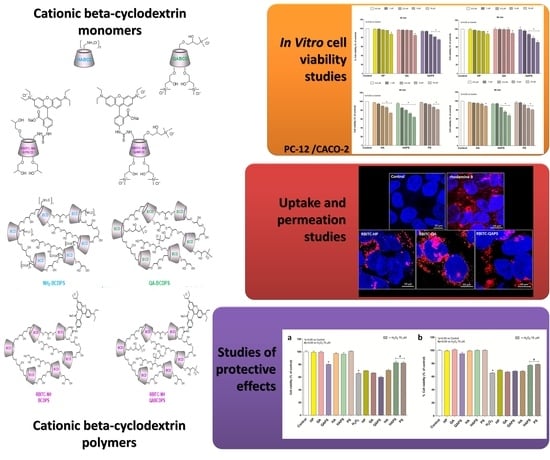
Investigation of Cytotoxicity and Cell Uptake of Cationic Beta-Cyclodextrins as Valid Tools in Nasal Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Beta-Cyclodextrins
2.3. Cell Culture and Cyclodextrins (CDs) Treatments
2.4. In Vitro Cell Viability Studies
2.5. Uptake and Permeation Studies
2.5.1. Confocal Microscopy
2.5.2. Relative Beta-Cyclodextrins Uptake
2.5.3. Uptake and Transepithelial Permeability Evaluation in Cell Monolayers
2.6. Studies of Protective Effects
2.7. Statistical Analysis
3. Results
3.1. In Vitro Cell Viability Studies
3.2. Uptake Studies by Confocal Microscopy
3.3. Relative Beta-Cyclodextrins Uptake
3.4. Uptake and Transepithelial Permeability Evaluation in Cell Monolayers
3.5. Studies of Protective Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Duchêne, D.; Bochot, A. Thirty years with cyclodextrins. Int. J. Pharm. 2016, 514, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, Z.; Khreich, N.; Auezova, L.; Fourmentin, S.; Elaissari, A.; Greige-Gerges, H. Cyclodextrin-membrane interaction in drug delivery and membrane structure maintenance. Int. J. Pharm. 2019, 564, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.S. Cyclodextrins: Emerging medicines of the new millennium. Biomolecules 2019, 9, 801. [Google Scholar] [CrossRef] [Green Version]
- Malanga, M.; Szemán, J.; Fenyvesi, É.; Puskás, I.; Csabai, K.; Gyémánt, G.; Fenyvesi, F.; Szente, L. “Back to the future”: A new look at hydroxypropyl beta-cyclodextrins. J. Pharm. Sci. 2016, 105, 2921–2931. [Google Scholar] [CrossRef] [Green Version]
- Váradi, J.; Hermenean, A.; Gesztelyi, R.; Jeney, V.; Balogh, E.; Majoros, L.; Malanga, M.; Fenyvesi, É.; Szente, L.; Bácskay, I. Pharmacokinetic properties of fluorescently labelled hydroxypropyl-beta-cyclodextrin. Biomolecules 2019, 9, 509. [Google Scholar] [CrossRef] [Green Version]
- Haimhoffer, Á.; Rusznyák, Á.; Réti-Nagy, K.; Vasvári, G.; Váradi, J.; Vecsernyés, M.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Fenyvesi, F.; et al. Cyclodextrins in drug delivery systems and their effects on biological barriers. Sci. Pharm. 2019, 87, 33. [Google Scholar] [CrossRef] [Green Version]
- Gavini, E.; Rassu, G.; Haukvik, T.; Lanni, C.; Racchi, M.; Giunchedi, P. Mucoadhesive microspheres for nasal administration of cyclodextrins. J. Drug Target. 2009, 17, 168–179. [Google Scholar] [CrossRef]
- Yalcin, A.; Soddu, E.; Turunc Bayrakdar, E.; Uyanikgil, Y.; Kanit, L.; Armagan, G.; Rassu, G.; Gavini, E.; Giunchedi, P. Neuroprotective effects of engineered polymeric nasal microspheres containing hydroxypropyl-β-cyclodextrin on β-amyloid (1-42)-induced toxicity. J. Pharm. Sci. 2016, 105, 2372–2380. [Google Scholar] [CrossRef]
- Rassu, G.; Gavini, E.; Carta, A.; Obinu, A.; Porcu, E.P.; Giunchedi, P. Hydroxypropyl-β-cyclodextrin formulated in nasal chitosan microspheres as candidate therapeutic agent in Alzheimer’s disease. Curr. Drug Deliv. 2018, 15, 746–748. [Google Scholar] [CrossRef]
- Vecsernyés, M.; Fenyvesi, F.; Bácskay, I.; Deli, M.A.; Szente, L.; Fenyvesi, É. Cyclodextrins, blood-brain barrier, and treatment of neurological diseases. Arch. Med. Res. 2014, 45, 711–729. [Google Scholar] [CrossRef] [PubMed]
- van de Manakker, F.; Vermonden, T.; van Nostrum, C.F.; Hennink, W.E. Cyclodextrin-based polymeric materials: Synthesis, properties, and pharmaceutical/biomedical applications. Biomacromolecules 2009, 10, 3157–3175. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. Synthesis, characterization and application of Epichlorohydrin-β-cyclodextrin polymer. Colloids Surf. B 2014, 114, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, J.; Jiang, W.; Ren, C.; Li, J.; Xin, J.; Li, K. Effective protection and controlled release of insulin by cationic beta-cyclodextrin polymers from alginate/chitosan nanoparticles. Int. J. Pharm. 2010, 393, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Park, T.G.; Jeong, J.H.; Kim, S.W. Current status of polymeric gene delivery systems. Adv. Drug Deliv. Rev. 2006, 58, 467–486. [Google Scholar] [CrossRef]
- Zuckerman, J.E.; Choi, C.H.J.; Han, H.; Davis, M.E. Polycation-siRNA nanoparticles can disassemble at the kidney glomerular basement membrane. PNAS 2012, 109, 3137–3142. [Google Scholar] [CrossRef] [Green Version]
- Bernkop-Schnürch, A. Mucoadhesive polymers: Basics, strategies and future trends. In Polymeric Biomaterials: Structure and Function; Dumitriu, S., Popa, V., Eds.; CRC Press: Boca Raton, FL, USA, 2013; Volume 1, pp. 202–206. [Google Scholar]
- Rassu, G.; Soddu, E.; Cossu, M.; Brundu, A.; Cerri, G.; Marchetti, N.; Ferraro, L.; Regan, R.F.; Giunchedi, P.; Gavini, E.; et al. Solid microparticles based on chitosan or methyl-β-cyclodextrin: A first formulative approach to increase the nose-to-brain transport of deferoxamine mesylate. J. Control. Release 2015, 201, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Thomsen, H.; Benkovics, G.; Fenyvesi, É.; Farewell, A.; Malanga, M.; Ericson, M.B. Delivery of cyclodextrin polymers to bacterial biofilms—An exploratory study using rhodamine labelled cyclodextrins and multiphoton microscopy. Int. J. Pharm. 2017, 531, 650–657. [Google Scholar] [CrossRef]
- Ashton, P.R.; Königer, R.; Stoddart, J.F.; Alker, D.; Harding, V.D. Amino acid derivatives of β-cyclodextrin. J. Org. Chem. 1996, 61, 903–908. [Google Scholar] [CrossRef]
- Malanga, M.; Bálint, M.; Puskás, I.; Tuza, K.; Sohajda, T.; Jicsinszky, L.; Szente, L.; Fenyvesi, É. Synthetic strategies for the fluorescent labeling of epichlorohydrin-branched cyclodextrin polymers. Beilstein J. Org. Chem. 2014, 10, 3007–3018. [Google Scholar] [CrossRef] [Green Version]
- Malanga, M.; Lászlo, J.; Fenyvesi, É. Rhodamine-labeled cyclodextrin derivatives. J. Drug Del. Sci. Tech. 2012, 22, 260–265. [Google Scholar] [CrossRef]
- Selvaraj, K.; Gowthamarajan, K.; Karri, V.V.S.R. Nose to brain transport pathways an overview: Potential of nanostructured lipid carriers in nose to brain targeting. Artif. Cells Nanomed. Biotechnol. 2018, 46, 2088–2095. [Google Scholar] [CrossRef] [PubMed]
- Zborowski, M.; Chalmers, J.J. Magnetic Cell Separation; Elsevier Science & Technology Books: Amsterdam, The Netherlands, 2007; p. 32. [Google Scholar]
- Mahmoudi, M.; Simchi, A.; Milani, A.S.; Stroeved, P. Cell toxicity of superparamagnetic iron oxide nanoparticles. J. Colloid Interface Sci. 2009, 336, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Horváth, T.; Bartos, C.; Bocsik, A.; Kiss, L.; Veszelka, S.; Deli, M.A.; Újhelyi, G.; Szabó-Révész, P.; Ambrus, R. Cytotoxicity of different excipients on RPMI 2650 human nasal epithelial cells. Molecules 2016, 21, 658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gänger, S.; Schindowski, K. Tailoring formulations for intranasal nose-to-brain delivery: A review on architecture, physico-chemical characteristics and mucociliary clearance of the nasal olfactory mucosa. Pharmaceutics 2018, 10, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, D.; Li, Y.; Ahlemeyer, B.; Krieglstein, J.; Kissel, T. In vitro cytotoxicity testing of polycations: Influence of polymer structure on cell viability and hemolysis. Biomaterials 2003, 24, 1121–1131. [Google Scholar] [CrossRef]
- Jeong, H.; Hwang, J.; Lee, H.; Hammond, P.T.; Choi, J.; Hong, J. In vitro blood cell viability profiling of polymers used in molecular assembly. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Ferruti, P.; Knobloch, S.; Ranucci, E.; Gianasi, E.; Duncan, R. A novel chemical modification of poly-l-lysine reducing toxicity while preserving cationic properties. Proc. Int. Symp. Control. Rel. Bioact. Mater. 1997, 24, 45–46. [Google Scholar]
- Cai, J.; Yue, Y.; Rui, D.; Zhang, Y.; Liu, S.; Wu, C. Effect of chain length on cytotoxicity and endocytosis of cationic polymers. Macromolecules 2011, 44, 2050–2057. [Google Scholar] [CrossRef]
- Fenyvesi, F.; Reti-Nagy, K.; Bacso, Z.; Gutay-Toth, Z.; Malanga, M.; Fenyvesi, E.; Szente, L.; Varadi, J.; Ujhelyi, Z.; Feher, P.; et al. Fluorescently labeled methyl-beta-cyclodextrin enters intestinal epithelial Caco-2 cells by fluid-phase endocytosis. PLoS ONE 2014, 9, e84856. [Google Scholar] [CrossRef] [Green Version]
- Réti-Nagy, K.; Malanga, M.; Fenyvesi, É.; Szente, L.; Vámosi, G.; Váradi, J.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Róka, E.; et al. Endocytosis of fluorescent cyclodextrins by intestinal Caco-2 cells and its role in paclitaxel drug delivery. Int. J. Pharm. 2015, 496, 509–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furubayashi, T.; Inoue, D.; Nishiyama, N.; Tanaka, A.; Yutani, R.; Kimura, S.; Katsumi, H.; Yamamoto, A.; Sakane, T. Comparison of various cell lines and three-dimensional mucociliary tissue model systems to estimate drug permeability using an in vitro transport study to predict nasal drug absorption in rats. Pharmaceutics 2020, 12, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Cyclodextrin | Code | DS 1 | MW 2 | CLR 3 | CD 4 | Analogue Fluorescent Cyclodextrin |
|---|---|---|---|---|---|---|
| (2-Hydroxy-3-N,N,N-trimethyl amino) propyl-beta-cyclodextrin chloride | QA | 3 | 1589.8 | 3 | RBITC-QA | |
| Quaternary-ammonium-beta-cyclodextrin soluble polymer crosslinked with epichlorohydrin | QAPS | 2.2 | 40,000 | ~11 | 2.2 | RBITC-QAPS |
| Heptakis (6-deoxy-6-amino)-beta-cyclodextrin heptahydrochloride | HA | - | 1383.3 | 7 | ||
| Soluble amino-beta-cyclodextrin polymer crosslinked with epichlorohydrin | HAPS | 1 | 25,000 | ~10 | 1 | |
| (2-Hydroxypropyl)-beta-cyclodextrin | HP | 4.5 | 1400 | RBITC-HP | ||
| Soluble β-cyclodextrin polymer crosslinked with epichlorohydrin | PS | - | 92,000 | ~11 | RBITC-PS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rassu, G.; Fancello, S.; Roldo, M.; Malanga, M.; Szente, L.; Migheli, R.; Gavini, E.; Giunchedi, P. Investigation of Cytotoxicity and Cell Uptake of Cationic Beta-Cyclodextrins as Valid Tools in Nasal Delivery. Pharmaceutics 2020, 12, 658. https://doi.org/10.3390/pharmaceutics12070658
Rassu G, Fancello S, Roldo M, Malanga M, Szente L, Migheli R, Gavini E, Giunchedi P. Investigation of Cytotoxicity and Cell Uptake of Cationic Beta-Cyclodextrins as Valid Tools in Nasal Delivery. Pharmaceutics. 2020; 12(7):658. https://doi.org/10.3390/pharmaceutics12070658
Chicago/Turabian StyleRassu, Giovanna, Silvia Fancello, Marta Roldo, Milo Malanga, Lajos Szente, Rossana Migheli, Elisabetta Gavini, and Paolo Giunchedi. 2020. "Investigation of Cytotoxicity and Cell Uptake of Cationic Beta-Cyclodextrins as Valid Tools in Nasal Delivery" Pharmaceutics 12, no. 7: 658. https://doi.org/10.3390/pharmaceutics12070658