Physicochemical Properties of Poly-vinyl Polymers and Their Influence on Ketoprofen Amorphous Solid Dispersion Performance: A Polymer Selection Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Solid Samples of Polymeric Solutions via Spray Drying
2.2.2. Cryo-Milling
2.2.3. Preparation of ASDs
2.2.4. Solid-State Characterization
Powder X-ray Diffraction (pXRD)
Modulated Differential Scanning Calorimetry (mDSC)
Prediction of Glass Transition Temperature of Polymer–Ketoprofen Systems
Determination of True Density by Helium Pycnometry
Determination of Relative Humidity Induced Glass Transition by DVS
Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopy (ATR-FTIR)
2.2.5. Solid State Stability
2.2.6. Solubility Testing
Determination of Crystalline Ketoprofen Equilibrium Solubility at pH 1.2
Determination of Ketoprofen–Polymer Solid Dispersion Dynamic Solubility at pH 1.2
2.2.7. HPLC Analysis
2.2.8. Tabletting: Tablet Hardness, Tensile Strength and Ejection Force Measurements
3. Results and Discussion
3.1. Characterization of Raw Materials
3.1.1. Thermal Properties
3.1.2. pXRD Analysis
3.2. Polymer–Ketoprofen System Characterization
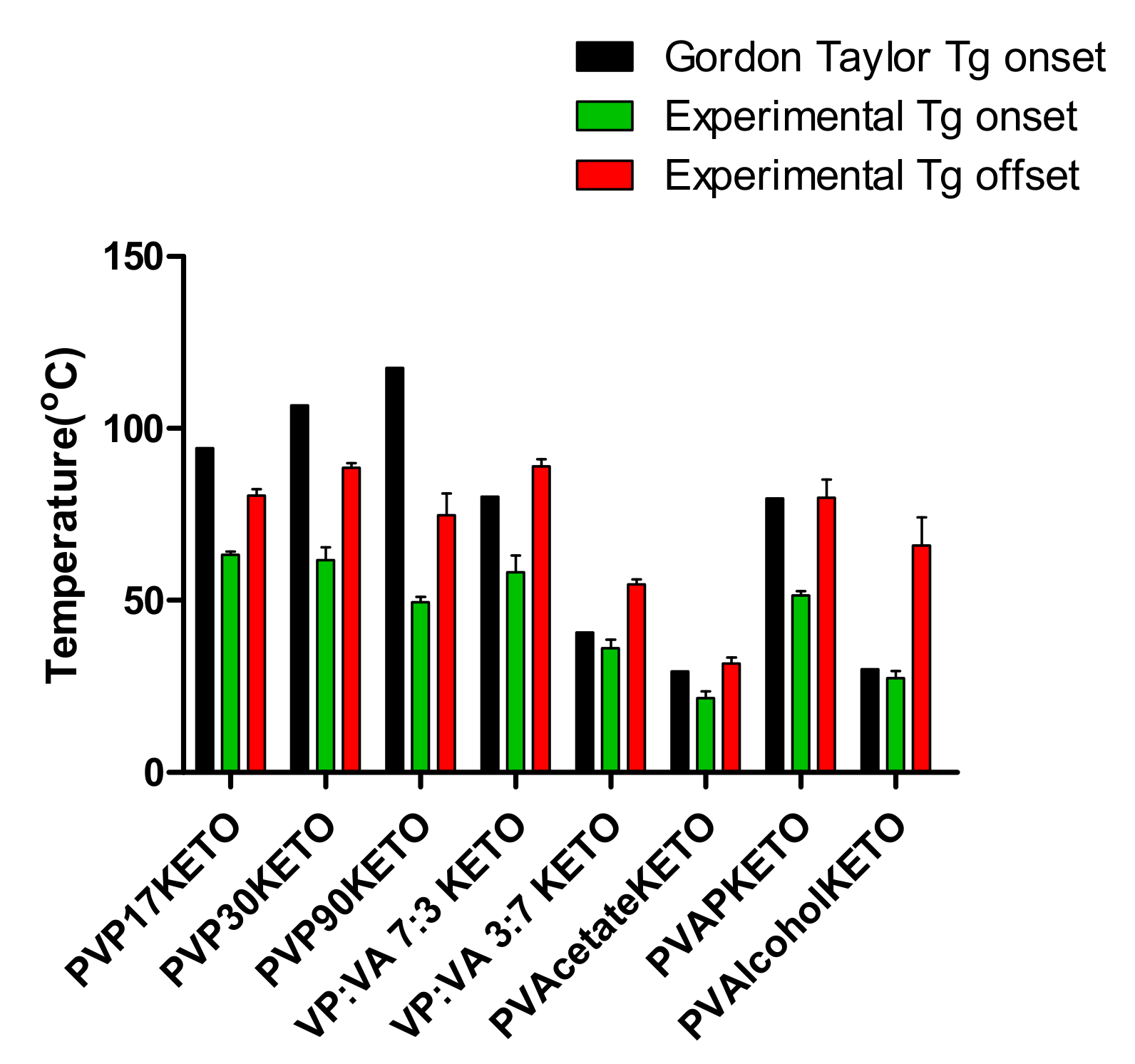
3.2.1. Thermal Properties
3.2.2. pXRD Analysis
3.2.3. ATR-FTIR Analysis
3.3. Influence of Polymer Choice on Water-Induced Phase Transition
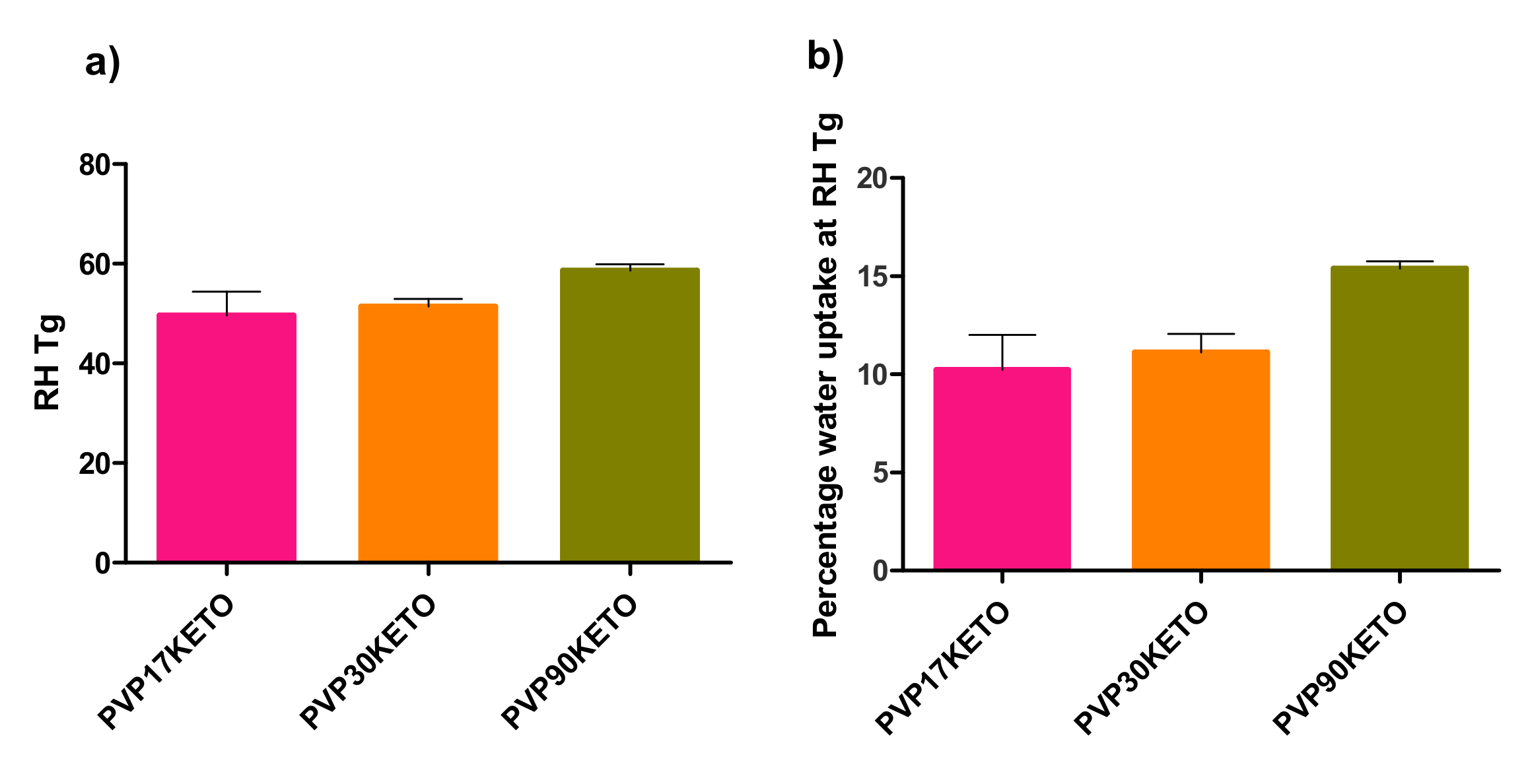
3.3.1. Influence of Polymer Molecular Weight on Water-Induced Phase Transition
3.3.2. Influence of Polymer Substitution Ratio on Water-Induced Phase Transition
3.4. Influence of Polymer Choice on Dynamic Solubility
3.5. Influence of Polymer Choice on the Stability of the Glassy State
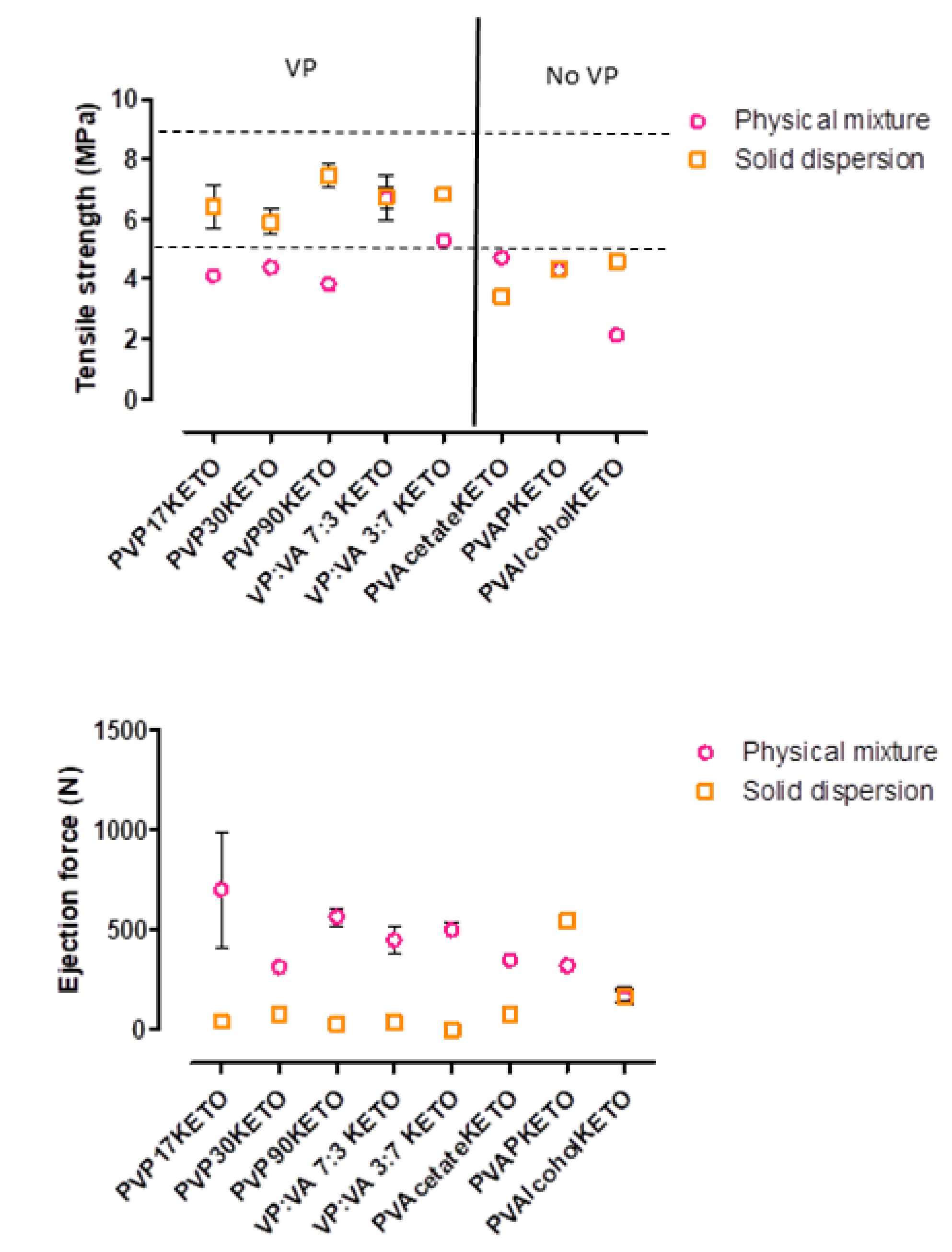
3.6. Influence of Polymer Choice on the Processability of ASDs—Tensile Strength and Ejection Force
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lipinski, C.A. Poor aqueous solubility–An industry wide problem in drug discovery. Am. Pharm. Rev. 2002, 5, 82–85. [Google Scholar]
- Lipp, R. The innovator pipeline: Bioavailability challenges and advanced oral drug delivery opportunities. Am. Pharm. Rev. 2013, 16, 14–16. [Google Scholar]
- Fahr, A.; Liu, X. Drug delivery strategies for poorly water-soluble drugs. Expert Opin. Drug Deliv. 2007, 4, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Caron, V.; Tajber, L.; Corrigan, O.I.; Healy, A.M. A comparison of spray drying and milling in the production of amorphous dispersions of sulfathiazole/polyvinylpyrrolidone and sulfadimidine/ polyvinylpyrrolidone. Mol. Pharm. 2011, 8, 532–542. [Google Scholar] [CrossRef]
- Agrawal, A.M.; Dudhedia, M.S.; Patel, A.D.; Raikes, M.S. Characterization and performance assessment of solid dispersions prepared by hot melt extrusion and spray drying process. Int. J. Pharm. 2013, 457, 71–81. [Google Scholar] [CrossRef]
- Školáková, T.; Slámová, M.; Školáková, A.; Kadeřábková, A.; Patera, J.; Zámostný, P. Investigation of Dissolution Mechanism and Release Kinetics of Poorly Water-Soluble Tadalafil from Amorphous Solid Dispersions Prepared by Various Methods. Pharmaceutics 2019, 11, 383. [Google Scholar] [CrossRef] [Green Version]
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Polymeric amorphous solid dispersions: A review of amorphization, crystallization, stabilization, solid-state characterization, and aqueous solubilization of biopharmaceutical classification system class II drugs. J. Pharm. Sci. 2016, 105, 2527–2544. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, N.N.; Majumdar, S.; Singh, A.; Deng, W.; Murthy, N.S.; Pinto, E.; Tewari, D.; Durig, T.; Repka, M.A. KlucelTM EF and ELF polymers for immediate-release oral dosage forms prepared by melt extrusion technology. AAPS PharmSciTech 2012, 13, 1158–1169. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.X.; Yang, M.; Van Den Berg, F.; Pajander, J.; Rades, T.; Rantanen, J. Influence of solvent evaporation rate and formulation factors on solid dispersion physical stability. Eur. J. Pharm. Sci. 2011, 44, 610–620. [Google Scholar] [CrossRef]
- Matsumoto, T.; Zografi, G. Physical properties of solid molecular dispersions of indomethacin with poly (vinylpyrrolidone) and poly (vinylpyrrolidone-co-vinyl-acetate) in relation to indomethacin crystallization. Pharm. Res. 1999, 16, 1722–1728. [Google Scholar] [CrossRef]
- Hilton, J.E.; Summers, M.P. The effect of wetting agents on the dissolution of indomethacin solid dispersion systems. Int. J. Pharm. 1986, 31, 157–164. [Google Scholar] [CrossRef]
- Knopp, M.M.; Olesen, N.E.; Holm, P.; Langguth, P.; Holm, R.; Rades, T. Influence of polymer molecular weight on drug-polymer solubility: A comparison between experimentally determined solubility in PVP and prediction derived from solubility in monomer. J. Pharm. Sci. 2015, 104, 2905–2912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rask, M.B.; Knopp, M.M.; Olesen, N.E.; Holm, R.; Rades, T. Influence of PVP/VA copolymer composition on drug-polymer solubility. Eur. J. Pharm. Sci. 2016, 85, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Rumondor, A.C.F.; Taylor, L.S. Effect of polymer hygroscopicity on the phase behavior of amorphous solid dispersions in the presence of moisture. Mol. Pharm. 2010, 7, 477–490. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: Washington, DC, USA, 2009; ISBN 0853696187. [Google Scholar]
- Kothari, K.; Ragoonanan, V.; Suryanarayanan, R. The role of drug-polymer hydrogen bonding interactions on the molecular mobility and physical stability of nifedipine solid dispersions. Mol. Pharm. 2015, 12, 162–170. [Google Scholar] [CrossRef]
- Broman, E.; Khoo, C.; Taylor, L.S. A comparison of alternative polymer excipients and processing methods for making solid dispersions of a poorly water soluble drug. Int. J. Pharm. 2001, 222, 139–151. [Google Scholar] [CrossRef]
- Worku, Z.A.; Paudel, A.; Van Den Mooter, G. Can compression induce demixing in amorphous solid dispersions? A case study of naproxen-PVP K25. Eur. J. Pharm. Biopharm. 2012, 81, 207–213. [Google Scholar]
- Worku, Z.A.; Aarts, J.; Van Den Mooter, G. Influence of compression forces on the structural stability of naproxen/PVP-VA 64 solid dispersions. Mol. Pharm. 2014, 11, 1102–1108. [Google Scholar] [CrossRef]
- Knopp, M.M.; Tajber, L.; Tian, Y.; Olesen, N.E.; Jones, D.S.; Kozyra, A.; Löbmann, K.; Paluch, K.; Brennan, C.M.; Holm, R.; et al. Comparative study of different methods for the prediction of drug-polymer solubility. Mol. Pharm. 2015, 12, 3408–3419. [Google Scholar] [CrossRef] [Green Version]
- Gordon, M.; Taylor, J.S. Ideal copolymers and the second-order transitions of synthetic rubbers. I. noncrystalline copolymers. J. Appl. Chem. 1952, 2, 493–500. [Google Scholar] [CrossRef]
- Burnett, D.J.; Thielmann, F.; Booth, J. Determining the critical relative humidity for moisture-induced phase transitions. Int. J. Pharm. 2004, 287, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Fox, T.G.; Flory, P.J. Second-order transition temperatures and related properties of polystyrene. I. Influence of molecular weight. J. Appl. Phys. 1950, 21, 581–591. [Google Scholar] [CrossRef]
- Bai, Q.; Zhang, G.; Xu, B.; Feng, X.; Jiang, H.; Li, H. Thermal and water dual-responsive shape memory poly(vinyl alcohol)/Al2O3 nanocomposite. RSC Adv. 2015, 5, 91213–91217. [Google Scholar] [CrossRef]
- Koosha, M.; Mirzadeh, H.; Shokrgozar, M.A.; Farokhi, M. Nanoclay-reinforced electrospun chitosan/PVA nanocomposite nanofibers for biomedical applications. RSC Adv. 2015, 5, 10479–10487. [Google Scholar] [CrossRef]
- Baird, J.A.; Van Eerdenbrugh, B.; Taylor, L.S. A classification system to assess the crystallization tendency of organic molecules from undercooled melts. J. Pharm. Sci. 2010, 99, 3787–3806. [Google Scholar] [CrossRef]
- Pal, K.; Banthia, A.K.; Majumdar, D.K. Preparation and characterization of polyvinyl alcohol-gelatin hydrogel membranes for biomedical applications. AAPS PharmSciTech 2007, 8, 21. [Google Scholar] [CrossRef]
- Hancock, B.C.; Zografi, G. Characteristics and significance of the amorphous state in pharmaceutical systems. J. Pharm. Sci. 1997, 86, 1–12. [Google Scholar] [CrossRef]
- Van den Mooter, G.; Wuyts, M.; Blaton, N.; Busson, R.; Grobet, P.; Augustijns, P.; Kinget, R. Physical stabilisation of amorphous ketoconazole in solid dispersions with polyvinylpyrrolidone K25. Eur. J. Pharm. Sci. 2001, 12, 261–269. [Google Scholar] [CrossRef]
- Yuan, X.; Xiang, T.X.; Anderson, B.D.; Munson, E.J. Hydrogen bonding interactions in amorphous indomethacin and its amorphous solid dispersions with poly(vinylpyrrolidone) and poly(vinylpyrrolidone-co-vinyl acetate) studied using 13C solid-state NMR. Mol. Pharm. 2015, 12, 4518–4528. [Google Scholar] [CrossRef]
- Taylor, L.S.; Zografi, G. Sugar-polymer hydrogen bond interactions in lyophilized amorphous mixtures. J. Pharm. Sci. 1998, 87, 1615–1621. [Google Scholar] [CrossRef]
- Di Martino, P.; Joiris, E.; Gobetto, R.; Masic, A.; Palmieri, G.F.; Martelli, S. Ketoprofen-poly(vinylpyrrolidone) physical interaction. J. Cryst. Growth 2004, 265, 302–308. [Google Scholar] [CrossRef]
- Sekizaki, H.; Danjo, K.; Eguchi, H.; Yonezawa, Y.; Sunada, H.; Otsuka, A. Solid-state interaction of ibuprofen with polyvinylpyrrolidone. Chem. Pharm. Bull. (Tokyo). 1995, 43, 988–993. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.Y.; Chung, Y.Y.; Cheah, X.Z.; Tan, E.Y.L.; Quah, J. The characterization and dissolution performances of spray dried solid dispersion of ketoprofen in hydrophilic carriers. Asian J. Pharm. Sci. 2015, 10, 372–385. [Google Scholar] [CrossRef] [Green Version]
- Rumondor, A.C.F.; Marsac, P.J.; Stanford, L.A.; Taylor, L.S. Phase behavior of poly(vinylpyrrolidone) containing amorphous solid dispersions in the presence of moisture. Mol. Pharm. 2009, 6, 1492–1505. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; Vanwert, A.; Bogner, R.H. Formation of physically stable amorphous drugs by milling with neusilin. J. Pharm. Sci. 2003, 92, 536–551. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S. Introduction to Spectroscopy, 5th ed.Cengage Learning: Stamford, CT, USA, 2015; ISBN 1-285-46012-X. [Google Scholar]
- Granero, G.E.; Ramachandran, C.; Amidon, G.L. Rapid in vivo dissolution of ketoprofen: Implications on the biopharmaceutics classification system. Pharmazie 2006, 61, 673–676. [Google Scholar]
- Knopp, M.M.; Nguyen, J.H.; Mu, H.; Langguth, P.; Rades, T.; Holm, R. Influence of Copolymer Composition on In Vitro and In Vivo Performance of Celecoxib-PVP/VA Amorphous Solid Dispersions. AAPS J. 2016, 18, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Ueda, K.; Yamazoe, C.; Yasuda, Y.; Higashi, K.; Kawakami, K.; Moribe, K. Mechanism of Enhanced Nifedipine Dissolution by Polymer-Blended Solid Dispersion through Molecular-Level Characterization. Mol. Pharm. 2018, 15, 4099–4109. [Google Scholar] [CrossRef]
- Saboo, S.; Mugheirbi, N.A.; Zemlyanov, D.Y.; Kestur, U.S.; Taylor, L.S. Congruent release of drug and polymer: A “sweet spot” in the dissolution of amorphous solid dispersions. J. Control. Release 2019, 298, 68–82. [Google Scholar] [CrossRef]
- Qian, F.; Huang, J.; Zhu, Q.; Haddadin, R.; Gawel, J.; Garmise, R.; Hussain, M. Is a distinctive single Tg a reliable indicator for the homogeneity of amorphous solid dispersion? Int. J. Pharm. 2010, 395, 232–235. [Google Scholar] [CrossRef]
- McKenna, A.; McCafferty, D.F. Effect of particle size on the compaction mechanism and tensile strength of tablets. J. Pharm. Pharmacol. 1982, 34, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Sebhatu, T.; Alderborn, G. Relationships between the effective interparticulate contact area and the tensile strength of tablets of amorphous and crystalline lactose of varying particle size. Eur. J. Pharm. Sci. 1999, 8, 235–242. [Google Scholar] [CrossRef]
- Démuth, B.; Nagy, Z.K.; Balogh, A.; Vigh, T.; Marosi, G.; Verreck, G.; Van Assche, I.; Brewster, M.E. Downstream processing of polymer-based amorphous solid dispersions to generate tablet formulations. Int. J. Pharm. 2015, 486, 268–286. [Google Scholar] [CrossRef] [PubMed]
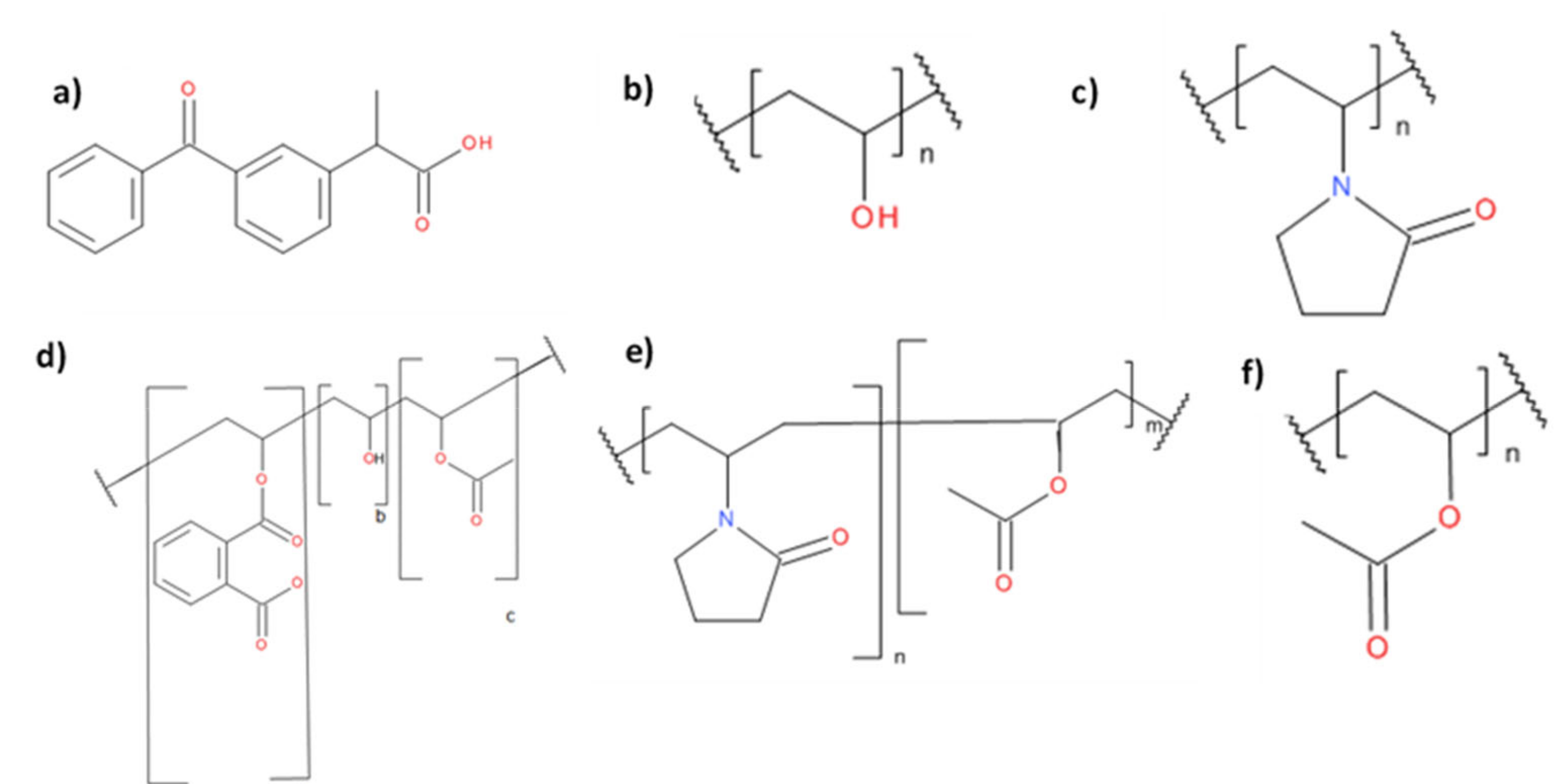








| Polymer | Supplier |
|---|---|
| Polyvinyl acetate (PVAcetate) Mw 100,000 g/mol | Acros Organics (Geel, Belgium) |
| Polyvinyl acetate phthalate (PVAP) Mw 47,000–61,000 g/mol [15] | Colorcon (Indianapolis, IN, USA) |
| Polyvinyl alcohol (PVAlcohol) Mw 15,000 g/mol | MP Biomedicals (Irvine, CA, USA) |
| Polyvinyl pyrrolidone K17 (PVP 17) Mw 7000–11,000 g/mol | BASF (Ludwigshafen, Germany) |
| Polyvinyl pyrrolidone K30 (PVP 30) Mw 54,000–55,000 g/mol | BASF (Ludwigshafen, Germany) |
| Polyvinyl pyrrolidone K90 (PVP 90) Mw 1,000,000–1,500,000 g/mol | BASF (Ludwigshafen, Germany) |
| Polyvinyl pyrrolidone vinyl acetate 3:7 E335 (VP:VA 3:7) Mw 28,000 g/mol [20] | Ashland (Lexington, KY, USA) |
| Polyvinyl pyrrolidone vinyl acetate 7:3 E735 (VP:VA 7:3) Mw 56,700 g/mol [20] | Ashland (Lexington, KY, USA) |
| Polymer | Tg | Tm (onset) |
|---|---|---|
| PVP 90 | 172.0 ± 1.2 °C | |
| PVP 30 | 153.6 ± 2.1 °C | |
| PVP 17 | 135.6 ± 0.3 °C | |
| PVPVA 7:3 | 114.2 ± 1.1 °C | |
| PVPVA 3:7 | 57.9 ± 4.3 °C | |
| PVAcetate | 38.6 ± 1.4 °C | |
| PVAP | 46.3 ± 0.7 °C / 116.0 ± 0.6 °C | |
| PVAlcohol 1st heating run | 41.1 ± 3.9 °C | 155.0 ± 0.1 °C |
| PVAlcohol 2nd heating run | 64.51 ± 15 °C |
| Material | True Density (g/cm3) |
|---|---|
| Crystalline ketoprofen | 1.25 ± 0.01 |
| Amorphous ketoprofen* | 1.19 ± 0.01 |
| PVP 17 | 1.20 ± 0.00 |
| PVP 30 | 1.20 ± 0.00 |
| PVP 90 | 1.24 ± 0.00 |
| VP:VA 7:3 | 1.21 ± 0.00 |
| VP:VA 3:7 | 1.23 ± 0.00 |
| PVAcetate | 1.22 ± 0.00 |
| PVAlcohol | 1.31 ± 0.00 |
| PVAP | 1.38 ± 0.02 |
| Poly-Vinyl Polymer Property | Effect on ASD Performance |
|---|---|
| High Mw |
|
| High VP content |
|
| High VA content |
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Browne, E.; Worku, Z.A.; Healy, A.M. Physicochemical Properties of Poly-vinyl Polymers and Their Influence on Ketoprofen Amorphous Solid Dispersion Performance: A Polymer Selection Case Study. Pharmaceutics 2020, 12, 433. https://doi.org/10.3390/pharmaceutics12050433
Browne E, Worku ZA, Healy AM. Physicochemical Properties of Poly-vinyl Polymers and Their Influence on Ketoprofen Amorphous Solid Dispersion Performance: A Polymer Selection Case Study. Pharmaceutics. 2020; 12(5):433. https://doi.org/10.3390/pharmaceutics12050433
Chicago/Turabian StyleBrowne, Emer, Zelalem A. Worku, and Anne Marie Healy. 2020. "Physicochemical Properties of Poly-vinyl Polymers and Their Influence on Ketoprofen Amorphous Solid Dispersion Performance: A Polymer Selection Case Study" Pharmaceutics 12, no. 5: 433. https://doi.org/10.3390/pharmaceutics12050433
APA StyleBrowne, E., Worku, Z. A., & Healy, A. M. (2020). Physicochemical Properties of Poly-vinyl Polymers and Their Influence on Ketoprofen Amorphous Solid Dispersion Performance: A Polymer Selection Case Study. Pharmaceutics, 12(5), 433. https://doi.org/10.3390/pharmaceutics12050433