Impact of Drug Loading Method on Drug Release from 3D-Printed Tablets Made from Filaments Fabricated by Hot-Melt Extrusion and Impregnation Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drug-Loaded Filament Preparation
2.1.1. HME Method
2.1.2. IMP Method
2.2. FDM 3D Printing Process
2.3. Characterization of Filaments and/or 3D-Printed Tablets
2.3.1. Differential Scanning Calorimetry (DSC)
2.3.2. Thermogravimetric Analysis (TGA)
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. Powder X-ray Diffraction (PXRD)
2.3.5. High Performance Liquid Chromatography (HPLC)
2.3.6. Swelling and Matrix Erosion Studies
2.3.7. Determination of Drug Content
2.3.8. Drug Release Studies
3. Results and Discussion
3.1. Characteristics of Filaments and 3D-Printed Tablets
3.2. Thermogravimetric Analysis
3.3. Differential Scanning Calorimetry
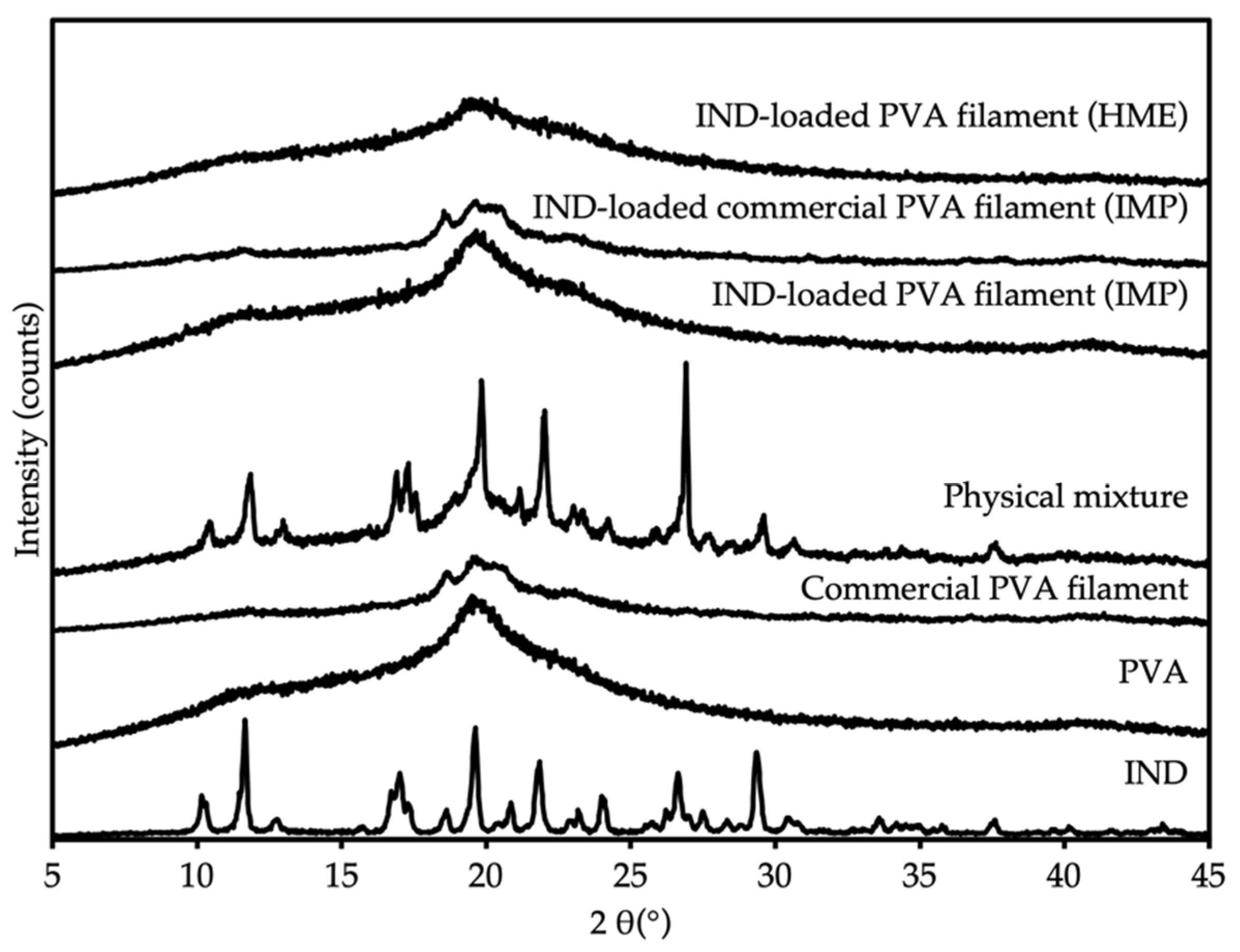
3.4. Powder X-ray Diffraction
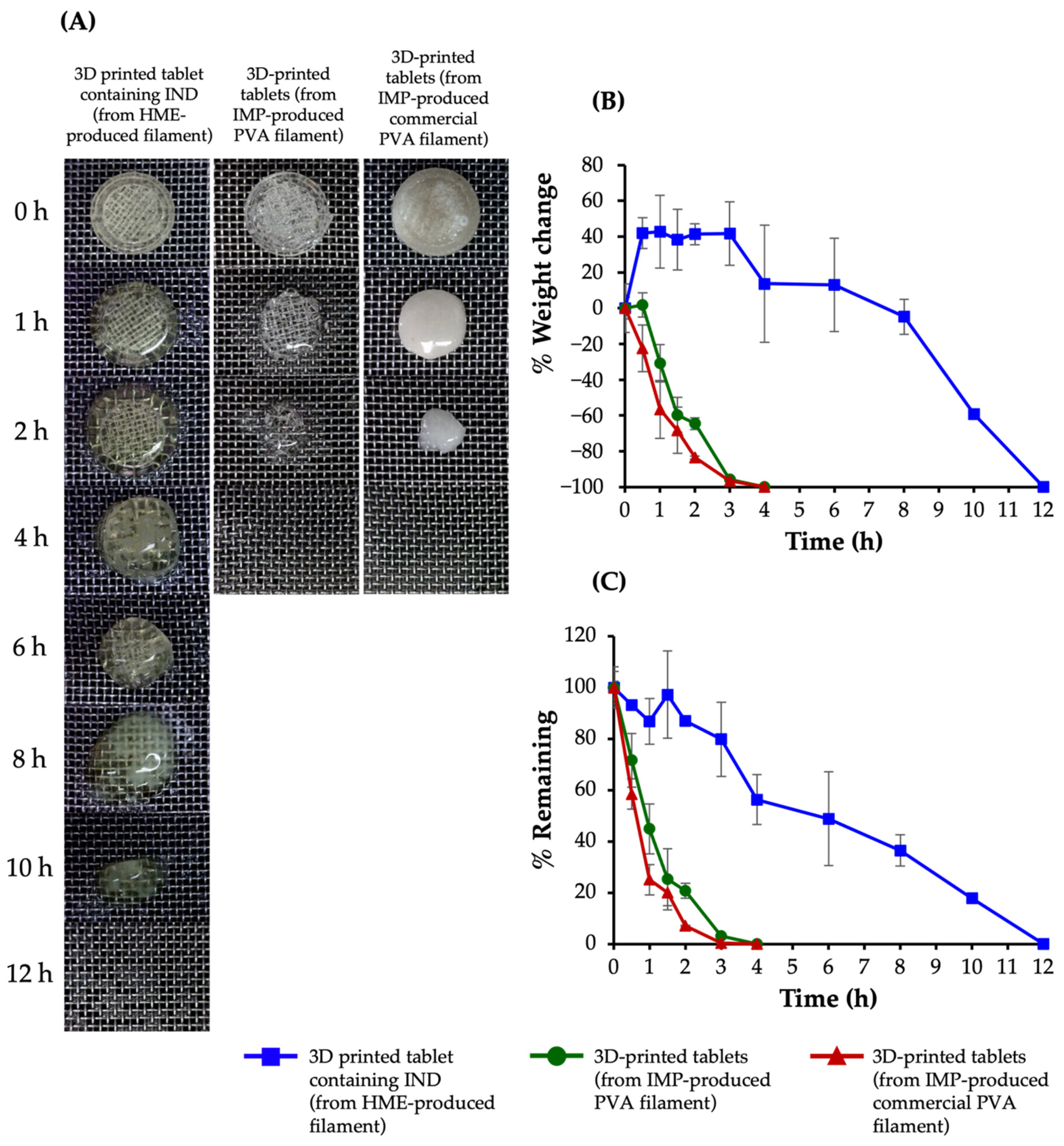
3.5. Swelling and Matrix Erosion Studies
3.6. Determination of Drug Content
3.7. Drug Release Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anonymous. 3D Systems to develop 3D printing for US aerospace and defense. Met. Powder Rep. 2015, 70, 152–153. [Google Scholar] [CrossRef]
- Nichols, M.R. How does the automotive industry benefit from 3D metal printing? Met. Powder Rep. 2019, 74, 257–258. [Google Scholar] [CrossRef]
- Klein, G.T.; Lu, Y.; Wang, M.Y. 3D Printing and Neurosurgery—Ready for Prime Time? World Neurosurg. 2013, 80, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Prasad, L.K.; Smyth, H. 3D Printing technologies for drug delivery: A review. Drug Dev. Ind. Pharm. 2016, 42, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Goole, J.; Amighi, K. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int. J. Pharm. 2016, 499, 376–394. [Google Scholar] [CrossRef]
- Roopavath, U.K.; Kalaskar, D.M. Introduction to 3D printing in medicine. In 3D Printing in Medicine; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–20. [Google Scholar]
- Awad, A.; Gaisford, S.; Basit, A.W. Fused Deposition Modelling: Advances in Engineering and Medicine. In Antibody-Drug Conjugates; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2018; pp. 107–132. [Google Scholar]
- Lepowsky, E.; Tasoglu, S. 3D Printing for drug manufacturing: A Perspective on the future of pharmaceuticals. Int. J. Bioprinting 2017, 4, 119. [Google Scholar] [CrossRef]
- Benjasirimongkol, P.; Ueda, K.; Higashi, K.; Sriamornsak, P.; Moribe, K. An Insight into stabilization mechanism of a solid dispersion of indomethacin/partially hydrolyzed polyvinyl alcohol prepared by hot–melt extrusion. Chem. Pharm. Bull. 2018, 66, 859–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, J.; Gholizadeh, H.; Lu, J.; Bunt, C.; Seyfoddin, A. Application of Fused Deposition Modelling (FDM) Method of 3D Printing in Drug Delivery. Curr. Pharm. Des. 2017, 23, 433–439. [Google Scholar] [CrossRef]
- Palo, M.; Holländer, J.; Suominen, J.; Yliruusi, J.; Sandler, N. 3D printed drug delivery devices: Perspectives and technical challenges. Expert Rev. Med. Devices 2017, 14, 685–696. [Google Scholar] [CrossRef]
- Karalia, D.; Siamidi, A.; Karalis, V.; Vlachou, M. 3D-Printed oral dosage forms: Mechanical properties, computational approaches and applications. Pharmaceutics 2021, 13, 1401. [Google Scholar] [CrossRef]
- Patil, H.; Tiwari, R.V.; Repka, M.A. Hot-melt extrusion: From theory to application in pharmaceutical formulation. AAPS PharmSciTech 2016, 17, 20–42. [Google Scholar] [CrossRef] [Green Version]
- Censi, R.; Gigliobianco, M.R.; Casadidio, C.; Di Martino, P. Hot melt extrusion: Highlighting physicochemical factors to be investigated while designing and optimizing a hot melt extrusion process. Pharmaceutics 2018, 10, 89. [Google Scholar] [CrossRef] [Green Version]
- Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. Advanced pharmaceutical applications of hot-melt extrusion coupled with fused deposition modelling (FDM) 3D printing for personalised drug delivery. Pharmaceutics 2018, 10, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sriamornsak, P.; Thirawong, N.; Weerapol, Y.; Nunthanid, J.; Sungthongjeen, S. Swelling and erosion of pectin matrix tablets and their impact on drug release behavior. Eur. J. Pharm. Biopharm. 2007, 67, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Indomethacin Extended-Release Capsules. Available online: https://online.uspnf.com/uspnf/document/1_GUID-11C71173-FC5C-4E9C-A4D4-6E8D68C97D62_5_en-US (accessed on 26 June 2021).
- Skowyra, J.; Pietrzak, K.; Alhnan, M.A. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur. J. Pharm. Sci. 2015, 68, 11–17. [Google Scholar] [CrossRef]
- Thanawuth, K.; Sriamornsak, P. Fabrication of indomethacin-loaded polyvinyl alcohol filaments through hot-melt extrusion. Key Eng. Mater. 2020, 859, 247–251. [Google Scholar] [CrossRef]
- Hong, S.; Shen, S.; Tan, D.C.T.; Ng, W.K.; Liu, X.; Chia, L.S.O.; Irwan, A.W.; Tan, R.; Nowak, S.A.; Marsh, K.; et al. High drug load, stable, manufacturable and bioavailable fenofibrate formulations in mesoporous silica: A comparison of spray drying versus solvent impregnation methods. Drug Deliv. 2016, 23, 316–327. [Google Scholar] [CrossRef]
- Gioumouxouzis, C.I.; Chatzitaki, A.-T.; Karavasili, C.; Katsamenis, O.; Tzetzis, D.; Mystiridou, E.; Bouropoulos, N.; Fatouros, D.G. Controlled release of 5-fluorouracil from alginate beads encapsulated in 3D printed pH-responsive solid dosage forms. AAPS PharmSciTech 2018, 19, 3362–3375. [Google Scholar] [CrossRef] [Green Version]
- Riza, S.H.; Masood, S.H.; Rashid, R.A.R.; Chandra, S. Selective laser sintering in biomedical manufacturing. In Metallic Biomaterials Processing and Medical Device Manufacturing; Elsevier: Amsterdam, The Netherlands, 2020; pp. 193–233. [Google Scholar]
- Bookwala, M.; Thipsay, P.; Ross, S.; Zhang, F.; Bandari, S.; Repka, M.A. Preparation of a crystalline salt of indomethacin and tromethamine by hot melt extrusion technology. Eur. J. Pharm. Biopharm. 2018, 131, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Nukala, P.K.; Palekar, S.; Solanki, N.; Fu, Y.; Patki, M.; Shohatee, A.A.; Trombetta, L.; Patel, K. Investigating the application of FDM 3D printing pattern in preparation of patient-tailored dosage forms. J. 3D Print. Med. 2019, 3, 23–37. [Google Scholar] [CrossRef]
- Monschke, M.; Wagner, K.G. Impact of HPMCAS on the Dissolution performance of polyvinyl alcohol celecoxib amorphous solid dispersions. Pharmaceutics 2020, 12, 541. [Google Scholar] [CrossRef]
- Goyanes, A.; Kobayashi, M.; Martínez-Pacheco, R.; Gaisford, S.; Basit, A.W. Fused-filament 3D printing of drug products: Microstructure analysis and drug release characteristics of PVA-based caplets. Int. J. Pharm. 2016, 514, 290–295. [Google Scholar] [CrossRef]
- Westedt, U.; Wittmar, M.; Hellwig, M.; Hanefeld, P.; Greiner, A.; Schaper, A.K.; Kissel, T. Paclitaxel releasing films consisting of poly(vinyl alcohol)-graft-poly(lactide-co-glycolide) and their potential as biodegradable stent coatings. J. Control. Release 2006, 111, 235–246. [Google Scholar] [CrossRef]
- Goyanes, A.; Martinez, P.R.; Buanz, A.; Basit, A.W.; Gaisford, S. Effect of geometry on drug release from 3D printed tablets. Int. J. Pharm. 2015, 494, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Fukushige, K.; Ogawa, E.; Hayashi, N.; Ozeki, T. 3D printing factors important for the fabrication of polyvinylalcohol filament-based tablets. Biol. Pharm. Bull. 2017, 40, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Benita, S.; Barkai, A.; Pathak, Y. Effect of drug loading extent on the in vitro release kinetic behaviour of nifedipine from polyacrylate microspheres. J. Control. Release 1990, 12, 213–222. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Bruschi, M.L., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 63–86. [Google Scholar]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Karthikeyan, M.; Deepa, M.K.; Bassim, E.; Rahna, C.S.; Raj, K.R.S. Investigation of kinetic drug release characteristics and in vitro evaluation of sustained-release matrix tablets of a selective COX-2 inhibitor for rheumatic diseases. J. Pharm. Innov. 2021, 16, 551–557. [Google Scholar] [CrossRef]



| Filament Used | Weight (mg) | Diameter (mm) | Thickness (mm) | IND Content (%) |
|---|---|---|---|---|
| Commercial PVA filaments (IMP) | 153.61 ± 0.46 | 7.11 ± 0.23 | 4.23 ± 0.01 | 1.44 ± 0.02 |
| PVA filaments (IMP) | 139.54 ± 4.42 | 6.98 ± 0.02 | 3.59 ± 0.16 | 4.86 ± 0.26 |
| PVA filaments (HME 5) * | 151.39 ± 1.86 | 7.07 ± 0.02 | 4.03 ± 0.04 | 4.35 ± 0.22 |
| PVA filaments (HME 30) * | 158.07 ± 0.05 | 7.01 ± 0.15 | 4.32 ± 0.20 | 27.94 ± 1.43 |
| Sample | Zero-Order | First-Order | Higuchi | Korsmeyer–Peppas | |
|---|---|---|---|---|---|
| (R2) | (R2) | (R2) | (R2) | (n) | |
| Filaments containing IND | |||||
| Commercial PVA filaments (IMP) | 0.9939 | 0.9811 | 0.9720 | 1.0000 | 0.83 |
| PVA filaments (IMP) | 0.9934 | 0.9369 | 0.8745 | 0.9936 | 1.02 |
| PVA filaments (HME 5) | 0.9547 | 0.9978 | 0.9695 | 0.9981 | 0.71 |
| PVA filaments (HME 30) | 0.9989 | 0.9722 | 0.8785 | 0.9992 | 0.97 |
| 3D-printed tablets containing IND | |||||
| Commercial PVA filaments (IMP) | 0.9532 | 0.9808 | 0.9547 | 0.9946 | 0.72 |
| PVA filaments (IMP) | 0.9411 | 0.9229 | 0.9297 | 0.9824 | 0.74 |
| PVA filaments (HME 5) | 0.9440 | 0.9758 | 0.9355 | 0.9866 | 0.74 |
| PVA filaments (HME 30) | 0.9881 | 0.9130 | 0.8520 | 0.9926 | 0.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thanawuth, K.; Sutthapitaksakul, L.; Konthong, S.; Suttiruengwong, S.; Huanbutta, K.; Dass, C.R.; Sriamornsak, P. Impact of Drug Loading Method on Drug Release from 3D-Printed Tablets Made from Filaments Fabricated by Hot-Melt Extrusion and Impregnation Processes. Pharmaceutics 2021, 13, 1607. https://doi.org/10.3390/pharmaceutics13101607
Thanawuth K, Sutthapitaksakul L, Konthong S, Suttiruengwong S, Huanbutta K, Dass CR, Sriamornsak P. Impact of Drug Loading Method on Drug Release from 3D-Printed Tablets Made from Filaments Fabricated by Hot-Melt Extrusion and Impregnation Processes. Pharmaceutics. 2021; 13(10):1607. https://doi.org/10.3390/pharmaceutics13101607
Chicago/Turabian StyleThanawuth, Kasitpong, Lalinthip Sutthapitaksakul, Srisuda Konthong, Supakij Suttiruengwong, Kampanart Huanbutta, Crispin R. Dass, and Pornsak Sriamornsak. 2021. "Impact of Drug Loading Method on Drug Release from 3D-Printed Tablets Made from Filaments Fabricated by Hot-Melt Extrusion and Impregnation Processes" Pharmaceutics 13, no. 10: 1607. https://doi.org/10.3390/pharmaceutics13101607
APA StyleThanawuth, K., Sutthapitaksakul, L., Konthong, S., Suttiruengwong, S., Huanbutta, K., Dass, C. R., & Sriamornsak, P. (2021). Impact of Drug Loading Method on Drug Release from 3D-Printed Tablets Made from Filaments Fabricated by Hot-Melt Extrusion and Impregnation Processes. Pharmaceutics, 13(10), 1607. https://doi.org/10.3390/pharmaceutics13101607










