Do Ophthalmic Solutions of Amphotericin B Solubilised in 2-Hydroxypropyl-?-Cyclodextrins Possess an Extended Physicochemical Stability?
Abstract
:1. Introduction
2. Materials and Methods
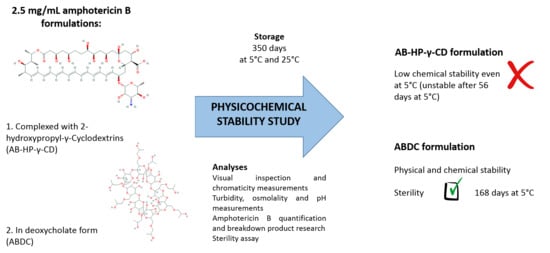
2.1. Preparation of the ABDC Formulation
2.2. Preparation of the AB-HP-γ-CD Formulation
2.3. Conditioning and Storage
2.4. Study Design
2.5. Analyses
2.5.1. Visual Inspection
2.5.2. Osmolality, pH and Turbidity Measurements
2.5.3. Amphotericin B Quantification and Breakdown Products Research
- Chemicals and instrumentation
- Method validation
2.5.4. Chromaticity Analysis
2.5.5. Sterility Assay
2.6. Data Analysi–Acceptability Criteria
3. Results
3.1. Amphotericin B Quantification and Breakdown Products Research
3.2. Physicochemical Stability of ABDC and AB-HP-γ-CD Formulations
3.3. Sterility Assay
3.4. Physicochemical Stability of AB-HP-ɣ-CD Additionned with 0.5 mg/mL Ascorbic Acid
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Green, M.D.; Apel, A.J.G.; Naduvilath, T.; Stapleton, F.J. Clinical outcomes of keratitis. Clin. Exp. Ophthalmol. 2007, 35, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Słowik, M.; Biernat, M.M.; Urbaniak-Kujda, D.; Kapelko-Słowik, K.; Misiuk-Hojło, M. Mycotic infections of the eye. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2015, 24, 1113–1117. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Satpathy, G.; Ahmed, N.H.; Nayak, N.; Tandon, R.; Sharma, N.; Agarwal, T.; Vanathi, M.; Titiyal, J.S. Spectrum of mycotic keratitis in north India: Sixteen years study from a tertiary care ophthalmic centre. J. Infect. Public Health 2019, 12, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-A.; Hsu, S.-L.; Hsiao, C.-H.; Ma, D.H.-K.; Sun, C.-C.; Yu, H.-J.; Fang, P.-C.; Kuo, M.-T. Comparison of fungal and bacterial keratitis between tropical and subtropical Taiwan: A prospective cohort study. Ann. Clin. Microbiol. Antimicrob. 2020, 19. [Google Scholar] [CrossRef] [Green Version]
- Khor, W.-B.; Prajna, V.N.; Garg, P.; Mehta, J.S.; Xie, L.; Liu, Z.; Padilla, M.D.B.; Joo, C.-K.; Inoue, Y.; Goseyarakwong, P.; et al. The Asia Cornea Society Infectious Keratitis Study: A Prospective Multicenter Study of Infectious Keratitis in Asia. Am. J. Ophthalmol. 2018, 195, 161–170. [Google Scholar] [CrossRef]
- Oliveira dos Santos, C.; Kolwijck, E.; van Rooij, J.; Stoutenbeek, R.; Visser, N.; Cheng, Y.Y.; Santana, N.T.Y.; Verweij, P.E.; Eggink, C.A. Epidemiology and Clinical Management of Fusarium keratitis in the Netherlands, 2005–2016. Front. Cell. Infect. Microbiol. 2020, 10. [Google Scholar] [CrossRef] [Green Version]
- Puig, M.; Weiss, M.; Salinas, R.; Johnson, D.A.; Kheirkhah, A. Etiology and Risk Factors for Infectious Keratitis in South Texas. J. Ophthalmic Vis. Res. 2020, 15, 128–137. [Google Scholar] [CrossRef]
- Chew, R.; Woods, M.L. Epidemiology of fungal keratitis in Queensland, Australia. Clin. Exp. Ophthalmol. 2019, 47, 26–32. [Google Scholar] [CrossRef]
- Bograd, A.; Seiler, T.; Droz, S.; Zimmerli, S.; Früh, B.; Tappeiner, C. Bacterial and Fungal Keratitis: A Retrospective Analysis at a University Hospital in Switzerland. Klin. Mon. Für Augenheilkd. 2019, 236, 358–365. [Google Scholar] [CrossRef]
- Thomas, B.; Audonneau, N.C.; Machouart, M.; Debourgogne, A. Fusarium infections: Epidemiological aspects over 10 years in a university hospital in France. J. Infect. Public Health 2020. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, W.-S.; Zhao, J.; Zhou, H.-Y. Review of clinical and basic approaches of fungal keratitis. Int. J. Ophthalmol. 2016, 9, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Sahay, P.; Singhal, D.; Nagpal, R.; Maharana, P.K.; Farid, M.; Gelman, R.; Sinha, R.; Agarwal, T.; Titiyal, J.S.; Sharma, N. Pharmacologic therapy of mycotic keratitis. Surv. Ophthalmol. 2019, 64, 380–400. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, P.; Abdel-Hadi, A.; Randhir Babu Singh, Y.; Revathi, R.; Anita, R.; Banawas, S.; Bin Dukhyil, A.A.; Alshehri, B.; Shobana, C.S.; Panneer Selvam, K.; et al. Fungal Keratitis: Epidemiology, Rapid Detection, and Antifungal Susceptibilities of Fusarium and Aspergillus Isolates from Corneal Scrapings. BioMed Res. Int. 2019, 2019, 6395840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourcier, T.; Sauer, A.; Dory, A.; Denis, J.; Sabou, M. Fungal keratitis. J. Fr. Ophtalmol. 2017, 40, e307–e313. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Masoomi, A.; Ahmadikia, K.; Tabatabaei, S.A.; Soleimani, M.; Rezaie, S.; Ghahvechian, H.; Banafsheafshan, A. Fungal keratitis: An overview of clinical and laboratory aspects. Mycoses 2018, 61, 916–930. [Google Scholar] [CrossRef]
- Al-Hatmi, A.M.S.; Bonifaz, A.; Ranque, S.; Sybren de Hoog, G.; Verweij, P.E.; Meis, J.F. Current antifungal treatment of fusariosis. Int. J. Antimicrob. Agents 2018, 51, 326–332. [Google Scholar] [CrossRef] [Green Version]
- Lakhani, P.; Patil, A.; Majumdar, S. Challenges in the Polyene- and Azole-Based Pharmacotherapy of Ocular Fungal Infections. J. Ocul. Pharmacol. Ther. 2019, 35, 6–22. [Google Scholar] [CrossRef]
- Peyron, F.; Elias, R.; Ibrahim, E.; Amarit-Combralier, V.; Bues-Charbit, M.; Balansard, G. Stability of amphotericin B in 5% dextrose ophthalmic solution. Int. J. Pharm. Compd. 1999, 3, 316–320. [Google Scholar]
- Curti, C.; Lamy, E.; Primas, N.; Fersing, C.; Jean, C.; Bertault-Peres, P.; Vanelle, P. Stability studies of five anti-infectious eye drops under exhaustive storage conditions. Die Pharm. 2017, 72, 741–746. [Google Scholar] [CrossRef]
- Vikmon, M.; Stadler-Szöke, Á.; Szejtli, J. Solubilization of amphotericin B with γ-cyclodextrin. J. Antibiot. 1985, 38, 1822–1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajagopalan, N.; Chen, S.C.; Chow, W.-S. A study of the inclusion complex of amphotericin-B with γ-cyclodextrin. Int. J. Pharm. 1986, 29, 161–168. [Google Scholar] [CrossRef]
- Jansook, P.; Kurkov, S.V.; Loftsson, T. Cyclodextrins as solubilizers: Formation of complex aggregates. J. Pharm. Sci. 2010, 99, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; Ruiz-Saldaña, H.K.; Molero, G.; Ballesteros, M.P.; Torrado, J.J. A novel formulation of solubilised amphotericin B designed for ophthalmic use. Int. J. Pharm. 2012, 437, 80–82. [Google Scholar] [CrossRef]
- Kajtár, M.; Vikmon, M.; Morlin, E.; Szejtli, J. Aggregation of amphotericin B in the presence of gamma-cyclodextrin. Biopolymers 1989, 28, 1585–1596. [Google Scholar] [CrossRef]
- He, J.; Chipot, C.; Shao, X.; Cai, W. Cyclodextrin-Mediated Recruitment and Delivery of Amphotericin B. J. Phys. Chem. C 2013, 117, 11750–11756. [Google Scholar] [CrossRef]
- Ruiz, H.K.; Serrano, D.R.; Dea-Ayuela, M.A.; Bilbao-Ramos, P.E.; Bolás-Fernández, F.; Torrado, J.J.; Molero, G. New amphotericin B-gamma cyclodextrin formulation for topical use with synergistic activity against diverse fungal species and Leishmania spp. Int. J. Pharm. 2014, 473, 148–157. [Google Scholar] [CrossRef]
- Jansook, P.; Maw, P.D.; Soe, H.M.S.H.; Chuangchunsong, R.; Saiborisuth, K.; Payonitikarn, N.; Autthateinchai, R.; Pruksakorn, P. Development of amphotericin B nanosuspensions for fungal keratitis therapy: Effect of self-assembled γ-cyclodextrin. J. Pharm. Investig. 2020, 1–13. [Google Scholar] [CrossRef]
- Pubchem. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 30 July 2020).
- Chang, Y.; Wang, Y.-H.; Hu, C.-Q. Simultaneous determination of purity and potency of amphotericin B by HPLC. J. Antibiot. 2011, 64, 735–739. [Google Scholar] [CrossRef]
- Hubert, P.; Nguyen-Huu, J.-J.; Boulanger, B.; Chapuzet, E.; Chiap, P.; Cohen, N.; Compagnon, P.-A.; Dewé, W.; Feinberg, M.; Lallier, M.; et al. Harmonization of strategies for the validation of quantitative analytical procedures: A SFSTP proposal—Part I. J. Pharm. Biomed. Anal. 2004, 36, 579–586. [Google Scholar] [CrossRef]
- Hubert, P.; Nguyen-Huu, J.-J.; Boulanger, B.; Chapuzet, E.; Chiap, P.; Cohen, N.; Compagnon, P.-A.; Dewé, W.; Feinberg, M.; Lallier, M.; et al. Harmonization of strategies for the validation of quantitative analytical procedures: A SFSTP proposal—Part II. J. Pharm. Biomed. Anal. 2007, 45, 70–81. [Google Scholar] [CrossRef]
- Hubert, P.; Nguyen-Huu, J.-J.; Boulanger, B.; Chapuzet, E.; Cohen, N.; Compagnon, P.-A.; Dewé, W.; Feinberg, M.; Laurentie, M.; Mercier, N.; et al. Harmonization of strategies for the validation of quantitative analytical procedures: A SFSTP proposal—Part III. J. Pharm. Biomed. Anal. 2007, 45, 82–96. [Google Scholar] [CrossRef]
- EDQM. Amphotericin B monography. In European Pharmacopeia, 10.2 ed.; EDQM, European Pharmacopoeia: Strasbourg, France, 2020. [Google Scholar]
- International Conference of Harmonization (ICH) Quality Guidelines: ICH. Guidelines for Stability Q1A to Q1f. Available online: http://www.ich.org/products/guidelines/%20quality/article/quality-guidelines.html (accessed on 18 June 2020).
- French Society of Clinical Pharmacy (SFPC); Evaluation and Research Group on Protection in Controlled Atmospher (GERPAC). Methodological Guidelines for Stability Studies of Hospital Pharmaceutical Preparations; Paris, France. 2013. Available online: https://www.gerpac.eu/IMG/pdf/guide_stabilite_anglais.pdf (accessed on 19 August 2020).
- Montenegro, M.B.; Souza, S.P.; Leão, R.A.C.; Rocha, H.V.A.; Rezende, C.M.; Souza, R.O.M.A. Methodology Development and Validation of Amphotericin B Stability by HPLC-DAD. J. Braz. Chem. Soc. 2020, 31, 916–926. [Google Scholar] [CrossRef]
- Teresa, L.-F.M.; Ferreira, V.F.N.; Adelaide, F.-A.; Schreier, S. Effect of Aggregation on the Kinetics of Autoxidation of the Polyene Antibiotic Amphotericin B. J. Pharm. Sci. 1993, 82, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, R.; Wasan, K.M.; Bizzotto, D. Fluorescence of Amphotericin B-Deoxycholate (Fungizone) Monomers and Aggregates and the Effect of Heat-Treatment. Langmuir 2007, 23, 8718–8725. [Google Scholar] [CrossRef]
- Sawangchan, P. The Effect of Aggregation State on the Degradation Kinetics of Amphotericin B in Aqueous Solution. Ph.D. Thesis, University of Iowa, Iowa City, IA, USA, 2017. [Google Scholar] [CrossRef] [Green Version]
- Hamilton-Miller, J.M.T. The effect of pH and of temperature on the stability and bioactivity of nystatin and amphotericin B. J. Pharm. Pharmacol. 1973, 25, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Beggs, W.H. Kinetics of amphotericin B decay in a liquid medium and characterization of the decay process. Curr. Microbiol. 1978, 1, 301–304. [Google Scholar] [CrossRef]
- Osaka, K.; Ritov, V.B.; Bernardo, J.F.; Branch, R.A.; Kagan, V.E. Amphotericin B protects cis-parinaric acid against peroxyl radical-induced oxidation: Amphotericin B as an antioxidant. Antimicrob. Agents Chemother. 1997, 41, 743–747. [Google Scholar] [CrossRef] [Green Version]
- Brajtburg, J.; Powderly, W.G.; Kobayashi, G.S.; Medoff, G. Amphotericin B: Current understanding of mechanisms of action. Antimicrob. Agents Chemother. 1990, 34, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Barwicz, J.; Gruda, I.; Tancrède, P. A kinetic study of the oxidation effects of amphotericin B on human low-density lipoproteins. FEBS Lett. 2000, 465, 83–86. [Google Scholar] [CrossRef] [Green Version]
- Belhachemi, M.H.; Boucherit, K.; Boucherit-Otmani, Z.; Belmir, S.; Benbekhti, Z. Effects of ascorbic acid and α-tocopherol on the therapeutic index of amphotericin B. J. Mycol. Méd. 2014, 24, e137–e142. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Kitagawa, Y.; Okumura, M.; Shigeta, Y. Accurate Standard Hydrogen Electrode Potential and Applications to the Redox Potentials of Vitamin C and NAD/NADH. J. Phys. Chem. A 2015, 119, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, P.; Cooksy, A. Novel, unifying mechanism for amphotericin B and other polyene drugs: Electron affinity, radicals, electron transfer, autoxidation, toxicity, and antifungal action. MedChemComm 2012, 3, 274–280. [Google Scholar] [CrossRef]
- Fustier, P.; St-Germain, F.; Lamarche, F.; Mondor, M. Non-enzymatic browning and ascorbic acid degradation of orange juice subjected to electroreduction and electro-oxidation treatments. Innov. Food Sci. Emerg. Technol. 2011, 12, 491–498. [Google Scholar] [CrossRef]
- European Pharmacopoeia. Degree of Coloration of Liquids; European Directorate for the Quality of Medecines and Healthcare: Strasbourg, France, 2020; Available online: https://www.drugfuture.com/Pharmacopoeia/EP7/DATA/20202E.PDF (accessed on 6 July 2020).
- United States Pharmacopeia USP <1061> Color-Instrumental Measurement, USP 43-NF38 2018; United States Pharmacopeia: New York, NY, USA, 2018; Available online: https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/usp-nf-commentary/usp-43-nf-38-index.pdf (accessed on 6 July 2020).
- European Pharmacopoeia Revises General Chapter on Degree of Coloration of Liquids|EDQ-European Directorate for the Quality of Medicines. Available online: https://www.edqm.eu/en/news/european-pharmacopoeia-revises-general-chapter-degree-coloration-liquids (accessed on 6 July 2020).
- Morand, K.; Bartoletti, A.C.; Bochot, A.; Barratt, G.; Brandely, M.L.; Chast, F. Liposomal amphotericin B eye drops to treat fungal keratitis: Physico-chemical and formulation stability. Int. J. Pharm. 2007, 344, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Chennell, P.; Delaborde, L.; Wasiak, M.; Jouannet, M.; Feschet-Chassot, E.; Chiambaretta, F.; Sautou, V. Stability of an ophthalmic micellar formulation of cyclosporine A in unopened multidose eyedroppers and in simulated use conditions. Eur. J. Pharm. Sci. 2017, 100, 230–237. [Google Scholar] [CrossRef]
- Ghiglioni, D.G.; Martino, P.A.; Bruschi, G.; Vitali, D.; Osnaghi, S.; Corti, M.G.; Beretta, G. Stability and Safety Traits of Novel Cyclosporine A and Tacrolimus Ophthalmic Galenic Formulations Involved in Vernal Keratoconjunctivitis Treatment by a High-Resolution Mass Spectrometry Approach. Pharmaceutics 2020, 12, 378. [Google Scholar] [CrossRef]






| Amphotericin B (Base) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | RRT Mean | Reference | 60 °C | HCl 1 h Contact | NaOH 1 h Contact | H2O2 10% | H2O2 30% | With UV | ||||||||||
| 1 h | 2 h | 4 h | 0.1 N | 0.5 N | 1 N | 0.1 N | 0.5 N | 1 N | 1 h | 2 h | 1 h | 2 h | 24 h | 48 h | 115 h | |||
| BP1 | 0.07 | 0.1% | ||||||||||||||||
| BP6 | 0.17 | 0.2% | 0.1% | |||||||||||||||
| BP7 | 0.23 | 0.1% | 0.1% | |||||||||||||||
| BP8 | 0.29 | 0.5% | 0.3% | 0.2% | 0.2% | 0.5% | 0.9% | 0.1% | 0.4% | 0.5% | 0.3% | 0.2% | 0.1% | |||||
| BP9 | 0.37 | 0.1% | 0.2% | 0.1% | 0.1% | 0.6% | 0.8% | |||||||||||
| BP10 | 0.49 | 0.1% | 0.2% | 0.2% | 0.1% | 0.3% | 0.1% | |||||||||||
| BP11 | 0.54 | 0.2% | 0.3% | 0.3% | 0.1% | 0.1% | 0.0% | |||||||||||
| BP12 | 0.59 | 0.9% | 0.6% | 0.6% | 0.6% | 0.7% | 0.2% | 0.1% | 0.1% | |||||||||
| BP13 | 0.70 | 0.6% | 0.8% | 0.3% | 0.2% | 0.5% | 0.1% | 1.1% | 2.3% | 11.3% | 17.5% | 0.1% | ||||||
| BP14 | 0.77 | 0.1% | 0.4% | 0.8% | 2.5% | 0.2% | 0.1% | 2.5% | 2.1% | 1.1% | 0.6% | |||||||
| BP15 | 0.85 | 0.3% | 0.3% | 0.8% | 0.6% | 0.2% | ||||||||||||
| Amphotericin B | 100.0% | 97.5% | 96.4% | 94.6% | 30.1% | 14.8% | 1.3% | 5.3% | 0.9% | 0.3% | 91.9% | 91.6% | 81.3% | 74.9% | 5.2% | 0.8% | 0.0% | |
| BP16 | 1.13 | 1.1% | 1.2% | 0.3% | 0.8% | |||||||||||||
| BP17 | 2.21 | 0.9% | 0.7% | 0.9% | ||||||||||||||
| BP18 | 2.59 | 1.1% | 0.8% | 1.4% | ||||||||||||||
| Amphotericin B (Deoxycholate) | ||||||||||||||||||
| Compound | RRT Mean | Reference | 60 °C | HCl 1 h Contact | NaOH 1 h Contact | H2O2 10% | H2O2 30% | With UV | ||||||||||
| 1h | 2h | 4h | 0.1 N | 0.5 N | 1 N | 0.1 N | 0.5 N | 1 N | 1 h | 2 h | 1 h | 2 h | 24 h | 48 h | 115 h | |||
| BP1 | 0.07 | 0.5% | 0.5% | 0.2% | ||||||||||||||
| BP6 | 0.17 | 0.1% | 0.1% | 0.1% | 5.8% | 46.6% | 43.4% | 0.1% | ||||||||||
| BP7 | 0.23 | 0.1% | 0.1% | 0.1% | 0.3% | 0.3% | ||||||||||||
| BP8 | 0.29 | 0.5% | 0.2% | 0.2% | 0.2% | 0.2% | 1.5% | 1.9% | 0.1% | 0.2% | 0.2% | 0.4% | 0.4% | 0.1% | ||||
| BP9 | 0.37 | 0.1% | 0.1% | 0.1% | 0.4% | 0.7% | ||||||||||||
| BP10 | 0.49 | 0.2% | 0.2% | 0.3% | ||||||||||||||
| BP11 | 0.54 | 0.3% | 0.4% | 0.5% | 0.1% | |||||||||||||
| BP12 | 0.59 | 0.2% | 0.5% | 0.6% | 0.6% | 0.4% | 0.2% | 0.3% | ||||||||||
| BP13 | 0.70 | 0.7% | 0.7% | 0.7% | 0.6% | 0.3% | 0.6% | 0.2% | 1.3% | 2.1% | 9.9% | 15.8% | 0.6% | |||||
| BP14 | 0.77 | 6.0% | 4.6% | 4.1% | 3.6% | 1.7% | 3.9% | 7.6% | 8.4% | 0.2% | 1.0% | 1.0% | 0.6% | 0.3% | 5.3% | 2.6% | 0.7% | |
| BP15 | 0.85 | 0.2% | 0.2% | 0.3% | 0.3% | 0.3% | 0.2% | 0.3% | 0.3% | 0.3% | ||||||||
| Amphotericin B | 100.0% | 97.7% | 97.3% | 96.0% | 45.2% | 36.1% | 30.9% | 33.8% | 0.9% | 0.0% | 93.6% | 93.1% | 85.8% | 79.3% | 6.5% | 1.8% | 0.0% | |
| BP16 | 1.13 | 0.9% | 0.2% | 1.0% | 1.0% | 0.6% | 0.8% | |||||||||||
| BP17 | 2.21 | 0.7% | 0.6% | 0.5% | ||||||||||||||
| BP18 | 2.59 | 1.4% | 0.9% | 0.9% | ||||||||||||||
| Storage Time (Days) | Amphotericin B Deoxycholate | Amphotericin B 2-Hydroxypropyl-γ-Cyclodextrin | ||||||
|---|---|---|---|---|---|---|---|---|
| 5 °C Storage | 25 °C Storage | 5°C Storage | 25°C Storage | |||||
| pH | Osmolality | pH | Osmolality | pH | Osmolality | pH | Osmolality | |
| 0 | 7.64 ± 0.02 | 320 ± 1 | 7.64 ± 0.02 | 320 ± 1 | 7.14 ± 0.02 | 465 ± 11 | 7.14 ± 0.02 | 465 ± 11 |
| 3 | 7.65 ± 0.00 | 323 ± 1 | 7.60 ± 0.05 | 320 ± 1 | 7.12 ± 0.01 | 458 ± 4 | 7.14 ± 0.02 | 463 ± 9 |
| 7 | 7.63 ± 0.01 | 320 ± 2 | 7.59 ± 0.00 | 321 ± 3 | 7.00 ± 0.00 | 457 ± 3 | 6.99 ± 0.00 | 458 ± 9 |
| 14 | 7.61 ± 0.01 | 321 ± 1 | 7.51 ± 0.01 | 320 ± 0 | 7.08 ± 0.01 | 471 ± 9 | 7.06 ± 0.02 | 457 ± 7 |
| 28 | 7.57 ± 0.00 | 322 ± 3 | 7.42 ± 0.02 | 320 ± 1 | 7.06 ± 0.00 | 456 ± 12 | 7.04 ± 0.00 | 463 ± 6 |
| 56 | 7.55 ± 0.01 | 325 ± 3 | 7.35 ± 0.00 | 326 ± 1 | 7.06 ± 0.01 | 466 ± 9 | 7.02 ± 0.00 | 465 ± 2 |
| 168 | 7.44 ± 0.01 | 323 ± 3 | 7.02 ± 0.01 | 327 ± 1 | 7.02 ± 0.00 | 468 ± 27 | 6.93 ± 0.01 | 468 ± 13 |
| 350 | 7.25 ± 0.01 | 335 ± 1 | 6.71 ± 0.01 | 330 ± 4 | 6.96 ± 0.00 | 492 ± 31 | 6.81 ± 0.00 | 478 ± 60 |
| Turbidity (FNU) * | pH | Osmolality (mOsmol/kg) | Concentration (mg/mL for Day 0 Then % of Day 0 Concentrations) | ||
|---|---|---|---|---|---|
| Before storage | Day 0 | 3.19 | 7.08 ± 0.00 | 423 ± 5 | 2.47 ± 0.05 |
| Storage at 5 °C | Day 7 | 3.20 | 7.09 ± 0.03 | 452 ± 4 | 96.54 ± 3.10 |
| Day 14 | 3.14 | 6.95 ± 0.02 | 449 ± 22 | 90.09 ± 2.31 | |
| Day 28 | 3.39 | 6.79 ± 0.01 | 450 ± 11 | 83.93 ± 1.21 | |
| Storage at 25 °C | Day 7 | 3.19 | 6.84 ± 0.00 | 453 ± 12 | 85.08 ± 1.58 |
| Day 14 | 3.23 | 6.64 ± 0.02 | 479 ± 4 | 72.41 ± 3.81 | |
| Day 28 | 3.32 | 6.38 ± 0.06 | 468 ± 7 | 55.32 ± 8.96 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chennell, P.; Yessaad, M.; Abd El Kader, F.; Jouannet, M.; Wasiak, M.; Bouattour, Y.; Sautou, V. Do Ophthalmic Solutions of Amphotericin B Solubilised in 2-Hydroxypropyl-?-Cyclodextrins Possess an Extended Physicochemical Stability? Pharmaceutics 2020, 12, 786. https://doi.org/10.3390/pharmaceutics12090786
Chennell P, Yessaad M, Abd El Kader F, Jouannet M, Wasiak M, Bouattour Y, Sautou V. Do Ophthalmic Solutions of Amphotericin B Solubilised in 2-Hydroxypropyl-?-Cyclodextrins Possess an Extended Physicochemical Stability? Pharmaceutics. 2020; 12(9):786. https://doi.org/10.3390/pharmaceutics12090786
Chicago/Turabian StyleChennell, Philip, Mouloud Yessaad, Florence Abd El Kader, Mireille Jouannet, Mathieu Wasiak, Yassine Bouattour, and Valérie Sautou. 2020. "Do Ophthalmic Solutions of Amphotericin B Solubilised in 2-Hydroxypropyl-?-Cyclodextrins Possess an Extended Physicochemical Stability?" Pharmaceutics 12, no. 9: 786. https://doi.org/10.3390/pharmaceutics12090786
APA StyleChennell, P., Yessaad, M., Abd El Kader, F., Jouannet, M., Wasiak, M., Bouattour, Y., & Sautou, V. (2020). Do Ophthalmic Solutions of Amphotericin B Solubilised in 2-Hydroxypropyl-?-Cyclodextrins Possess an Extended Physicochemical Stability? Pharmaceutics, 12(9), 786. https://doi.org/10.3390/pharmaceutics12090786