservation of particle fineness. Chem. Ing. Tech. 2017, 89, 1034–1040. [Google Scholar] [CrossRef]
- Bitterlich, A. Nanozerkleinerung pharmazeutischer Wirkstoffe in Mahlkörpermühlen. Ph.D. Thesis, Technische Universität Carolo-Wilhelmina zu Braunschweig, Braunschweig, Germany, 2015. [Google Scholar]
- Bouffard, J.; Kaster, M.; Dumont, H. Influence of process variable and physicochemical properties on the granulation mechanism of mannitol in a fluid bed top spray granulator. Drug Dev. Ind. Pharm. 2005, 31, 923–933. [Google Scholar] [CrossRef]
- Abberger, T.; Seo, A.; Schaefer, T. The effect of droplet size and powder particle size on the mechanisms of nucleation and growth in fluid bed melt granulation. Int. J. Pharm. 2002, 249, 185–197. [Google Scholar] [CrossRef]











| Carrier Material | X10 | X50 | X90 | Sm | ρsolid |
|---|---|---|---|---|---|
| [µm] | [µm] | [µm] | [m2·g−1] | [g·cm−3] | |
| lactose | 14.6 | 57.7 | 139.0 | 0.266 | 1.5366 |
| mannitol | 10.4 | 71.5 | 238.0 | 0.263 | 1.4839 |
| sucrose (air jet sieved) | 16.2 | 44.5 | 111.0 | 0.248 | 1.5852 |
| Ratio Polymer/API [-] | cm,carrier1 [-] | cm,polymer1 [-] | cm,API1 [-] |
|---|---|---|---|
| 0.250 | 0.889 | 0.022 | 0.089 |
| 0.375 | 0.878 | 0.033 | 0.089 |
| 0.500 | 0.867 | 0.044 | 0.089 |
| 0.625 | 0.856 | 0.055 | 0.089 |
| 0.750 | 0.844 | 0.067 | 0.089 |
| 0.875 2 | 0.833 | 0.078 | 0.089 |
| Carrier Material | εcompact [-] |
|---|---|
| sucrose | 0.118 ± 0.004 |
| mannitol | 0.113 ± 0.002 |
| lactose | 0.120 ± 0.008 |
| Stabilizers | After Milling | 3 Days of Storage | 7 Days of Storage | |||
|---|---|---|---|---|---|---|
| z-avg. | PdI | z-avg. | PdI | z-avg. | PdI | |
| [nm] | [-] | [nm] | [-] | [nm] | [-] | |
| 2.50 wt% PVP/VA + 0.25 wt% SDS | 132.7 | 0.13 | 147.5 | 0.13 | 154.1 | 0.11 |
| 2.50 wt% HPMC + 0.25 wt% SDS | 141.6 | 0.12 | 153.2 | 0.14 | 155.9 | 0.14 |
| Carrier Material | Apparent IDR [mg·cm−2·min−1] | R2 [-] |
|---|---|---|
| sucrose | 1.623 ± 0.062 | 0.994 |
| mannitol | 0.640 ± 0.029 | 0.992 |
| lactose | 0.290 ± 0.031 | 0.956 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wewers, M.; Czyz, S.; Finke, J.H.; John, E.; Van Eerdenbrugh, B.; Juhnke, M.; Bunjes, H.; Kwade, A. Influence of Formulation Parameters on Redispersibility of Naproxen Nanoparticles from Granules Produced in a Fluidized Bed Process. Pharmaceutics 2020, 12, 363. https://doi.org/10.3390/pharmaceutics12040363
Wewers M, Czyz S, Finke JH, John E, Van Eerdenbrugh B, Juhnke M, Bunjes H, Kwade A. Influence of Formulation Parameters on Redispersibility of Naproxen Nanoparticles from Granules Produced in a Fluidized Bed Process. Pharmaceutics. 2020; 12(4):363. https://doi.org/10.3390/pharmaceutics12040363
Chicago/Turabian StyleWewers, Martin, Stefan Czyz, Jan Henrik Finke, Edgar John, Bernard Van Eerdenbrugh, Michael Juhnke, Heike Bunjes, and Arno Kwade. 2020. "Influence of Formulation Parameters on Redispersibility of Naproxen Nanoparticles from Granules Produced in a Fluidized Bed Process" Pharmaceutics 12, no. 4: 363. https://doi.org/10.3390/pharmaceutics12040363
APA StyleWewers, M., Czyz, S., Finke, J. H., John, E., Van Eerdenbrugh, B., Juhnke, M., Bunjes, H., & Kwade, A. (2020). Influence of Formulation Parameters on Redispersibility of Naproxen Nanoparticles from Granules Produced in a Fluidized Bed Process. Pharmaceutics, 12(4), 363. https://doi.org/10.3390/pharmaceutics12040363