In Vitro Evaluation of Enteric-Coated HPMC Capsules—Effect of Formulation Factors on Product Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
2.2.1. Coating Formulations
2.2.2. Disintegration Test
2.2.3. Acid Uptake Test
2.2.4. Scanning Electron Microscopy (SEM)
2.2.5. Statistical Analysis
2.2.6. Mechanical Properties of the Capsules
3. Results and Discussion
3.1. Surface Structure of the Enteric-Coated Capsules
3.2. Disintegration Test and Acid Uptake
3.3. Comparison of Formulation-Specific Factors on Disintegration Time
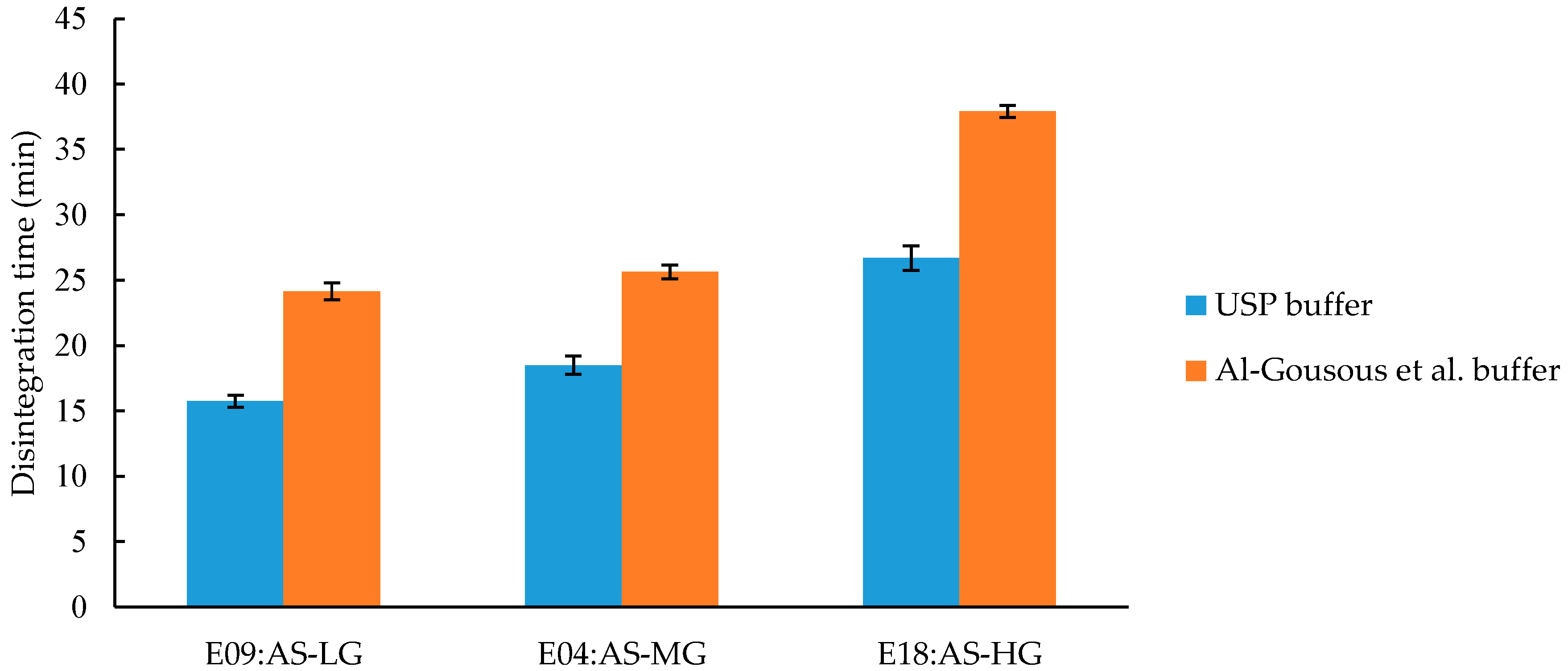
3.3.1. Effect of Different Polymer Grades on Disintegration Time
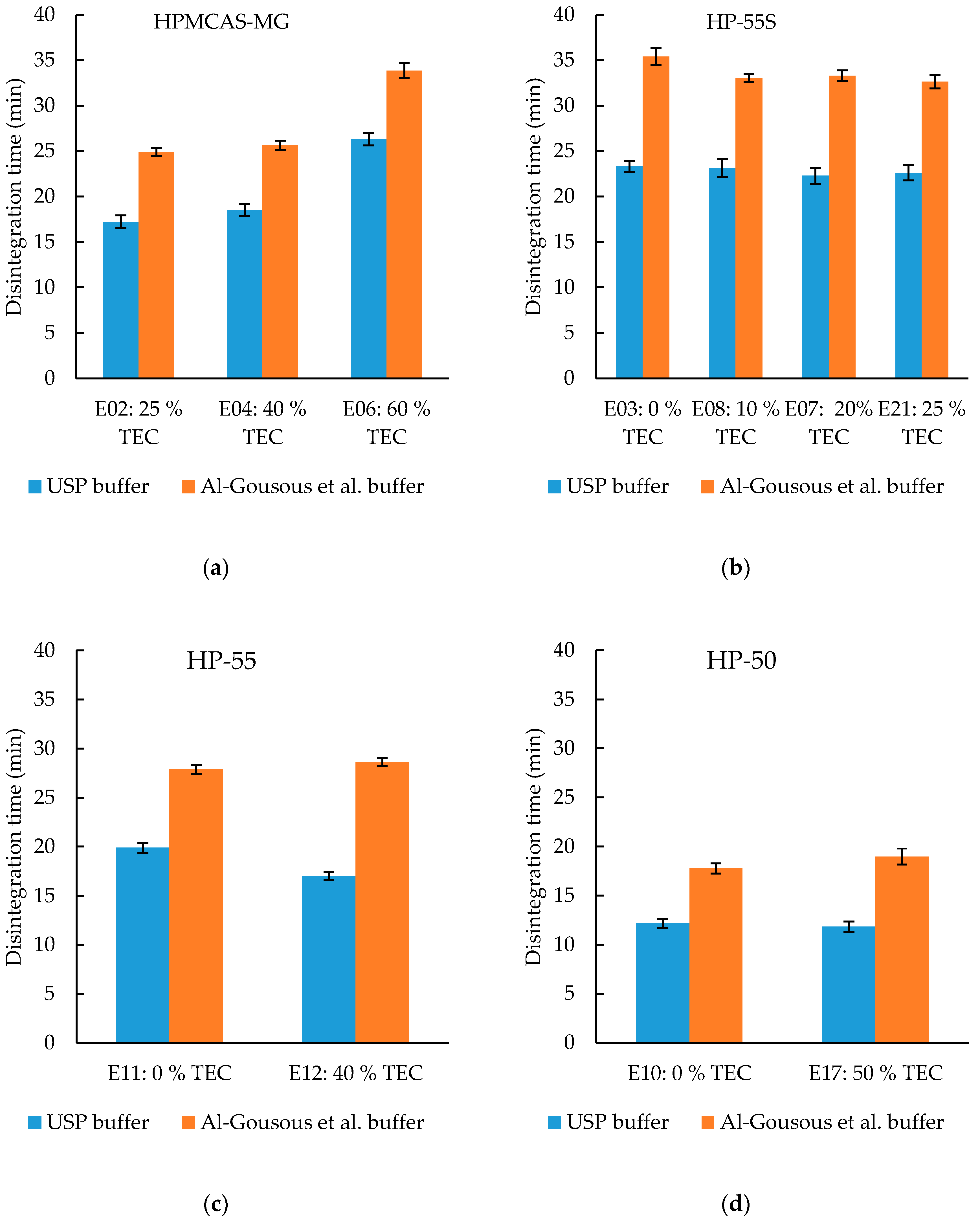
3.3.2. Effect of Plasticizer Content on Disintegration Time
3.3.3. Effect of Talc-Content on C-A-P Formulations on Disintegration Time
3.3.4. Effect of Different Processing Parameters on the Disintegration Time of Eudragit L100-55 Formulations
3.3.5. Difference between Disintegration Test Performed with Disc vs. without Disc but with Sinker
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, F.; Basit, A.W. A paradigm shift in enteric coating: Achieving rapid release in the proximal small intestine of man. J. Control. Release 2010, 147, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Al-Gousous, J.; Ruan, H.; Blechar, J.A.; Sun, K.X.; Salehi, N.; Langguth, P.; Job, N.M.; Lipka, E.; Loebenberg, R.; Bermejo, M.; et al. Mechanistic analysis and experimental verification of bicarbonate-controlled enteric coat dissolution: Potential in vivo implications. Eur. J. Pharm. Biopharm. 2019, 139, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Adeyeye, M.C.; Adebayo, A. Hard Shell Capsules in Clinical Trials. In Pharmaceutical Dosage Forms; CRC Press: Boca Raton, FL, USA, 2017; pp. 31–74. [Google Scholar]
- Chattoraj, S.; Daugherity, P.; McDermott, T.; Olsofsky, A.; Roth, W.J.; Tobyn, M. Sticking and Picking in Pharmaceutical Tablet Compression: An IQ Consortium Review. J. Pharm. Sci. 2018, 107, 2267–2282. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Taylor, L.J.; Murphy, B.; Krzyzaniak, J.; Dawson, N.; Mullarney, M.P.; Meenan, P.; Sun, C.C. Mechanism and Kinetics of Punch Sticking of Pharmaceuticals. J. Pharm. Sci. 2017, 106, 151–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, E.T.; Cadé, D.; Benameur, H. Challenges and opportunities in the encapsulation of liquid and semi-solid formulations into capsules for oral administration. Adv. Drug Deliv. Rev. 2008, 60, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Blair, T.C.; Buckton, G.; Bloomfield, S.F. On the mechanism of kill of microbial contaminants during tablet compression. Int. J. Pharm. 1991. [Google Scholar] [CrossRef]
- Thoma, K.; Bechtold, K. Enteric Coated Hard Gelatin Capsules. Available online: https://s3.amazonaws.com/cpsl-web/kc/library/enteric-coated-hard-gelatin-capsules.pdf (accessed on 16 June 2020).
- Cole, E.T. Liquid filled and sealed hard gelatin capsules. Bull. Tech. Gattefosse 1999, 92, 67–78. [Google Scholar]
- Cole, E.T.; Scott, R.A.; Connor, A.L.; Wilding, I.R.; Petereit, H.U.; Schminke, C.; Beckert, T.; Cadé, D. Enteric coated HPMC capsules designed to achieve intestinal targeting. Int. J. Pharm. 2002, 231, 83–95. [Google Scholar] [CrossRef]
- Smith, A.M.; Ingham, A.; Grover, L.M.; Perrie, Y. Polymer film formulations for the preparation of enteric pharmaceutical capsules. J. Pharm. Pharmacol. 2010, 62, 167–172. [Google Scholar] [CrossRef]
- Capsugel DRcaps Capsules Brochure. Available online: https://s3.amazonaws.com/cpsl-web/kc/library/c1a-32029_DRCaps-A4_FIN.PDF (accessed on 17 June 2020).
- Fu, M.; Al-Gousous, J.; Blechar, J.A.; Langguth, P. Enteric Hard Capsules for Targeting the Small Intestine: Positive Correlation between In Vitro Disintegration and Dissolution Times. Pharmaceutics 2020, 12, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.B.; Gupta, R.; Kumria, R.; Jacob, S.; Attimarad, M. Formulation and Evaluation of Enteric Coated Tablets of Proton Pump Inhibitor. J. Basic Clin. Pharm. 2010, 1, 215–221. [Google Scholar] [PubMed]
- Liu, F.; Lizio, R.; Meier, C.; Petereit, H.-U.; Blakey, P.; Basit, A.W. A novel concept in enteric coating: A double-coating system providing rapid drug release in the proximal small intestine. J. Control. Release 2009, 133, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Bozdag, S.; Çalis, S.; Sumnu, M. Formulation and stability evaluation of enteric-coated omeprazole formulations. STP Pharma Sci. 1999, 9, 321–327. [Google Scholar]
- Shin-Etsu Chemical Co. Ltd. Shin-Etsu AQOAT®—For Pharmaceutical: Shin-Etsu Cellulose. Available online: https://www.metolose.jp/en/pharmaceutical/aqoat.html (accessed on 26 March 2020).
- Shin-Etsu Chemical Co. Ltd. HPMCP—For Pharmaceutical: Shin-Etsu Cellulose. Available online: https://www.metolose.jp/en/pharmaceutical/hpmcp.html (accessed on 26 March 2020).
- Al-Gousous, J.; Amidon, G.L.; Langguth, P. Toward Biopredictive Dissolution for Enteric Coated Dosage Forms. Mol. Pharm. 2016, 13, 1927–1936. [Google Scholar] [CrossRef] [PubMed]
- Al-Tabakha, M.M.; Arida, A.I.; Fahelelbom, K.M.S.; Sadek, B.; Jarad, R.A.A. Performances of new generation of delayed release capsules. J. Young Pharm. 2015. [Google Scholar] [CrossRef] [Green Version]
- Vassarstats. Available online: http://vassarstats.net/ (accessed on 7 April 2020).
- Dowling, N.E. Mechanical Behavior of Materials: Engineering Methods for Deformation, Fracture, and Fatigue, 4th ed.; Pearson: Harlow, UK, 2012; ISBN 0273764551. [Google Scholar]
- Missaghi, S.; Young, C.; Fegely, K.; Rajabi-Siahboomi, A.R. Delayed release film coating applications on oral solid dosage forms of proton pump inhibitors: Case studies Delayed release solid dosage forms of proton pump inhibitors. Drug Dev. Ind. Pharm. 2010. [Google Scholar] [CrossRef] [PubMed]
- Al-Gousous, J.; Tsume, Y.; Fu, M.; Salem, I.I.; Langguth, P. Unpredictable Performance of pH-Dependent Coatings Accentuates the Need for Improved Predictive in Vitro Test Systems. Mol. Pharm. 2017, 14, 4209–4219. [Google Scholar] [CrossRef] [PubMed]
- Shin-Etsu Chemical Co. Ltd. Shin-Etsu Pharmaceutical Excipients. Available online: www.metolose.jp/en/pharmaceutical/ (accessed on 30 March 2020).
- Obara, S.; Kokubo, H. Application of HPMC and HPMCAS to Aqueous Film Coating of Pharmaceutical Dosage Forms. In Aqueous Polymeric coatings for Pharmaceutical Dosage Forms; McGinity, J.W., Felton, L.A., Eds.; Informa Healthcare USA, Inc.: New York, NY, USA, 2008; pp. 304–305. [Google Scholar]
- Florence, A.T.; Attwood, D. Polymers and macromolecules. In Physicochemical Principles of Pharmacy In Manufacture, Formulation and Clinical Use; Florence, A.T., Attwood, D., Eds.; Pharmaceutical Press: London, UK, 2015; pp. 301–302. [Google Scholar]
- FDA SCOGS (Select Committee on GRAS Substances). Available online: https://www.accessdata.fda.gov/scripts/fdcc/?set=SCOGS (accessed on 1 April 2020).
- Fadda, H.M.; Hernández, M.C.; Margetson, D.N.; Mcallister, S.M.; Basit, A.W.; Brocchini, S.; Suárez, N. The molecular interactions that influence the plasticizer dependent dissolution of acrylic polymer films. J. Pharm. Sci. 2008. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siepmann, F.; Ornlaksana, P.; Bodmeier, R. Process and Formulation Factors Affecting Drug Release from Pellets Coated with Ethylcellulose Pseudolatex Aquacoat®. In Aqueous Polymeric Coatings for Pharmaceutical Dosage Forms; McGinity, J.W., Felton, L.A., Eds.; Informa Healthcare USA, Inc.: New York, NY, USA, 2008; p. 220. [Google Scholar]
- Evonik EUDRAGIT® Application Guidelines; Evonik Nutrition & Care GmbH: Darmstadt, Germany, 2020; Available online: https://oncare.evonik.com/ (accessed on 26 March 2020).









| Polymer | Name | Grade | Function Related Characteristic | Opening pH Value |
|---|---|---|---|---|
| Hypromellose acetate succinate, HPMCAS | Shin-Etsu AQOAT® | AS-LG | Acetyl: 8.2% Succinoyl: 14.9% | >5.5 |
| Hypromellose acetate succinate, HPMCAS | Shin-Etsu AQOAT® | AS-MG | Acetyl: 9.3% Succinoyl: 11.3% | >6.0 |
| Hypromellose acetate succinate, HPMCAS | Shin-Etsu AQOAT® | AS-HG | Acetyl: 11.7% Succinoyl: 7.5% | >6.5 |
| Hypromellose Phthalate | HPMCP | HP-50 | Phthalyl: 23.1% Viscosity: 55 mPas | >5.0 |
| Hypromellose Phthalate | HPMCP | HP-55 | Phthalyl: 32.9% Viscosity: 43 mPas | >5.5 |
| Hypromellose Phthalate | HPMCP | HP-55S | Phthalyl: 33.2% Viscosity: 167 mPas | >5.5 |
| Methacrylic acid and ethyl acrylate copolymer | Eudragit® | L100-55 | Ratio of methacylic acid to ethyl acrylate ~1:1 | >5.5 |
| Cellulose acetate phthalate | EastmanTM C-A-P | C-A-P Cellulose Ester NF | Acetyl: 21.5–26% Phthalyl: 30–36% | >6.0 |
| HPMCAS | |||||
|---|---|---|---|---|---|
| Batch No. | E02 | E06 | E04 | E09 | E18 |
| Polymer type | AS-MG | AS-MG | AS-MG | AS-LG | AS-HG |
| Content | 4.99 | 5.00 | 5.00 | 5.00 | 5.00 |
| Triethyl citrate (TEC) | 1.25 | 3.00 | 2.00 | 2.00 | 2.00 |
| Talc | 7.49 | 7.50 | 7.50 | 7.50 | 7.50 |
| Water | 17.23 | 16.90 | 16.50 | 16.50 | 16.50 |
| Ethanol 96% (v/v) | 68.95 | 67.60 | 69.00 | 69.00 | 69.00 |
| Food color | 0.09 | 0 | 0 | 0 | 0 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| HPMCP | ||||||||
|---|---|---|---|---|---|---|---|---|
| Batch No. | E03 | E08 | E07 | E21 | E11 | E12 | E10 | E17 |
| Polymer type | HP-55S | HP-55S | HP-55S | HP-55S | HP-55 | HP-55 | HP-50 | HP-50 |
| content | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 |
| TEC | 0.00 | 0.60 | 1.20 | 1.50 | 0.00 | 2.40 | 0.00 | 3.00 |
| Talc | 7.50 | 7.50 | 7.50 | 7.50 | 7.50 | 7.50 | 7.50 | 7.50 |
| Water | 12.97 | 17.90 | 17.00 | 17.00 | 12.97 | 17.90 | 12.97 | 16.70 |
| Ethanol 96% (v/v) | 73.53 | 68.00 | 68.30 | 68.00 | 73.53 | 68.00 | 73.53 | 66.80 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Methacrylic Acid and Ethyl Acrylate Copolymer | ||
|---|---|---|
| Batch No. | E15-02 | E15-03 |
| Polymer type | EU 100-55 | EU 100-55 |
| content | 6.00 | 6.00 |
| TEC | 1.20 | 1.20 |
| Talc | 3.00 | 3.00 |
| Water | 14.00 | 14.00 |
| Ethanol 96% (v/v) | 75.80 | 75.80 |
| Total | 100.00 | 100.00 |
| Cellulose Acetate Phthalate | ||
|---|---|---|
| Batch No. | E19 | E20 |
| Polymer type | C-A-P | C-A-P |
| content | 6.00 | 6.00 |
| TEC | 2.00 | 2.00 |
| Talc | 0.00 | 3.00 |
| Water | 46.00 | 44.50 |
| Ethanol 96% (v/v) | 46.00 | 44.50 |
| Total | 100.00 | 100.00 |
| Shin-Etsu AQOAT®/HPMCP | Eudragit® L100-55 | Aquateric® | |
|---|---|---|---|
| Before coating | |||
| Preheating to °C | 30 | 25 | 30 |
| Coating | |||
| Nozzle diameter (mm) | 0.5 | 0.5 | 0.5 |
| Spray rate (g/min) | 6.5–7 | 0.75–0.85 | 7 |
| Atomizing pressure (bar) | 2.0 | 0.5 | 2.0 |
| Inlet air volume (m3/h) | 55 | 60 | 50 |
| Inlet air temperature (°C) | 58–60 | 40–75 | 55 |
| Product temperature (°C) | 35–38 | 25–40 | 37 |
| Coating time (min) | 65 | 181–310 | 80 |
| Drum speed (rpm) | 30 | 30 | 30 |
| Batch Name | Viscosity (mPas) | Spray Rate (g/min) | Inlet Temp. (°C) | Outlet Temp. (°C) | Weight Gain (%) |
|---|---|---|---|---|---|
| E02 | 32.6 ± 0.1 | 6.5–7.0 | 57–64 | 38–39 | 7.10 |
| E06 | 32.6 ± 0.1 | 6.9–8.0 | 59–62 | 39–41 | 7.20 |
| E04 | 32.6 ± 0.1 | 6.5–8.5 | 58–60 | 39–40 | 7.15 |
| E09 | 29.6 ± 0.1 | 6.3–7.4 | 58–60 | 39–40 | 7.26 |
| E18 | 40.2 ± 0.2 | 8.6–10 | 57–60 | 38–40 | 7.23 |
| E03 | 99.2 ± 0.2 | 6.5–7.3 | 58–60 | 39–40 | 6.95 |
| E08 | 99.2 ± 0.2 | 7.2–9.0 | 59–60 | 39–41 | 7.21 |
| E07 | 99.2 ± 0.2 | 6.6–8.2 | 59–64 | 39–42 | 7.06 |
| E21 | 99.2 ± 0.2 | 7.1–7.9 | 58–60 | 40–41 | 7.12 |
| E10 | 48.1 ± 0.2 | 6.0–7.1 | 59–61 | 39–42 | 7.17 |
| E11 | 46.0 ± 0.2 | 5.3–6.7 | 59–64 | 39–40 | 7.28 |
| E12 | 47.5 ± 0.2 | 6.7–8.2 | 58–62 | 38–41 | 7.25 |
| E17 | 48.8 ± 0.2 | 7.0–8.9 | 58–60 | 39–40 | 7.09 |
| E15-02 | 45.5 ± 0.2 | 2.7–2.8 | 73–80 | 45–48 | 7.40 |
| E15-03 | 45.2 ± 0.2 | 1.2–1.3 | 35–40 | 24–31 | 7.50 |
| E19 | 41.5 ± 0.2 | 4.4–4.9 | 55–62 | 39–40 | 7.05 |
| E20 | 47.2 ± 0.2 | 4.6–5.0 | 46–52 | 35–38 | 7.13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, M.; Blechar, J.A.; Sauer, A.; Al-Gousous, J.; Langguth, P. In Vitro Evaluation of Enteric-Coated HPMC Capsules—Effect of Formulation Factors on Product Performance. Pharmaceutics 2020, 12, 696. https://doi.org/10.3390/pharmaceutics12080696
Fu M, Blechar JA, Sauer A, Al-Gousous J, Langguth P. In Vitro Evaluation of Enteric-Coated HPMC Capsules—Effect of Formulation Factors on Product Performance. Pharmaceutics. 2020; 12(8):696. https://doi.org/10.3390/pharmaceutics12080696
Chicago/Turabian StyleFu, Maoqi, Johannes Andreas Blechar, Andreas Sauer, Jozef Al-Gousous, and Peter Langguth. 2020. "In Vitro Evaluation of Enteric-Coated HPMC Capsules—Effect of Formulation Factors on Product Performance" Pharmaceutics 12, no. 8: 696. https://doi.org/10.3390/pharmaceutics12080696
APA StyleFu, M., Blechar, J. A., Sauer, A., Al-Gousous, J., & Langguth, P. (2020). In Vitro Evaluation of Enteric-Coated HPMC Capsules—Effect of Formulation Factors on Product Performance. Pharmaceutics, 12(8), 696. https://doi.org/10.3390/pharmaceutics12080696





