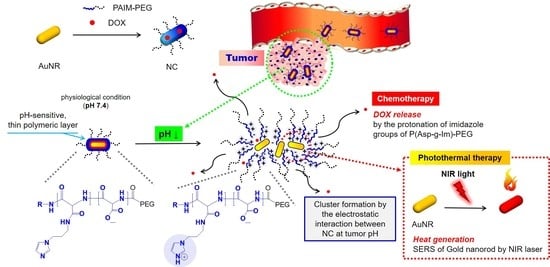
An On-Demand pH-Sensitive Nanocluster for Cancer Treatment by Combining Photothermal Therapy and Chemotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PAIM-PEG, a pH-Sensitive Polymer
2.3. Preparation of Nanocluster (NC) Systems
2.4. Measurement of DOX and Gold Concentration
2.5. Absorption Spectra
2.6. Particle Size and Zeta Measurement by Dynamic Light Scattering (DLS)
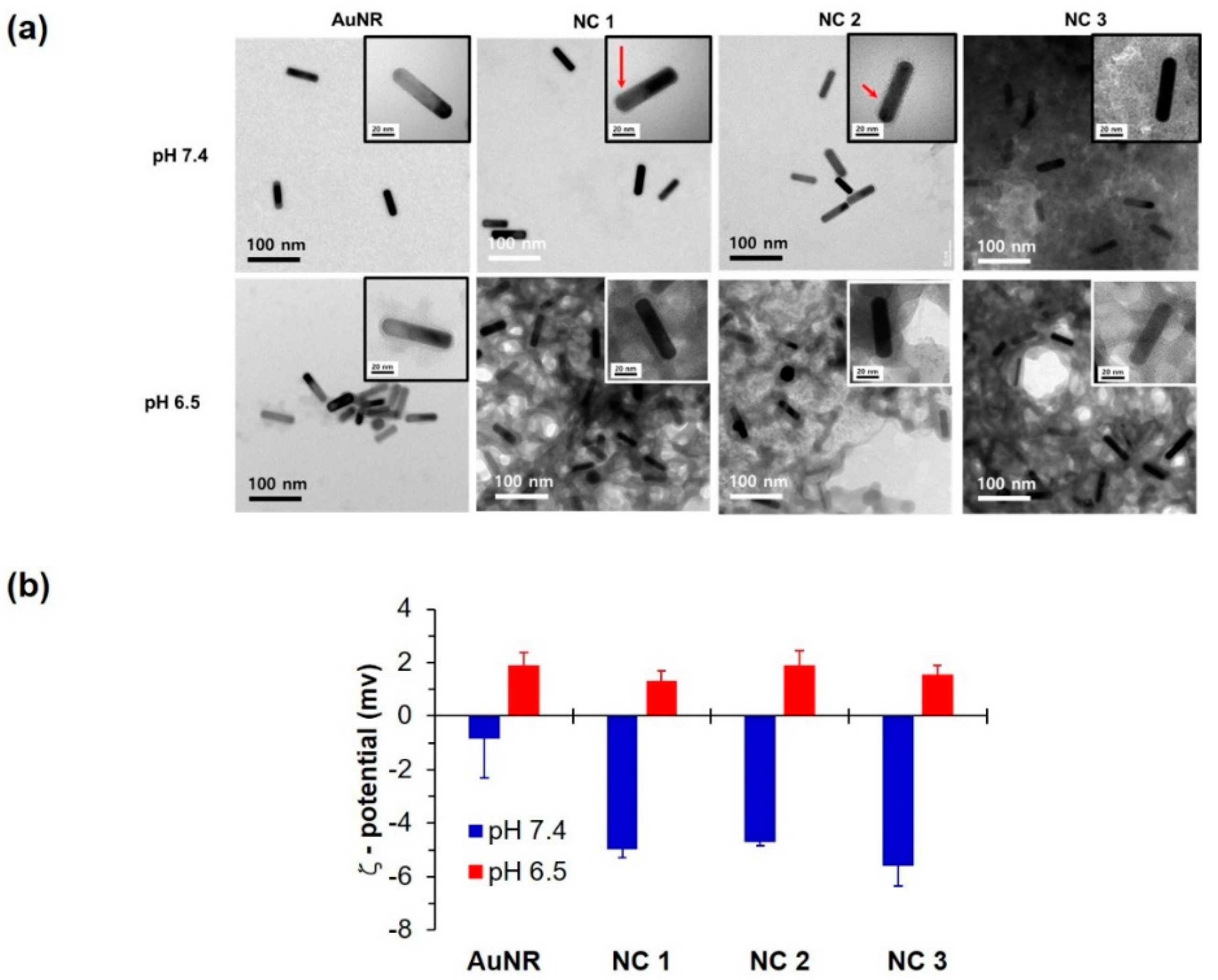
2.7. Morphology of NC Systems
2.8. DOX Release Profile of NC Systems
2.9. Photothermal Effect
2.10. In Vitro Anti-Cancer Effect
2.11. Cellular Uptake
2.12. Animal Care
2.13. Biodistribution of NC Systems
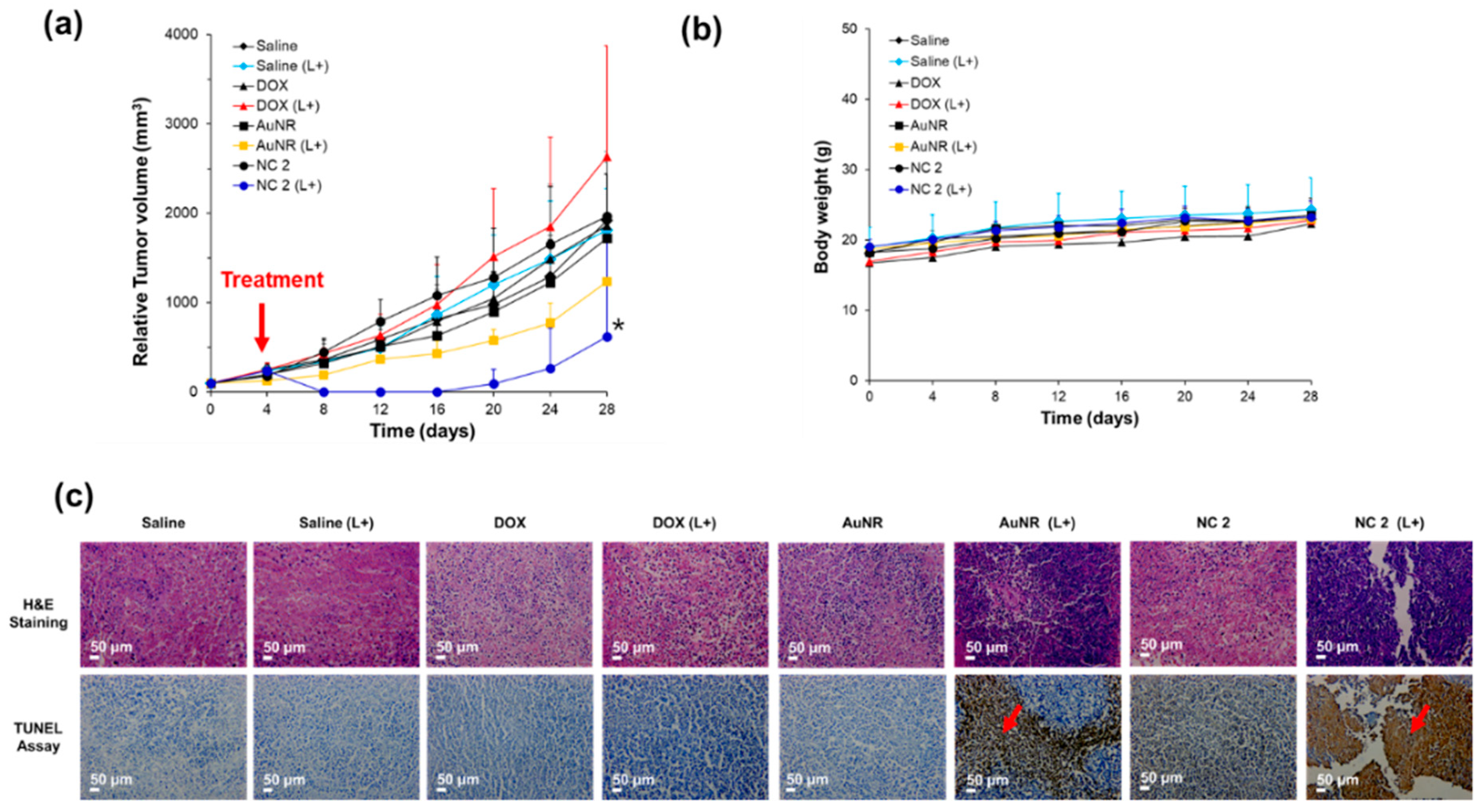
2.14. In Vivo Study: Anti-Cancer Efficacy, Toxicity, and Histological Analysis
3. Results and Discussion
3.1. Preparation and Optimization of NC Systems
3.2. NC Characterization
3.3. In Vitro Study for Cytotoxicity and Cellular Uptake
3.4. In Vivo Evaluation of NC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liao, J.; Li, W.; Peng, J.; Yang, Q.; Li, H.; Wei, Y.; Zhang, X.; Qian, Z. Combined cancer photothermal-chemotherapy based on doxorubicin/gold nanorod-loaded polymersomes. Theranostics 2015, 5, 345. [Google Scholar] [CrossRef]
- Hou, G.; Qian, J.; Xu, W.; Sun, T.; Wang, Y.; Wang, J.; Ji, L.; Suo, A. A novel pH-sensitive targeting polysaccharide-gold nanorod conjugate for combined photothermal-chemotherapy of breast cancer. Carbohydr. Polym. 2019, 212, 334–344. [Google Scholar] [CrossRef]
- Zeng, J.Y.; Zhang, M.K.; Peng, M.Y.; Gong, D.; Zhang, X.Z. Porphyrinic metal–organic frameworks coated gold nanorods as a versatile nanoplatform for combined photodynamic/photothermal/chemotherapy of tumor. Adv. Funct. Mater. 2018, 28, 1705451. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Sun, Y.; Wang, L.; Ding, L.; Zhu, W.-H.; Di, W.; Duan, Y.-R. Gold-caged copolymer nanoparticles as multimodal synergistic photodynamic/photothermal/chemotherapy platform against lethality androgen-resistant prostate cancer. Biomaterials 2019, 212, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Yung, B.C.; Zhou, Z.; Mao, Z.; Chen, X. Artificial molecular machines in nanotheranostics. ACS Nano 2017, 12, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Yung, B.; Huang, P.; Chen, X. Nanotechnology for multimodal synergistic cancer therapy. Chem. Rev. 2017, 117, 13566–13638. [Google Scholar] [CrossRef]
- Yu, G.; Yang, Z.; Fu, X.; Yung, B.C.; Yang, J.; Mao, Z.; Shao, L.; Hua, B.; Liu, Y.; Zhang, F. Polyrotaxane-based supramolecular theranostics. Nat. Commun. 2018, 9, 766. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, M.; Saha, M.L.; Mao, Z.; Chen, J.; Yao, Y.; Zhou, Z.; Liu, Y.; Gao, C.; Huang, F. Antitumor activity of a unique polymer that incorporates a fluorescent self-assembled metallacycle. J. Am. Chem. Soc. 2017, 139, 15940–15949. [Google Scholar] [CrossRef]
- Lee, C.; Hwang, H.S.; Lee, S.; Kim, B.; Kim, J.O.; Oh, K.T.; Lee, E.S.; Choi, H.-G.; Youn, Y.S. Rabies virus-inspired silica-coated gold nanorods as a photothermal therapeutic platform for treating brain tumors. Adv. Mater. 2017, 29, 1605563. [Google Scholar] [CrossRef]
- Lim, C.; Moon, J.; Sim, T.; Won, W.R.; Lee, E.S.; Youn, Y.S.; Oh, K.T. A nano-complex system to overcome antagonistic photo-chemo combination cancer therapy. J. Control. Release 2019, 295, 164–173. [Google Scholar] [CrossRef]
- Yoo, E.; Choi, J.H.; Hoang, N.H.; Lee, J.S.; Vuong, S.; Hur, B.; Han, P.; Oh, K.T.; Fahmy, T.; Kim, D. Particle-in-Particle Platform for Nanoconfinement-Induced Oncothermia. ACS Appl. Bio Mater. 2018, 1, 1927–1941. [Google Scholar] [CrossRef]
- Welsher, K.; Sherlock, S.P.; Dai, H. Deep-tissue anatomical imaging of mice using carbon nanotube fluorophores in the second near-infrared window. Proc. Natl. Acad. Sci. USA 2011, 108, 8943–8948. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kim, S.; Nam, J.-M. Plasmonically engineered nanoprobes for biomedical applications. J. Am. Chem. Soc. 2016, 138, 14509–14525. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, C.; Feng, L.; Yang, K.; Liu, Z. Functional nanomaterials for phototherapies of cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Tran, T.T.P.; Jin, S.G.; Yong, C.S.; Truong, D.H.; Tran, T.H.; Kim, J.O. Combined hyperthermia and chemotherapy as a synergistic anticancer treatment. J. Pharm. Investig. 2019, 49, 519–526. [Google Scholar]
- Vankayala, R.; Lin, C.-C.; Kalluru, P.; Chiang, C.-S.; Hwang, K.C. Gold nanoshells-mediated bimodal photodynamic and photothermal cancer treatment using ultra-low doses of near infra-red light. Biomaterials 2014, 35, 5527–5538. [Google Scholar] [CrossRef]
- Parchur, A.K.; Sharma, G.; Jagtap, J.M.; Gogineni, V.R.; LaViolette, P.S.; Flister, M.J.; White, S.B.; Joshi, A. Vascular interventional radiology-guided photothermal therapy of colorectal cancer liver metastasis with theranostic gold nanorods. ACS Nano 2018, 12, 6597–6611. [Google Scholar] [CrossRef]
- Wu, Y.; Ali, M.R.; Dong, B.; Han, T.; Chen, K.; Chen, J.; Tang, Y.; Fang, N.; Wang, F.; El-Sayed, M.A. Gold nanorod photothermal therapy alters cell junctions and actin network in inhibiting cancer cell collective migration. ACS Nano 2018, 12, 9279–9290. [Google Scholar] [CrossRef]
- Ma, X.; Jin, Y.; Wang, Y.; Zhang, S.; Peng, D.; Yang, X.; Wei, S.; Chai, W.; Li, X.; Tian, J. Multimodality molecular imaging-guided tumor border delineation and photothermal therapy analysis based on graphene oxide-conjugated gold nanoparticles chelated with Gd. Contrast Media Mol. Imaging 2018. [Google Scholar] [CrossRef]
- Grabinski, C.; Schaeublin, N.; Wijaya, A.; D’Couto, H.; Baxamusa, S.H.; Hamad-Schifferli, K.; Hussain, S.M. Effect of gold nanorod surface chemistry on cellular response. ACS Nano 2011, 5, 2870–2879. [Google Scholar] [CrossRef]
- Sheng, G.; Chen, Y.; Han, L.; Huang, Y.; Liu, X.; Li, L.; Mao, Z. Encapsulation of indocyanine green into cell membrane capsules for photothermal cancer therapy. Acta Biomater. 2016, 43, 251–261. [Google Scholar] [CrossRef]
- Mirhadi, E.; Nassirli, H.; Malaekeh-Nikouei, B. An updated review on therapeutic effects of nanoparticle-based formulations of saffron components (safranal, crocin, and crocetin). J. Pharm. Investig. 2019, 50, 47–58. [Google Scholar] [CrossRef]
- Hoffman, A.S. “Intelligent” polymers in medicine and biotechnology. Artif. Organs 1995, 19, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef]
- Masood, F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater. Sci. Eng. C 2016, 60, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in biomaterials for drug delivery. Adv. Mater. 2018, 30, 1705328. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Cobo, I.; Li, M.; Sumerlin, B.S.; Perrier, S. Smart hybrid materials by conjugation of responsive polymers to biomacromolecules. Nat. Mater. 2015, 14, 143. [Google Scholar] [CrossRef]
- Roth, P.J.; Lowe, A.B. Stimulus-responsive polymers. Polym. Chem. 2017, 8, 10–11. [Google Scholar] [CrossRef]
- Taghizadeh, B.; Taranejoo, S.; Monemian, S.A.; Salehi Moghaddam, Z.; Daliri, K.; Derakhshankhah, H.; Derakhshani, Z. Classification of stimuli–responsive polymers as anticancer drug delivery systems. Drug Deliv. 2015, 22, 145–155. [Google Scholar] [CrossRef]
- Lim, E.-K.; Chung, B.H.; Chung, S.J. Recent advances in ph-sensitive polymeric nanoparticles for smart drug delivery in cancer therapy. J. Pharm. Investig. 2018, 19, 300–317. [Google Scholar] [CrossRef] [PubMed]
- Sim, T.; Lim, C.; Hoang, N.H.; Oh, K.T. Recent advance of pH-sensitive nanocarriers targeting solid tumors. J. Pharm. Investig. 2017. [Google Scholar] [CrossRef]
- Ma, X.; Williams, R.O. Polymeric nanomedicines for poorly soluble drugs in oral delivery systems: An update. J. Pharm. Investig. 2018, 48, 61–75. [Google Scholar] [CrossRef]
- Choi, Y.H.; Han, H.-K. Nanomedicines: Current status and future perspectives in aspect of drug delivery and pharmacokinetics. J. Pharm. Investig. 2018, 48, 1–18. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, Y.J.; Kim, D. Image-guided nanomedicine for cancer. J. Pharm. Investig. 2017, 47, 51–64. [Google Scholar] [CrossRef]
- Priya, B.; Viness, P.; Yahya, E.C.; Lisa, C.d.T. Stimuli-responsive polymers and their applications in drug delivery. Biomed. Mater. 2009, 4, 022001. [Google Scholar]
- Lim, C.; Youn, Y.S.; Lee, K.S.; Hoang, N.H.; Sim, T.; Lee, E.S.; Oh, K.T. Development of a robust pH-sensitive polyelectrolyte ionomer complex for anticancer nanocarriers. Int. J. Nanomed. 2016, 11, 703. [Google Scholar]
- Sim, T.; Park, G.; Min, H.; Kang, S.; Lim, C.; Bae, S.; Lee, E.S.; Youn, Y.S.; Oh, K.T. Development of a gene carrier using a triblock co-polyelectrolyte with poly(ethylene imine)-poly(lactic acid)-poly(ethylene glycol). J. Bioact. Compat. Polym. 2016, 32, 280–292. [Google Scholar] [CrossRef]
- Song, H.-T.; Hoang, N.H.; Yun, J.M.; Park, Y.J.; Song, E.H.; Lee, E.S.; Youn, Y.S.; Oh, K.T. Development of a new tri-block copolymer with a functional end and its feasibility for treatment of metastatic breast cancer. Colloids Surf. B Biointerfaces 2016, 144, 73–80. [Google Scholar] [CrossRef]
- Lim, C.; Sim, T.; Hoang, N.H.; Oh, K.T. A stable nanoplatform for antitumor activity using PEG-PLL-PLA triblock co-polyelectrolyte. Colloids Surf. B: Biointerfaces 2017, 153, 10–18. [Google Scholar] [CrossRef]
- Tran, P.; Lee, S.-E.; Kim, D.-H.; Pyo, Y.-C.; Park, J.-S. Recent advances of nanotechnology for the delivery of anticancer drugs for breast cancer treatment. J. Pharm. Investig. 2019, 50, 1–10. [Google Scholar] [CrossRef]
- Lee, E.S.; Gao, Z.; Kim, D.; Park, K.; Kwon, I.C.; Bae, Y.H. Super pH-sensitive multifunctional polymeric micelle for tumor pHe specific TAT exposure and multidrug resistance. J. Control. Release 2008, 129, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Oh, Y.T.; Lee, K.S.; Yun, J.M.; Park, B.T.; Oh, K.T. Development of a pH-sensitive polymer using poly(aspartic acid-graft-imidazole)-block-poly(ethylene glycol) for acidic pH targeting systems. Macromol. Res. 2011, 19, 453–460. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, J.; Bi, Y.; Xu, X.; Zhou, H.; Gao, J.; Hu, Y.; Zhao, Y.; Chai, Z. Near-infrared light remote-controlled intracellular anti-cancer drug delivery using thermo/pH sensitive nanovehicle. Acta Biomater. 2015, 17, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Fan, X.; Li, L. pH-sensitive polymeric micelles formed by doxorubicin conjugated prodrugs for co-delivery of doxorubicin and paclitaxel. Carbohydr. Polym. 2016, 137, 19–29. [Google Scholar] [CrossRef]
- Lee, E.-S.; Kim, J.-H.; Yun, J.-M.; Lee, K.-S.; Park, G.-Y.; Lee, B.-J.; Oh, K.-T. Functional polymers for drug delivery systems in nanomedicines. J. Pharm. Investig. 2010, 40, 45–61. [Google Scholar] [CrossRef]
- Sim, T.; Lim, C.; Cho, Y.H.; Lee, E.S.; Youn, Y.S.; Oh, K.T. Development of pH-sensitive nanogels for cancer treatment using crosslinked poly(aspartic acid-graft-imidazole)-block-poly(ethylene glycol). J. Appl. Polym. Sci. 2018, 135, 46268. [Google Scholar] [CrossRef]
- Sim, T.; Lim, C.; Hoang, N.H.; Kim, J.E.; Lee, E.S.; Youn, Y.S.; Oh, K.T. Synergistic photodynamic therapeutic effect of indole-3-acetic acid using a pH sensitive nano-carrier based on poly(aspartic acid-graft-imidazole)-poly(ethylene glycol). J. Mater. Chem. B 2017, 5, 8498–8505. [Google Scholar] [CrossRef]
- Lee, E.S.; Kim, J.H.; Sim, T.; Youn, Y.S.; Lee, B.-J.; Oh, Y.T.; Oh, K.T. A feasibility study of a pH sensitive nanomedicine using doxorubicin loaded poly(aspartic acid-graft-imidazole)-block-poly(ethylene glycol) micelles. J. Mater. Chem. 2014, 2, 1152–1159. [Google Scholar] [CrossRef]
- Kalia, J.; Raines, R.T. Hydrolytic stability of hydrazones and oximes. Angew. Chem. Int. Ed. 2008, 47, 7523–7526. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Zhao, X.; Wu, Q.; Zhu, H.; Mao, Z.; Gao, C. Doxorubicin-conjugated pH-responsive gold nanorods for combined photothermal therapy and chemotherapy of cancer. Bioact. Mater. 2018, 3, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.M.; Park, S.-y.; Lee, E.S.; Youn, Y.S.; Park, G.Y.; Lim, C.; Lee, B.-J.; Song, H.-T.; Oh, Y.T.; Oh, K.T. Physicochemical characterizations of amphiphilic block copolymers with different MWs and micelles for development of anticancer drug nanocarriers. Macromol. Res. 2012, 20, 944–953. [Google Scholar] [CrossRef]
- Kwag, D.S.; Oh, K.T.; Lee, E.S. Facile synthesis of multilayered polysaccharidic vesicles. J. Control. Release 2014, 187, 83–90. [Google Scholar] [CrossRef]
- Park, C.; Vo, C.L.-N.; Kang, T.; Oh, E.; Lee, B.-J. New method and characterization of self-assembled gelatin–oleic nanoparticles using a desolvation method via carbodiimide/N-hydroxysuccinimide (EDC/NHS) reaction. Eur. J. Pharm. Biopharm. 2015, 89, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.M.; Oh, K.T.; Youn, Y.S.; Lee, E.S. Artificial nano-pin as a temporal molecular glue for the targeting of acidic tumor cells. Polym. Adv. Technol. 2014, 25, 842–850. [Google Scholar] [CrossRef]
- Oh, N.M.; Oh, K.T.; Youn, Y.S.; Lee, D.K.; Cha, K.H.; Lee, D.H.; Lee, E.S. Poly(L-aspartic acid) nanogels for lysosome-selective antitumor drug delivery. Colloids Surf. B Biointerfaces 2013, 101, 298–306. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Das, A. Structure of indole imidazole heterodimer in a supersonic jet: A gas phase study on the interaction between the aromatic side chains of tryptophan and histidine residues in proteins. J. Phys. Chem. A 2012, 116, 11573–11580. [Google Scholar] [CrossRef]
- Lee, E.S.; Na, K.; Bae, Y.H. Polymeric micelle for tumor pH and folate-mediated targeting. J. Control. Release 2003, 91, 103–113. [Google Scholar] [CrossRef]
- Kim, G.M.; Bae, Y.H.; Jo, W.H. pH-induced Micelle Formation of Poly(histidine-co-phenylalanine)-block-Poly(ethylene glycol) in Aqueous Media. Macromol. Biosci. 2005, 5, 1118–1124. [Google Scholar] [CrossRef]
- Lee, E.S.; Kim, D.; Youn, Y.S.; Oh, K.T.; Bae, Y.H. A virus-mimetic nanogel vehicle. Angew. Chem. Int. Ed. Engl. 2008, 47, 2418–2421. [Google Scholar] [CrossRef] [PubMed]
- Rajendrakumar, S.K.; Chang, N.-C.; Mohapatra, A.; Uthaman, S.; Lee, B.-I.; Tsai, W.-b.; Park, I.-K. A lipophilic ir-780 dye-encapsulated zwitterionic polymer-lipid micellar nanoparticle for enhanced photothermal therapy and nir-based fluorescence imaging in a cervical tumor mouse model. Int. J. Mol. Sci. 2018, 19, 1189. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fu, L.; Lin, W.; Zhang, W.; Pei, Q.; Zheng, X.; Liu, S.; Zhang, T.; Xie, Z. Vaginal delivery of mucus-penetrating organic nanoparticles for photothermal therapy against cervical intraepithelial neoplasia in mice. J. Mater. Chem. B 2019, 7, 4528–4537. [Google Scholar] [CrossRef]
- Patel, S.; Kim, J.; Herrera, M.; Mukherjee, A.; Kabanov, A.V.; Sahay, G. Brief update on endocytosis of nanomedicines. Adv. Drug Deliv. Rev. 2019, 144, 90–111. [Google Scholar] [CrossRef]
- Kim, D.; Lee, E.S.; Oh, K.T.; Gao, Z.G.; Bae, Y.H. Doxorubicin-Loaded Polymeric Micelle Overcomes Multidrug Resistance of Cancer by Double-Targeting Folate Receptor and Early Endosomal pH. Small 2008, 4, 2043–2050. [Google Scholar] [CrossRef]
- Kim, D.; Lee, E.S.; Park, K.; Kwon, I.C.; Bae, Y.H. Doxorubicin loaded pH-sensitive micelle: Antitumoral efficacy against ovarian A2780/DOXR tumor. Pharm. Res. 2008, 25, 2074–2082. [Google Scholar] [CrossRef]
- Darrigues, E.; Nima, Z.A.; Nedosekin, D.A.; Watanabe, F.; Alghazali, K.M.; Zharov, V.P.; Biris, A.S. Tracking Gold Nanorods’ Interaction with Large 3D Pancreatic-Stromal Tumor Spheroids by Multimodal Imaging: Fluorescence, Photoacoustic, and Photothermal Microscopies. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Jin, S.; Ma, X.; Ma, H.; Zheng, K.; Liu, J.; Hou, S.; Meng, J.; Wang, P.C.; Wu, X.; Liang, X.-J. Surface chemistry-mediated penetration and gold nanorod thermotherapy in multicellular tumor spheroids. Nanoscale 2013, 5, 143–146. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, L.; Zhu, X.; Chen, J.; Zhao, M.; Li, S.; Yu, C.; Hu, L.; Qiao, H.; Guo, Z. Hyaluronic acid functionalized gold nanorods combined with copper-based therapeutic agents for chemo-photothermal cancer therapy. J. Mater. Chem. B 2020, 8, 4841–4845. [Google Scholar] [CrossRef]
- Bailly, A.-L.; Correard, F.; Popov, A.; Tselikov, G.; Chaspoul, F.; Appay, R.; Al-Kattan, A.; Kabashin, A.V.; Braguer, D.; Esteve, M.-A. In vivo evaluation of safety, biodistribution and pharmacokinetics of laser-synthesized gold nanoparticles. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]



| Materials | AuNR | DOX | * G/D/P Ratio | ||||
|---|---|---|---|---|---|---|---|
| Code | Target Content (wt %) | Loading Content (wt %) | Loading Efficiency (wt %) | Target Amount (wt %) | Loading Content (wt %) | Loading Efficiency (wt %) | |
| NC 1 | 50 | 32.9 | 65.8 | 25 | 23.9 | 95.5 | 2:1:4 |
| NC 2 | 25 | 22.6 | 90.3 | 25 | 24.5 | 98.1 | 1:1:4 |
| NC 3 | 12.5 | 11.2 | 89.5 | 25 | 22.7 | 90.6 | 0.5:1:4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, T.; Lim, C.; Hoang, N.H.; Shin, Y.; Kim, J.C.; Park, J.Y.; Her, J.; Lee, E.S.; Youn, Y.S.; Oh, K.T. An On-Demand pH-Sensitive Nanocluster for Cancer Treatment by Combining Photothermal Therapy and Chemotherapy. Pharmaceutics 2020, 12, 839. https://doi.org/10.3390/pharmaceutics12090839
Sim T, Lim C, Hoang NH, Shin Y, Kim JC, Park JY, Her J, Lee ES, Youn YS, Oh KT. An On-Demand pH-Sensitive Nanocluster for Cancer Treatment by Combining Photothermal Therapy and Chemotherapy. Pharmaceutics. 2020; 12(9):839. https://doi.org/10.3390/pharmaceutics12090839
Chicago/Turabian StyleSim, Taehoon, Chaemin Lim, Ngoc Ha Hoang, Yuseon Shin, Jae Chang Kim, June Yong Park, Jaewon Her, Eun Seong Lee, Yu Seok Youn, and Kyung Taek Oh. 2020. "An On-Demand pH-Sensitive Nanocluster for Cancer Treatment by Combining Photothermal Therapy and Chemotherapy" Pharmaceutics 12, no. 9: 839. https://doi.org/10.3390/pharmaceutics12090839
APA StyleSim, T., Lim, C., Hoang, N. H., Shin, Y., Kim, J. C., Park, J. Y., Her, J., Lee, E. S., Youn, Y. S., & Oh, K. T. (2020). An On-Demand pH-Sensitive Nanocluster for Cancer Treatment by Combining Photothermal Therapy and Chemotherapy. Pharmaceutics, 12(9), 839. https://doi.org/10.3390/pharmaceutics12090839