Compatible Stability and Aerosol Characteristics of Atrovent® (Ipratropium Bromide) Mixed with Salbutamol Sulfate, Terbutaline Sulfate, Budesonide, and Acetylcysteine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
2.3. Physical Compatibility Assessments
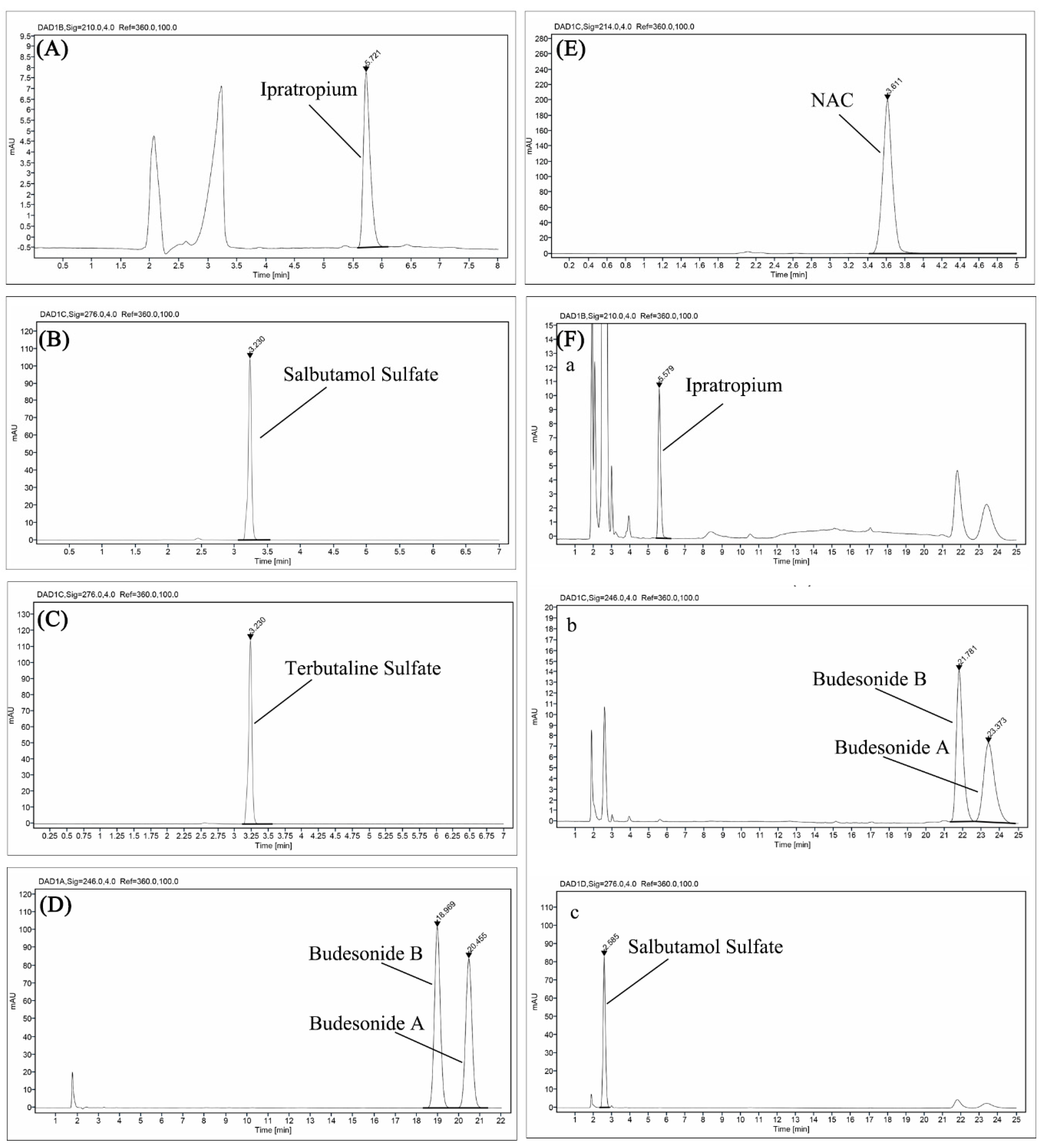
2.4. Chemical Stability Assessments
2.5. Particle Size Distribution Characterization by Laser Diffraction
2.6. Aerosol Particle Distribution Measurement by the Next-Generation Impactor
2.7. Active Drug Delivery Rate and Total Active Drug Delivered
3. Results
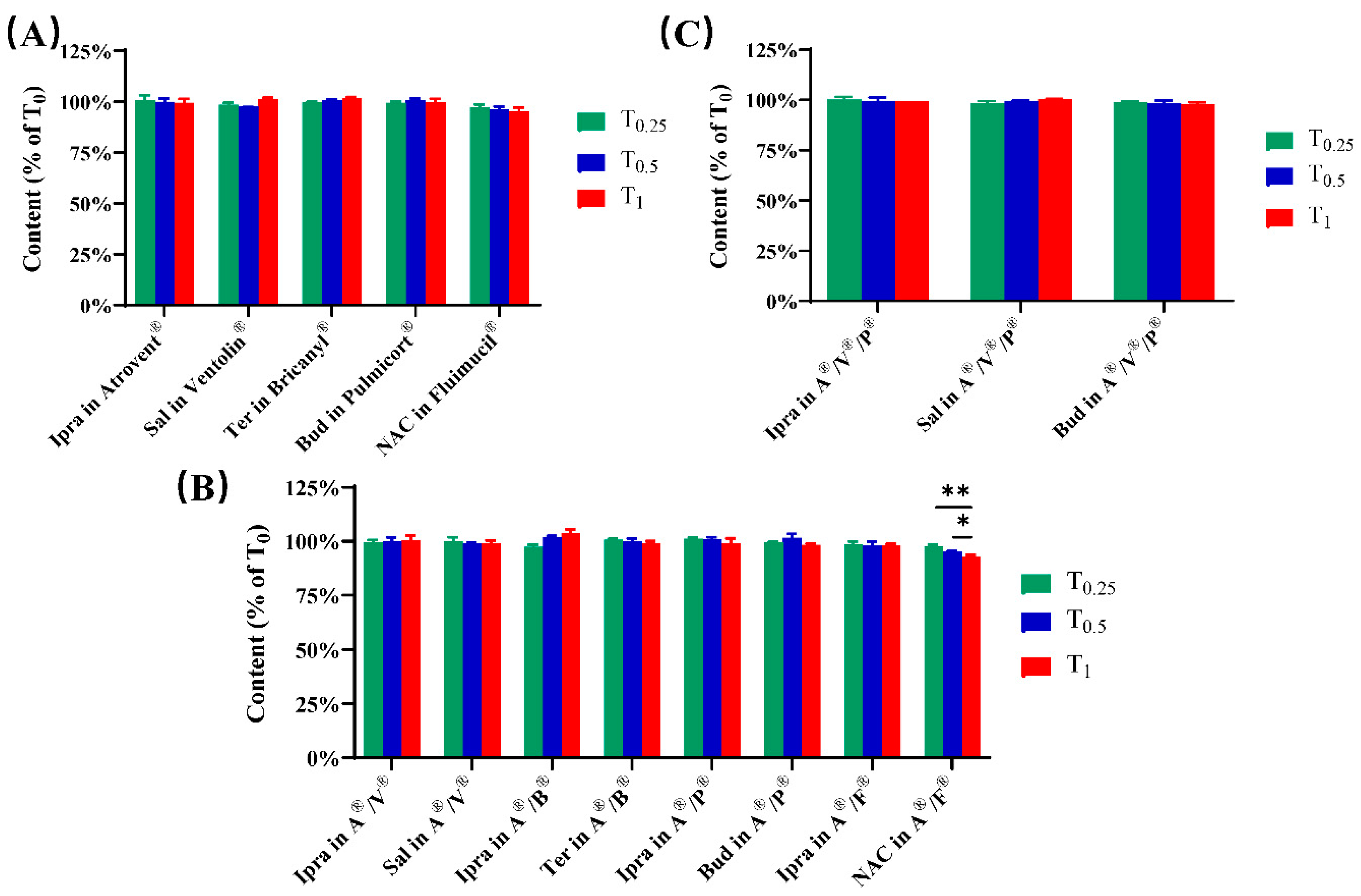
3.1. Physicochemical Compatibility
3.2. Geometrical Particle Size Distribution
3.3. Aerosol Particle Size Distribution
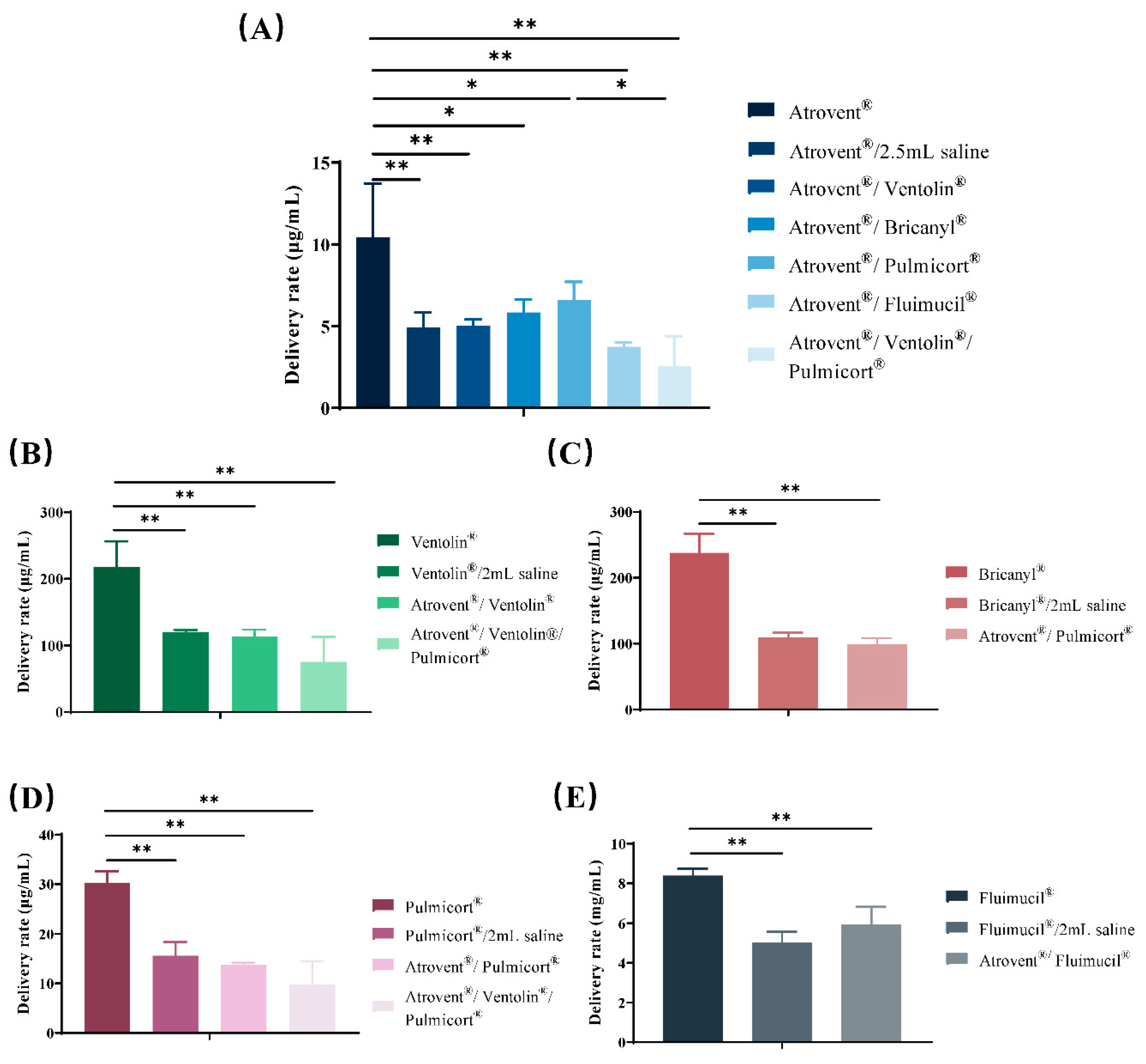
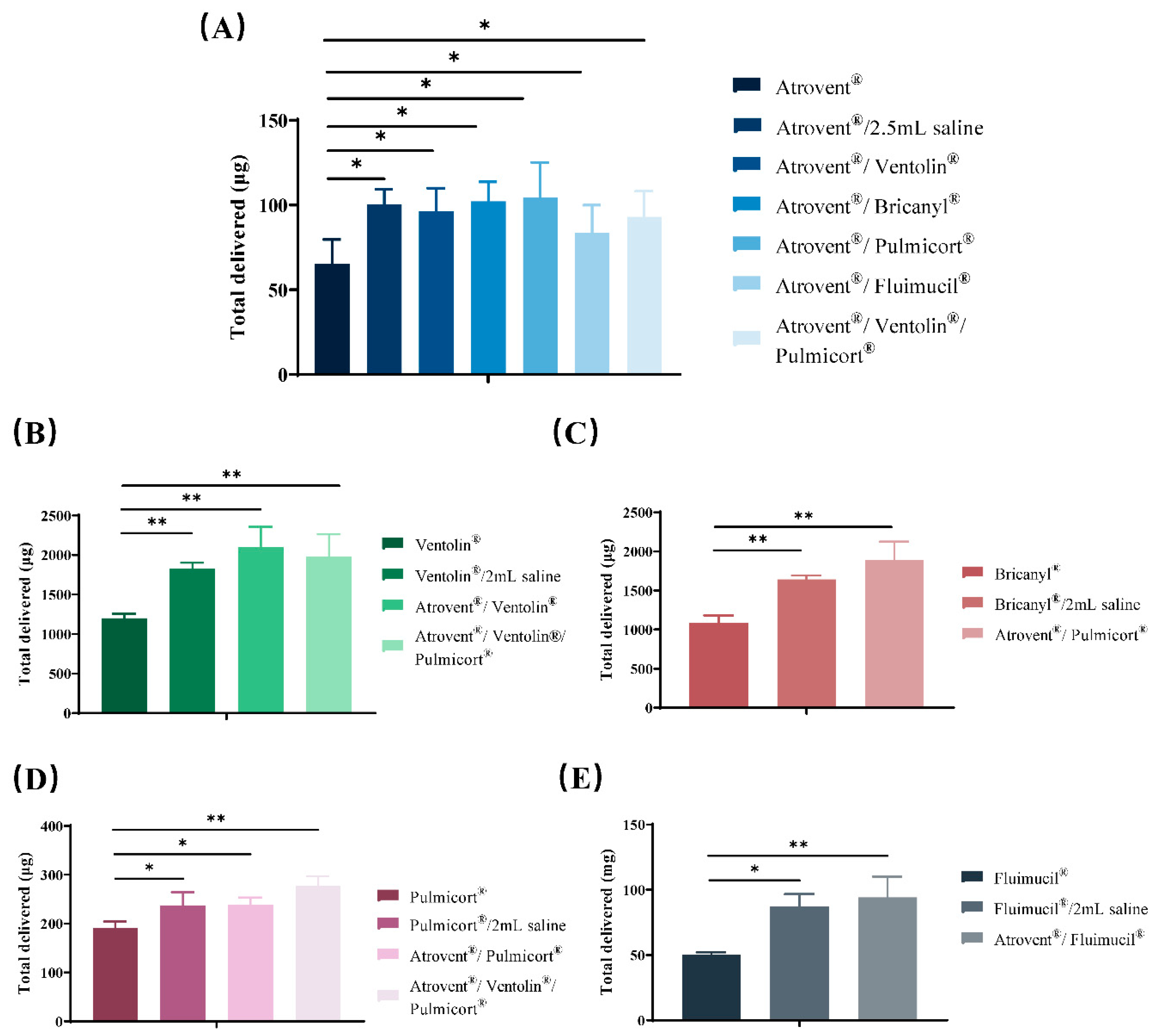
3.4. Active Drug Delivery Rate and Total Active Drug Delivered
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martin, A.R.; Finlay, W.H. Nebulizers for drug delivery to the lungs. Expert Opin. Drug Deliv. 2015, 12, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Patton, J.S.; Fishburn, C.S.; Weers, J.G. The lungs as a portal of entry for systemic drug delivery. Proc. Am. Thorac. Soc. 2004, 1, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Currie, G.P.; Lipworth, B.J. Inhaled treatment for chronic obstructive pulmonary disease: What’s new and how does it fit? QJM 2016, 109, 505–512. [Google Scholar] [CrossRef] [Green Version]
- Mirza, S.; Clay, R.D.; Koslow, M.A.; Scanlon, P.D. COPD Guidelines: A Review of the 2018 GOLD Report. Mayo Clin. Proc. 2018, 93, 1488–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GOLD: Global Initiative for Chronic Obstructive Lung Disease. Available online: https://goldcopd.org/gold-reports/ (accessed on 3 April 2020).
- Aziz, M.I.A.; Tan, L.E.; Wu, D.B.; Pearce, F.; Chua, G.S.W.; Lin, L.; Tan, P.T.; Ng, K. Comparative efficacy of inhaled medications (ICS/LABA, LAMA, LAMA/LABA and SAMA) for COPD: A systematic review and network meta-analysis. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 3203–3231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sion, K.Y.J.; Huisman, E.L.; Punekar, Y.S.; Naya, I.; Ismaila, A.S. A Network Meta-Analysis of Long-Acting Muscarinic Antagonist (LAMA) and Long-Acting beta2-Agonist (LABA) Combinations in COPD. Pulm. Ther. 2017, 3, 297–316. [Google Scholar] [CrossRef]
- Cazzola, M.; Page, C.P.; Calzetta, L.; Matera, M.G. Pharmacology and therapeutics of bronchodilators. Pharmacol. Rev. 2012, 64, 450–504. [Google Scholar] [CrossRef]
- Calzetta, L.; Di Marco, F.; Blasi, F.; Cazzola, M.; Centanni, S.; Micheletto, C.; Rossi, A.; Rogliani, P. Impact of ICS/LABA and LABA/LAMA FDCs on functional and clinical outcomes in COPD: A network meta-analysis. Pulm. Pharmacol. Ther. 2019, 59, 101855. [Google Scholar] [CrossRef]
- Kamin, W.; Schwabe, A.; Kramer, I. Inhalation solutions: Which one are allowed to be mixed? Physico-chemical compatibility of drug solutions in nebulizers. J. Cyst. Fibros. 2006, 5, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Burchett, D.K.; Darko, W.; Zahra, J.; Noviasky, J.; Probst, L.; Smith, A. Mixing and compatibility guide for commonly used aerosolized medications. Am. J. Health Syst. Pharm. 2010, 67, 227–230. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Hu, J.; Deng, L.; Li, S.; Chen, X.; Liu, F.; Wang, S.; Mohammed Abdul, K.S.; Beng, H.; Tan, W. Aerosol Characteristics and Physico-Chemical Compatibility of Combivent((R)) (Containing Salbutamol and Ipratropium Bromide) Mixed with Three Other Inhalants: Budesonide, Beclomethasone or N-Acetylcysteine. Pharmaceutics 2020, 12, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akapo, S.; Gupta, J.; Martinez, E.; McCrea, C.; Ye, L.; Roach, M. Compatibility and aerosol characteristics of formoterol fumarate mixed with other nebulizing solutions. Ann. Pharmacother. 2008, 42, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Itazawa, T.; Adachi, Y.; Ito, Y.; Higuchi, O.; Mochizuki, H.; Shimojo, N.; Inoue, T. Aerosol characteristics of admixture of budesonide inhalation suspension with a beta2-agonist, procaterol. Allergol. Int. 2013, 62, 131–135. [Google Scholar] [CrossRef] [Green Version]
- Kramer, I.; Schwabe, A.; Lichtinghagen, R.; Kamin, W. Physicochemical compatibility of nebulizable drug mixtures containing dornase alfa and ipratropium and/or albuterol. Pharm. Sci. 2007, 62, 760–766. [Google Scholar]
- Desager, K.N.; Van Bever, H.P.; Stevens, W.J. Osmolality and pH of anti-asthmatic drug solutions. Agents Actions 1990, 31, 225–228. [Google Scholar] [CrossRef]
- Lowry, R.H.; Wood, A.M.; Higenbottam, T.W. Effects of pH and osmolarity on aerosol-induced cough in normal volunteers. Clin. Sci. (Lond.) 1988, 74, 373–376. [Google Scholar] [CrossRef] [Green Version]
- Chrystyn, H. Anatomy and physiology in delivery: Can we define our targets? Allergy 1999, 54 (Suppl. 49), 82–87. [Google Scholar] [CrossRef]
- Usmani, O.S.; Biddiscombe, M.F.; Barnes, P.J. Regional lung deposition and bronchodilator response as a function of beta2-agonist particle size. Am. J. Respir. Crit. Care Med. 2005, 172, 1497–1504. [Google Scholar] [CrossRef]
- Nikander, K.; von Hollen, D.; Larhrib, H. The size and behavior of the human upper airway during inhalation of aerosols. Expert Opin. Drug Deliv. 2017, 14, 621–630. [Google Scholar] [CrossRef]
- Cheng, Y.S. Mechanisms of pharmaceutical aerosol deposition in the respiratory tract. AAPS PharmSciTech 2014, 15, 630–640. [Google Scholar] [CrossRef]
- Lip Kwok, P.C. Electrostatics of Pharmaceutical Aerosols for Pulmonary Delivery. Curr. Pharm. Des. 2015, 21, 3945–3954. [Google Scholar] [CrossRef] [PubMed]
- Berg, E.B.; Picard, R.J. In vitro delivery of budesonide from 30 jet nebulizer/compressor combinations using infant and child breathing patterns. Respir. Care 2009, 54, 1671–1678. [Google Scholar] [PubMed]
- De Boer, A.H.; Gjaltema, D.; Hagedoorn, P.; Frijlink, H.W. Characterization of inhalation aerosols: A critical evaluation of cascade impactor analysis and laser diffraction technique. Int. J. Pharm. 2002, 249, 219–231. [Google Scholar] [CrossRef]
- Melani, A.S. Effects on aerosol performance of mixing of either budesonide or beclomethasone dipropionate with albuterol and ipratropium bromide. Respir. Care 2011, 56, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Ejiofor, S.; Turner, A.M. Pharmacotherapies for COPD. Clin. Med. Insights Circ. Respir. Pulm. Med. 2013, 7, 17–34. [Google Scholar] [CrossRef]
- Albertson, T.E.; Chenoweth, J.A.; Pearson, S.J.; Murin, S. The pharmacological management of asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS). Expert Opin. Pharmacother. 2020, 21, 213–231. [Google Scholar] [CrossRef]
- Appleton, S.; Jones, T.; Poole, P.; Pilotto, L.; Adams, R.; Lasserson, T.J.; Smith, B.; Muhammad, J. Ipratropium bromide versus long-acting beta-2 agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2006. [Google Scholar] [CrossRef]
- Bonasia, P.; Cook, C.; Cheng, Y.; Ong, S. Compatibility of arformoterol tartrate inhalation solution with three nebulized drugs. Curr. Med. Res. Opin. 2007, 23, 2477–2483. [Google Scholar] [CrossRef]
- Kasawar, G.B.; Farooqui, M. Development and validation of a stability indicating RP-HPLC method for the simultaneous determination of related substances of albuterol sulfate and ipratropium bromide in nasal solution. J. Pharm. Biomed. Anal. 2010, 52, 19–29. [Google Scholar] [CrossRef]
- Lee, T.Y.; Chen, C.M.; Lee, C.N.; Chiang, Y.C.; Chen, H.Y. Compatibility and osmolality of inhaled N-acetylcysteine nebulizing solution with fenoterol and ipratropium. Am. J. Health Syst. Pharm. 2005, 62, 828–833. [Google Scholar] [CrossRef]
- Nikander, K.; Denyer, J.; Smaldone, G.C. Effects of equipment dead space and pediatric breathing patterns on inhaled mass of nebulized budesonide. J. Aerosol Med. 1999, 12, 67–73. [Google Scholar] [CrossRef]
- Hess, D.; Fisher, D.; Williams, P.; Pooler, S.; Kacmarek, R.M. Medication nebulizer performance. Effects of diluent volume, nebulizer flow, and nebulizer brand. Chest 1996, 110, 498–505. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Zhang, R.; Beng, H.; Deng, L.; Ke, Q.; Tan, W. Effects of flow pattern, device and formulation on particle size distribution of nebulized aerosol. Int. J. Pharm. 2019, 560, 35–46. [Google Scholar] [CrossRef]
- Walz-Jung, H.; Kramer, I.; Kamin, W. Drug Output and Aerosol Characteristics of Different Jet Nebulisers for Adults While Simulating the Nebulisation of Salbutamol. Pneumologie 2018, 72, 820–831. [Google Scholar] [CrossRef]
- Ochowiak, M.; Matuszak, M.; Wlodarczak, S. The analysis of pneumatic atomization of Newtonian and non-Newtonian fluids for different medical nebulizers. Drug Dev. Ind. Pharm. 2017, 43, 1999–2010. [Google Scholar] [CrossRef]



| Samples | Volume (mL) | Nebulization Time (min) |
|---|---|---|
| Atrovent® | 2 | 8 |
| Ventolin® | 2.5 | 9 |
| Bricanyl® | 2 | 8 |
| Pulmicort® | 2 | 8 |
| Fluimucil® | 3 | 10 |
| Atrovent®/Ventolin® | 4.5 | 13 |
| Atrovent®/Bricanyl® | 4 | 13 |
| Atrovent®/Pulmicort® | 4 | 14 |
| Atrovent®/Fluimucil® | 5 | 14 |
| Atrovent®/Ventolin®/Pulmicort® | 6.5 | 19 |
| Admixtures | Active Components | Ratio of Mobile Phase (A/B) by Volume | Injection Volume (μL) | Run Time (min) | Detection Wavelength (nm) |
|---|---|---|---|---|---|
| Atrovent® | ipratropium bromide | 75/25 | 80 | 7.5 | 210 |
| Ventolin® | salbutamol sulfate | 75/25 | 20 | 4.5 | 276 |
| Bricanyl® | terbutaline sulfate | 75/25 | 20 | 4.5 | 276 |
| Pulmicort® | budesonide | 62/38 | 20 | 22 | 246 |
| Fluimucil® | NAC | 91/9 | 5 | 5 | 214 |
| Atrovent®/Ventolin® | ipratropium bromide | 75/25 | 80 | 7.5 | 210 |
| salbutamol sulfate | 276 | ||||
| Atrovent®/Bricanyl® | ipratropium bromide | 75/25 | 80 | 7.5 | 210 |
| terbutaline sulfate | 276 | ||||
| Atrovent®/Pulmicort® | ipratropium bromide | 0 min 75/25 8 min 75/25 12 min 55/45 16.5 min 55/45 19.5 min 65/35 | 80 | 25 | 210 |
| budesonide | 246 | ||||
| Atrovent®/Fluimucil® | ipratropium bromide | 75/25 | 80 | 7.5 | 210 |
| NAC | 0 min 91/9 5 min 91/9 12 min 40/60 | 5 | 14.5 | 214 | |
| Atrovent®/Ventolin®/Pulmicort® | ipratropium bromide | 0 min 75/25 8 min 75/25 12 min 55/45 16.5 min 55/45 19.5 min 65/35 | 80 | 25 | 210 |
| salbutamol sulfate | 276 | ||||
| budesonide | 246 |
| Samples | X10 (μm) | X50 (μm) | X90 (μm) | VMD (μm) |
|---|---|---|---|---|
| Atrovent® | 1.67 ± 0.20 | 4.19 ± 0.08 | 7.01 ± 0.17 | 4.26 ± 0.14 |
| Ventolin® | 1.68 ± 0.11 | 4.32 ± 0.13 | 6.75 ± 0.16 | 4.24 ± 0.05 |
| Bricanyl® | 1.66 ± 0.06 | 4.40 ± 0.05 | 7.64 ± 0.18 | 4.55 ± 0.08 |
| Pulmicort® | 1.87 ± 0.09 | 4.37 ± 0.02 | 7.19 ± 0.17 | 4.97 ± 0.07 |
| Fluimucil® | 1.54 ± 0.13 | 4.03 ± 0.09 | 6.69 ± 0.11 | 4.18 ± 0.11 |
| Atrovent®/Ventolin® | 1.69 ± 0.10 | 3.93 ± 0.04 | 6.25 ± 0.16 | 3.95 ± 0.16 |
| Atrovent®/Bricanyl® | 1.68 ± 0.11 | 4.10 ± 0.12 | 6.82 ± 0.09 | 4.20 ± 0.04 |
| Atrovent®/Pulmicort® | 1.73 ± 0.07 | 4.02 ± 0.18 | 6.38 ± 0.04 | 4.03 ± 0.00 |
| Atrovent®/Fluimucil® | 1.55 ± 0.15 | 3.95 ± 0.08 | 6.89 ± 0.17 | 4.10 ± 0.13 |
| Atrovent®/Ventolin®/Pulmicort® | 1.70 ± 0.11 | 4.09 ± 0.13 | 6.61 ± 0.09 | 4.13 ± 0.12 |
| Samples | Active Components | FPD 1 | FPF (%) | MMAD (μm) |
|---|---|---|---|---|
| Atrovent® | Ipratropium bromide | 68.98 ± 0.28 | 66.40 ± 1.01 | 3.07 ± 0.11 |
| Ventolin® | salbutamol sulfate | 856.07 ± 16.23 | 59.90 ± 1.40 | 4.12 ± 0.13 |
| Bricanyl® | terbutaline sulfate | 1004.28 ± 9.35 | 58.33 ± 2.27 | 3.82 ± 0.20 |
| Pulmicort® | budesonide | 193.14 ± 19.80 | 47.65 ± 1.77 | 5.06 ± 0.16 |
| Fluimucil® | NAC | 78.91 ± 11.04 | 55.38 ± 7.21 | 4.44 ± 0.58 |
| Atrovent®/2.5 mL saline | Ipratropium bromide | 100.72 ± 6.37 | 63.92 ± 2.49 | 3.39 ± 0.03 |
| Ventolin®/2 mL saline | salbutamol sulfate | 1934.29 ± 103.40 | 63.02 ± 1.25 | 3.42 ± 0.18 |
| Bricanyl®/2 mL saline | terbutaline sulfate | 1630.02 ± 180.39 | 60.32 ± 1.96 | 3.28 ± 0.28 |
| Pulmicort®/2 mL saline | budesonide | 282.29 ± 27.93 | 46.20 ± 3.05 | 5.30 ± 0.84 |
| Fluimucil®/2 mL saline | NAC | 120.83 ± 4.07 | 52.40 ± 2.04 | 4.38 ± 0.06 |
| Atrovent®/Ventolin® | ipratropium bromide | 97.23 ± 6.37 | 65.54 ± 1.80 | 3.15 ± 0.06 |
| salbutamol sulfate | 2112.95 ± 217.01 | 65.27 ± 0.98 | 3.18 ± 0.07 | |
| Atrovent®/Bricanyl® | ipratropium bromide | 99.28 ± 0.17 | 64.71 ± 0.04 | 3.63 ± 0.02 |
| terbutaline sulfate | 1491.85 ± 276.90 | 61.79 ± 2.80 | 3.46 ± 0.17 | |
| Atrovent®/Pulmicort® | ipratropium bromide | 95.90 ± 25.08 | 50.50 ± 8.94 | 3.89 ± 0.77 |
| budesonide | 274.23 ± 23.13 | 47.01 ± 4.91 | 6.45 ± 0.49 | |
| Atrovent®/Fluimucil® | ipratropium bromide | 96.17 ± 5.81 | 54.73 ± 1.30 | 4.48 ± 0.15 |
| NAC | 111.61 ± 2.86 | 53.39 ± 1.64 | 4.55 ± 0.17 | |
| Atrovent®/Ventolin®/Pulmicort® | ipratropium bromide | 120.16 ± 4.18 | 57.97 ± 5.32 | 4.68 ± 0.43 |
| salbutamol sulfate | 2359.09 ± 198.30 | 52.66 ± 4.23 | 4.68 ± 0.33 | |
| budesonide | 279.87 ± 10.79 | 44.78 ± 1.76 | 6.29 ± 0.27 |
| Samples | Active Components | Active Drug Delivery Rate 1 | Total Active Drug Delivered 2 |
|---|---|---|---|
| Atrovent® | Ipratropium bromide | 10.43 ± 3.30 | 65.34 ± 14.37 |
| Ventolin® | salbutamol sulfate | 218 ± 38.50 | 1201.00 ± 56.20 |
| Bricanyl® | terbutaline sulfate | 237.96 ± 29.20 | 1084.73 ± 95.26 |
| Pulmicort® | budesonide | 30.27 ± 2.33 | 190.46 ± 14.13 |
| Fluimucil® | NAC | 8.39 ± 0.35 | 50.14 ± 1.91 |
| Atrovent®/2.5 mL saline | Ipratropium bromide | 4.93 ± 0.92 | 100.39 ± 8.92 |
| Ventolin®/2 mL saline | salbutamol sulfate | 120.37 ± 3.24 | 1830.47 ± 72.39 |
| Bricanyl®/2 mL saline | terbutaline sulfate | 109.49 ± 7.34 | 1640.28 ± 50.76 |
| Pulmicort®/2 mL saline | budesonide | 15.57 ± 2.78 | 237.33 ± 27.07 |
| Fluimucil®/2 mL saline | NAC | 5.04 ± 0.53 | 86.99 ± 9.67 |
| Atrovent®/Ventolin® | ipratropium bromide | 5.05 ± 0.36 | 96.32 ± 13.57 |
| salbutamol sulfate | 113.53 ± 10.27 | 1936 ± 53.87 | |
| Atrovent®/Bricanyl® | ipratropium bromide | 5.83 ± 0.80 | 102.22 ± 11.48 |
| terbutaline sulfate | 99.41 ± 9.29 | 1694.74 ± 29.66 | |
| Atrovent®/Pulmicort® | ipratropium bromide | 6.58 ± 1.14 | 104.27 ± 20.94 |
| budesonide | 13.74 ± 0.44 | 237.87 ± 35.50 | |
| Atrovent®/Fluimucil® | ipratropium bromide | 3.73 ± 0.29 | 83.39 ± 16.60 |
| NAC | 5.93 ± 0.91 | 94.02 ± 16.09 | |
| Atrovent®/Ventolin®/Pulmicort® | ipratropium bromide | 2.55 ± 1.84 | 92.90 ± 15.32 |
| salbutamol sulfate | 75.01 ± 37.75 | 1981.51 ± 283.01 | |
| budesonide | 9.76 ± 4.77 | 277.82 ± 19.42 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Du, S.; Zhang, Z.; He, W.; Lu, E.; Wang, R.; Sha, X.; Ma, Y. Compatible Stability and Aerosol Characteristics of Atrovent® (Ipratropium Bromide) Mixed with Salbutamol Sulfate, Terbutaline Sulfate, Budesonide, and Acetylcysteine. Pharmaceutics 2020, 12, 776. https://doi.org/10.3390/pharmaceutics12080776
Chen Y, Du S, Zhang Z, He W, Lu E, Wang R, Sha X, Ma Y. Compatible Stability and Aerosol Characteristics of Atrovent® (Ipratropium Bromide) Mixed with Salbutamol Sulfate, Terbutaline Sulfate, Budesonide, and Acetylcysteine. Pharmaceutics. 2020; 12(8):776. https://doi.org/10.3390/pharmaceutics12080776
Chicago/Turabian StyleChen, Yiting, Shilin Du, Zhirui Zhang, Wenxiu He, Enhao Lu, Rui Wang, Xianyi Sha, and Yan Ma. 2020. "Compatible Stability and Aerosol Characteristics of Atrovent® (Ipratropium Bromide) Mixed with Salbutamol Sulfate, Terbutaline Sulfate, Budesonide, and Acetylcysteine" Pharmaceutics 12, no. 8: 776. https://doi.org/10.3390/pharmaceutics12080776
APA StyleChen, Y., Du, S., Zhang, Z., He, W., Lu, E., Wang, R., Sha, X., & Ma, Y. (2020). Compatible Stability and Aerosol Characteristics of Atrovent® (Ipratropium Bromide) Mixed with Salbutamol Sulfate, Terbutaline Sulfate, Budesonide, and Acetylcysteine. Pharmaceutics, 12(8), 776. https://doi.org/10.3390/pharmaceutics12080776





