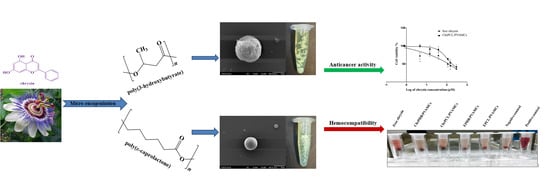
Evaluation of the Hemocompatibility and Anticancer Potential of Poly(ε-Caprolactone) and Poly(3-Hydroxybutyrate) Microcarriers with Encapsulated Chrysin
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
2.2.1. Fourier-Transform Infrared Spectrometry
2.2.2. Field Emission Scanning Electron Microscopy
2.2.3. Dynamic Light Scattering
2.2.4. Zeta-Potential
2.2.5. High Performance Liquid Chromatography
2.3. Synthesis of Chrysin-Loaded MCs
2.3.1. Synthesis of ChrPCL/PVAMCs
2.3.2. Synthesis of ChrPHB/PVAMCs
2.4. Determination of Chrysin Entrapment Efficiency and Loading Capacity
2.5. In Vitro Chrysin Release Study
2.6. Biological Evaluation
2.6.1. Cell Lines and Culture Conditions
2.6.2. Cell Viability of Human Breast Cancer Cell Line (MDA-MB-231)
2.7. Blood Sample Collection and Handling
2.7.1. Blood Profile Analysis
2.7.2. Hemocompatibility Studies
2.7.3. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of Chrysin-Loaded MCs
3.2. FT-IR Spectroscopy
3.3. FESEM Analyses
3.4. Particle Size Analysis And Z-Potential
3.5. Entrapment Efficiency and Loading Capacity
3.6. Release Study
3.7. Breast Cancer Cell Viability after Exposure to Chrysin-Loaded MCs
3.8. Effect of Chrysin-Loaded MCs on Blood Profile Analysis
3.9. Hemolysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GIT | Gastrointestinal tract |
| PHAs | Polyhydroxyalkanoates |
| PCLs | Poly(ε-caprolactones) |
| PHB | Poly(3-hydroxybutyric acid) |
| PLGA | Poly(lactide-co-glycolide) |
| PLA | Polylactate |
| PGA | Polyglycolate |
| PVA | Poly(vinyl alcohol) |
| Chr | Chrysin |
| MCs | Microcarriers |
| EPCL/PVAMCs | Empty PVA-stabilized PCL microcarriers |
| EPHB/PVAMCs | Empty PVA-stabilized PHB microcarriers |
| ChrPCL/PVAMCs | Chrysin-loaded PVA-stabilized PCL microcarriers |
| ChrPHB/PVAMCs | Chrysin-loaded PVA-stabilized PHB microcarriers |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| NaOH | Sodium hydroxide |
| PBS | Phosphate buffered saline |
| IMBB | Institute of Molecular Biology and Biotechnology |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| DMSO | Dimethyl sulfoxide |
| FT-IR | Fourier-transform infrared |
| FESEM | Field emission scanning electron microscopy |
| DLS | Dynamic Light Scattering |
| SD | Standard deviation |
| HPLC | High Performance Liquid Chromatography |
| DAD | Diode array detector |
| O/W | Oil-in-water |
| DMEM | Dulbecco′s Modified Eagle′s—Medium |
| FBS | Fetal Bovine Serum |
| RBCs | Red blood cells |
| HGB | Hemoglobin |
| HCT | Hematocrit |
| MCV | Mean corpuscular volume |
| fl | Femtoliters |
| MCH | Mean corpuscular hemoglobin |
| MCHC | Mean corpuscular hemoglobin concentration |
| RDW | Red cell distribution width |
| WBCs | White blood cells |
| NE | Neutrophils |
| LY | Lymphocytes |
| MO | Monocytes |
| EO | Eosinophils |
| BA | Basophils |
| PLTs | Platelets |
| EDTA | Ethylenediamine tetraacetic acid |
| TNBC | Triple-negative breast cancer |
| ER | Estrogen receptor |
| PR | Progesterone receptor |
| HER2 | Human epidermal growth factor receptor 2 |
| ASTM | American Society for Testing and Material |
References
- Shrivastav, A.; Kim, H.-Y.; Kim, Y.-R. Advances in the applications of polyhydroxyalkanoate nanoparticles for novel drug delivery system. Biomed. Res. Int. 2013, 2013, 581684. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.N.; Hemant, K.S.Y.; Ram, M.; Shivakumar, H.G. Microencapsulation: A promising technique for controlled drug delivery. Res. Pharm. Sci. 2010, 5, 65–77. [Google Scholar] [PubMed]
- Tomaro-Duchesneau, C.; Saha, S.; Malhotra, M.; Kahouli, I.; Prakash, S. Microencapsulation for the therapeutic delivery of drugs, live mammalian and bacterial cells, and other biopharmaceutics: Current status and future directions. J. Pharm. 2013, 2013, 103527. [Google Scholar] [CrossRef] [PubMed]
- Felder, C.B.; Blanco-Prieto, M.J.; Heizmann, J.; Merkle, H.P.; Gander, B. Ultrasonic atomization and subsequent polymer desolvation for peptide and protein microencapsulation into biodegradable polyesters. J. Microencapsul. 2003, 20, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Kiyoyama, S.; Shiomori, K.; Kawano, Y.; Hatate, Y. Preparation of microcapsules and control of their morphology. J. Microencapsul. 2003, 20, 497–508. [Google Scholar] [CrossRef]
- Sinha, V.R.; Trehan, A. Biodegradable microspheres for protein delivery. J. Control. Release 2003, 90, 261–280. [Google Scholar] [CrossRef]
- Sinha, V.R.; Goyal, V.; Bhinge, J.R.; Mittal, B.R.; Trehan, A. Diagnostic microspheres: An overview. Crit. Rev. Ther. Drug Carrier Syst. 2003, 20, 431–460. [Google Scholar] [CrossRef]
- Wang, J.; Chua, K.M.; Wang, C.H. Stabilization and encapsulation of human immunoglobulin G into biodegradable microspheres. J. Colloid Interface Sci. 2004, 271, 92–101. [Google Scholar] [CrossRef]
- Pekarek, K.J.; Jacob, J.S.; Mathiowitz, E. Double-walled polymer microspheres for controlled drug release. Nature 1994, 367, 258–260. [Google Scholar] [CrossRef]
- Jeong, B.; Bae, Y.H.; Lee, D.S.; Kim, S.W. Biodegradable block copolymers as injectable drug-delivery systems. Nature 1997, 388, 860–862. [Google Scholar] [CrossRef]
- Ulbrich, K.; Pechar, M.; Strohalm, J.; Subr, V.; Rihova, B. Synthesis of biodegradable polymers for controlled drug release. Ann. N. Y. Acad. Sci. 1997, 831, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, R.; Amiji, M. Chitosan-based gastrointestinal delivery systems. J. Control. Release 2003, 89, 151–165. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, J.; Mao, H.Q.; Leong, K.W. Polyphosphoesters in drug and gene delivery. Adv. Drug Deliv. Rev. 2003, 55, 483–499. [Google Scholar] [CrossRef]
- Zhang, L.; Schwendeman, S.P. Injectable biodegradable polymer depots for minimally invasive delivery Of peptides and proteins. Adv. Exp. Med. Biol. 2009, 611, 611–613. [Google Scholar] [PubMed] [Green Version]
- Park, J.H.; Ye, M.; Park, K. Biodegradable polymers for microencapsulation of drugs. Molecules 2005, 10, 146–161. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.H.; Lee, D.J. Slow release of drug through deformed coating film: Effects of morphology and drug diffusivity in the coating film. J. Pharm. Sci. 2001, 90, 1478–1496. [Google Scholar] [CrossRef]
- Tunon, A.; Grasjo, J.; Alderborn, G. Effect of intragranular porosity on compression behaviour of and drug release from reservoir pellets. Eur. J. Pharm. Sci. 2003, 19, 333–344. [Google Scholar] [CrossRef]
- Fulzele, S.V.; Satturwar, P.M.; Kasliwal, R.H.; Dorle, A.K. Preparation and evaluation of microcapsules using polymerized rosin as a novel wall forming material. J. Microencapsul. 2004, 21, 83–89. [Google Scholar] [CrossRef]
- Abraham, G.A.; Gallardo, A.; San Roman, J.; Fernandez-Mayoralas, A.; Zurita, M.; Vaquero, J. Polymeric matrices based on graft copolymers of PCL onto acrylic backbones for releasing antitumoral drugs. J. Biomed. Mater. Res. 2003, 64, 638–647. [Google Scholar] [CrossRef]
- Calandrelli, L.; De Rosa, G.; Errico, M.E.; La Rotonda, M.I.; Laurienzo, P.; Malinconico, M.; Oliva, A.; Quaglia, F. Novel graft PLLA-based copolymers: Potential of their application to particle technology. J Biomed. Mater. Res. 2002, 62, 244–253. [Google Scholar] [CrossRef]
- Cicek, H.; Tuncel, A.; Tuncel, M.; Piskin, E. Degradation and drug release characteristics of monosize polyethylcyanoacrylate microspheres. J. Biomater. Sci. Polym. Ed. 1995, 6, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Mi, F.L.; Lin, Y.M.; Wu, Y.B.; Shyu, S.S.; Tsai, Y.H. Chitin/PLGA blend microspheres as a biodegradable drug-delivery system: Phase-separation, degradation and release behavior. Biomaterials 2002, 23, 3257–3267. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, C.C. In vitro release behavior of insulin from biodegradable hybrid hydrogel networks of polysaccharide and synthetic biodegradable polyester. J. Biomater. Appl. 2002, 16, 305–325. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Y.; Allen, C. Polymer-drug compatibility: A guide to the development of delivery systems for the anticancer agent, ellipticine. J. Pharm. Sci. 2004, 93, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Shabina, M.; Afzal, M.; Hameed, S. Bacterial polyhydroxyalkanoates-eco-friendly next generation plastic: Production, biocompatibility, biodegradation, physical properties and applications. Green Chem. Lett. Rev. 2015, 8, 56–77. [Google Scholar]
- Errico, C.; Bartoli, C.; Chiellini, F.; Chiellini, E. Poly(hydroxyalkanoates)-based polymeric nanoparticles for drug delivery. J. Biomed. Biotechnol. 2009, 2009, 571702. [Google Scholar] [CrossRef]
- Jenkins, M.J.; Harrison, K.L. The effect of molecular weight on the crystallization kinetics of polycaprolactone. Polym. Adv. Technol. 2006, 17, 474–478. [Google Scholar] [CrossRef]
- Kulkarni, A.; Reiche, J.; Kratz, K.; Kamusewitz, H.; Sokolov, I.M.; Lendlein, A. Enzymatic chain scission kinetics of poly(ε-caprolactone) monolayers. Langmuir 2007, 23, 12202–12207. [Google Scholar] [CrossRef]
- Jia, W.; Gu, Y.C.; Gou, M.L.; Dai, M.; Li, X.Y.; Kan, B.; Yang, J.L.; Song, Q.F.; Wei, Y.Q.; Qian, Z.Y. Preparation of biodegradable polycaprolactone/poly (ethylene glycol)/polycaprolactone (PCEC) nanoparticles. Drug Deliv. 2008, 15, 409–416. [Google Scholar] [CrossRef]
- Sisson, A.L.; Ekinci, D.; Lendlein, A. The contemporary role of ε-caprolactone chemistry to create advanced polymer architectures. Polymer 2013, 54, 4333–4350. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, F.A.; Barakat, N.A.M.; Kanjwal, M.A.; Aryal, S.; Khil, M.S.; Kim, H.-Y. Novel self-assembled amphiphilic poly(e-caprolactone)-grafted-poly(vinyl alcohol) nanoparticles: Hydrophobic and hydrophilic drugs carrier nanoparticles. J. Mater. Sci. Mater. Med. 2009, 20, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Morissette, M.; Litim, N.; Di Paolo, T. Discovery and Development of Neuroprotective Agents from Natural Products; Brahmachari, G., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2017; p. 32. [Google Scholar]
- Zeinali, M.; Rezaee, S.A.; Hosseinzadeh, H. An overview on immunoregulatory and anti-inflammatory properties of chrysin and flavonoids substances. Biomed. Pharmacother. 2017, 92, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Ren, J.; Cheng, H.; Xin, W.Q.; Chen, X.; Hu, K. Induction of apoptosis by 7-piperazinethylchrysin in HCT-116 human colon cancer cells. Oncol. Rep. 2012, 28, 1719–1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahadori, M.; Bahadara, J.; Amini, E. Anticancer properties of chrysin on colon cancer cells, in vitro and in vivo with modulation of caspase-3, -9, Bax and Sall4. Iran. J. Biotechnol. 2016, 14, 177–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.Z.; Xiang, H.L.; Quan, M.F.; He, L.H. Inhibition of cell growth by BrMC through inactivation of Akt in HER-2/neu-overexpressing breast cancer cells. Oncol. Lett. 2014, 7, 1632–1638. [Google Scholar] [CrossRef] [Green Version]
- Sulaiman, G.M.; Jabir, M.S.; Hameed, A.H. Nanoscale modification of chrysin for improved of therapeutic efficiency and cytotoxicity. Artif. Cells Nanomed. Biotechnol. 2018, 46, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Lim, H.K.; Shim, S.H.; Jung, J. Improved chemotherapeutic efficacy of injectable chrysin encapsulated by copolymer nanoparticles. Int. J. Nanomed. 2017, 12, 1917–1925. [Google Scholar] [CrossRef] [Green Version]
- Mohammadinejad, S.; Akbarzadeh, A.; Rahmati-Yamchi, M.; Hatam, S.; Kachalaki, S.; Zohreh, S.; Zarghami, N. Preparation and evaluation of chrysin encapsulated in PLGA-PEG nanoparticles in the T47-D breast cancer cell line. Asian Pac. J. Cancer Prev. 2015, 16, 3753–3758. [Google Scholar] [CrossRef] [Green Version]
- Mohammadian, F.; Abhari, A.; Dariushnejad, H.; Zarghami, F.; Nikanfar, A.; Pilehvar-Soltanahmadi, Y.; Zarghami, N. Upregulation of Mir-34a in AGS gastric cancer cells by a PLGA-PEG-PLGA chrysin nano formulation. Asian Pac. J. Cancer Prev. 2015, 16, 8259–8263. [Google Scholar] [CrossRef]
- Nday, C.M.; Eleftheriadou, D.; Jackson, G. Magnetic chrysin silica nanomaterials behavior in an amyloidogenic environment. Hell. J. Nucl. Med. 2019, 22, 42–50. [Google Scholar] [PubMed]
- Firouzi-Amandi, A.; Dadashpour, M.; Nouri, M.; Zarghami, N.; Serati-Nouri, H.; Jafari-Gharabaghlou, D.; Karzar, B.H.; Mellatyar, H.; Aghebati-Maleki, L.; Babaloo, Z.; et al. Chrysin-nanoencapsulated PLGA-PEG for macrophage repolarization: Possible application in tissue regeneration. Biomed. Pharmacother. 2018, 105, 773–780. [Google Scholar] [CrossRef] [PubMed]
- De Abreu, L.C.; Ottoni, M.H.F.; Dos Santos, M.G.; Meireles, A.B.; de Almeida, V.G.; De Fátima Pereira, W.; de Avelar-Freitas, B.A.; Brito-Melo, G.E.A. Dimethyl sulfoxide (DMSO) decreases cell proliferation and TNF-α, IFN-γ, and IL-2 cytokines production in cultures of peripheral blood lymphocytes. Molecules 2017, 22, 1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, S.; Prestidge, C.A.; Miesel, L.; Sweeney, D.; Shinabarger, D.L.; Boulos, R.A. Preclinical development of Ramizol, an antibiotic belonging to a new class, for the treatment of Clostridium difficile colitis. J. Antibiot. 2016, 69, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Nojavan, S.; Tahmasebi, Z.; Davarani, S.S.H. Effect of type of stirring on hollow fiber liquid phase microextraction and electromembrane extraction of basic drugs: Speed up extraction time and enhancement of extraction efficiency. RSC Adv. 2016, 6, 110221–110228. [Google Scholar] [CrossRef]
- Mezzour, H.; Zerouale, K.; Neffati, F.; Douki, W.; Ben Amor, A.; Najjar, M.F. Evaluation of a spectrophotometric technique for plasmatic haemoglobin determination on Konelab 30. Ann. Biol. Clin. 2006, 64, 319–326. [Google Scholar]
- Ammar, S.H. Preparation of polyvinyl alcohol from local raw material. IJCPE 2008, 9, 15–21. [Google Scholar]
- Kemala, T.; Budianto, E.; Soegiyono, B. Preparation and characterization of microspheres based on blend of poly(lactic acid) and poly(ɛ-caprolactone) with poly(vinyl alcohol) as emulsifier. Arab. J. Chem. 2012, 5, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Calve, E.; de Malmazet, E.; Risso, F.; Masbernat, O. Coalescence of water drops at an oil–water interface loaded with microparticles and surfactants. Ind. Eng. Chem. Res. 2019, 58, 15573–15587. [Google Scholar] [CrossRef] [Green Version]
- Irfan, M.; Seiler, M. Encapsulation using hyperbranched polymers: From research and technologies to emerging applications. Ind. Eng. Chem. Res. 2010, 49, 1169–1196. [Google Scholar] [CrossRef]
- Halevas, E.; Mitrakas, A.; Mavroidi, B.; Athanasiou, D.; Gkika, P.; Antoniou, K.; Samaras, G.; Lialiaris, E.; Hatzidimitriou, A.; Pantazaki, A.; et al. Structurally characterized copper-chrysin complexes display genotoxic and cytotoxic activity in human cells. Inorg. Chim. Acta 2020, 515, 120062. [Google Scholar] [CrossRef]
- Halevas, E.; Mavroidi, B.; Antonoglou, O.; Hatzidimitriou, A.; Sagnou, M.; Pantazaki, A.A.; Litsardakis, G.; Pelecanou, M. Structurally characterized gallium–chrysin complexes with anticancer potential. Dalton Trans. 2020, 49, 2734–2746. [Google Scholar] [CrossRef]
- Elzein, T.; Nasser-Eddine, M.; Delaite, C.; Bistac, S.; Dumas, P. FTIR study of polycaprolactone chain organization at interfaces. J. Colloid Interface Sci. 2004, 273, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Ramezani, M.; Amoozegar, M.A.; Ventosa, A. Screening and comparative assay of poly-hydroxyalkanoates produced by bacteria isolated from the Gavkhooni Wetland in Iran and evaluation of poly-β-hydroxybutyrate production by halotolerant bacterium Oceanimonas sp. GK1. Ann. Microbiol. 2015, 65, 517–526. [Google Scholar] [CrossRef]
- Sindhu, R.; Ammu, B.; Binod, P.; Deepthi, S.K.; Ramachandran, K.B.; Soccol, C.R.; Pandey, A. Production and characterization of poly-3-hydroxybutyrate from crude glycerol by Bacillus sphaericus NII 0838 and improving its thermal properties by blending with other polymers. Braz. Arch. Biol. Technol. 2011, 54, 783–794. [Google Scholar] [CrossRef] [Green Version]
- Michailidou, G.; Ainali, N.M.; Xanthopoulou, E.; Nanaki, S.; Kostoglou, M.; Koukaras, E.N.; Bikiaris, D.N. Effect of poly(vinyl alcohol) on nanoencapsulation of budesonide in chitosan nanoparticles via ionic gelation and its improved bioavailability. Polymers 2020, 12, 1101. [Google Scholar] [CrossRef]
- Natarajan, V.; Krithica, N.; Madhan, B.; Sehgal, P.K. Formulation and evaluation of quercetin polycaprolactone microspheres for the treatment of rheumatoid arthritis. J. Pharm. Sci. 2011, 100, 195–205. [Google Scholar] [CrossRef]
- Aguilar-Rabiela, A.E.; Hernández-Cooper, E.M.; Otero, J.A.; Vergara-Porras, B. Modeling the release of curcumin from microparticles of poly(hydroxybutyrate) [PHB]. Int. J. Biol. Macromol. 2020, 140, 47–52. [Google Scholar] [CrossRef]
- Alex, A.T.; Joseph, A.; Shavi, G.; Rao, J.V.; Udupa, N. Development and evaluation of carboplatin-loaded PCL nanoparticles for intranasal delivery. Drug Deliv. 2016, 23, 2144–2153. [Google Scholar] [CrossRef]
- Nel, A.E.; Madler, L.; Velegol, D.; Xia, T.; Hoek, E.M.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Ma, G.; Yuan, Z.; Qian, H.; Xu, L.; Sidransky, E.; Chen, S. Development of zwitterionic polypeptide nanoformulation with high doxorubicin loading content for targeted drug delivery. Langmuir 2019, 35, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Ma, G.; Kampf, N.; Yuan, Z.; Chen, S. Development of long-circulating zwitterionic cross-linked micelles for active-targeted drug delivery. Biomacromolecules 2016, 17, 2010–2018. [Google Scholar] [CrossRef] [PubMed]
- Eatemadia, A.; Daraee, H.; Aiyelabegan, H.T.; Negahdari, B.; Rajeian, B.; Nosratollah, Z. Synthesis and characterization of chrysin-loaded PCL-PEG-PCL nanoparticle and its effect on breast cancer cell line. Biomed. Pharmacother. 2016, 84, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Anari, E.; Akbarzadeh, A.; Zarghami, N. Chrysin-loaded PLGA-PEG nanoparticles designed for enhanced effect on the breast cancer cell line. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1410–1416. [Google Scholar] [CrossRef]
- Maia, J.L.; Santana, M.H.A.; Ré, M.I. The effect of some processing conditions on the characteristics of biodegradable microspheres obtained by an emulsion solvent evaporation process. Braz. J. Chem. Eng. 2004, 21, 1–12. [Google Scholar] [CrossRef]
- Ahuja, G.; Pathak, K. Porous carriers for controlled/modulated drug delivery. Indian J. Pharm. Sci. 2009, 71, 599–607. [Google Scholar] [CrossRef] [Green Version]
- Papaneophytou, C.; Katsipis, G.; Halevas, E.; Pantazaki, A.A. Polyhydroxyalkanoates applications in drug carriers. In Biotechnological Applications of Polyhydroxyalkanoates; Kalia, V., Ed.; Springer: Singapore, 2019. [Google Scholar]
- Mrakovcic, M.; Absenger, M.; Riedl, R.; Smole, C.; Roblegg, E.; Fröhlich, L.F.; Fröhlich, E. Assessment of long-term effects of nanoparticles in a microcarrier cell culture system. PLoS ONE 2013, 8, e56791. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.; Herman, C.; Razdan, S.; Abedin, M.R.; Van Stoecker, W.; Barua, S. Microparticles for suspension culture of mammalian cells. ACS Appl. Bio Mater. 2019, 2, 2791–2801. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Xie, W.; He, A.D.; Da, X.W.; Liang, M.L.; Yao, G.Q.; Xiang, J.Z.; Gao, C.J.; Ming, Z.Y. Antiplatelet activity of chrysin via inhibiting platelet αIIbβ3-mediated signaling pathway. Mol. Nutr. Food Res. 2016, 60, 1984–1993. [Google Scholar] [CrossRef]
- Szopa, J.; Wróbel-Kwiatkowska, M.; Kulma, A.; Zuk, M.; Skórkowska-Telichowska, K.; Dymińska, L.; Mączka, M.; Hanuza, J.; Zebrowski, J.; Preisner, M. Chemical composition and molecular structure of fibers from transgenic flax producing polyhydroxybutyrate, and mechanical properties and platelet aggregation of composite materials containing these fibers. Compos. Sci. Technol. 2009, 69, 2438–2446. [Google Scholar] [CrossRef]
- Erkurta, M.A.; Kaya, E.; Berber, I.; Koroglu, M.; Kuku, I. Thrombocytopenia in adults: Review article. J. Hematol. 2012, 1, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Dobrovolskaia, M.A.; Clogston, J.D.; Neun, B.W.; Hall, J.B.; Patri, A.K.; McNeil, S.E. Method for analysis of nanoparticle hemolytic properties in vitro. Nano Lett. 2008, 8, 2180–2187. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, Y.; Cui, Y.; Xu, C.; Ji, X.; Luan, Y. Cytarabine-AOT catanionic vesicle-loaded biodegradable thermosensitive hydrogel as an efficient cytarabine delivery system. Int. J. Pharm. 2014, 473, 560–571. [Google Scholar] [CrossRef] [PubMed]
- ASTM E2524-08(2013). In Standard Test Method for Analysis of Hemolytic Properties of Nanoparticles; ASTM International: West Conshohocken, PA, USA, 2013; Available online: www.astm.org (accessed on 10 January 2021).
- Choi, J.; Reipa, V.; Hitchins, V.M.; Goering, P.L.; Malinauskas, R.A. Physicochemical characterization and in vitro hemolysis evaluation of silver nanoparticles. Toxicol. Sci. 2011, 123, 133–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, N.; Sahoo, S.K.; Kumar, R. Hemolysis tendency of anticancer nanoparticles changes with type of blood group antigen: An insight into blood nanoparticle interactions. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110645. [Google Scholar] [CrossRef]









| Micro-Formulation | dFESEM a (µm) | dDLS b (µm) | PDI | Z-Potential (mV) | Entrapment Efficiency (%) | Loading Capacity (%) | In Vitro Release (%) |
|---|---|---|---|---|---|---|---|
| EPCL/PVAMCs | 2 | 2.4 ± 1.3 | 2.03 | −16.2 ± 3.8 | - | - | - |
| EPHB/PVAMCs | 10.9 | 10.4 ± 4.4 | 1.95 | −14.1 ± 3.1 | - | - | - |
| ChrPCL/PVAMCs | 1.1–12.1 | 11.8 ± 4.7 | 2.11 | −18.1 ± 4.1 | 58.10 | 3.79 | 23.10 |
| ChrPHB/PVAMCs | 21.3 | 24.7 ± 8.5 | 1.93 | −16.3 ± 4.0 | 43.63 | 15.85 | 18.01 |
| Type of Chrysin-Loaded Nano-Formulation | Cell Line | Treatment Duration (Hours) | IC50 | References |
|---|---|---|---|---|
| Methoxy PEG-β-PCL nanoparticles | A549 non-small-cell lung cancer | 48 | 2.5 µM | [39] |
| PLGA-PEG-PLGA nanoparticles | AGS gastric cancer | 24, 48, 72 | 58.2, 44.2, 36.8 µM | [41] |
| PCL-PEG-PCL nanoparticles | T47D breast cancer | 24, 48, 72 | 2, 10, 10 µM | [63] |
| PLGA-PEG nanoparticles | T47D breast cancer | 24, 48, 72 | 40.19, 35.75, 31.28 µM | [64] |
| MCF-7 breast cancer | 66.41, 56.80, 42.54 µM |
| Concentration (µg·mL−1) | Free Chrysin * | ChrPHB/PVAMCs | ChrPCL/PVAMCs | EPHB/PVAMCs | EPCL/PVAMCs |
|---|---|---|---|---|---|
| Percentage of Hemolysis (%) | |||||
| 5 | 1.2 | 0.2 | 0.1 | 0.03 | 0.02 |
| 20 | 2.1 | 0.3 | 0.1 | 0.04 | 0.03 |
| 40 | 2.7 | 0.5 | 0.3 | 0.03 | 0.03 |
| 60 | 3.0 | 0.6 | 0.5 | 0.05 | 0.6 |
| 80 | 3.5 | 0.7 | 0.7 | 0.07 | 0.06 |
| 100 | 6.8 | 1.1 | 1.0 | 0.1 | 0.1 |
| 200 | 7.0 | 1.4 | 1.2 | 0.3 | 0.3 |
| 300 | 7.3 | 1.4 | 1.3 | 0.5 | 0.6 |
| 400 | 7.9 | 1.6 | 1.5 | 0.6 | 0.6 |
| 500 | 8.2 | 2.0 | 1.8 | 0.6 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halevas, E.; Kokotidou, C.; Zaimai, E.; Moschona, A.; Lialiaris, E.; Mitraki, A.; Lialiaris, T.; Pantazaki, A. Evaluation of the Hemocompatibility and Anticancer Potential of Poly(ε-Caprolactone) and Poly(3-Hydroxybutyrate) Microcarriers with Encapsulated Chrysin. Pharmaceutics 2021, 13, 109. https://doi.org/10.3390/pharmaceutics13010109
Halevas E, Kokotidou C, Zaimai E, Moschona A, Lialiaris E, Mitraki A, Lialiaris T, Pantazaki A. Evaluation of the Hemocompatibility and Anticancer Potential of Poly(ε-Caprolactone) and Poly(3-Hydroxybutyrate) Microcarriers with Encapsulated Chrysin. Pharmaceutics. 2021; 13(1):109. https://doi.org/10.3390/pharmaceutics13010109
Chicago/Turabian StyleHalevas, Eleftherios, Chrysoula Kokotidou, Elda Zaimai, Alexandra Moschona, Efstratios Lialiaris, Anna Mitraki, Theodore Lialiaris, and Anastasia Pantazaki. 2021. "Evaluation of the Hemocompatibility and Anticancer Potential of Poly(ε-Caprolactone) and Poly(3-Hydroxybutyrate) Microcarriers with Encapsulated Chrysin" Pharmaceutics 13, no. 1: 109. https://doi.org/10.3390/pharmaceutics13010109
APA StyleHalevas, E., Kokotidou, C., Zaimai, E., Moschona, A., Lialiaris, E., Mitraki, A., Lialiaris, T., & Pantazaki, A. (2021). Evaluation of the Hemocompatibility and Anticancer Potential of Poly(ε-Caprolactone) and Poly(3-Hydroxybutyrate) Microcarriers with Encapsulated Chrysin. Pharmaceutics, 13(1), 109. https://doi.org/10.3390/pharmaceutics13010109