What Are the Current Approaches to Optimising Antimicrobial Dosing in the Intensive Care Unit?
Abstract
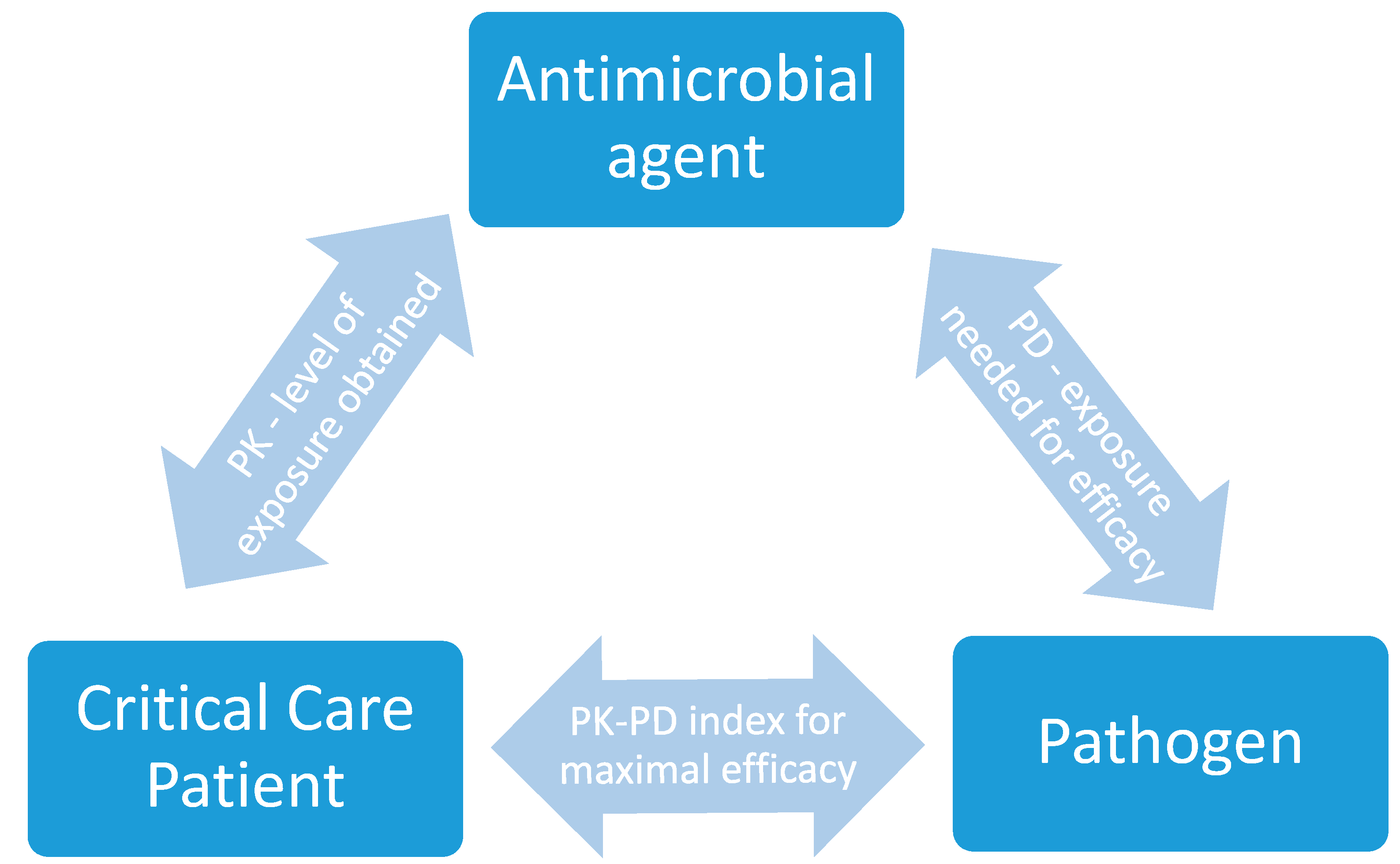
1. Introduction
1.1. Altered Pharmacokinetics
1.2. Pharmacodynamic Considerations
2. Dosing Nomograms
3. Therapeutic Drug Monitoring in the Intensive Care Unit (ICU)
4. Dosing Software
4.1. Linear Regression Based Dosing Software
4.2. Population PK-Based Dosing Software
4.3. Bayesian Forecasting Dosing Software
4.4. Artificial Intelligence Software
4.5. Challenges ahead for Widespread Dosing Software
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Antimicrobial | Study Profile | Reference |
|---|---|---|
| Aminoglycosides |
| Rea RS et al. [118] |
| Amoxicillin and clavulanic acid |
| Carlier M et al. [119] |
| Cefazolin |
| Roberts JA et al. [120] |
| Cefepime |
| Nicasio AM et al. [121] |
| Cefotaxime |
| Beranger A et al. [122] |
| Ceftazidime |
| Georges B et al. [123] |
| Ceftazidime (pediatric) |
| Shi ZR et al. Shi, Chen [124] |
| Ceftolazone and tazobactam |
| Sime FB et al. [125] |
| Ceftriaxone |
| Garot D et al. [126] Leegwater E et al. [127] [126,127] |
| Ciprofloxacin |
| Khachman D et al. [128] |
| Doripenem |
| Abdul-Aziz MH et al. [129] |
| Flucloxacillin |
| Ulldemolins M et al. [130] |
| Fluconazole |
| Aoyama T et al. [131] |
| Fosfomycin |
| Parker SL et al. [132] |
| Ganciclovir |
| Kren SD et al. [133] |
| Imipenem |
| De Velde F [134] |
| Levofloxacin |
| Roberts JA et al. [135] |
| Linezolid |
| Soraluce A et al. [136] |
| Meropenem |
| Crandon Jl et al. [137] Braune S et al. [138] Rapp M et al. [139] |
| Micafungin |
| Maseda E et al. [140] |
| Piperacillin and tazobactam |
| Felton TW et al. [141] |
| Polymyxin B |
| Sandri AM et al. [142] |
| Posaconazole |
| Sime FB et al. [143] |
| Tigecycline |
| Xie J et al. [144] |
| Vancomycin |
| Neely MN et al. [90] |
References
- Ferrer, R.; Martin-Loeches, I.; Phillips, G.; Osborn, T.M.; Townsend, S.; Dellinger, R.P.; Artigas, A.; Schorr, C.; Levy, M.M. Empiric Antibiotic Treatment Reduces Mortality in Severe Sepsis and Septic Shock From the First Hour: Results From a Guideline-Based Performance Improvement Program. Crit. Care Med. 2014, 42, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ellis, P.; Arabi, Y.; Roberts, D.; Light, B.; Parrillo, J.E.; Dodek, P.; Wood, G.; Kumar, A.; Simon, D.; et al. Initiation of Inappropriate Antimicrobial Therapy Results in a Fivefold Reduction of Survival in Human Septic Shock. Chest 2009, 136, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med. 2017, 45, 486–552. [Google Scholar] [CrossRef]
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef]
- Bhattaram, V.A.; Bonapace, C.; Chilukuri, D.M.; Duan, J.Z.; Garnett, C.; Gobburu, J.V.S.; Jang, S.H.; Kenna, L.; Lesko, L.J.; Madabushi, R.; et al. Impact of Pharmacometric Reviews on New Drug Approval and Labeling Decisions—A Survey of 31 New Drug Applications Submitted Between 2005 and 2006. Clin. Pharmacol. Ther. 2007, 81, 213–221. [Google Scholar] [CrossRef]
- Tuntland, T.; Ethell, B.; Kosaka, T.; Blasco, F.; Zang, R.X.; Jain, M.; Gould, T.; Hoffmaster, K. Implementation of pharmacokinetic and pharmacodynamic strategies in early research phases of drug discovery and development at Novartis Institute of Biomedical Research. Front. Pharmacol. 2014, 5, 174. [Google Scholar] [CrossRef]
- Roberts, J.A. Using PK/PD to optimize antibiotic dosing for critically ill patients. Curr. Pharm. Biotechnol. 2011, 12, 2070–2079. [Google Scholar] [CrossRef]
- Sime, F.B.; Roberts, M.S.; Peake, S.L.; Lipman, J.; Roberts, J.A. Does Beta-lactam Pharmacokinetic Variability in Critically Ill Patients Justify Therapeutic Drug Monitoring? A Systematic Review. Ann. Intensive Care. 2012, 2, 35. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. DALI: Defining antibiotic levels in intensive care unit patients: Are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef]
- Sinnollareddy, M.G.; Roberts, M.S.; Lipman, J.; Roberts, J.A. β-Lactam pharmacokinetics and pharmacodynamics in critically ill patients and strategies for dose optimization: A structured review. Clin. Exp. Pharmacol. Physiol. 2012, 39, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Aspasia Soulatati, S.P.D. Liver dysfunction in the intensive care unit. Ann. Gastroenterol. 2005, 18, 35–45. [Google Scholar]
- Kramer, L.; Jordan, B.; Druml, W.; Bauer, P.; Metnitz, P.G. Incidence and prognosis of early hepatic dysfunction in critically ill patients—A prospective multicenter study. Crit Care Med. 2007, 35, 1099–1104. [Google Scholar] [CrossRef]
- Saloojee, A.; Skinner, D.L.; Loots, E.; Hardcastle, T.C.; Muckart, D.J.J. Hepatic dysfunction: A common occurrence in severely injured patients. Injury 2017, 48, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, J.; Acharya, A.; Cerda, J.; Maccariello, E.R.; Madarasu, R.C.; Tolwani, A.J.; Liang, X.; Fu, P.; Liu, Z.H.; Mehta, R.L. A Prospective International Multicenter Study of AKI in the Intensive Care Unit. Clin. J. Am. Soc. Nephrol. CJASN 2015, 10, 1324–1331. [Google Scholar] [CrossRef]
- Hoste, E.A.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef]
- Mehta, R.L.; Pascual, M.T.; Soroko, S.; Savage, B.R.; Himmelfarb, J.; Ikizler, T.A.; Paganini, E.P.; Chertow, G.M. Spectrum of acute renal failure in the intensive care unit: The PICARD experience. Kidney Int. 2004, 66, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Uchino, S.; Kellum, J.A.; Bellomo, R.; Doig, G.S.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; et al. Acute renal failure in critically ill patients: A multinational, multicenter study. Jama 2005, 294, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Blot, S.I.; Pea, F.; Lipman, J. The effect of pathophysiology on pharmacokinetics in the critically ill patient—Concepts appraised by the example of antimicrobial agents. Adv. Drug Deliv. Rev. 2014, 77, 3–11. [Google Scholar] [CrossRef]
- Halilovic, J.; Heintz, B.H. Antibiotic dosing in cirrhosis. Am. J. Health Syst. Pharm. AJHP Off. J. Am. Soc. Health Syst. Pharm. 2014, 71, 1621–1634. [Google Scholar] [CrossRef] [PubMed]
- Bilbao-Meseguer, I.; Rodríguez-Gascón, A.; Barrasa, H.; Isla, A.; Solinís, M. Augmented Renal Clearance in Critically Ill Patients: A Systematic Review. Clin. Pharmacokinet. 2018, 57, 1107–1121. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.A.; Putt, M.T.; Shanmugathasan, S.; Roberts, J.A.; Lipman, J. Augmented renal clearance in the Intensive Care Unit: An illustrative case series. Int. J. Antimicrob. Agents 2010, 35, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Sime, F.B.; Udy, A.A.; Roberts, J.A. Augmented renal clearance in critically ill patients: Etiology, definition and implications for beta-lactam dose optimization. Curr. Opin. Pharmacol. 2015, 24, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ulldemolins, M.; Roberts, J.A.; Rello, J.; Paterson, D.L.; Lipman, J. The Effects of Hypoalbuminaemia on Optimizing Antibacterial Dosing in Critically Ill Patients. Clin. Pharmacokinet. 2011, 50, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.M.; Roberts, J.A.; Lipman, J. Pharmacokinetics and pharmacodynamics in critically ill patients. Curr. Opin. Anaesthesiol. 2010, 23, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Tsai, D.; Lipman, J.; Roberts, J.A. Pharmacokinetic/pharmacodynamic considerations for the optimization of antimicrobial delivery in the critically ill. Curr. Opin. Crit. Care 2015, 21, 412–420. [Google Scholar] [CrossRef]
- Boucher, B.A.; Wood, G.C.; Swanson, J.M. Pharmacokinetic changes in critical illness. Crit. Care Clin. 2006, 22, 255–271. [Google Scholar] [CrossRef]
- Smith, B.S.; Yogaratnam, D.; Levasseur-Franklin, K.E.; Forni, A.; Fong, J. Introduction to drug pharmacokinetics in the critically ill patient. Chest 2012, 141, 1327–1336. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Roberts, J.A. Antibiotic dosing during extracorporeal membrane oxygenation: Does the system matter? Curr. Opin. Anaesthesiol. 2020, 33, 71–82. [Google Scholar] [CrossRef]
- Zamoner, W.; de Freitas, F.M.; Garms, D.S.S.; de Oliveira, M.G.; Balbi, A.L.; Ponce, D. Pharmacokinetics and pharmacodynamics of antibiotics in critically ill acute kidney injury patients. Pharmacol. Res. Perspect. 2016, 4, e00280. [Google Scholar] [CrossRef]
- Cheng, V.; Abdul-Aziz, M.-H.; Roberts, J.A.; Shekar, K. Optimising drug dosing in patients receiving extracorporeal membrane oxygenation. J. Thorac Dis. 2018, 10 (Suppl. 5), S629–S641. [Google Scholar] [CrossRef] [PubMed]
- Fissell, W.H. Antimicrobial dosing in acute renal replacement. Adv. Chronic Kidney Dis. 2013, 20, 85–93. [Google Scholar] [CrossRef]
- Forrest, A.; Nix, D.E.; Ballow, C.H.; Goss, T.F.; Birmingham, M.C.; Schentag, J.J. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob. Agents Chemother. 1993, 37, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, P.S.; Paladino, J.A.; Schentag, J.J. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int. J. Antimicrob. Agents 2008, 31, 345–351. [Google Scholar] [CrossRef]
- Carrie, C.; Petit, L.; d’Houdain, N.; Sauvage, N.; Cottenceau, V.; Lafitte, M.; Foumenteze, C.; Hisz, Q.; Menu, D.; Legeron, R.; et al. Association between augmented renal clearance, antibiotic exposure and clinical outcome in critically ill septic patients receiving high doses of beta-lactams administered by continuous infusion: A prospective observational study. Int. J. Antimicrob. Agents 2018, 51, 443–449. [Google Scholar] [CrossRef]
- Moise-Broder, P.A.; Forrest, A.; Birmingham, M.C.; Schentag, J.J. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin. Pharmacokinet. 2004, 43, 925–942. [Google Scholar] [CrossRef]
- Li, C.; Du, X.; Kuti, J.L.; Nicolau, D.P. Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob. Agents Chemother. 2007, 51, 1725–1730. [Google Scholar] [CrossRef]
- Mouton, J.W.; Muller, A.E.; Canton, R.; Giske, C.G.; Kahlmeter, G.; Turnidge, J. MIC-based dose adjustment: Facts and fables. J. Antimicrob. Chemother. 2018, 73, 564–568. [Google Scholar] [CrossRef]
- Roberts, J.A.; Lipman, J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 2009, 37, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Rea, R.S.; Capitano, B. Optimizing use of aminoglycosides in the critically ill. Semin Respir. Crit. Care Med. 2007, 28, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.E.; Huttner, B.; Huttner, A. Therapeutic Drug Monitoring of Beta-Lactams and Other Antibiotics in the Intensive Care Unit: Which Agents, Which Patients and Which Infections? Drugs 2018, 78, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Zelenitsky, S.A.; Ariano, R.E. Support for higher ciprofloxacin AUC 24/MIC targets in treating Enterobacteriaceae bloodstream infection. J. Antimicrob. Chemother. 2010, 65, 1725–1732. [Google Scholar] [CrossRef]
- Rybak, M.; Lomaestro, B.; Rotschafer, J.C.; Moellering, R., Jr.; Craig, W.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharmacy 2009, 66, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Meagher, A.K.; Passarell, J.A.; Cirincione, B.B.; Van Wart, S.A.; Liolios, K.; Babinchak, T.; Ellis-Grosse, E.J.; Ambrose, P.G. Exposure-response analyses of tigecycline efficacy in patients with complicated skin and skin-structure infections. Antimicrob. Agents Chemother. 2007, 51, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Passarell, J.A.; Meagher, A.K.; Liolios, K.; Cirincione, B.B.; Van Wart, S.A.; Babinchak, T.; Ellis-Grosse, E.J.; Ambrose, P.G. Exposure-response analyses of tigecycline efficacy in patients with complicated intra-abdominal infections. Antimicrob. Agents Chemother. 2008, 52, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.A. Does the dose matter? Clin Infect Dis. 2001, 33 (Suppl. 3), S233–S237. [Google Scholar] [CrossRef]
- Louie, A.; Kaw, P.; Liu, W.; Jumbe, N.; Miller, M.H.; Drusano, G.L. Pharmacodynamics of daptomycin in a murine thigh model of Staphylococcus aureus infection. Antimicrob. Agents Chemother. 2001, 45, 845–851. [Google Scholar] [CrossRef]
- Girard, D.; Finegan, S.M.; Dunne, M.W.; Lame, M.E. Enhanced efficacy of single-dose versus multi-dose azithromycin regimens in preclinical infection models. J. Antimicrob. Chemother. 2005, 56, 365–371. [Google Scholar] [CrossRef]
- Vogelman, B.; Gudmundsson, S.; Leggett, J.; Turnidge, J.; Ebert, S.; Craig, W.A. Correlation of Antimicrobial Pharmacokinetic Parameters with Therapeutic Efficacy in an Animal Model. J. Infect. Dis. 1988, 158, 831–847. [Google Scholar] [CrossRef]
- Pea, F.; Viale, P.; Cojutti, P.; Del Pin, B.; Zamparini, E.; Furlanut, M. Therapeutic drug monitoring may improve safety outcomes of long-term treatment with linezolid in adult patients. J. Antimicrob. Chemother. 2012, 67, 2034–2042. [Google Scholar] [CrossRef]
- Bergen, P.J.; Li, J.; Nation, R.L. Dosing of colistin-back to basic PK/PD. Curr. Opin. Pharmacol. 2011, 11, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Lepak, A.J.; Andes, D.R. Antifungal pharmacokinetics and pharmacodynamics. Cold Spring Harb. Perspect. Med. 2014, 5, a019653. [Google Scholar] [CrossRef]
- Perez-Pitarch, A.; Ferriols-Lisart, R.; Aguilar, G.; Ezquer-Garin, C.; Belda, F.J.; Guglieri-Lopez, B. Dosing of caspofungin based on a pharmacokinetic/pharmacodynamic index for the treatment of invasive fungal infections in critically ill patients on continuous venovenous haemodiafiltration. Int. J. Antimicrob. Agents 2018, 51, 115–121. [Google Scholar] [CrossRef]
- Hong, Y.; Shaw, P.J.; Nath, C.E.; Yadav, S.P.; Stephen, K.R.; Earl, J.W.; McLachlan, A.J. Population pharmacokinetics of liposomal amphotericin B in pediatric patients with malignant diseases. Antimicrob. Agents Chemother. 2006, 50, 935–942. [Google Scholar] [CrossRef]
- Leblebicioglu, H.; Cakir, N.; Celen, M.; Kurt, H.; Baris, H.; Laeuffer, J. Comparative activity of carbapenem testing (the COMPACT study) in Turkey. BMC Infect Dis. 2012, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Kiratisin, P.; Chongthaleong, A.; Tan, T.Y.; Lagamayo, E.; Roberts, S.; Garcia, J.; Davies, T. Comparative in vitro activity of carbapenems against major Gram-negative pathogens: Results of Asia-Pacific surveillance from the COMPACT II study. Int. J. Antimicrob. Agents 2012, 39, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Valenza, G.; Seifert, H.; Decker-Burgard, S.; Laeuffer, J.; Morrissey, I.; Mutters, R. Comparative Activity of Carbapenem Testing (COMPACT) study in Germany. Int. J. Antimicrob. Agents 2012, 39, 255–258. [Google Scholar] [CrossRef]
- Hidron, A.I.; Edwards, J.R.; Patel, J.; Horan, T.C.; Sievert, D.M.; Pollock, D.A.; Fridkin, S.K. NHSN annual update: Antimicrobial-resistant pathogens associated with healthcare-associated infections: Annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect. Control Hosp. Epidemiol. 2008, 29, 996–1011. [Google Scholar] [CrossRef]
- National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1992-April 2000, issued June 2000. Am. J. Infect. Control 2000, 28, 429–448. [CrossRef]
- Vincent, J.L.; Bihari, D.J.; Suter, P.M.; Bruining, H.A.; White, J.; Nicolas-Chanoin, M.H.; Wolff, M.; Spencer, R.C.; Hemmer, M. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. Jama 1995, 274, 639–644. [Google Scholar] [CrossRef]
- Hanberger, H.; Garcia-Rodriguez, J.A.; Gobernado, M.; Goossens, H.; Nilsson, L.E.; Struelens, M.J. Antibiotic susceptibility among aerobic gram-negative bacilli in intensive care units in 5 European countries. French and Portuguese ICU Study Groups. Jama 1999, 281, 67–71. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Levison, M.E.; Levison, J.H. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect. Dis. Clin. North Am. 2009, 23, 791–815. [Google Scholar] [CrossRef]
- Roberts, J.A.; Kruger, P.; Paterson, D.L.; Lipman, J. Antibiotic resistance—What’s dosing got to do with it? Crit Care Med. 2008, 36, 2433–2440. [Google Scholar] [CrossRef]
- Chennavasin, P.; Brater, D.C. Nomograms for Drug Use in Renal Disease. Clin. Pharmacokinet. 1981, 6, 193–214. [Google Scholar] [CrossRef] [PubMed]
- Burton, M.E. Applied Pharmacokinetics and Pharmacodynamics: Principles of Therapeutic Drug Monitoring, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006. [Google Scholar]
- Lewis, S.J.; Mueller, B.A. Development of a vancomycin dosing approach for critically ill patients receiving hybrid hemodialysis using Monte Carlo simulation. SAGE Open Med. 2018, 6, 2050312118773257. [Google Scholar] [CrossRef] [PubMed]
- Baptista, J.P.; Roberts, J.A.; Sousa, E.; Freitas, R.; Deveza, N.; Pimentel, J. Decreasing the time to achieve therapeutic vancomycin concentrations in critically ill patients: Developing and testing of a dosing nomogram. Crit. Care 2014, 18, 654. [Google Scholar] [CrossRef]
- Medellin-Garibay, S.E.; Romano-Moreno, S.; Tejedor-Prado, P.; Rubio-Alvaro, N.; Rueda-Naharro, A.; Blasco-Navalpotro, M.A.; Garcia, B.; Barcia, E. Influence of Mechanical Ventilation on the Pharmacokinetics of Vancomycin Administered by Continuous Infusion in Critically Ill Patients. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Pea, F.; Furlanut, M.; Negri, C.; Pavan, F.; Crapis, M.; Cristini, F.; Viale, P. Prospectively validated dosing nomograms for maximizing the pharmacodynamics of vancomycin administered by continuous infusion in critically ill patients. Antimicrob. Agents Chemother. 2009, 53, 1863–1867. [Google Scholar] [CrossRef]
- Crumby, T.; Rinehart, E.; Carby, M.C.; Kuhl, D.; Talati, A.J. Pharmacokinetic comparison of nomogram-based and individualized vancomycin regimens in neonates. Am. J. Health Syst. Pharm. AJHP. Off. J. Am. Soc. Health Syst. Pharm. 2009, 66, 149–153. [Google Scholar] [CrossRef]
- Miloslavsky, M.; Galler, M.F.; Moawad, I.; Actis, J.; Cummings, B.M.; El Saleeby, C.M. The Impact of Pediatric-Specific Vancomycin Dosing Guidelines: A Quality Improvement Initiative. Pediatrics 2017, 139, e20162423. [Google Scholar] [CrossRef] [PubMed]
- Watling, S.M.; Kisor, D.F. Population pharmacokinetics: Development of a medical intensive care unit-specific gentamicin dosing nomogram. Ann. Pharmacother. 1993, 27, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Pea, F.; Viale, P.; Cojutti, P.; Furlanut, M. Dosing Nomograms for Attaining Optimum Concentrations of Meropenem by Continuous Infusion in Critically Ill Patients with Severe Gram-Negative Infections: A Pharmacokinetics/Pharmacodynamics-Based Approach. Antimicrob. Agents Chemother. 2012, 56, 6343. [Google Scholar] [CrossRef]
- Minichmayr, I.K.; Roberts, J.A.; Frey, O.R.; Roehr, A.C.; Kloft, C.; Brinkmann, A. Development of a dosing nomogram for continuous-infusion meropenem in critically ill patients based on a validated population pharmacokinetic model. J. Antimicrob. Chemoth. 2018, 73, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Reeves, D.; Lovering, A.; Thomson, A. Therapeutic drug monitoring in the past 40 years of the Journal of Antimicrobial Chemotherapy. J. Antimicrob. Chemother. 2016, 71, 3330–3332. [Google Scholar] [CrossRef] [PubMed]
- Prins, J.M.; Weverling, G.J.; de Blok, K.; van Ketel, R.J.; Speelman, P. Validation and nephrotoxicity of a simplified once-daily aminoglycoside dosing schedule and guidelines for monitoring therapy. Antimicrob. Agents Chemother. 1996, 40, 2494–2499. [Google Scholar] [CrossRef] [PubMed]
- De Waele, J.J.; Carrette, S.; Carlier, M.; Stove, V.; Boelens, J.; Claeys, G.; Leroux-Roels, I.; Hoste, E.; Depuydt, P.; Decruyenaere, J.; et al. Therapeutic drug monitoring-based dose optimisation of piperacillin and meropenem: A randomised controlled trial. Intensive Care Med. 2014, 40, 380–987. [Google Scholar] [CrossRef]
- Kadambari, S.; Heath, P.T.; Sharland, M.; Lewis, S.; Nichols, A.; Turner, M.A. Variation in gentamicin and vancomycin dosage and monitoring in UK neonatal units. J. Antimicrob. Chemother. 2011, 66, 2647–2650. [Google Scholar] [CrossRef]
- Wong, G.; Brinkman, A.; Benefield, R.J.; Carlier, M.; De Waele, J.J.; El Helali, N.; Frey, O.; Harbarth, S.; Huttner, A.; McWhinney, B.; et al. An international, multicentre survey of β-lactam antibiotic therapeutic drug monitoring practice in intensive care units. J. Antimicrob. Chemother. 2014, 69, 1416–1423. [Google Scholar] [CrossRef]
- Tabah, A.; De Waele, J.; Lipman, J.; Zahar, J.R.; Cotta, M.O.; Barton, G.; Timsit, J.F.; Roberts, J.A. The ADMIN-ICU survey: A survey on antimicrobial dosing and monitoring in ICUs. J. Antimicrob. Chemother. 2015, 70, 2671–2677. [Google Scholar] [CrossRef]
- Wong, G.; Sime, F.B.; Lipman, J.; Roberts, J.A. How do we use therapeutic drug monitoring to improve outcomes from severe infections in critically ill patients? BMC Infect. Dis. 2014, 14, 288. [Google Scholar] [CrossRef]
- Mouton, J.W.; Theuretzbacher, U.; Craig, W.A.; Tulkens, P.M.; Derendorf, H.; Cars, O. Tissue concentrations: Do we ever learn? J. Antimicrob. Chemother. 2007, 61, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Kiem, S.; Schentag, J.J. Interpretation of Antibiotic Concentration Ratios Measured in Epithelial Lining Fluid. Antimicrob. Agents Chemother. 2008, 52, 24–36. [Google Scholar] [CrossRef]
- Aulin, L.B.S.; Valitalo, P.A.; Rizk, M.L.; Visser, S.A.G.; Rao, G.; van der Graaf, P.H.; van Hasselt, J.G.C. Validation of a Model Predicting Anti-infective Lung Penetration in the Epithelial Lining Fluid of Humans. Pharm. Res. 2018, 35, 26. [Google Scholar] [CrossRef] [PubMed]
- Jager, N.G.L.; van Hest, R.M.; Lipman, J.; Roberts, J.A.; Cotta, M.O. Antibiotic exposure at the site of infection: Principles and assessment of tissue penetration. Expert Rev. Clin. Pharmacol. 2019, 12, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Nau, R.; Sorgel, F.; Eiffert, H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin. Microbiol. Rev. 2010, 23, 858–883. [Google Scholar] [CrossRef] [PubMed]
- Musteata, F.M. Clinical Utility of Free Drug Monitoring. Ther. Drug Monit. Newer Drugs Biomarkers 2012, 75–101. [Google Scholar] [CrossRef]
- Briscoe, S.E.; McWhinney, B.C.; Lipman, J.; Roberts, J.A.; Ungerer, J.P. A method for determining the free (unbound) concentration of ten beta-lactam antibiotics in human plasma using high performance liquid chromatography with ultraviolet detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2012, 907, 178–184. [Google Scholar] [CrossRef]
- Wong, G.; Briscoe, S.; Adnan, S.; McWhinney, B.; Ungerer, J.; Lipman, J.; Roberts, J.A. Protein binding of beta-lactam antibiotics in critically ill patients: Can we successfully predict unbound concentrations? Antimicrob. Agents Chemother. 2013, 57, 6165–6170. [Google Scholar] [CrossRef]
- Neely, M.N.; Youn, G.; Jones, B.; Jelliffe, R.W.; Drusano, G.L.; Rodvold, K.A.; Lodise, T.P. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob. Agents Chemother. 2014, 58, 309–316. [Google Scholar] [CrossRef]
- Turnidge, J. Pharmacodynamics and dosing of aminoglycosides. Infect. Dis. Clin. North Am. 2003, 17, 503–528. [Google Scholar] [CrossRef]
- Paterson, D.L.; Robson, J.M.; Wagener, M.M.; Peters, M. Monitoring of serum aminoglycoside levels with once-daily dosing. Pathology 1998, 30, 289–294. [Google Scholar] [CrossRef]
- Mohan, M.; Batty, K.T.; Cooper, J.A.; Wojnar-Horton, R.E.; Ilett, K.F. Comparison of gentamicin dose estimates derived from manual calculations, the Australian ‘Therapeutic Guidelines: Antibiotic’ nomogram and the SeBA-GEN and DoseCalc software programs. Br. J. Clin. Pharmacol. 2004, 58, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Donagher, J.; Martin, J.H.; Barras, M.A. Individualised medicine: Why we need Bayesian dosing. Int. Med. J. 2017, 47, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Avent, M.L.; Rogers, B.A. Optimising antimicrobial therapy through the use of Bayesian dosing programs. Int. J. Clin. Pharmacy 2019, 41, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Sheiner, L.B.; Beal, S.; Rosenberg, B.; Marathe, V.V. Forecasting individual pharmacokinetics. Clin. Pharmacol. Therapeut. 1979, 26, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.B.; Kojiro, K.; Shephard, E.A.; Won, R.; Chang, E.; Chan, D.; Elbarbry, F. Review and Validation of Bayesian Dose-Optimizing Software and Equations for Calculation of the Vancomycin Area Under the Curve in Critically Ill Patients. Pharmacotherapy 2018, 38, 1174–1183. [Google Scholar] [CrossRef]
- Sheiner, L.B.; Beal, S.L. Bayesian individualization of pharmacokinetics: Simple implementation and comparison with non-Bayesian methods. J. Pharm. Sci. 1982, 71, 1344–1348. [Google Scholar] [CrossRef]
- Heil, E.L.; Nicolau, D.P.; Farkas, A.; Roberts, J.A.; Thom, K.A. Pharmacodynamic Target Attainment for Cefepime, Meropenem, and Piperacillin-Tazobactam Using a Pharmacokinetic/Pharmacodynamic-Based Dosing Calculator in Critically Ill Patients. Antimicrob. Agents Chemother. 2018, 62, e01008-18. [Google Scholar] [CrossRef]
- Crandon, J.L.; Bulik, C.C.; Kuti, J.L.; Nicolau, D.P. Clinical pharmacodynamics of cefepime in patients infected with Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2010, 54, 1111–1116. [Google Scholar] [CrossRef]
- Muller, A.E.; Punt, N.; Mouton, J.W. Optimal exposures of ceftazidime predict the probability of microbiological and clinical outcome in the treatment of nosocomial pneumonia. J. Antimicrob. Chemother. 2013, 68, 900–906. [Google Scholar] [CrossRef]
- MacVane, S.H.; Kuti, J.L.; Nicolau, D.P. Clinical pharmacodynamics of antipseudomonal cephalosporins in patients with ventilator-associated pneumonia. Antimicrob. Agents Chemother. 2014, 58, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, N.J.; Kuti, J.L.; Nicolau, D.P.; Van Wart, S.; Nicasio, A.M.; Liu, J.; Lee, B.J.; Neely, M.N.; Scheetz, M.H. Defining Clinical Exposures of Cefepime for Gram-Negative Bloodstream Infections That Are Associated with Improved Survival. Antimicrob. Agents Chemother. 2015, 60, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, O.; Johansson, F.; Komorowski, M.; Faisal, A.; Sontag, D.; Doshi-Velez, F.; Celi, L.A. Guidelines for reinforcement learning in healthcare. Nat. Med. 2019, 25, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.C.; Hauser, K. Artificial intelligence framework for simulating clinical decision-making: A Markov decision process approach. Artif. Intell. Med. 2013, 57, 9–19. [Google Scholar] [CrossRef]
- Sutton, R.S.; Barto, A.G. Reinforcement Learning: An Introduction, 2nd ed.; MIT Press: Cambridge, MA, USA, 2018; pp. 1–526. [Google Scholar]
- Komorowski, M.; Celi, L.A.; Badawi, O.; Gordon, A.C.; Faisal, A.A. The Artificial Intelligence Clinician learns optimal treatment strategies for sepsis in intensive care. Nat. Med. 2018, 24, 1716–1720. [Google Scholar] [CrossRef]
- Kantasiripitak, W.; Van Daele, R.; Gijsen, M.; Ferrante, M.; Spriet, I.; Dreesen, E. Software Tools for Model-Informed Precision Dosing: How Well Do They Satisfy the Needs? Front. Pharmacol. 2020, 11, 620. [Google Scholar] [CrossRef]
- Ryu, J.Y.; Kim, H.U.; Lee, S.Y. Deep learning improves prediction of drug-drug and drug-food interactions. Proc. Natl. Acad. Sci. USA 2018, 115, E4304–E4311. [Google Scholar] [CrossRef] [PubMed]
- Polasek, T.M.; Kirkpatrick, C.M.J.; Rostami-Hodjegan, A. Precision dosing to avoid adverse drug reactions. Ther. Adv. Drug Saf. 2019, 10, 2042098619894147. [Google Scholar] [CrossRef]
- Vinks, A.A.; Peck, R.W.; Neely, M.; Mould, D.R. Development and Implementation of Electronic Health Record-Integrated Model-Informed Clinical Decision Support Tools for the Precision Dosing of Drugs. Clin. Pharmacol. Ther. 2020, 107, 129–135. [Google Scholar] [CrossRef]
- Skodvin, B.; Aase, K.; Brekken, A.L.; Charani, E.; Lindemann, P.C.; Smith, I. Addressing the key communication barriers between microbiology laboratories and clinical units: A qualitative study. J. Antimicrob. Chemother. 2017, 72, 2666–2672. [Google Scholar] [CrossRef]
- Darwich, A.S.; Ogungbenro, K.; Vinks, A.A.; Powell, J.R.; Reny, J.L.; Marsousi, N.; Daali, Y.; Fairman, D.; Cook, J.; Lesko, L.J.; et al. Why has model-informed precision dosing not yet become common clinical reality? lessons from the past and a roadmap for the future. Clin. Pharmacol. Ther. 2017, 101, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, S.M.; Breitkreutz, M.L.; Bi, C.; Matzuka, B.J.; Dalal, J.; Casey, K.L.; Garg, U.; Winkle, S.; Leeder, J.S.; Breedlove, J.; et al. Design and Testing of an EHR-Integrated, Busulfan Pharmacokinetic Decision Support Tool for the Point-of-Care Clinician. Front. Pharmacol. 2016, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Karnik, K. FDA regulation of clinical decision support software. J. Law Biosci. 2014, 1, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Van Lent-Evers, N.A.; Mathot, R.A.; Geus, W.P.; van Hout, B.A.; Vinks, A.A. Impact of goal-oriented and model-based clinical pharmacokinetic dosing of aminoglycosides on clinical outcome: A cost-effectiveness analysis. Ther. Drug Monit. 1999, 21, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Jowett, S.; Bryan, S.; Poller, L.; Van Den Besselaar, A.M.; Van Den Meer, F.J.; Palareti, G.; Shiach, C.; Tripodi, A.; Keown, M.; Ibrahim, S.; et al. The cost-effectiveness of computer-assisted anticoagulant dosage: Results from the European Action on Anticoagulation (EAA) multicentre study. J. Thromb. Haemost. 2009, 7, 1482–1490. [Google Scholar] [CrossRef]
- Rea, R.S.; Capitano, B.; Bies, R.; Bigos, K.L.; Smith, R.; Lee, H. Suboptimal aminoglycoside dosing in critically ill patients. Ther Drug Monit. 2008, 30, 674–681. [Google Scholar] [CrossRef]
- Carlier, M.; Noe, M.; De Waele, J.J.; Stove, V.; Verstraete, A.G.; Lipman, J.; Roberts, J.A. Population pharmacokinetics and dosing simulations of amoxicillin/clavulanic acid in critically ill patients. J. Antimicrob. Chemother. 2013, 68, 2600–2608. [Google Scholar] [CrossRef]
- Roberts, J.A.; Udy, A.A.; Jarrett, P.; Wallis, S.C.; Hope, W.W.; Sharma, R.; Kirkpatrick, C.M.; Kruger, P.S.; Roberts, M.S.; Lipman, J. Plasma and target-site subcutaneous tissue population pharmacokinetics and dosing simulations of cefazolin in post-trauma critically ill patients. J. Antimicrob. Chemother. 2015, 70, 1495–1502. [Google Scholar] [CrossRef]
- Nicasio, A.M.; Ariano, R.E.; Zelenitsky, S.A.; Kim, A.; Crandon, J.L.; Kuti, J.L.; Nicolau, D.P. Population pharmacokinetics of high-dose, prolonged-infusion cefepime in adult critically ill patients with ventilator-associated pneumonia. Antimicrob. Agents Chemother. 2009, 53, 1476–1481. [Google Scholar] [CrossRef]
- Beranger, A.; Oualha, M.; Urien, S.; Genuini, M.; Renolleau, S.; Aboura, R.; Hirt, D.; Heilbronner, C.; Toubiana, J.; Treluyer, J.M.; et al. Population Pharmacokinetic Model to Optimize Cefotaxime Dosing Regimen in Critically Ill Children. Clin. Pharmacokinet. 2018, 57, 867–875. [Google Scholar] [CrossRef]
- Georges, B.; Conil, J.M.; Seguin, T.; Ruiz, S.; Minville, V.; Cougot, P.; Decun, J.F.; Gonzalez, H.; Houin, G.; Fourcade, O.; et al. Population pharmacokinetics of ceftazidime in intensive care unit patients: Influence of glomerular filtration rate, mechanical ventilation, and reason for admission. Antimicrob. Agents Chemother. 2009, 53, 4483–4489. [Google Scholar] [CrossRef]
- Shi, Z.R.; Chen, X.K.; Tian, L.Y.; Wang, Y.K.; Zhang, G.Y.; Dong, L.; Jirasomprasert, T.; Jacqz-Aigrain, E.; Zhao, W. Population Pharmacokinetics and Dosing Optimization of Ceftazidime in Infants. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Sime, F.B.; Lassig-Smith, M.; Starr, T.; Stuart, J.; Pandey, S.; Parker, S.L.; Wallis, S.C.; Lipman, J.; Roberts, J.A. Population Pharmacokinetics of Unbound Ceftolozane and Tazobactam in Critically Ill Patients without Renal Dysfunction. Antimicrob. Agents Chemother. 2019, 63, e01265-19. [Google Scholar] [CrossRef]
- Garot, D.; Respaud, R.; Lanotte, P.; Simon, N.; Mercier, E.; Ehrmann, S.; Perrotin, D.; Dequin, P.F.; Le Guellec, C. Population pharmacokinetics of ceftriaxone in critically ill septic patients: A reappraisal. Br. J. Clin. Pharmacol. 2011, 72, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Leegwater, E.; Kraaijenbrink, B.V.C.; Moes, D.; Purmer, I.M.; Wilms, E.B. Population pharmacokinetics of ceftriaxone administered as continuous or intermittent infusion in critically ill patients. J. Antimicrob. Chemother. 2020, 75, 1554–1558. [Google Scholar] [CrossRef] [PubMed]
- Khachman, D.; Conil, J.M.; Georges, B.; Saivin, S.; Houin, G.; Toutain, P.L.; Laffont, C.M. Optimizing ciprofloxacin dosing in intensive care unit patients through the use of population pharmacokinetic-pharmacodynamic analysis and Monte Carlo simulations. J. Antimicrob. Chemother. 2011, 66, 1798–1809. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Abd Rahman, A.N.; Mat-Nor, M.B.; Sulaiman, H.; Wallis, S.C.; Lipman, J.; Roberts, J.A.; Staatz, C.E. Population Pharmacokinetics of Doripenem in Critically Ill Patients with Sepsis in a Malaysian Intensive Care Unit. Antimicrob. Agents Chemother. 2016, 60, 206–214. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ulldemolins, M.; Roberts, J.A.; Wallis, S.C.; Rello, J.; Lipman, J. Flucloxacillin dosing in critically ill patients with hypoalbuminaemia: Special emphasis on unbound pharmacokinetics. J. Antimicrob. Chemother. 2010, 65, 1771–1778. [Google Scholar] [CrossRef]
- Aoyama, T.; Hirata, K.; Hirata, R.; Yamazaki, H.; Yamamoto, Y.; Hayashi, H.; Matsumoto, Y. Population pharmacokinetics of fluconazole after administration of fosfluconazole and fluconazole in critically ill patients. J. Clin. Pharm. Ther. 2012, 37, 356–363. [Google Scholar] [CrossRef]
- Parker, S.L.; Frantzeskaki, F.; Wallis, S.C.; Diakaki, C.; Giamarellou, H.; Koulenti, D.; Karaiskos, I.; Lipman, J.; Dimopoulos, G.; Roberts, J.A. Population Pharmacokinetics of Fosfomycin in Critically Ill Patients. Antimicrobial. Agents Chemother. 2015, 59, 6471–6476. [Google Scholar] [CrossRef]
- Krens, S.D.; Hodiamont, C.J.; Juffermans, N.P.; Mathot, R.A.A.; van Hest, R.M. Population Pharmacokinetics of Ganciclovir in Critically Ill Patients. Ther. Drug Monit. 2019, 42, 295–301. [Google Scholar] [CrossRef]
- De Velde, F.; de Winter, B.C.M.; Neely, M.N.; Yamada, W.M.; Koch, B.C.P.; Harbarth, S.; von Dach, E.; van Gelder, T.; Huttner, A.; Mouton, J.W. Population Pharmacokinetics of Imipenem in Critically Ill Patients: A Parametric and Nonparametric Model Converge on CKD-EPI Estimated Glomerular Filtration Rate as an Impactful Covariate. Clin. Pharmacokinet. 2020. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Cotta, M.O.; Cojutti, P.; Lugano, M.; Rocca, G.D.; Pea, F. Does Critical Illness Change Levofloxacin Pharmacokinetics? Antimicrobial. Agents Chemother. 2016, 60, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Soraluce, A.; Barrasa, H.; Asin-Prieto, E.; Sanchez-Izquierdo, J.A.; Maynar, J.; Isla, A.; Rodriguez-Gascon, A. Novel Population Pharmacokinetic Model for Linezolid in Critically Ill Patients and Evaluation of the Adequacy of the Current Dosing Recommendation. Pharmaceutics 2020, 12, 54. [Google Scholar] [CrossRef]
- Crandon, J.L.; Ariano, R.E.; Zelenitsky, S.A.; Nicasio, A.M.; Kuti, J.L.; Nicolau, D.P. Optimization of meropenem dosage in the critically ill population based on renal function. Intensive Care Med. 2011, 37, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Braune, S.; Konig, C.; Roberts, J.A.; Nierhaus, A.; Steinmetz, O.; Baehr, M.; Kluge, S.; Langebrake, C. Pharmacokinetics of meropenem in septic patients on sustained low-efficiency dialysis: A population pharmacokinetic study. Crit Care 2018, 22, 25. [Google Scholar] [CrossRef]
- Rapp, M.; Urien, S.; Foissac, F.; Beranger, A.; Bouazza, N.; Benaboud, S.; Bille, E.; Zheng, Y.; Gana, I.; Moulin, F.; et al. Population pharmacokinetics of meropenem in critically ill children with different renal functions. Eur. J. Clin. Pharmacol. 2020, 76, 61–71. [Google Scholar] [CrossRef]
- Maseda, E.; Grau, S.; Luque, S.; Castillo-Mafla, M.-P.; Suárez-de-la-Rica, A.; Montero-Feijoo, A.; Salgado, P.; Gimenez, M.-J.; García-Bernedo, C.A.; Gilsanz, F.; et al. Population pharmacokinetics/pharmacodynamics of micafungin against Candida species in obese, critically ill, and morbidly obese critically ill patients. Crit. Care 2018, 22, 94. [Google Scholar] [CrossRef]
- Felton, T.W.; Roberts, J.A.; Lodise, T.P.; Van Guilder, M.; Boselli, E.; Neely, M.N.; Hope, W.W. Individualization of piperacillin dosing for critically ill patients: Dosing software to optimize antimicrobial therapy. Antimicrobial. Agents Chemother. 2014, 58, 4094–4102. [Google Scholar] [CrossRef] [PubMed]
- Sandri, A.M.; Landersdorfer, C.B.; Jacob, J.; Boniatti, M.M.; Dalarosa, M.G.; Falci, D.R.; Behle, T.F.; Bordinhao, R.C.; Wang, J.; Forrest, A.; et al. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: Implications for selection of dosage regimens. Clin. Infect Dis. 2013, 57, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Sime, F.B.; Byrne, C.J.; Parker, S.; Stuart, J.; Butler, J.; Starr, T.; Pandey, S.; Wallis, S.C.; Lipman, J.; Roberts, J.A. Population pharmacokinetics of total and unbound concentrations of intravenous posaconazole in adult critically ill patients. Crit. Care. 2019, 23, 205. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Roberts, J.A.; Alobaid, A.S.; Roger, C.; Wang, Y.; Yang, Q.; Sun, J.; Dong, H.; Wang, X.; Xing, J.; et al. Population Pharmacokinetics of Tigecycline in Critically Ill Patients with Severe Infections. Antimicrobial. Agents Chemother. 2017, 61, e00345-17. [Google Scholar] [CrossRef] [PubMed]


| Class | PK-PD Index | Reference |
|---|---|---|
| Aminoglycosides | Cmax/MIC AUC0–24/MIC | [40] |
| Beta-Lactams | fT > MIC | [41] |
| Fluoroquinolones | Cmax/MIC AUC0–24/MIC | [33,42] |
| Glycopeptides | AUC0–24/MIC | [43] |
| Glycylcyclines | AUC0–24/MIC | [44,45] |
| Lincosamides | fT > MIC | [46] |
| Lipopeptides | Cmax/MIC AUC0–24/MIC | [47] |
| Macrolides | fT > MIC AUC0–24/MIC (azithromycin) | [48,49] |
| Oxazolidinones | fT > MIC AUC0–24/MIC | [50] |
| Polymyxins | AUC0–24/MIC | [51] |
| Triazoles antifungals | AUC0–24/MIC | [52] |
| Echinocandins | AUC0–24/MIC Cmax/MIC | [53] |
| Polyenes | Cmax/MIC | [54] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, M.G.; Cotta, M.O.; Abdul-Aziz, M.H.; Roberts, J.A. What Are the Current Approaches to Optimising Antimicrobial Dosing in the Intensive Care Unit? Pharmaceutics 2020, 12, 638. https://doi.org/10.3390/pharmaceutics12070638
Chai MG, Cotta MO, Abdul-Aziz MH, Roberts JA. What Are the Current Approaches to Optimising Antimicrobial Dosing in the Intensive Care Unit? Pharmaceutics. 2020; 12(7):638. https://doi.org/10.3390/pharmaceutics12070638
Chicago/Turabian StyleChai, Ming G., Menino O. Cotta, Mohd H. Abdul-Aziz, and Jason A. Roberts. 2020. "What Are the Current Approaches to Optimising Antimicrobial Dosing in the Intensive Care Unit?" Pharmaceutics 12, no. 7: 638. https://doi.org/10.3390/pharmaceutics12070638
APA StyleChai, M. G., Cotta, M. O., Abdul-Aziz, M. H., & Roberts, J. A. (2020). What Are the Current Approaches to Optimising Antimicrobial Dosing in the Intensive Care Unit? Pharmaceutics, 12(7), 638. https://doi.org/10.3390/pharmaceutics12070638





