Safety Evaluation of Nanotechnology Products
Abstract
:1. Introduction
2. Mechanism of Toxicity
2.1. Oxidative Stress
2.2. Cell Death Mechanisms
2.2.1. Apoptosis
2.2.2. Autophagy
2.2.3. Necrosis
2.3. Genotoxicity
2.4. Immune Response
3. Toxicity Assessment of Nanomaterials
3.1. In Vitro Characterization
3.1.1. Electron Microscopy
3.1.2. Atomic Force Microscopy
3.1.3. Confocal Laser Scanning Microscopy
3.1.4. Dark-Field Microscopy
3.1.5. Light-Scattering Microscopy
3.2. In Vitro Cell-Based Cytotoxicity Assay
3.2.1. Cell Viability Assays
3.2.2. Cell Cytotoxicity Assays
3.3. In Vivo Studies
3.3.1. Blood Circulation
3.3.2. Pharmacokinetics
Absorption
Distribution
Metabolism
Clearance
4. Physicochemical Properties of NPs Affecting Toxicity
4.1. Effect of Size
4.2. Effect of Shape
4.3. Effect of Surface Charge
4.4. Effect of Surface Functionality
4.5. Effect of Hydrophobicity
4.6. Effect of Aggregation
4.7. Effect of Solubility
5. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary
| Oxidative stress | an imbalance between the production of reactive oxygen species (ROS) and antioxidant mechanisms |
| Apoptosis | alternatively known as type-1 cell death, apoptosis is a process of programmed cell death |
| Autophagy | a vital lysosome-dependent metabolic process to remove dysfunctional organelles and unfold proteins |
| Necrosis | a non-programmed and unregulated cell death process |
| Genotoxicity | the destruction of the genetic material of the cell due to toxicity |
| QSAR | quantitative structure–activity relationships, enabling the prediction of the toxicity of nanomaterials based on their biological and physicochemical properties |
| Metabolism In vivo | metabolism of NPs in tissues after systemic administration |
| Pharmacokinetics | demonstrates how organisms affect particular materials (e.g., nanostructures) after entering the body via several processes (e.g., absorption, distribution, metabolism, and elimination) |
| Physicochemical properties | the various physicochemical properties of NPs include size, shape, surface functionality, surface charge, composition, hydrophobicity, aggregation, and solubility |
| Biohazards | infectious agents or hazardous biological materials that present a risk or potential risk to the health of people, animals, or the environment |
References
- Yun, Q.; Li, L.; Hu, Z.; Lu, Q.; Chen, B.; Zhang, H. Layered Transition Metal Dichalcogenide-Based Nanomaterials for Electrochemical Energy Storage. Adv. Mater. 2020, 32, 1903826. [Google Scholar] [CrossRef] [PubMed]
- Lizundiaab, E.; Pugliac, D.; Nguyend, T.-D.; Armentanoe, I. Cellulose nanocrystal based multifunctional nanohybrids. Prog. Mater. Sci. 2020, 112, 100668. [Google Scholar] [CrossRef]
- Joo, J.I.; Choi, M.; Jang, S.; Choi, S.; Park, S.; Shin, D.; Cho, K. Realizing Cancer Precision Medicine by Integrating Systems Biology and Nanomaterial Engineering. Adv. Mater. 2020, 32, e1906783. [Google Scholar] [CrossRef]
- Ding, B.; Zheng, P.; Ma, P.; Lin, J. Manganese Oxide Nanomaterials: Synthesis, Properties, and Theranostic Applications. Adv. Mater. 2020, 32, e1905823. [Google Scholar] [CrossRef]
- Xu, G.; Zeng, S.; Zhang, B.; Swihart, M.T.; Yong, K.-T.; Prasad, P.N. New Generation Cadmium-Free Quantum Dots for Biophotonics and Nanomedicine. Chem. Rev. 2016, 116, 12234–12327. [Google Scholar] [CrossRef]
- Poon, W.; Zhang, Y.-N.; Ouyang, B.; Kingston, B.R.; Wu, J.L.Y.; Wilhelm, S.; Chan, W.C.W. Elimination Pathways of Nanoparticles. ACS Nano 2019, 13, 5785–5798. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Halprin, K.M.; Ohkawara, A. The Measurement of Glutathione in Human Epidermis Using Glutathione Reductase. J. Investig. Dermatol. 1967, 48, 149–152. [Google Scholar] [CrossRef] [Green Version]
- Soenen, S.J.; Rivera-Gil, P.; Montenegro, J.M.; Parak, W.J.; De Smedt, S.C.; Braeckmans, K. Cellular Toxicity of Inorganic Nanoparti-cles: Common Aspects and Guidelines for Improved Nanotoxicity Evaluation. Nano Today 2011, 6, 446–465. [Google Scholar] [CrossRef]
- Pagliari, F.; Mandoli, C.; Forte, G.; Magnani, E.; Pagliari, S.; Nardone, G. Cerium Oxide Nanoparticles Protect Cardiac Pro-genitor Cells from Oxidative Stress. ACS Nano 2012, 6, 3767–3775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, J.; Azfer, A.; Rogers, L.M.; Wang, X.; Kolattukudy, P.E. Cardioprotective Effects of Cerium Oxide Nanoparticles in a Trans-genic Murine Model of Cardiomyopathy. Cardiovasc. Res. 2007, 73, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, A.; Jana, N.R. Vitamin C Conjugated Nanoparticle Protects Cells from Oxidative Stress at Low Dose but Induces Oxidative Stress and Cell Death at High Dose. ACS Appl. Mater. Interfaces 2017, 9, 41807–41817. [Google Scholar] [CrossRef]
- Anathy, V.; Roberson, E.; Guala, A.; Godburn, K.E.; Budd, R.C.; Janssen-Heininger, Y.M. Redox-Based Regulation of Apoptosis: S-Glutathionylation As a Regulatory Mechanism to Control Cell Death. Antioxid. Redox Signal. 2012, 16, 496–505. [Google Scholar] [CrossRef] [Green Version]
- Dalle-Donne, I.; Rossi, R.; Colombo, G.; Giustarini, D.; Milzani, A. Protein S-Glutathionylation: A Regulatory Device from Bacte-ria to Humans. Trends Biochem. Sci. 2009, 34, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Shelton, M.D.; Chock, P.B.; Mieyal, J.J. Glutaredoxin: Role in Reversible Protein S-Glutathionylation and Regulation of Redox Sig-nal Transduction and Protein Translocation. Antioxid. Redox Signal. 2005, 7, 348–366. [Google Scholar] [CrossRef]
- Allen, E.M.; Mieyal, J.J. Protein-Thiol Oxidation and Cell Death: Regulatory Role of Glutaredoxins. Antioxid. Redox Signal. 2012, 17, 1748–1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Gaffrey, M.J.; Su, D.; Liu, T.; Camp, D.G.; Smith, R.D.; Qian, W.J. Resin-Assisted Enrichment of Thiols as a General Strategy for Proteomic Profiling of Cysteine-Based Reversible Modifications. Nat. Protoc. 2014, 9, 64–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, J.; Kodali, V.K.; Gaffrey, M.J.; Guo, J.; Chu, R.K.; Camp, D.G. Quantitative Profiling of Protein S-Glutathionylation Re-veals Redox-Dependent Regulation of Macrophage Function during Nanoparticle-Induced Oxidative Stress. ACS Nano 2016, 10, 524–538. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Pedro, J.M.B.-S.; Kroemer, G. Organelle-specific initiation of cell death. Nat. Cell Biol. 2014, 16, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Fadeel, B.; Orrenius, S. Apoptosis: A basic biological phenomenon with wide-ranging implications in human disease. J. Intern. Med. 2005, 258, 479–517. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Hao, H.; Cai, M.; Wang, S.; Ma, J. In Vitro and in vivo Mechanism of Bone Tumor Inhibition by Seleni-um-Doped Bone Mineral Nanoparticles. ACS Nano 2016, 10, 9927–9937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravichandran, K.S. Find-me and eat-me signals in apoptotic cell clearance: Progress and conundrums. J. Exp. Med. 2010, 207, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Hernández, M.; del Pino, P.; Mitchell, S.G.; Moros, M.; Stepien, G.; Pelaz, B.; Parak, W.J.; Galvez, E.; Pardo, J.; De La Fuente, J.M. Dissecting the Molecular Mechanism of Apoptosis during Photothermal Therapy Using Gold Nanoprisms. ACS Nano 2015, 9, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Zhang, Y.; Deng, K.; Chen, Y.; Li, X.; Deng, X. UV-Emitting Upconversion-Based TiO2 Photosensitizing Nanoplat-form: Near-Infrared Light Mediated in Vivo Photodynamic Therapy via Mitochondria-Involved Apoptosis Pathway. ACS Nano 2015, 9, 2584–2599. [Google Scholar] [CrossRef]
- Shi, S.; Lin, S.; Li, Y.; Zhang, T.; Shao, X.; Tian, T. Effects of Tetrahedral DNA Nanostructures on Autophagy in Chondro-cytes. Chem. Commun. 2018, 54, 1327–1330. [Google Scholar] [CrossRef]
- Peynshaert, K.; Manshian, B.B.; Joris, F.; Braeckmans, K.; De Smedt, S.; Demeester, J.; Soenen, S. Exploiting Intrinsic Nanoparticle Toxicity: The Pros and Cons of Nanoparticle-Induced Autophagy in Biomedical Research. Chem. Rev. 2014, 114, 7581–7609. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Fan, J.; Wang, Z.; Zeng, X.; Sun, Y.; Song, P.; Ju, D. The role of autophagy in the neurotoxicity of cationic PAMAM dendrimers. Biomaterials 2014, 35, 7588–7597. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, Y.; Hou, L.; Chang, J.; Zhang, Z.; Zhang, H. Positioning Remodeling Nanogels Mediated Codelivery of Anti-vascular Drug and Autophagy Inhibitor for Cooperative Tumor Therapy. ACS Appl. Mater. Interfaces 2020, 12, 6978–6990. [Google Scholar] [CrossRef]
- Xie, Y.; Jiang, J.; Tang, Q.; Zou, H.; Zhao, X. Iron Oxide Nanoparticles as Autophagy Intervention Agents Suppress He-patoma Growth by Enhancing Tumoricidal Autophagy. Adv. Sci. 2020, 7, 1903323. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Q.; Song, B.; Wu, S.; Su, Y.; Zhang, H.; He, Y. A real-time documentation and mechanistic investigation of quantum dots-induced autophagy in live Caenorhabditis elegans. Biomaterials 2015, 72, 38–48. [Google Scholar] [CrossRef]
- Li, J.; Yang, S.; Deng, Y.; Chai, P.; Yang, Y.; He, X. Emancipating Target-Functionalized Carbon Dots from Autophagy Vesi-cles for a Novel Visualized Tumor Therapy. Adv. Funct. Mater. 2018, 28, 1800881. [Google Scholar] [CrossRef]
- Qu, G.; Liu, S.; Zhang, S.; Wang, L.; Wang, X.; Sun, B. Graphene Oxide Induces Toll-like Receptor 4 (TLR4)-Dependent Ne-crosis in Macrophages. ACS Nano 2013, 7, 5732–5745. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Shi, Y.; Liang, Y.; Yang, A.; Fan, Z.; Yuan, L.; Zou, X.; Chang, X.; Zhang, H.; Wang, X.; et al. Single-walled carbon-nanohorns improve biocompatibility over nanotubes by triggering less protein-initiated pyroptosis and apoptosis in macrophages. Nat. Commun. 2018, 9, 2393. [Google Scholar] [CrossRef] [Green Version]
- Cabada, T.F.; de Pablo, C.S.; Serrano, A.M.; Guerrero, F.; Olmedo, J.J.; Gomez, M.R. Induction of Cell Death in a Glio-blastoma Line by Hyperthermic Therapy Based on Gold Nanorods. Int. J. Nanomed. 2012, 7, 1511–1523. [Google Scholar]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Gong, Y.; Liu, L.; Zhou, Y.; Fang, X.; Zhang, C.; Li, Y.; Li, J. The use of human umbilical vein endothelial cells (HUVECs) as an in vitro model to assess the toxicity of nanoparticles to endothelium: A review. J. Appl. Toxicol. 2017, 37, 1359–1369. [Google Scholar] [CrossRef]
- Zeiger, E. Genetic Toxicology Testing. In Comprehensive Toxicology; McQueen, C.A., Ed.; Elsevier: Oxford, UK, 2010; pp. 139–158. [Google Scholar]
- Lord, C.; Ashworth, A. The DNA damage response and cancer therapy. Nat. Cell Biol. 2012, 481, 287–294. [Google Scholar] [CrossRef]
- Sargent, L.M.; Reynolds, S.H.; Castranova, V. Potential Pulmonary Effects of Engineered Carbon Nanotubes: In Vitro Genotoxic Effects. Nanotoxicology 2010, 4, 396–408. [Google Scholar] [CrossRef]
- Lindberg, H.K.; Falck, G.C.-M.; Singh, R.; Suhonen, S.; Järventaus, H.; Vanhala, E.; Catalán, J.; Farmer, P.B.; Savolainen, K.M.; Norppa, H. Genotoxicity of short single-wall and multi-wall carbon nanotubes in human bronchial epithelial and mesothelial cells in vitro. Toxicology 2013, 313, 24–37. [Google Scholar] [CrossRef]
- Lindberg, H.K.; Falck, G.C.-M.; Suhonen, S.; Vippola, M.; Vanhala, E.; Catalán, J.; Savolainen, K.; Norppa, H. Genotoxicity of nanomaterials: DNA damage and micronuclei induced by carbon nanotubes and graphite nanofibres in human bronchial epithelial cells in vitro. Toxicol. Lett. 2009, 186, 166–173. [Google Scholar] [CrossRef]
- Møller, P.; Jacobsen, N.R.; Folkmann, J.K.; Danielsen, P.H.; Mikkelsen, L.; Hemmingsen, J.G.; Vesterdal, L.K.; Forchhammer, L.; Wallin, H.; Loft, S. Role of oxidative damage in toxicity of particulates. Free. Radic. Res. 2009, 44, 1–46. [Google Scholar] [CrossRef]
- Burgum, M.J.; Clift, M.J.D.; Evans, S.; Hondow, N.; Miller, M.; Lopez, S.B.; Williams, A.; Tarat, A.; Jenkins, G.J.; Doak, S.H. In Vitro Primary-Indirect Genotoxicity in Bronchial Epithelial Cells Promoted by Industrially Relevant Few-Layer Graphene. Small 2021, 17, 2002551. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.; Barguilla, I.; Domenech, J.; Marcos, R.; Hernández, A. Biological effects, including oxidative stress and genotoxic damage, of polystyrene nanoparticles in different human hematopoietic cell lines. J. Hazard. Mater. 2020, 398, 122900. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S. Genotoxicity and carcinogenicity risk of carbon nanotubes. Adv. Drug Deliv. Rev. 2013, 65, 2098–2110. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, D.H.; Yan, J.; Chen, Y.; Mittelstaedt, R.A.; Zhang, Y.; Biris, A.S.; Heflich, R.H.; Chen, T. Genotoxicity of silver nanoparticles evaluated using the Ames test and in vitro micronucleus assay. Mutat. Res. Toxicol. Environ. Mutagen. 2012, 745, 4–10. [Google Scholar] [CrossRef]
- Landsiedel, R.; Kapp, M.D.; Schulz, M.; Wiench, K.; Oesch, F. Genotoxicity investigations on nanomaterials: Methods, preparation and characterization of test material, potential artifacts and limitations—Many questions, some answers. Mutat. Res. Mutat. Res. 2009, 681, 241–258. [Google Scholar] [CrossRef]
- Magdolenova, Z.; Collins, A.; Kumar, A.; Dhawan, A.; Stone, V.; Dusinska, M. Mechanisms of Genotoxicity: A Review of In Vitro and In Vivo Studies with Engineered Nanoparticles. Nanotoxicology 2013, 8, 233–278. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ma, P.; Luo, Q.; Chen, J.; Gan, Y.; Du, J.; Ding, S.; Xi, Z. Intraperitoneal injection of magnetic Fe3O4-nanoparticle induces hepatic and renal tissue injury via oxidative stress in mice. Int. J. Nanomed. 2012, 7, 4809–4818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, O.; Mandina, T.; Lamadrid, A.I.; Diaz, A.; Remigio, A.; Gonzalez, Y. Sensitivity and Variability of Visual Scoring in the Comet Assay. Results of an Inter-Laboratory Scoring Exercise with the Use of Silver Staining. Mutat. Res. 2004, 556, 25–34. [Google Scholar] [PubMed]
- Watson, C.; Ge, J.; Cohen, J.; Pyrgiotakis, G.; Engelward, B.P.; Demokritou, P. High-Throughput Screening Platform for Engineered Nanoparticle-Mediated Genotoxicity Using CometChip Technology. ACS Nano 2014, 8, 2118–2133. [Google Scholar] [CrossRef] [Green Version]
- Hassan, H.A.F.M.; Smyth, L.; Rubio, N.; Ratnasothy, K.; Wang, J.T.W.; Bansal, S.S. Carbon Nanotubes’ Surface Chemistry De-termines their Potency as Vaccine Nanocarriers In Vitro and In Vivo. J. Control. Release 2016, 225, 205–216. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Irvine, D.J. Guiding Principles in the Design of Molecular Bioconjugates for Vaccine Applications. Bioconjugate Chem. 2015, 26, 791–801. [Google Scholar] [CrossRef] [Green Version]
- Sexton, A.; Whitney, P.; Chong, S.-F.; Zelikin, A.; Johnston, A.; De Rose, R.; Brooks, A.; Caruso, F.; Kent, S.J. A Protective Vaccine Delivery System for In Vivo T Cell Stimulation Using Nanoengineered Polymer Hydrogel Capsules. ACS Nano 2009, 3, 3391–3400. [Google Scholar] [CrossRef]
- Niikura, K.; Matsunaga, T.; Suzuki, T.; Kobayashi, S.; Yamaguchi, H.; Orba, Y.; Kawaguchi, A.; Hasegawa, H.; Kajino, K.; Ninomiya, T.; et al. Gold Nanoparticles as a Vaccine Platform: Influence of Size and Shape on Immunological Responses in Vitro and in Vivo. ACS Nano 2013, 7, 3926–3938. [Google Scholar] [CrossRef]
- Ji, Z.; Wang, X.; Zhang, H.; Lin, S.; Meng, H.; Sun, B.; George, S.; Xia, T.; Nel, A.E.; Zink, J.I. Designed Synthesis of CeO2 Nanorods and Nanowires for Studying Toxicological Effects of High Aspect Ratio Nanomaterials. ACS Nano 2012, 6, 5366–5380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Feng, Z.; Wang, C.; Su, Q.; Song, H.; Zhang, C.; Huang, P.; Liang, X.-J.; Dong, A.; Kong, D.; et al. Co-localized delivery of nanomedicine and nanovaccine augments the postoperative cancer immunotherapy by amplifying T-cell responses. Biomaterials 2020, 230, 119649. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Bhatia, E.; Sharma, S.; Ahamad, N.; Banerjee, R. Advancements in prophylactic and therapeutic nanovaccines. Acta Biomater. 2020, 108, 1–21. [Google Scholar] [CrossRef]
- Zhou, W.; Moguche, A.O.; Chiu, D.; Murali-Krishna, K.; Baneyx, F. Just-in-time vaccines: Biomineralized calcium phosphate core-immunogen shell nanoparticles induce long-lasting CD8+ T cell responses in mice. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 571–578. [Google Scholar] [CrossRef] [Green Version]
- Kasturi, S.P.; Skountzou, I.; Albrecht, R.A.; Koutsonanos, D.; Hua, T.; Nakaya, H.I. Programming the Magnitude and Persis-tence of Antibody Responses with Innate Immunity. Nature 2011, 470, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Zhou, F.; Hou, B.; Wang, D.; Wang, T.; Fu, Y.; Ma, Y.; Yu, H.; Li, Y. Binary Cooperative Prodrug Nanoparticles Improve Immunotherapy by Synergistically Modulating Immune Tumor Microenvironment. Adv. Mater. 2018, 30, e1803001. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Khan, A.R.; Ji, J.; Lin, G.; Zhao, X.; Zhai, G. Crosslinked Self-Assembled Nanoparticles for Chemo-Sonodynamic Combi-nation Therapy Favoring Antitumor, Antimetastasis Management and Immune Responses. J. Control. Release 2018, 290, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Horváth, L.; Magrez, A.; Golberg, D.; Zhi, C.; Bando, Y.; Smajda, R.; Horváth, E.; Forró, L.; Schwaller, B. In Vitro Investigation of the Cellular Toxicity of Boron Nitride Nanotubes. ACS Nano 2011, 5, 3800–3810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plascencia-Villa, G.; Starr, C.R.; Armstrong, L.S.; Poncea, A.; Jose’-Yacaman, M. Imaging Interactions of Metal Oxide Nanoparti-cles with Macrophage Cells by Ultra-High Resolution Scanning Electron Microscopy Techniques. Integr. Biol. 2012, 4, 1358–1366. [Google Scholar] [CrossRef] [Green Version]
- Ankri, R.; Ashkenazy, A.; Milstein, Y.; Brami, Y.; Olshinka, A.; Goldenberg-Cohen, N.; Popovtzer, A.; Fixler, D.; Hirshberg, A. Gold Nanorods Based Air Scanning Electron Microscopy and Diffusion Reflection Imaging for Mapping Tumor Margins in Squamous Cell Carcinoma. ACS Nano 2016, 10, 2349–2356. [Google Scholar] [CrossRef]
- Guarnieri, D.; Sabella, S.; Muscetti, O.; Belli, V.; Malvindi, M.A.; Fusco, S. Transport Across the Cell-Membrane Dictates Na-noparticle Fate and Toxicity: A New Paradigm in Nanotoxicology. Nanoscale 2014, 6, 10264–10273. [Google Scholar] [CrossRef] [PubMed]
- Love, S.A.; Liu, Z.; Haynes, C. Examining changes in cellular communication in neuroendocrine cells after noble metal nanoparticle exposure. Analyst 2012, 137, 3004–3010. [Google Scholar] [CrossRef] [PubMed]
- Yazdimamaghani, M.; Moos, P.J.; Ghandehari, H. Global Gene Expression Analysis of Macrophage Response Induced by Non-porous and Porous Silica Nanoparticles. Nanomedicine 2018, 14, 533–545. [Google Scholar] [CrossRef]
- Asharani, P.V.; Mun, G.L.K.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Peckys, D.B.; de Jonge, N. Visualizing Gold Nanoparticle Uptake in Live Cells with Liquid Scanning Transmission Electron Microscopy. Nano Lett. 2011, 11, 1733–1738. [Google Scholar] [CrossRef]
- Brown, C.P. Advancing musculoskeletal research with nanoscience. Nat. Rev. Rheumatol. 2013, 9, 614–623. [Google Scholar] [CrossRef]
- Khanal, D.; Kondyurin, A.; Hau, H.; Knowles, J.C.; Levinson, O.; Ramzan, I. Biospectroscopy of Nanodiamond-Induced Al-terations in Conformation of Intra- and Extracellular Proteins—A Nanoscale IR Study. Anal. Chem. 2016, 88, 7530–7538. [Google Scholar] [CrossRef] [Green Version]
- Govindaraju, S.; Rengaraj, A.; Arivazhagan, R.; Huh, Y.S.; Yun, K. Curcumin-Conjugated Gold Clusters for Bioimaging and An-ticancer Applications. Bioconjugate Chem. 2018, 29, 363–370. [Google Scholar] [CrossRef]
- Reggente, M.; Passeri, D.; Angeloni, L.; Scaramuzzo, F.A.; Barteri, M.; De Angelis, F.; Persiconi, I.; De Stefano, M.E.; Rossi, M. Detection of stiff nanoparticles within cellular structures by contact resonance atomic force microscopy subsurface nanomechanical imaging. Nanoscale 2017, 9, 5671–5676. [Google Scholar] [CrossRef]
- Smith, T.T.; Stephan, S.B.; Moffett, H.; McKnight, L.; Ji, W.; Reiman, D.; Bonagofski, E.; Wohlfahrt, M.E.; Pillai, S.P.S.; Stephan, M. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat. Nanotechnol. 2017, 12, 813–820. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Shi, G.; Zhang, J.; Song, H.; Niu, J.; Shi, S.; Huang, P.; Wang, Y.; Wang, W.; Li, C.; et al. Targeted antigen delivery to dendritic cell via functionalized alginate nanoparticles for cancer immunotherapy. J. Control. Release 2017, 256, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Meng, Q.; Sun, H.; Yin, Q.; Yu, H.; Zhang, Z.; Cao, M.; Zhang, Y.; Li, Y. Shrapnel nanoparticles loading docetaxel inhibit metastasis and growth of breast cancer. Biomaterials 2015, 64, 10–20. [Google Scholar] [CrossRef]
- He, B.; Lin, P.; Jia, Z.; Du, W.; Qu, W.; Yuan, L.; Dai, W.; Zhang, H.; Wang, X.; Wang, J.; et al. The transport mechanisms of polymer nanoparticles in Caco-2 epithelial cells. Biomaterials 2013, 34, 6082–6098. [Google Scholar] [CrossRef]
- Xia, D.; He, Y.; Li, Q.; Hu, C.; Huang, W.; Zhang, Y. Transport Mechanism of Lipid Covered Saquinavir Pure Drug Nano-particles in Intestinal Epithelium. J. Control. Release 2018, 269, 159–170. [Google Scholar] [CrossRef]
- Syed, A.M.; Sindhwani, S.; Wilhelm, S.; Kingston, B.R.; Lee, D.S.W.; Gommerman, J.L.; Chan, W.C.W. Three-Dimensional Imaging of Transparent Tissues via Metal Nanoparticle Labeling. J. Am. Chem. Soc. 2017, 139, 9961–9971. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cui, Y.; Irudayaraj, J. Single-Cell Quantification of Cytosine Modifications by Hyperspectral Dark-Field Imaging. ACS Nano 2015, 9, 11924–11932. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Wang, X.; Ren, W.; Liu, J.; Irudayaraj, J. Optical Clearing Delivers Ultrasensitive Hyperspectral Dark-Field Imaging for Single-Cell Evaluation. ACS Nano 2016, 10, 3132–3143. [Google Scholar] [CrossRef] [Green Version]
- Zhai, S.; Hu, X.; Hu, Y.; Wu, B.; Xing, D. Visible light-induced crosslinking and physiological stabilization of diselenide-rich nanoparticles for redox-responsive drug release and combination chemotherapy. Biomaterials 2017, 121, 41–54. [Google Scholar] [CrossRef]
- Afonin, K.A.; Bindewald, E.; Voss, N.; Jacovetty, E.; Shapiro, B.A.; Jaeger, L. In vitro assembly of cubic RNA-based scaffolds de-signed in silico. Nat. Nanotechnol. 2010, 5, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, C.F.; Grainger, D.W. In vitro assessments of nanomaterial toxicity. Adv. Drug Deliv. Rev. 2009, 61, 438–456. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, S.; Raghavan, G.; Yang, G.; Cohen-Karni, T. Effect of Graphene on Nonneuronal and Neuronal Cell Viability and Stress. Nano Lett. 2017, 17, 3297–3301. [Google Scholar] [CrossRef]
- Sokolova, V.; Shi, Z.; Huang, S.; Du, Y.; Kopp, M.; Frede, A.; Knuschke, T.; Buer, J.; Yang, D.; Wu, J.; et al. Delivery of the TLR ligand poly(I:C) to liver cells in vitro and in vivo by calcium phosphate nanoparticles leads to a pronounced immunostimulation. Acta Biomater. 2017, 64, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Wörle-Knirsch, J.M.; Pulskamp, K.; Krug, H.F. Oops They Did It Again! Carbon Nanotubes Hoax Scientists in Viability Assays. Nano Lett. 2006, 6, 1261–1268. [Google Scholar] [CrossRef]
- Marquis, B.; Love, S.A.; Braun, K.L.; Haynes, C. Analytical methods to assess nanoparticle toxicity. Analyst 2009, 134, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Brandão, F.; Bessa, M.J.; Costa, S.; Valdiglesias, V.; Kiliç, G. In Vitro Cytotoxicity of Superparamagnetic Iron Oxide Nanoparticles on Neuronal and Glial Cells. Evaluation of Nanoparticle Interference with Viability Tests. J. Appl. Toxicol. 2016, 36, 361–372. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Vitale, I.; Abrams, J.M.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; Dawson, T.M.; Dawson, V.L.; El-Deiry, W.S.; Fulda, S.; et al. Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2012, 19, 107–120. [Google Scholar] [CrossRef]
- Andón, F.T.; Fadeel, B. Programmed Cell Death: Molecular Mechanisms and Implications for Safety Assessment of Nano-materials. Acc. Chem. Res. 2013, 46, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Bussy, C.; Al-Jamal, K.T.; Boczkowskim, J.; Lanonem, S.; Pratom, M.; Biancom, A. Microglia Determine Brain Region-Specific Neuro-toxic Responses to Chemically Functionalized Carbon Nanotubes. ACS Nano 2015, 9, 7815–7830. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Behzadi, S.; Laurent, S.; Forrestm, M.L.; Stroeve, P.; Mahmoudi, M. Toxicity of Nanomaterials. Chem. Soc. Rev. 2012, 41, 2323–2343. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pacheco, R.; Marquina, C.; Valdivia, J.G.; Gutiérrez, M.; Romero, M.S.; Cornudella, R.; Laborda, A.; Viloria, A.; Higuera, T.; García, A.; et al. Magnetic nanoparticles for local drug delivery using magnetic implants. J. Magn. Magn. Mater. 2007, 311, 318–322. [Google Scholar] [CrossRef]
- Zhu, M.-T.; Feng, W.-Y.; Wang, B.; Wang, T.-C.; Gu, Y.-Q.; Wang, M.; Wang, Y.; Ouyang, H.; Zhao, Y.-L.; Chai, Z.-F. Comparative study of pulmonary responses to nano- and submicron-sized ferric oxide in rats. Toxicology 2008, 247, 102–111. [Google Scholar] [CrossRef]
- Dai, C.; Yuan, Y.; Liu, C.; Wei, J.; Hong, H.; Li, X.; Pan, X. Degradable, antibacterial silver exchanged mesoporous silica spheres for hemorrhage control. Biomaterials 2009, 30, 5364–5375. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Aggarwal, P.; Hall, J.B.; McNeil, S.E. Preclinical Studies To Understand Nanoparticle Interaction with the Immune System and Its Potential Effects on Nanoparticle Biodistribution. Mol. Pharm. 2008, 5, 487–495. [Google Scholar] [CrossRef] [Green Version]
- Cavadas, M.; Gonzalez-Fernandez, A.; Franco, R. Pathogen-Mimetic Stealth Nanocarriers for Drug Delivery: A Future Possi-bility. Nanomedicine 2011, 7, 730–743. [Google Scholar] [CrossRef]
- Jones, C.F.; Campbell, R.A.; Brooks, A.E.; Assemi, S.; Tadjiki, S.; Thiagarajan, G.; Mulcock, C.; Weyrich, A.S.; Brooks, B.D.; Ghandehari, H.; et al. Cationic PAMAM Dendrimers Aggressively Initiate Blood Clot Formation. ACS Nano 2012, 6, 9900–9910. [Google Scholar] [CrossRef] [Green Version]
- Delfino, R.J.; Sioutas, C.; Malik, S. Potential Role of Ultrafine Particles in Associations between Airborne Particle Mass and Cardiovascular Health. Environ. Heal. Perspect. 2005, 113, 934–946. [Google Scholar] [CrossRef] [Green Version]
- Jun, E.-A.; Lim, K.-M.; Kim, K.; Bae, O.-N.; Noh, J.-Y.; Chung, K.-H.; Chung, J.-H. Silver nanoparticles enhance thrombus formation through increased platelet aggregation and procoagulant activity. Nanotoxicology 2010, 5, 157–167. [Google Scholar] [CrossRef]
- Radomski, A.; Jurasz, P.; Alonso-Escolano, D.; Drews, M.; Morandi, M.; Malinski, T. Nanoparticle-Induced Platelet Aggrega-tion and Vascular Thrombosis. J. Pharmacol. 2005, 146, 882–893. [Google Scholar]
- Sahu, S.C.; Casciano, D.A. Nanotoxicity: From In Vivo and In Vitro Models to Health Risks; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Ruiz, A.; Ali, L.M.A.; Cáceres-Vélez, P.R.; Cornudella, R.; Gutiérrez, M.; Moreno, J.A. Hematotoxicity of Magnetite Nanoparti-cles Coated with Polyethylene Glycol: In Vitro and In Vivo Studies. Toxicol. Res. 2015, 4, 1555–1564. [Google Scholar] [CrossRef] [Green Version]
- Borm, P.; Klaessig, F.C.; Landry, T.D.; Moudgil, B.; Pauluhn, J.; Thomas, K.; Trottier, R.; Wood, S. Research Strategies for Safety Evaluation of Nanomaterials, Part V: Role of Dissolution in Biological Fate and Effects of Nanoscale Particles. Toxicol. Sci. 2006, 90, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Shan, W.; Zhu, X.; Liu, M.; Li, L.; Zhong, J.; Sun, W.; Zhang, Z.; Huang, Y. Overcoming the Diffusion Barrier of Mucus and Absorption Barrier of Epithelium by Self-Assembled Nanoparticles for Oral Delivery of Insulin. ACS Nano 2015, 9, 2345–2356. [Google Scholar] [CrossRef]
- Miller, M.R.; Raftis, J.B.; Langrish, J.P.; McLean, S.G.; Samutrtai, P.; Connell, S.; Wilson, S.; Vesey, A.T.; Fokkens, P.H.B.; Boere, A.J.F.; et al. Inhaled Nanoparticles Accumulate at Sites of Vascular Disease. ACS Nano 2017, 11, 4542–4552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Zheng, Y.; Liu, M.; Shan, W.; Zhang, Z.; Huang, Y. Biomimetic Viruslike and Charge Reversible Nanoparticles to Sequen-tially Overcome Mucus and Epithelial Barriers for Oral Insulin Delivery. ACS Appl. Mater. Interfaces 2018, 10, 9916–9928. [Google Scholar] [CrossRef]
- Almeida, J.P.M.; Lin, A.Y.; Langsner, R.J.; Eckels, P.; Foster, A.E.; Drezek, R.A. In vivo immune cell distribution of gold nanoparticles in naïve and tumor bearing mice. Small 2013, 10, 812–819. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Ensign, L.M.; Boylan, N.J.; Schön, A.; Gong, X.; Yang, J.C. Impact of Surface Polyethylene Glycol (PEG) Density on Bio-degradable Nanoparticle Transport in Mucus Ex Vivo and Distribution In Vivo. ACS Nano 2015, 9, 9217–9227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaghini, E.; Turner, H.; Pilling, A.; Naasani, I.; MacRobert, A.J. In vivo biodistribution and toxicology studies of cadmium-free indium-based quantum dot nanoparticles in a rat model. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2644–2655. [Google Scholar] [CrossRef]
- Park, J.-H.; Gu, L.; Von Maltzahn, G.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat. Mater. 2009, 8, 331–336. [Google Scholar] [CrossRef]
- Levy, M.; Luciani, N.; Alloyeau, D.; Elgrabli, D.; Deveaux, V.; Pechoux, C.; Chat, S.; Wang, G.; Vats, N.; Gendron, F.; et al. Long term in vivo biotransformation of iron oxide nanoparticles. Biomaterials 2011, 32, 3988–3999. [Google Scholar] [CrossRef]
- Mohammad, A.K.; Reineke, J.J. Quantitative Detection of PLGA Nanoparticle Degradation in Tissues following Intravenous Administration. Mol. Pharm. 2013, 10, 2183–2189. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Cheng, X.; Chen, M.; Liu, C.; Zhao, P.; Huang, W.; He, J.; Zhou, Z.; Miao, L. Hypotoxic and Rapidly Metabolic PEG-PCL-C3-ICG Nanoparticles for Fluorescence-Guided Photothermal/Photodynamic Therapy against OSCC. ACS Appl. Mater. Interfaces 2017, 9, 31509–31518. [Google Scholar] [CrossRef]
- Guoming, H.; Wu, Y.; Lin, Y.; Xu, X.; Lian, H.; Huang-Hao, Y.; Liu, J.-Z.; Wu, X.; Yang, H.-H. Black Phosphorus Quantum Dots with Renal Clearance Property for Efficient Photodynamic Therapy. Small 2018, 14. [Google Scholar] [CrossRef]
- Xu, J.; Yu, M.; Carter, P.; Hernandez, E.; Dang, A.; Kapur, P.; Hsieh, J.; Zheng, J. In Vivo X-ray Imaging of Transport of Renal Clearable Gold Nanoparticles in the Kidneys. Angew. Chem. Int. Ed. 2017, 56, 13356–13360. [Google Scholar] [CrossRef]
- Tan, L.; Wan, J.; Guo, W.; Ou, C.; Liu, T.; Fu, C. Renal-Clearable Quaternary Chalcogenide Nanocrystal for Photoacous-tic/Magnetic Resonance Imaging Guided Tumor Photothermal Therapy. Biomaterials 2018, 159, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, Y.; Wu, P.; Zhou, Y.; Yu, F.; Zhu, C.; Li, Z.; Hang, Y.; Wang, K.; Li, J.; et al. Reversibly Stabilized Polycation Nanoparticles for Combination Treatment of Early- and Late-Stage Metastatic Breast Cancer. ACS Nano 2018, 12, 6620–6636. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Qiu, Y.; Ding, D.; Lin, H.; Sun, W.; Wang, G.D.; Huang, W.; Zhang, W.; Lee, D.; Liu, G.; et al. Gadolinium-Encapsulated Graphene Carbon Nanotheranostics for Imaging-Guided Photodynamic Therapy. Adv. Mater. 2018, 30, e1802748. [Google Scholar] [CrossRef] [PubMed]
- Stern, S.T.; McNeil, S.E. Nanotechnology Safety Concerns Revisited. Toxicol. Sci. 2007, 101, 4–21. [Google Scholar] [CrossRef]
- Shen, A.M.; Minko, T. Pharmacokinetics of inhaled nanotherapeutics for pulmonary delivery. J. Control. Release 2020, 326, 222–244. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Maynard, A.; Donaldson, K.; Castranova, V.; Fitzpatrick, J.; Ausman, K.; Carter, J.; Karn, B.; Kreyling, W.; Lai, D.; et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: Elements of a screening strategy. Part. Fibre Toxicol. 2005, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G. Pulmonary effects of inhaled ultrafine particles. Int. Arch. Occup. Environ. Heal. 2000, 74, 1–8. [Google Scholar] [CrossRef]
- Peters, A.; Veronesi, B.; Calderón-Garcidueñas, L.; Gehr, P.; Chen, L.C.; Geiser, M.; Reed, W.; Rothen-Rutishauser, B.; Schürch, S.; Schulz, H. Translocation and potential neurological effects of fine and ultrafine particles a critical update. Part. Fibre Toxicol. 2006, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- Oberdörster, G.; Ferin, J.; Lehnert, B.E. Correlation between Particle Size, In Vivo Particle Persistence, and Lung Injury. Environ. Health Perspect. 1994, 102, 173. [Google Scholar]
- Tang, Y.; Wang, F.; Jin, C.; Liang, H.; Zhong, X.; Yang, Y. Mitochondrial injury induced by nanosized titanium dioxide in A549 cells and rats. Environ. Toxicol. Pharmacol. 2013, 36, 66–72. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Yang, Z.; Huang, R.; Chen, J.; Wang, R.; Lin, Y. Toxicity of Carbon Nanotubes. Curr. Drug Metab. 2013, 14, 891–899. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Ba, T.; Pu, J.; Chen, T.; Song, Y.; Gu, Y.; Qian, Q.; Xu, Y.; Xiang, K.; et al. Susceptibility of Young and Adult Rats to the Oral Toxicity of Titanium Dioxide Nanoparticles. Small 2013, 9, 1742–1752. [Google Scholar] [CrossRef]
- Bennett, W.D. Rapid Translocation of Nanoparticles From the Lung to the Bloodstream? Am. J. Respir. Crit. Care Med. 2002, 165, 1671–1672. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Shan, W.; Zhang, Z.; Huang, Y. Engineering nanomaterials to overcome the mucosal barrier by modulating surface properties. Adv. Drug Deliv. Rev. 2018, 124, 150–163. [Google Scholar] [CrossRef]
- Choi, H.S.; Ashitate, Y.; Lee, J.H.; Kim, S.H.; Matsui, A.; Insin, N. Rapid Translocation of Nanoparticles from the Lung Air-spaces to the Body. Nat. Biotechnol. 2010, 28, 1300–1303. [Google Scholar] [CrossRef] [Green Version]
- Kreyling, W.G.; Hirn, S.; Möller, W.; Schleh, C.; Wenk, A.; Celik, G.; Lipka, J.; Schäffler, M.; Haberl, N.; Johnston, B.D.; et al. Air–Blood Barrier Translocation of Tracheally Instilled Gold Nanoparticles Inversely Depends on Particle Size. ACS Nano 2014, 8, 222–233. [Google Scholar] [CrossRef] [Green Version]
- Mühlfeld, C.; Gehr, P.; Rothen-Rutishauser, B. Translocation and Cellular Entering Mechanisms of Nanoparticles in the Respir-atory Tract. Swiss Med. Wkly 2008, 138, 387–391. [Google Scholar]
- Semmler-Behnke, M.; Takenaka, S.; Fertsch, S.; Wenk, A.; Seitz, J.; Mayer, P.; Oberdörster, G.; Kreyling, W. Efficient Elimination of Inhaled Nanoparticles from the Alveolar Region: Evidence for Interstitial Uptake and Subsequent Reentrainment onto Airways Epithelium. Environ. Heal. Perspect. 2007, 115, 728–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borm, P.J.; Müller-Schulte, D. Nanoparticles in drug delivery and environmental exposure: Same size, same risks? Nanomedicine 2006, 1, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Tinkle, S.S.; Antonini, J.M.; Rich, B.A.; Roberts, J.R.; Salmen, R.; DePree, K.; Adkins, E.J. Skin as a route of exposure and sensitization in chronic beryllium disease. Environ. Heal. Perspect. 2003, 111, 1202–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toll, R.; Jacobi, U.; Richter, H.; Lademann, J.; Schaefer, H.; Blume-Peytavi, U. Penetration Profile of Microspheres in Follicular Tar-geting of Terminal Hair Follicles. J. Investig. Dermatol. 2004, 123, 168–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuji, J.S.; Maynard, A.D.; Howard, P.C.; James, J.T.; Lam, C.W.; Warheit, D.B. Research Strategies for Safety Evaluation of Na-nomaterials, Part IV: Risk Assessment of Nanoparticles. Toxicol. Sci. 2006, 89, 42–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperling, R.; Gil, P.R.; Zhang, F.; Zanella, M.; Parak, W.J. Biological applications of gold nanoparticles. Chem. Soc. Rev. 2008, 37, 1896–1908. [Google Scholar] [CrossRef]
- Ryman-Rasmussen, J.P.; Riviere, J.E.; Monteiro-Riviere, N.A. Penetration of Intact Skin by Quantum Dots with Diverse Physico-chemical Properties. Toxicol. Sci. 2006, 91, 159–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouse, J.G.; Yang, J.; Ryman-Rasmussen, J.P.; Barron, A.R.; Monteiro-Riviere, N.A. Effects of Mechanical Flexion on the Penetration of Fullerene Amino Acid-Derivatized Peptide Nanoparticles through Skin. Nano Lett. 2007, 7, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Ryman-Rasmussen, J.P.; Riviere, J.E.; Monteiro-Riviere, N.A. Surface Coatings Determine Cytotoxicity and Irritation Potential of Quantum Dot Nanoparticles in Epidermal Keratinocytes. J. Investig. Dermatol. 2007, 127, 143–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, M.Q.; Wu, Q.; Wang, J.X.; Hou, S.K.; Miao, Y.; Peng, J.L. In Vitro and In Vivo Transdermal Delivery Capacity of Quan-tum Dots through Mouse Skin. Nanotechnology 2007, 18, 455103. [Google Scholar] [CrossRef]
- Mortensen, L.; Oberdörster, G.; Pentland, A.P.; DeLouise, L.A. In Vivo Skin Penetration of Quantum Dot Nanoparticles in the Murine Model: The Effect of UVR. Nano Lett. 2008, 8, 2779–2787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsaesser, A.; Howard, C.V. Toxicology of Nanoparticles. Adv. Drug. Deliv. Rev. 2012, 64, 129–137. [Google Scholar] [CrossRef]
- Hoet, P.H.; Brüske-Hohlfeld, I.; Salata, O.V. Nanoparticles-Known and Unknown Health Risks. J. Nanobiotechnology 2004, 2, 12. [Google Scholar] [CrossRef] [Green Version]
- Florence, A.T. Nanoparticle uptake by the oral route: Fulfilling its potential? Drug Discov. Today Technol. 2005, 2, 75–81. [Google Scholar] [CrossRef]
- Jani, P.; Halbert, G.W.; Langridge, J.; Florence, A.T. Nanoparticle Uptake by the Rat Gastrointestinal Mucosa: Quantitation and Particle Size Dependency. J. Pharm. Pharmacol. 2011, 42, 821–826. [Google Scholar] [CrossRef]
- Balasubramanian, S.K.; Jittiwat, J.; Manikandan, J.; Ong, C.N.; Yu, L.; Ong, W.-Y. Biodistribution of gold nanoparticles and gene expression changes in the liver and spleen after intravenous administration in rats. Biomaterials 2010, 31, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Texier, I.; Josser, V. In Vivo Imaging of Quantum Dots. Adv. Struct. Saf. Stud. 2009, 544, 393–406. [Google Scholar] [CrossRef]
- Shokeen, M.; Fettig, N.M.; Rossin, R. Synthesis, in vitro and in vivo evaluation of radiolabeled nanoparticles. Q. J. Nucl. Med. Mol. Imaging 2008, 52, 267–277. [Google Scholar] [PubMed]
- Matsumoto, Y.; Nichols, J.W.; Toh, K.; Nomoto, T.; Cabral, H.; Miura, Y.; Christie, R.J.; Yamada, N.; Ogura, T.; Kano, M.R.; et al. Vascular bursts enhance permeability of tumour blood vessels and improve nanoparticle delivery. Nat. Nanotechnol. 2016, 11, 533–538. [Google Scholar] [CrossRef]
- Campbell, F.; Bos, F.L.; Sieber, S.; Arias-Alpizar, G.; Koch, B.E.V.; Huwyler, J.; Kros, A.; Bussmann, J. Directing Nanoparticle Biodistribution through Evasion and Exploitation of Stab2-Dependent Nanoparticle Uptake. ACS Nano 2018, 12, 2138–2150. [Google Scholar] [CrossRef] [PubMed]
- Soenen, S.J.; Parak, W.J.; Rejman, J.; Manshian, B. (Intra)Cellular Stability of Inorganic Nanoparticles: Effects on Cytotoxicity, Particle Functionality, and Biomedical Applications. Chem. Rev. 2015, 115, 2109–2135. [Google Scholar] [CrossRef]
- Mejias, R.; Gutierrez, L.; Salas, G.; Perez-Yague, S.; Zotes, T.M.; Lazaro, F.J. Long Term Biotransformation and Toxicity of Di-mercaptosuccinic Acid-Coated Magnetic Nanoparticles Support their Use in Biomedical Applications. J. Control. Release 2013, 171, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Freund, B.; Tromsdorf, U.I.; Bruns, O.T.; Heine, M.; Giemsa, A.; Bartelt, A.; Salmen, S.C.; Raabe, N.; Heeren, J.; Ittrich, H.; et al. A Simple and Widely Applicable Method to 59Fe-Radiolabel Monodisperse Superparamagnetic Iron Oxide Nanoparticles for In Vivo Quantification Studies. ACS Nano 2012, 6, 7318–7325. [Google Scholar] [CrossRef] [PubMed]
- Feliu, N.; Docter, D.; Heine, M.; del Pino, P.; Ashraf, S.; Kolosnjaj-Tabi, J.; Macchiarini, P.; Nielsen, P.; Alloyeau, D.; Gazeau, F.; et al. In vivo degeneration and the fate of inorganic nanoparticles. Chem. Soc. Rev. 2016, 45, 2440–2457. [Google Scholar] [CrossRef] [Green Version]
- Kolosnjaj-Tabi, J.; Javed, Y.; Lartigue, L.; Volatron, J.; Elgrabli, D.; Marangon, I.; Pugliese, G.; Caron, B.; Figuerola, A.; Luciani, N.; et al. The One Year Fate of Iron Oxide Coated Gold Nanoparticles in Mice. ACS Nano 2015, 9, 7925–7939. [Google Scholar] [CrossRef] [Green Version]
- Lartigue, L.; Alloyeau, D.; Kolosnjaj-Tabi, J.; Javed, Y.; Guardia, P.; Riedinger, A.; Péchoux, C.; Pellegrino, T.; Wilhelm, C.; Gazeau, F. Biodegradation of Iron Oxide Nanocubes: High-Resolution In Situ Monitoring. ACS Nano 2013, 7, 3939–3952. [Google Scholar] [CrossRef]
- Tsoi, K.M.; MacParland, S.; Ma, X.-Z.; Spetzler, V.N.; Echeverri, J.; Ouyang, B.; Fadel, S.M.; Sykes, E.A.; Goldaracena, N.; Kaths, J.M.; et al. Mechanism of hard-nanomaterial clearance by the liver. Nat. Mater. 2016, 15, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Abdelhalim, M.A.K.; Jarrar, B.M. Gold nanoparticles administration induced prominent inflammatory, central vein intima disruption, fatty change and Kupffer cells hyperplasia. Lipids Heal. Dis. 2011, 10, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Feng, W.; Wang, M.; Wang, T.; Gu, Y.; Zhu, M.; Ouyang, H.; Shi, J.; Zhang, F.; Zhao, Y.; et al. Acute toxicological impact of nano- and submicro-scaled zinc oxide powder on healthy adult mice. J. Nanoparticle Res. 2008, 10, 263–276. [Google Scholar] [CrossRef]
- Chen, Z.; Meng, H.; Xing, G.; Chen, C.; Zhao, Y.; Jia, G.; Wang, T.; Yuan, H.; Ye, C.; Zhao, F.; et al. Acute toxicological effects of copper nanoparticles in vivo. Toxicol. Lett. 2006, 163, 109–120. [Google Scholar] [CrossRef]
- Schluep, T.; Hwang, J.; Hildebrandt, I.; Czernin, J.; Choi, C.; Alabi, C. Pharmacokinetics and Tumor Dynamics of the Nano-particle IT-101 from PET Imaging and Tumor Histological Measurements. Proc. Natl. Acad. Sci. USA 2009, 106, 11394–11399. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Yuan, S.-X.; Zhao, L.-H.; Wang, C.; Ni, J.-S.; Wang, Z.-G.; Ling-Hao, Z.; Wu, M.-C.; Zhou, W.-P. Ligand-Directed Stearic Acid Grafted Chitosan Micelles to Increase Therapeutic Efficacy in Hepatic Cancer. Mol. Pharm. 2015, 12, 644–652. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.-F.; Zhou, L.; Liu, Y.; Meng, L.; Zhang, K.; Wu, X.; Zhang, L.; Li, B.; Chen, C. Characterization of gold nanorods in vivo by integrated analytical techniques: Their uptake, retention, and chemical forms. Anal. Bioanal. Chem. 2010, 396, 1105–1114. [Google Scholar] [CrossRef]
- Poelstra, K.; Prakash, J.; Beljaars, L. Drug targeting to the diseased liver. J. Control. Release 2012, 161, 188–197. [Google Scholar] [CrossRef]
- Wang, B.; He, X.; Zhang, Z.; Zhao, Y.; Feng, W. Metabolism of Nanomaterials in Vivo: Blood Circulation and Organ Clearance. Acc. Chem. Res. 2013, 46, 761–769. [Google Scholar] [CrossRef]
- Heyder, J.; Gebhart, J.; Rudolf, G.; Schiller, C.; Stahlhofen, W. Deposition of particles in the human respiratory tract in the size range 0.005–15 μm. J. Aerosol Sci. 1986, 17, 811–825. [Google Scholar] [CrossRef]
- Knowles, M.R.; Boucher, R.C. Mucus clearance as a primary innate defense mechanism for mammalian airways. J. Clin. Investig. 2002, 109, 571–577. [Google Scholar] [CrossRef]
- Faraj, A.A.; Shaik, A.P.; Shaik, A.S. Effect of Surface Coating on the Biocompatibility and In Vivo MRI Detection of Iron Oxide Nanoparticles after Intrapulmonary Administration. Nanotoxicology 2015, 9, 825–834. [Google Scholar] [CrossRef]
- Heilig, E.A.; Thompson, K.J.; Molina, R.M.; Ivanov, A.R.; Brain, J.D.; Wessling-Resnick, M. Manganese and iron transport across pulmonary epithelium. Am. J. Physiol. Cell. Mol. Physiol. 2006, 290, L1247–L1259. [Google Scholar] [CrossRef] [PubMed]
- Turi, J.L.; Yang, F.; Garrick, M.; Piantadosi, C.A.; Ghio, A.J. The iron cycle and oxidative stress in the lung. Free. Radic. Biol. Med. 2004, 36, 850–857. [Google Scholar] [CrossRef]
- Valois, C.R.A.; Braz, J.M.; Nunes, E.S.; Vinolo, M.A.R.; Lima, E.C.D.; Curi, R. The Effect of DMSA-Functionalized Magnetic Na-noparticles on Transendothelial Migration of Monocytes in the Murine Lung via a β2 Integrin-Dependent Pathway. Biomaterials 2010, 31, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.S.; Cho, M.; Kim, S.R.; Choi, M.; Lee, J.Y.; Han, B.S.; Park, S.N.; Yu, M.K.; Jon, S.; Jeong, J. Pulmonary Toxicity and Kinetic Study of Cy5.5-Conjugated Super-paramagnetic Iron Oxide Nanoparticles by Optical Imaging. Toxicol. Appl. Pharmacol. 2009, 239, 106–115. [Google Scholar] [CrossRef]
- Haque, S.; Whittaker, M.; McIntosh, M.P.; Pouton, C.; Phipps, S.; Kaminskas, L.M. A comparison of the lung clearance kinetics of solid lipid nanoparticles and liposomes by following the 3H-labelled structural lipids after pulmonary delivery in rats. Eur. J. Pharm. Biopharm. 2018, 125, 1–12. [Google Scholar] [CrossRef]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zheng, J. Clearance Pathways and Tumor Targeting of Imaging Nanoparticles. ACS Nano 2015, 9, 6655–6674. [Google Scholar] [CrossRef] [Green Version]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Yu, M.; Peng, C.; Carter, P.; Tian, J.; Ning, X. Dose Dependencies and Biocompatibility of Renal Clearable Gold Nano-particles: From Mice to Non-human Primates. Angew. Chem. Int. Ed. Engl. 2018, 57, 266–271. [Google Scholar] [CrossRef]
- Cheng, L.; Jiang, D.; Kamkaew, A.; Valdovinos, H.; Im, H.-J.; Feng, L.; England, C.G.; Goel, S.; Barnhart, T.E.; Liu, Z.; et al. Renal-Clearable PEGylated Porphyrin Nanoparticles for Image-Guided Photodynamic Cancer Therapy. Adv. Funct. Mater. 2017, 27, 1702928. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J. Urine Formation and Excretion. In Physiology, 7th ed.; Zhu, D.N., Ed.; People’s Medical Publishing House: Beijing, China, 2008; pp. 212–239. [Google Scholar]
- Ye, L.; Yong, K.-T.; Liu, L.; Roy, I.; Hu, R.; Zhu, J.; Cai, H.; Law, W.-C.; Liu, J.; Wang, K.; et al. A pilot study in non-human primates shows no adverse response to intravenous injection of quantum dots. Nat. Nanotechnol. 2012, 7, 453–458. [Google Scholar] [CrossRef]
- Haraldsson, B.; Nyström, J.; Deen, W.M. Properties of the Glomerular Barrier and Mechanisms of Proteinuria. Physiol. Rev. 2008, 88, 451–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, J.E. Guyton and Hall Textbook of Medical Physiology, 13th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Du, B.; Jiang, X.; Das, A.; Zhou, Q.; Yu, M.; Jin, R.; Zheng, J. Glomerular barrier behaves as an atomically precise bandpass filter in a sub-nanometre regime. Nat. Nanotechnol. 2017, 12, 1096–1102. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, Y.; Luehmann, H.; Xia, X.; Brown, P.; Jarreau, C. Evaluating the Pharmacokinetics and In Vivo Cancer Tar-geting Capability of Au Nanocages by Positron Emission Tomography Imaging. ACS Nano 2012, 6, 5880–5888. [Google Scholar] [CrossRef] [Green Version]
- Iyer, A.K.; Khaled, G.; Fang, J.; Maeda, H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov. Today 2006, 11, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Sato, N.; Hiraga, A.; Saga, T.; Nakamoto, Y.; Ueda, H. 3D-Micro-MR Angiography of Mice Using Macromo-lecular MR Contrast Agents with Polyamidoamine Dendrimer Core with Reference to their Pharmacokinetic Properties. Magn. Reson. Med. 2001, 45, 454–460. [Google Scholar] [CrossRef]
- Liu, R.S.; Chou, T.K.; Chang, C.H.; Wu, C.Y.; Chang, C.W.; Chang, T.J. Biodistribution, Pharmacokinetics and PET Imaging of [(18)F]FMISO, [(18)F]FDG and [(18)F]FAc in a Sarcoma- and Inflammation-Bearing Mouse Model. Nucl. Med. Biol. 2009, 36, 305–312. [Google Scholar] [CrossRef]
- Zamboni, W.C. Liposomal, Nanoparticle, and Conjugated Formulations of Anticancer Agents. Clin. Cancer Res. 2005, 11, 8230–8234. [Google Scholar] [CrossRef] [Green Version]
- Kareem, H.; Sandstrom, K.; Elia, R.; Gedda, L.; Anniko, M.; Lundqvist, H. Blocking EGFR in the Liver Improves the Tu-mor-to-Liver Uptake Ratio of Radiolabeled EGF. Tumor. Biol. 2010, 31, 79–87. [Google Scholar] [CrossRef]
- Yang, R.H.; Chang, L.W.; Wu, J.P.; Tsai, M.H.; Wang, H.J.; Kuo, Y.C. Persistent Tissue Kinetics and Redistribution of Nanoparti-cles, Quantum Dot 705, in Mice: ICP-MS Quantitative Assessment. Environ. Health Persp. 2007, 115, 1339–1343. [Google Scholar] [CrossRef]
- Kobayashi, H.; Longmire, M.R.; Ogawa, M.; Choyke, P.L. Rational chemical design of the next generation of molecular imaging probes based on physics and biology: Mixing modalities, colors and signals. Chem. Soc. Rev. 2011, 40, 4626–4648. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Yu, M.; Zhou, C.; Yang, S.; Ning, X.; Zheng, J. Passive Tumor Targeting of Renal-Clearable Luminescent Gold Nanoparticles: Long Tumor Retention and Fast Normal Tissue Clearance. J. Am. Chem. Soc. 2013, 135, 4978–4981. [Google Scholar] [CrossRef] [Green Version]
- Mo, J.; He, L.; Ma, B.; Chen, T. Tailoring Particle Size of Mesoporous Silica Nanosystem To Antagonize Glioblastoma and Overcome Blood–Brain Barrier. ACS Appl. Mater. Interfaces 2016, 8, 6811–6825. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Z.; Wang, L.; Cun, D.; Tong, H.H.Y.; Yan, R.; Chen, X.; Wang, R.; Zheng, Y. Enhanced topical penetration, system exposure and anti-psoriasis activity of two particle-sized, curcumin-loaded PLGA nanoparticles in hydrogel. J. Control. Release 2017, 254, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Senut, M.; Zhang, Y.; Liu, F.; Sen, A.; Ruden, D.M.; Mao, G. Size-Dependent Toxicity of Gold Nanoparticles on Human Embryonic Stem Cells and Their Neural Derivatives. Small 2015, 12, 631–646. [Google Scholar] [CrossRef] [Green Version]
- Ji, C.; Gao, Q.; Dong, X.; Yin, W.; Gu, Z.; Gan, Z. A Size-Reducible Nanodrug With An Aggregation-Enhanced Photody-namic Effect for Deep Chemo-Photodynamic Therapy. Angew. Chem. Int. Ed. Engl. 2018, 57, 11384–11388. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Yang, X.; Du, X.; Wang, H.; Li, H.; Liu, W.; Yao, Y.; Zhu, Y.; Ma, Y.; Song, E. Optimizing the Size of Micellar Nanoparticles for Efficient siRNA Delivery. Adv. Funct. Mater. 2015, 25, 4778–4787. [Google Scholar] [CrossRef]
- Caster, J.M.; Yu, S.K.; Patel, A.N.; Newman, N.J.; Lee, Z.J.; Warner, S.; Wagner, K.T.; Roche, K.C.; Tian, X.; Min, Y.; et al. Effect of particle size on the biodistribution, toxicity, and efficacy of drug-loaded polymeric nanoparticles in chemoradiotherapy. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-H.; Bai, J.; Deng, J.; Fang, C.-J.; Chen, X. TAT-Modified Gold Nanoparticle Carrier with Enhanced Anticancer Activity and Size Effect on Overcoming Multidrug Resistance. ACS Appl. Mater. Interfaces 2017, 9, 5828–5837. [Google Scholar] [CrossRef]
- Chaudhuri, P.; Harfouche, R.; Soni, S.; Hentschel, D.M.; Sengupta, S. Shape Effect of Carbon Nanovectors on Angiogenesis. ACS Nano 2010, 4, 574–582. [Google Scholar] [CrossRef]
- Chauhan, V.P.; Popovic’, Z.; Chen, O.; Cui, J.; Fukumura, D.; Bawendi, M.G. Fluorescent Nanorods and Nanospheres for Real-Time In Vivo Probing of Nanoparticle Shape-Dependent Tumor Penetration. Angew. Chem. Int. Ed. 2011, 50, 11417–11420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Yang, G.; Wang, Y.; Du, Y.; Liu, H.; Zhu, Y.; Mao, C.; Zhang, S. Chimeric Protein Template-Induced Shape Control of Bone Mineral Nanoparticles and Its Impact on Mesenchymal Stem Cell Fate. Biomacromolecules 2015, 16, 1987–1996. [Google Scholar] [CrossRef] [Green Version]
- Shao, A.; Xie, Y.; Zhu, S.; Guo, Z.; Zhu, S.; Guo, J.; Shi, P.; James, T.D.; Tian, H.; Zhu, W.-H. Far-Red and Near-IR AIE-Active Fluorescent Organic Nanoprobes with Enhanced Tumor-Targeting Efficacy: Shape-Specific Effects. Angew. Chem. Int. Ed. 2015, 54, 7275–7280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florez, L.; Herrmann, C.; Cramer, J.M.; Hauser, C.P.; Koynov, K.; Landfester, K. How Shape Infl uences Uptake: Interactions of Anisotropic Polymer Nanoparticles and Human Mesenchymal Stem Cells. Small 2012, 8, 2222–2230. [Google Scholar] [CrossRef]
- Huang, X.; Li, L.; Liu, T.; Hao, N.; Liu, H.; Chen, D.; Tang, F. The Shape Effect of Mesoporous Silica Nanoparticles on Biodistribution, Clearance, and Biocompatibility in Vivo. ACS Nano 2011, 5, 5390–5399. [Google Scholar] [CrossRef]
- Yue, J.; Feliciano, T.J.; Li, W.; Lee, A.; Odom, T.W. Gold Nanoparticle Size and Shape Effects on Cellular Uptake and Intracellular Distribution of siRNA Nanoconstructs. Bioconjugate Chem. 2017, 28, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Souris, J.S.; Lee, C.-H.; Cheng, S.-H.; Chen, C.-T.; Yang, C.-S.; Ho, J.-A.A.; Mou, C.-Y.; Lo, L.-W. Surface charge-mediated rapid hepatobiliary excretion of mesoporous silica nanoparticles. Biomaterials 2010, 31, 5564–5574. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, K.; Mena, J.A.; Male, K.B.; Hrapovic, S.; Kamen, A.; Luong, J. Effect of Surface Charge on the Cellular Uptake and Cytotoxicity of Fluorescent Labeled Cellulose Nanocrystals. ACS Appl. Mater. Interfaces 2010, 2, 2924–2932. [Google Scholar] [CrossRef] [Green Version]
- Calatayud, M.P.; Sanz, B.; Raffa, V.; Riggio, C.; Ibarra, M.R.; Goya, G.F. The Effect of Surface Charge of Functionalized Fe3O4 Nano-particles on Protein Adsorption and Cell Uptake. Biomaterials 2014, 35, 6389–6399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahbazi, M.-A.; Hamidi, M.; Mäkilä, E.; Zhang, H.; Almeida, P.V.; Kaasalainen, M.; Salonen, J.J.; Hirvonen, J.T.; Santos, H.A. The mechanisms of surface chemistry effects of mesoporous silicon nanoparticles on immunotoxicity and biocompatibility. Biomaterials 2013, 34, 7776–7789. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, H.; Wu, C.; Wang, J.; Zhang, S.; Gao, J. Inhibition of Intrinsic Coagulation Improves Safety and Tu-mor-Targeted Drug Delivery of Cationic Solid Lipid Nanoparticles. Biomaterials 2018, 156, 77–87. [Google Scholar] [CrossRef]
- Yang, K.J.; Son, J.; Jung, S.Y.; Yi, G.; Yoo, J.; Kim, D.K. Optimized Phospholipid-Based Nanoparticles for Inner Ear Drug De-livery and Therapy. Biomaterials 2018, 171, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Loos, C.; Syrovets, T.; Musyanovych, A.; Mailänder, V.; Landfester, K.; Simmet, T. Amino-Functionalized Nanoparticles as Inhib-itors of mTOR and Inducers of Cell Cycle Arrest in Leukemia Cells. Biomaterials 2014, 35, 1944–1953. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Parker, C.L.; Lin, Y.; Press, O.W.; Park, S.I.; Lai, S.K. Pretargeting with Bispecific Fusion Proteins Facilitates Delivery of Na-noparticles to Tumor Cells with Distinct Surface Antigens. J. Control. Release 2017, 255, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Guo, Q.; Zhao, Y.; Zhang, P.; Zhang, T.; Zhang, X.; Li, C. Functional Silver Nanoparticle as a Benign Antimicrobial Agent That Eradicates Antibiotic-Resistant Bacteria and Promotes Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 25798–25807. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, T.; Alarçin, E.; Byambaa, B.; Guan, X.; Ding, J. Porous Electrospun Fibers with Self-Sealing Functionality: An Enabling Strategy for Trapping Biomacromolecules. Small 2017, 13, 1701949. [Google Scholar] [CrossRef]
- Ning, W.; Di, Z.; Yu, Y.; Zeng, P.; Di, C.; Chen, D.; Kong, X.; Nie, G.; Zhao, Y.; Li, L. Imparting Designer Biorecognition Functionality to Metal-Organic Frameworks by a DNA-Mediated Surface Engineering Strategy. Small 2018, 14, e1703812. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.; Park, J.; Willis, K.; Park, J.E.; Lyle, L.T.; Lee, W. Surface Modification of Polymer Nanoparticles with Native Albu-min for Enhancing Drug Delivery to Solid Tumors. Biomaterials 2018, 180, 206–224. [Google Scholar] [CrossRef]
- He, Y.; Huang, Y.; Huang, Z.; Jiang, Y.; Sun, X.; Shen, Y. Bisphosphonate-Functionalized Coordination Polymer Nanoparti-cles for the Treatment of Bone Metastatic Breast Cancer. J. Control. Release 2017, 264, 76–88. [Google Scholar] [CrossRef]
- Zhou, Y.; Deng, R.; Zhen, M.; Li, J.; Guan, M.; Jia, W.; Li, X.; Zhang, Y.; Yu, T.; Zou, T.; et al. Amino acid functionalized gadofullerene nanoparticles with superior antitumor activity via destruction of tumor vasculature in vivo. Biomaterials 2017, 133, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.; Yang, J.; Yoon, D.; Kim, S.; Joo, S.W.; Choi, J. Differential Crosstalk between Global DNA Methylation and Metab-olomics Associated with Cell Type Specific Stress Response by Pristine and Functionalized MWCNT. Biomaterials 2017, 115, 167–180. [Google Scholar] [CrossRef]
- Shahabi, S.; Döscher, S.; Bollhorst, T.; Treccani, L.; Maas, M.; Dringen, R. Enhancing Cellular Uptake and Doxorubicin Deliv-ery of Mesoporous Silica Nanoparticles via Surface Functionalization: Effects of Serum. ACS Appl. Mater Interfaces 2015, 7, 26880–26891. [Google Scholar] [CrossRef]
- Shima, F.; Akagi, T.; Uto, T.; Akashi, M. Manipulating the Antigen-Specific Immune Response by the Hydrophobicity of Am-phiphilic Poly(γ-Glutamic Acid) Nanoparticles. Biomaterials 2013, 34, 9709–9716. [Google Scholar] [CrossRef]
- Manshian, B.B.; Moyano, D.F.; Corthout, N.; Munck, S.; Himmelreich, U.; Rotello, V.M. High-Content Imaging and Gene Ex-pression Analysis to Study Cell- Nanomaterial Interactions: The Effect of Surface Hydrophobicity. Biomaterials 2014, 35, 9941–9950. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.R.; Gnopo, Y.D.M.; Gambinossi, F.; Mylon, S.E.; Ferri, J.K. Modulation of Cell Responses to Ag-(MeO2MA-co-OEGMA): Effects of Nanoparticle Surface Hydrophobicity and Serum Proteins on Cellular Uptake and Toxicity. J. Biomed. Mater. Res. A 2018, 106, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Fayol, D.; Luciani, N.; Lartigue, L.; Gazeau, F.; Wilhelm, C. Managing Magnetic Nanoparticle Aggregation and Cellular Uptake: A Precondition for Efficient Stem-Cell Differentiation and MRI Tracking. Adv. Heal. Mater. 2013, 2, 313–325. [Google Scholar] [CrossRef]
- Mejías, R.; Flores, P.H.; Talelli, M.; Tajada-Herráiz, J.L.; Brollo, M.; Portilla, Y. Cell-Promoted Nanoparticle Aggregation De-creases Nanoparticle-Induced Hyperthermia under an Alternating Magnetic Field Independently of Nanoparticle Coating, Core Size, and Subcellular Localization. ACS Appl. Mater. Interfaces 2019, 11, 340–355. [Google Scholar] [CrossRef] [PubMed]
- Sugikawa, K.; Matsuo, K.; Ikeda, A. Suppression of Gold Nanoparticle Aggregation on Lipid Membranes Using Nanosized Liposomes To Increase Steric Hindrance. Langmuir 2018, 35, 229–236. [Google Scholar] [CrossRef]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; Macmillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The entry of nanoparticles into solid tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Pouton, C.W.; McIntosh, M.P.; Ascher, D.B.; Keizer, D.W.; Whittaker, M.R.; Kaminskas, L.M. The impact of size and charge on the pulmonary pharmacokinetics and immunological response of the lungs to PLGA nanoparticles after intratracheal administration to rats. Nanomed. Nanotechnol. Biol. Med. 2020, 30, 102291. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Chen, X.; Li, X.; Liu, Y.; Jia, F.; Jin, Q.; Ji, J. Structure-Switchable DNA Programmed Disassembly of Nanoparticles for Smart Size Tunability and Cancer-Specific Drug Release. ACS Appl. Mater. Interfaces 2020, 12, 22560–22571. [Google Scholar] [CrossRef]
- Cheng, Z.; Al Zaki, A.; Hui, J.Z.; Muzykantov, V.R.; Tsourkas, A. Multifunctional Nanoparticles: Cost Versus Benefit of Adding Targeting and Imaging Capabilities. Science 2012, 338, 903–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreher, M.R.; Liu, W.; Michelich, C.R.; Dewhirst, M.W.; Yuan, F.; Chilkoti, A. Tumor Vascular Permeability, Accumulation, and Penetration of Macromolecular Drug Carriers. J. Natl. Cancer Inst. 2006, 98, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Lankveld, D.P.K.; Oomen, A.G.; Krystek, P.; Neigh, A.; De Jong, A.T.; Noorlander, C.W.; Van Eijkeren, J.; Geertsma, R.E.; De Jong, W.H. The kinetics of the tissue distribution of silver nanoparticles of different sizes. Biomaterials 2010, 31, 8350–8361. [Google Scholar] [CrossRef]
- Yuan, Y.-Y.; Mao, C.-Q.; Du, X.-J.; Du, J.-Z.; Wang, F.; Wang, J. Surface Charge Switchable Nanoparticles Based on Zwitterionic Polymer for Enhanced Drug Delivery to Tumor. Adv. Mater. 2012, 24, 5476–5480. [Google Scholar] [CrossRef]
- Cong, Z.; Zhang, L.; Ma, S.-Q.; Lam, K.S.; Yang, F.-F.; Liao, Y.-H. Size-Transformable Hyaluronan Stacked Self-Assembling Peptide Nanoparticles for Improved Transcellular Tumor Penetration and Photo–Chemo Combination Therapy. ACS Nano 2020, 14, 1958–1970. [Google Scholar] [CrossRef]
- Peng, S.; Ouyang, B.; Men, Y.; Du, Y.; Cao, Y.; Xie, R.; Pang, Z.; Shen, S.; Yang, W. Biodegradable zwitterionic polymer membrane coating endowing nanoparticles with ultra-long circulation and enhanced tumor photothermal therapy. Biomaterials 2020, 231, 119680. [Google Scholar] [CrossRef]
- Wang, J.; Wu, W.; Zhang, Y.; Wang, X.; Qian, H.; Liu, B.; Jiang, X. The combined effects of size and surface chemistry on the accumulation of boronic acid-rich protein nanoparticles in tumors. Biomaterials 2014, 35, 866–878. [Google Scholar] [CrossRef]
- Guo, X.; Wei, X.; Jing, Y.; Zhou, S. Size Changeable Nanocarriers with Nuclear Targeting for Effectively Overcoming Multidrug Resistance in Cancer Therapy. Adv. Mater. 2015, 27, 6450–6456. [Google Scholar] [CrossRef] [PubMed]
- Sykes, E.A.; Chen, J.; Zheng, G.; Chan, W.C. Investigating the Impact of Nanoparticle Size on Active and Passive Tumor Targeting Efficiency. ACS Nano 2014, 8, 5696–5706. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mao, W.; Lock, L.L.; Tang, J.; Sui, M.; Sun, W.; Cui, H.; Xu, D.; Shen, Y. The Role of Micelle Size in Tumor Accumulation, Penetration, and Treatment. ACS Nano 2015, 9, 7195–7206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Wua, D.; Shen, X.; Chen, J.; Sun, Y.M.; Liu, P.X. Size-Dependent Radiosensitization of PEG-Coated Gold Nanoparticles for Cancer Radiation Therapy. Biomaterials 2012, 33, 6408–6419. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Tian, R.; Wu, J.; Fan, Q.; Yung, B.C.; Niu, G.; Jacobson, O.; Wang, Z.; Liu, G.; Yu, G.; et al. Impact of Semiconducting Perylene Diimide Nanoparticle Size on Lymph Node Mapping and Cancer Imaging. ACS Nano 2017, 11, 4247–4255. [Google Scholar] [CrossRef]
- Popović, Z.; Liu, W.; Chauhan, V.; Lee, J.; Wong, C.; Greytak, A.; Insin, N.; Nocera, D.G.; Fukumura, D.; Jain, R.K.; et al. A Nanoparticle Size Series for In Vivo Fluorescence Imaging. Angew. Chem. Int. Ed. 2010, 49, 8649–8652. [Google Scholar] [CrossRef] [Green Version]
- Hickey, J.; Santos, J.L.; Williford, J.-M.; Mao, H.-Q. Control of polymeric nanoparticle size to improve therapeutic delivery. J. Control. Release 2015, 219, 536–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kibria, G.; Hatakeyama, H.; Ohga, N.; Hida, K.; Harashima, H. The effect of liposomal size on the targeted delivery of doxorubicin to Integrin αvβ3-expressing tumor endothelial cells. Biomaterials 2013, 34, 5617–5627. [Google Scholar] [CrossRef]
- Tang, L.; Yang, X.; Yin, Q.; Cai, K.; Wang, H.; Chaudhury, I.; Yao, C.; Zhou, Q.; Kwon, M.; Hartman, J.A.; et al. Investigating the optimal size of anticancer nanomedicine. Proc. Natl. Acad. Sci. USA 2014, 111, 15344–15349. [Google Scholar] [CrossRef] [Green Version]
- Ruan, S.; Hu, C.; Tang, X.; Cun, X.; Xiao, W.; Shi, K.; He, Q.; Gao, H. Increased Gold Nanoparticle Retention in Brain Tumors by in Situ Enzyme-Induced Aggregation. ACS Nano 2016, 10, 10086–10098. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; He, Q.; Gao, H. Matrix metalloproteinase triggered size-shrinkable gelatin-gold fabricated nanoparticles for tumor microenvironment sensitive penetration and diagnosis of glioma. Nanoscale 2015, 7, 9487–9496. [Google Scholar] [CrossRef]
- Hu, C.; Cun, X.; Ruan, S.; Liu, R.; Xiao, W.; Yang, X.; Yang, Y.; Yang, C.; Gao, H. Enzyme-triggered size shrink and laser-enhanced NO release nanoparticles for deep tumor penetration and combination therapy. Biomaterials 2018, 168, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xiao, W.; Hu, C.; Xie, R.; Gao, H. Theranostic size-reducible and no donor conjugated gold nanocluster fabricated hyaluronic acid nanoparticle with optimal size for combinational treatment of breast cancer and lung metastasis. J. Control. Release 2018, 278, 127–139. [Google Scholar] [CrossRef]
- Zhu, X.; Vo, C.; Taylor, M.; Smith, B.R. Non-spherical micro- and nanoparticles in nanomedicine. Mater. Horizons 2019, 6, 1094–1121. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, Z.; Song, J.; Chen, X. Anisotropic nanomaterials for shape-dependent physicochemical and biomedical applications. Chem. Soc. Rev. 2019, 48, 5140–5176. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, R.; Singhb, V.; Jurneyb, P.; Shib, L.; Sreenivasanb, S.V.; Roy, K. Mammalian Cells Preferentially Internalize Hydrogel Nanodiscs over Nanorods and Use Shape-Specific Uptake Mechanisms. Proc. Natl. Acad. Sci. USA 2013, 110, 17247–17252. [Google Scholar] [CrossRef] [Green Version]
- Toy, R.; Peiris, P.M.; Ghaghada, K.B.; Karathanasis, E. Shaping cancer nanomedicine: The effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine 2014, 9, 121–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Chang, Z.; Lu, M.; Shao, D.; Yue, J.; Yang, D.; Zheng, X.; Li, M.; He, K.; Zhang, M.; et al. Shape-controlled magnetic mesoporous silica nanoparticles for magnetically-mediated suicide gene therapy of hepatocellular carcinoma. Biomaterials 2018, 154, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.-M.; Wang, Z.; Shao, D.; Yue, J.; Xing, H.; Li, L.; Ge, M.; Li, M.; Yan, H.; Hu, H.; et al. Shape Engineering Boosts Magnetic Mesoporous Silica Nanoparticle-Based Isolation and Detection of Circulating Tumor Cells. ACS Appl. Mater. Interfaces 2018, 10, 10656–10663. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Nie, T.; Chen, Y.; Wang, Z.; Huang, H.; Liu, L.; Chen, Y. Molecular bottlebrush as a unimolecular vehicle with tunable shape for photothermal cancer therapy. Biomaterials 2018, 178, 620–629. [Google Scholar] [CrossRef]
- Rampersaud, S.; Fang, J.; Wei, Z.; Fabijanic, K.; Silver, S.; Jaikaran, T.; Ruiz, Y.; Houssou, M.; Yin, Z.; Zheng, S.; et al. The Effect of Cage Shape on Nanoparticle-Based Drug Carriers: Anticancer Drug Release and Efficacy via Receptor Blockade Using Dextran-Coated Iron Oxide Nanocages. Nano Lett. 2016, 16, 7357–7363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kankala, R.K.; Han, Y.; Na, J.; Lee, C.; Sun, Z.; Wang, S.; Kimura, T.; Ok, Y.S.; Yamauchi, Y.; Chen, A.; et al. Nanoarchitectured Structure and Surface Biofunctionality of Mesoporous Silica Nanoparticles. Adv. Mater. 2020, 32, e1907035. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Lyu, Z.; Louis, B.; Tintaru, A.; Laurini, E.; Marson, D.; Zhang, M.; Shao, W.; Jiang, Y.; Bouhlel, A.; et al. Surface Charge of Supramolecular Nanosystems for In Vivo Biodistribution: A MicroSPECT/CT Imaging Study. Small 2020, 16. [Google Scholar] [CrossRef]
- Xiao, K.; Li, Y.; Luo, J.; Lee, J.S.; Xiao, W.; Gonik, A.M.; Agarwal, R.G.; Lam, K.S. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials 2011, 32, 3435–3446. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Wang, L.; Zhang, M.; Tao, K.; Wang, B.; Lin, M.; Zhang, X.; Liu, Y.; Hou, Y.; Zhang, H.; et al. Tumor Microenvironment-Responsive Nanoshuttles with Sodium Citrate Modification for Hierarchical Targeting and Improved Tumor Theranostics. ACS Appl. Mater. Interfaces 2019, 11, 25730–25739. [Google Scholar] [CrossRef]
- Verma, A.; Stellacci, F. Effect of Surface Properties on Nanoparticle–Cell Interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.; Forcada, J.; Hidalgo-Alvarez, R. Cationic Polymer Nanoparticles and Nanogels: From Synthesis to Biotechnological Applications. Chem. Rev. 2014, 114, 367–428. [Google Scholar] [CrossRef]
- Hühn, D.; Kantner, K.; Geidel, C.; Brandholt, S.; De Cock, I.; Soenen, S.J.H.; Rivera_Gil, P.; Montenegro, J.-M.; Braeckmans, K.; Müllen, K.; et al. Polymer-Coated Nanoparticles Interacting with Proteins and Cells: Focusing on the Sign of the Net Charge. ACS Nano 2013, 7, 3253–3263. [Google Scholar] [CrossRef] [PubMed]
- Hillaireau, H.; Couvreur, P. Nanocarriers’ entry into the cell: Relevance to drug delivery. Cell. Mol. Life Sci. 2009, 66, 2873–2896. [Google Scholar] [CrossRef]
- Fleischer, C.C.; Payne, C.K. Nanoparticle Surface Charge Mediates the Cellular Receptors Used by Protein–Nanoparticle Complexes. J. Phys. Chem. B 2012, 116, 8901–8907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jallouli, Y.; Paillard, A.; Chang, J.; Sevin, E.; Betbeder, D. Influence of surface charge and inner composition of porous nanoparticles to cross blood–brain barrier in vitro. Int. J. Pharm. 2007, 344, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Dante, S.; Petrelli, A.; Petrini, E.M.; Marotta, R.; Maccione, A.; Alabastri, A.; Quarta, A.; De Donato, F.; Ravasenga, T.; Sathya, A.; et al. Selective Targeting of Neurons with Inorganic Nanoparticles: Revealing the Crucial Role of Nanoparticle Surface Charge. ACS Nano 2017, 11, 6630–6640. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Steinbrück, A.; Gao, J.; Doggett, N.; Hollingsworth, J.A.; Iyer, R. Comprehensive Analysis of the Effects of CdSe Quantum Dot Size, Surface Charge, and Functionalization on Primary Human Lung Cells. ACS Nano 2012, 6, 4748–4762. [Google Scholar] [CrossRef]
- Gai, S.; Yang, G.; Yang, P.; He, F.; Lin, J. Recent advances in functional nanomaterials for light–triggeredcancer therapy. Nano Today 2018, 19, 146–187. [Google Scholar] [CrossRef]
- Song, B.; He, Y. Fluorescent silicon nanomaterials: From synthesis to functionalization and application. Nano Today 2019, 26, 149–163. [Google Scholar] [CrossRef]
- Wu, X.A.; Choi, C.H.J.; Zhang, C.; Hao, L.; Mirkin, C.A. Intracellular Fate of Spherical Nucleic Acid Nanoparticle Conjugates. J. Am. Chem. Soc. 2014, 136, 7726–7733. [Google Scholar] [CrossRef]
- Harris, J.M.; Chess, R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef]
- Hamilton, A.; Biganzoli, L.; Coleman, R.; Mauriac, L.; Hennebert, P.; Awada, A.; Nooij, M.; Beex, L.; Piccart, M.; Van Hoorebeeck, I.; et al. EORTC 10968: A phase I clinical and pharmacokinetic study of polyethylene glycol liposomal doxorubicin (Caelyx®, Doxil®) at a 6-week interval in patients with metastatic breast cancer. Ann. Oncol. 2002, 13, 910–918. [Google Scholar] [CrossRef]
- Guo, S.; Huang, L. Nanoparticles Escaping RES and Endosome: Challenges for siRNA Delivery for Cancer Therapy. J. Nanomater. 2011, 2011, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Danhier, F.; Kouhé, T.T.B.; Duhem, N.; Ucakar, B.; Staub, A.; Draoui, N.; Feron, O.; Préat, V. Vitamin E-based micelles enhance the anticancer activity of doxorubicin. Int. J. Pharm. 2014, 476, 9–15. [Google Scholar] [CrossRef]
- Duhem, N.; Danhier, F.; Préat, V. Vitamin E-based nanomedicines for anti-cancer drug delivery. J. Control. Release 2014, 182, 33–44. [Google Scholar] [CrossRef]
- Chen, G.; Wang, F.; Trachootham, D.; Huang, P. Preferential killing of cancer cells with mitochondrial dysfunction by natural compounds. Mitochondrion 2010, 10, 614–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, D.; Wang, A.-T.; Yang, Z.-Z.; Liu, Y.-J.; Qi, X.-R. Enhance Cancer Cell Recognition and Overcome Drug Resistance Using Hyaluronic Acid and α-Tocopheryl Succinate Based Multifunctional Nanoparticles. Mol. Pharm. 2015, 12, 2189–2202. [Google Scholar] [CrossRef]
- Liang, N.; Sun, S.; Li, X.; Piao, H.; Piao, H.; Cui, F.; Fang, L. α-Tocopherol succinate-modified chitosan as a micellar delivery system for paclitaxel: Preparation, characterization and in vitro/in vivo evaluations. Int. J. Pharm. 2012, 423, 480–488. [Google Scholar] [CrossRef]
- Tao, Y.; Han, J.; Wang, X.; Dou, H. Nano-formulation of paclitaxel by vitamin E succinate functionalized pluronic micelles for enhanced encapsulation, stability and cytotoxicity. Colloids Surf. B Biointerfaces 2013, 102, 604–610. [Google Scholar] [CrossRef]
- Liang, D.-S.; Su, H.-T.; Liu, Y.-J.; Wang, A.-T.; Qi, X.-R. Tumor-specific penetrating peptides-functionalized hyaluronic acid- d -α-tocopheryl succinate based nanoparticles for multi-task delivery to invasive cancers. Biomaterials 2015, 71, 11–23. [Google Scholar] [CrossRef]
- Xiang, Z.; Yang, X.; Xu, J.; Lai, W.; Wang, Z.; Hu, Z.; Tian, J.; Geng, L.; Fang, Q. Tumor detection using magnetosome nanoparticles functionalized with a newly screened EGFR/HER2 targeting peptide. Biomaterials 2017, 115, 53–64. [Google Scholar] [CrossRef]
- Kinnari, P.J.; Hyvönen, M.L.; Mäkilä, E.M.; Kaasalainen, M.H.; Rivinoja, A.; Salonen, J.J.; Hirvonen, J.T.; Laakkonen, P.M.; Santos, H.A. Tumour homing peptide-functionalized porous silicon nanovectors for cancer therapy. Biomaterials 2013, 34, 9134–9141. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Low, P.S. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Adv. Drug Deliv. Rev. 2002, 54, 675–693. [Google Scholar] [CrossRef]
- Adams, G.P.; Weiner, L.M. Monoclonal antibody therapy of cancer. Nat. Biotechnol. 2005, 23, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.C. Peptidic Tumor Targeting Agents: The Road from Phage Display Peptide Selections to Clinical Applications. Curr. Pharm. Des. 2010, 16, 1040–1054. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2016, 17, 20–37. [Google Scholar] [CrossRef]
- Wang, B.; Lv, L.; Wang, Z.; Zhao, Y.; Wu, L.; Fang, X.; Xu, Q.; Xin, H. Nanoparticles functionalized with Pep-1 as potential glioma targeting delivery system via interleukin 13 receptor α2-mediated endocytosis. Biomaterials 2014, 35, 5897–5907. [Google Scholar] [CrossRef]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnology 2014, 12, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moyano, D.F.; Goldsmith, M.; Solfiell, D.J.; Landesman-Milo, D.; Miranda, O.R.; Peer, D.; Rotello, V.M. Nanoparticle Hydrophobicity Dictates Immune Response. J. Am. Chem. Soc. 2012, 134, 3965–3967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnaud, C.; Monnier, C.; Demurtas, D.; Jud, C.; Vanhecke, D.; Montet, X.; Hovius, R.; Lattuada, M.; Rothen-Rutishauser, B.; Fink, A. Insertion of Nanoparticle Clusters into Vesicle Bilayers. ACS Nano 2014, 8, 3451–3460. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, X.; Gu, N. Computational Investigation of Interaction between Nanoparticles and Membranes: Hydrophobic/Hydrophilic Effect. J. Phys. Chem. B 2008, 112, 16647–16653. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Y.; Drubin, D.G.; Oster, G.F. The Mechanochemistry of Endocytosis. PLoS Biol. 2009, 7, e1000204. [Google Scholar] [CrossRef] [Green Version]
- Orsi, M.; Essex, J.W. Permeability of Drugs and Hormones through a Lipid Bilayer: Insights from Dual-Resolution Molecular Dynamics. Soft Matter 2010, 6, 3797–3808. [Google Scholar] [CrossRef]
- Paula, S.; Volkov, A.; Van Hoek, A.; Haines, T.; Deamer, D. Permeation of protons, potassium ions, and small polar molecules through phospholipid bilayers as a function of membrane thickness. Biophys. J. 1996, 70, 339–348. [Google Scholar] [CrossRef] [Green Version]
- Bedrov, D.; Smith, G.D.; Davande, H.; Li, L. Passive Transport of C60Fullerenes through a Lipid Membrane: A Molecular Dynamics Simulation Study. J. Phys. Chem. B 2008, 112, 2078–2084. [Google Scholar] [CrossRef]
- Qiao, R.; Roberts, A.P.; Mount, A.; Klaine, S.J.; Ke, P.C. Translocation of C60 and Its Derivatives Across a Lipid Bilayer. Nano Lett. 2007, 7, 614–619. [Google Scholar] [CrossRef]
- Ding, H.-M.; Tian, W.-D.; Ma, Y.-Q. Designing Nanoparticle Translocation through Membranes by Computer Simulations. ACS Nano 2012, 6, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.; Lewis, D.J.; Watson, S.P.; Thomas, S.G.; Pikramenou, Z. pH-Controlled Delivery of Luminescent Europium Coated Nanoparticles into Platelets. Proc. Natl. Acad. Sci. USA 2012, 109, 1862–1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Lehn, R.C.; Ricci, M.; Silva, P.H.J.; Andreozzi, P.; Reguera, J.; Voïtchovsky, K. Lipid Tail Protrusions Mediate the Insertion of Nanoparticles into Model Cell Membranes. Nat. Commun. 2014, 5, 4482. [Google Scholar] [CrossRef]
- Yang, K.; Ma, Y.-Q. Computer simulation of the translocation of nanoparticles with different shapes across a lipid bilayer. Nat. Nanotechnol. 2010, 5, 579–583. [Google Scholar] [CrossRef]
- Guo, Y.; Terazzi, E.; Seemann, R.; Fleury, J.B.; Baulin, V.A. Direct proof of spontaneous translocation of lipid-covered hydrophobic nanoparticles through a phospholipid bilayer. Sci. Adv. 2016, 2, e1600261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, K.; Rahimi, M.; Yazdani, M.; Kim, S.T.; Moyano, D.F.; Hou, S.; Das, R.; Mout, R.; Rezaee, F.; Mahmoudi, M.; et al. Regulation of Macrophage Recognition through the Interplay of Nanoparticle Surface Functionality and Protein Corona. ACS Nano 2016, 10, 4421–4430. [Google Scholar] [CrossRef] [Green Version]
- Moyano, D.F.; Saha, K.; Prakash, G.; Yan, B.; Kong, H.; Yazdani, M.; Rotello, V.M. Fabrication of Corona-Free Nanoparticles with Tunable Hydrophobicity. ACS Nano 2014, 8, 6748–6755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porret, E.; Sancey, L.; Martín-Serrano, A.; Montañez, M.I.; Seeman, R.; Yahia-Ammar, A.; Okuno, H.; Gomez, F.; Ariza, A.; Hildebrandt, N.; et al. Hydrophobicity of Gold Nanoclusters Influences Their Interactions with Biological Barriers. Chem. Mater. 2017, 29, 7497–7506. [Google Scholar] [CrossRef]
- Du, X.-J.; Wang, J.-L.; Liu, W.-W.; Yang, J.-X.; Sun, C.-Y.; Sun, R.; Li, H.; Shen, S.; Luo, Y.-L.; Ye, X.-D.; et al. Regulating the surface poly(ethylene glycol) density of polymeric nanoparticles and evaluating its role in drug delivery in vivo. Biomaterials 2015, 69, 1–11. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, Q.; Mu, K.; Xie, H.; Zhu, Y.; Zhu, W.; Zhao, Y.; Xu, H.; Yang, X. pH/temperature sensitive magnetic nanogels conjugated with Cy5.5-labled lactoferrin for MR and fluorescence imaging of glioma in rats. Biomaterials 2013, 34, 7418–7428. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Q.; Li, Z.; Li, F.; Wu, D.; Fan, M.; Zheng, A.; Huang, B.; Gan, L.; Zhao, Y.; et al. Hydrophobicity-Adaptive Nanogels for Programmed Anticancer Drug Delivery. Nano Lett. 2018, 18, 7909–7918. [Google Scholar] [CrossRef]
- Sadhukha, T.; Wiedmann, T.S.; Panyam, J. Enhancing therapeutic efficacy through designed aggregation of nanoparticles. Biomaterials 2014, 35, 7860–7869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Wang, D. Controlling the Growth of Charged-Nanoparticle Chains through Interparticle Electrostatic Repulsion. Angew. Chem. Int. Ed. 2008, 47, 3984–3987. [Google Scholar] [CrossRef] [PubMed]
- Derjaguin, B.; Landau, L. Theory of the Stability of Strongly Charged Liphobic Sols and of Electrolytes. Prog. Surf. Sci. 1993, 43, 30–59. [Google Scholar] [CrossRef]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef] [Green Version]
- Rausch, K.; Reuter, A.; Fischer, K.; Schmidt, M. Evaluation of Nanoparticle Aggregation in Human Blood Serum. Biomacromolecules 2010, 11, 2836–2839. [Google Scholar] [CrossRef]
- Van Landeghem, F.K.H.; Maier-Hauff, K.; Jordan, A.; Hoffmann, K.T.; Gneveckow, U.; Scholz, R. Post-Mortem Studies in Glioblastoma Patients Treated with Thermotherapy Using Magnetic Nanoparticles. Biomaterials 2009, 30, 52–57. [Google Scholar] [CrossRef]
- Albanese, A.; Chan, W.C. Effect of Gold Nanoparticle Aggregation on Cell Uptake and Toxicity. ACS Nano 2011, 5, 5478–5489. [Google Scholar] [CrossRef]
- Zhou, D.; Keller, A.A. Role of morphology in the aggregation kinetics of ZnO nanoparticles. Water Res. 2010, 44, 2948–2956. [Google Scholar] [CrossRef]
- Bian, S.-W.; Mudunkotuwa, I.A.; Rupasinghe, T.; Grassian, V.H. Aggregation and Dissolution of 4 nm ZnO Nanoparticles in Aqueous Environments: Influence of pH, Ionic Strength, Size, and Adsorption of Humic Acid. Langmuir 2011, 27, 6059–6068. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lenhart, J.J.; Walker, H.W. Aggregation Kinetics and Dissolution of Coated Silver Nanoparticles. Langmuir 2011, 28, 1095–1104. [Google Scholar] [CrossRef]
- Tripathy, N.; Hong, T.-K.; Ha, K.-T.; Jeong, H.-S.; Hahn, Y.-B. Effect of ZnO nanoparticles aggregation on the toxicity in RAW 264.7 murine macrophage. J. Hazard. Mater. 2014, 270, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Sabuncu, A.C.; Grubbs, J.; Qian, S.; Abdel-Fattah, T.M.; Stacey, M.W.; Beskok, A. Probing nanoparticle interactions in cell culture media. Colloids Surf. B 2012, 95, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Ojea-Jiménez, I.; Puntes, V.J. Instability of cationic gold nanoparticle bioconjugates: The role of citrate ions. Am. Chem. Soc. 2009, 131, 13320–13327. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.A.; Lohse, S.E.; Murphy, C.J. Tuning Cellular Response to Nanoparticles via Surface Chemistry and Aggregation. Small 2014, 10, 1642–1651. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Li, H.; Huang, N.; Jin, Q.; Ren, K.; Ji, J. Enhanced Retention and Cellular Uptake of Nanoparticles in Tumors by Controlling Their Aggregation Behavior. ACS Nano 2013, 7, 6244–6257. [Google Scholar] [CrossRef]
- Ferrari, M. Nanogeometry: Beyond Drug Delivery. Nat. Nanotechnol. 2008, 3, 131–132. [Google Scholar] [CrossRef]


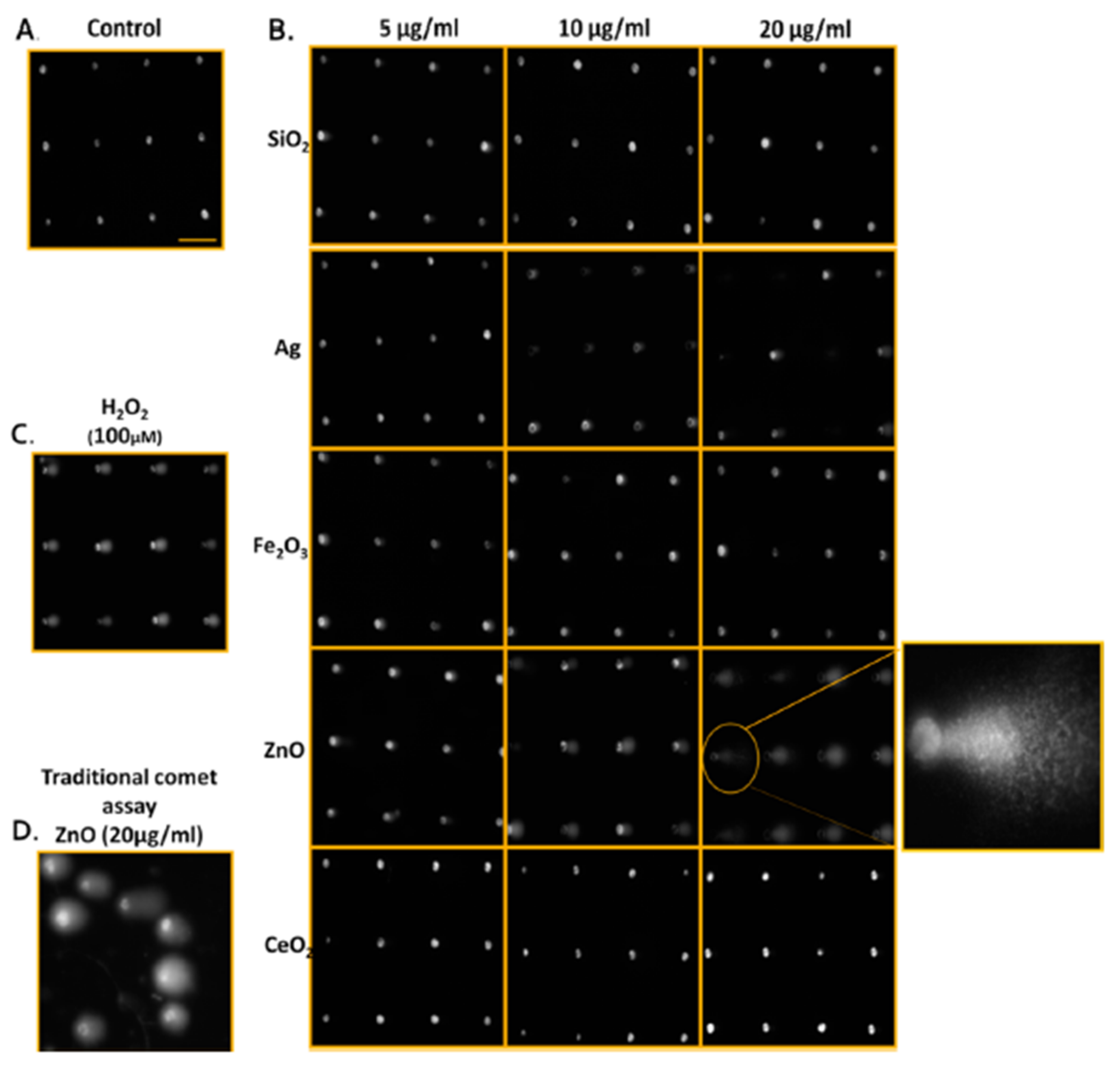
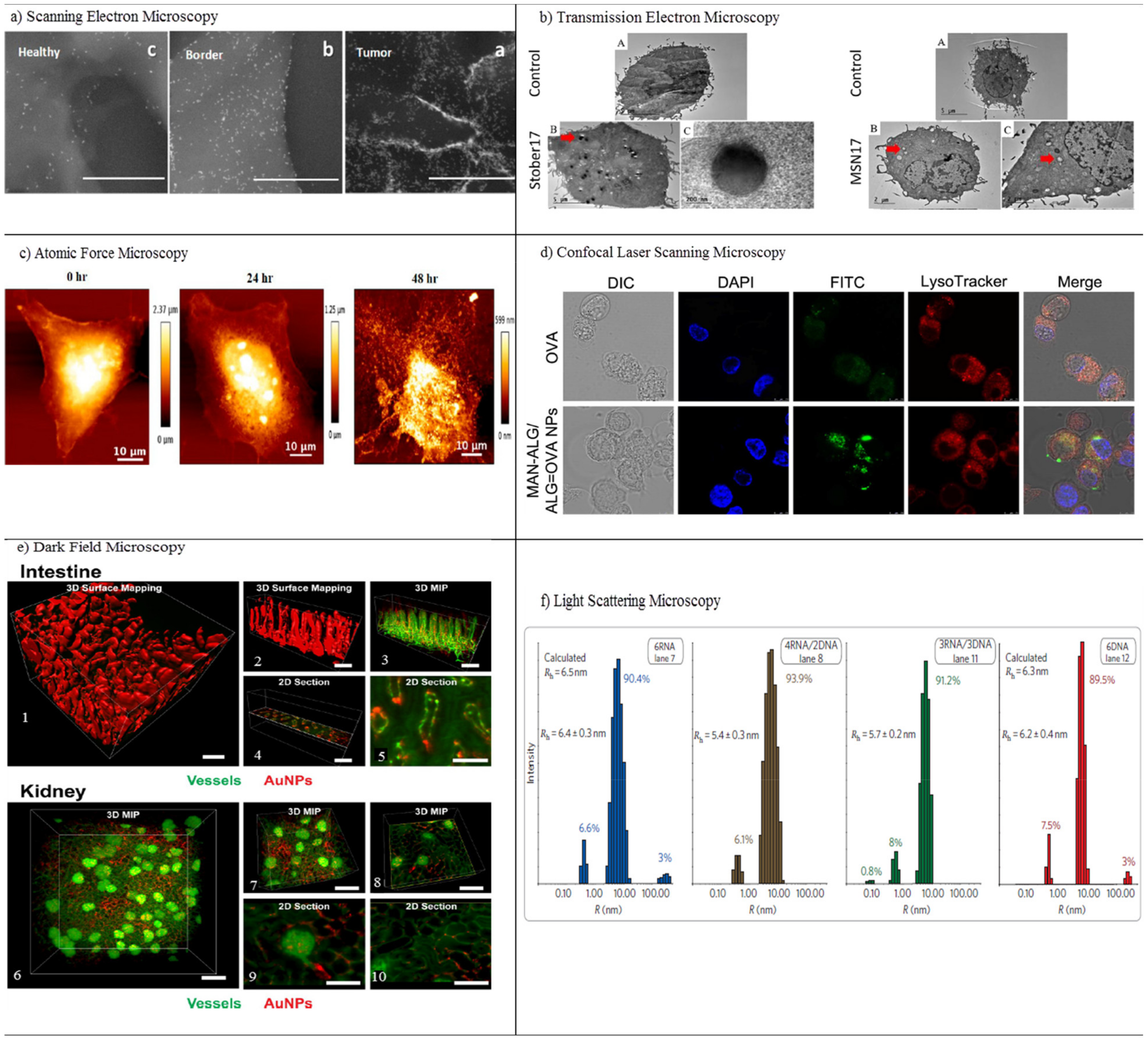





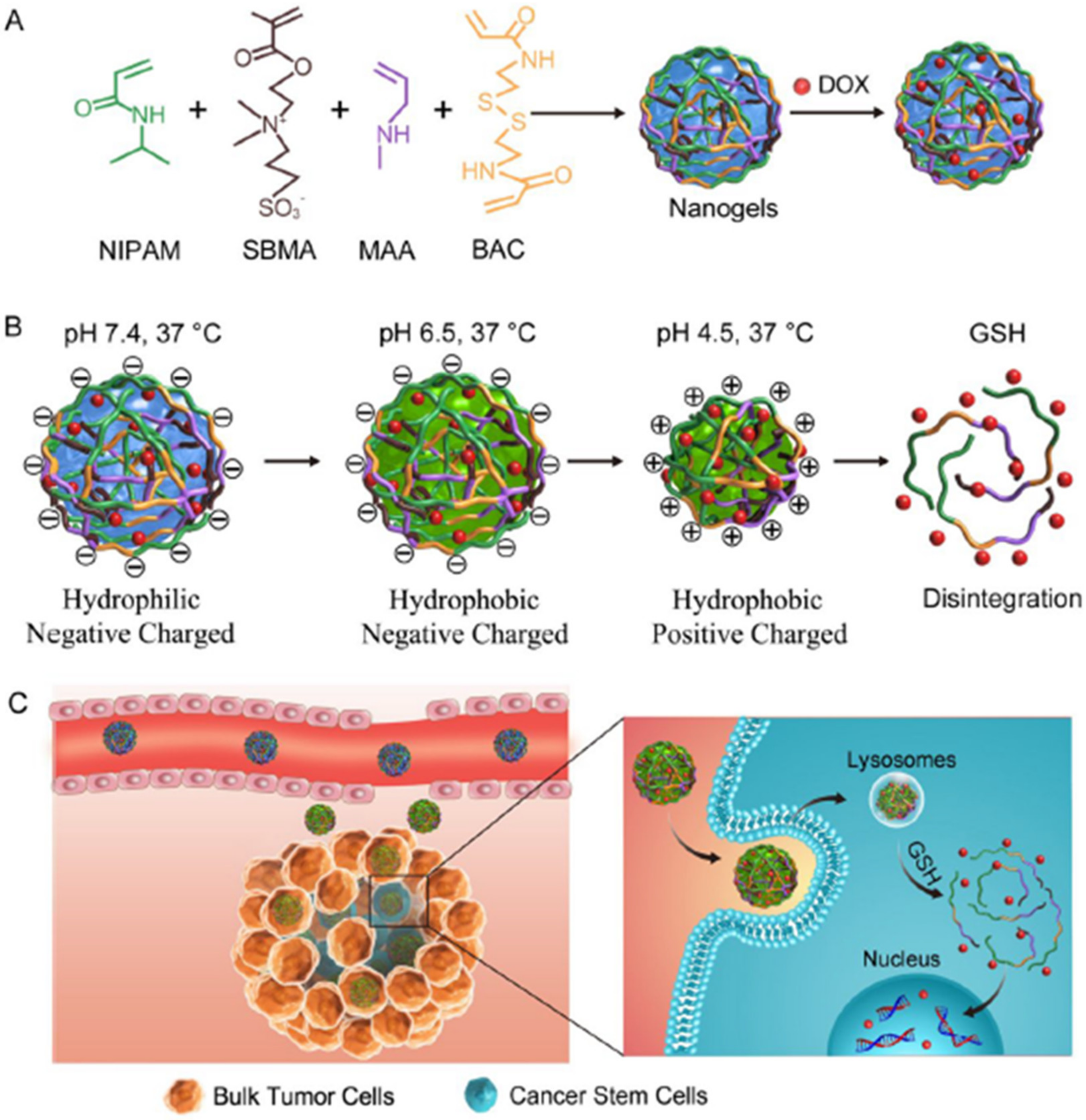
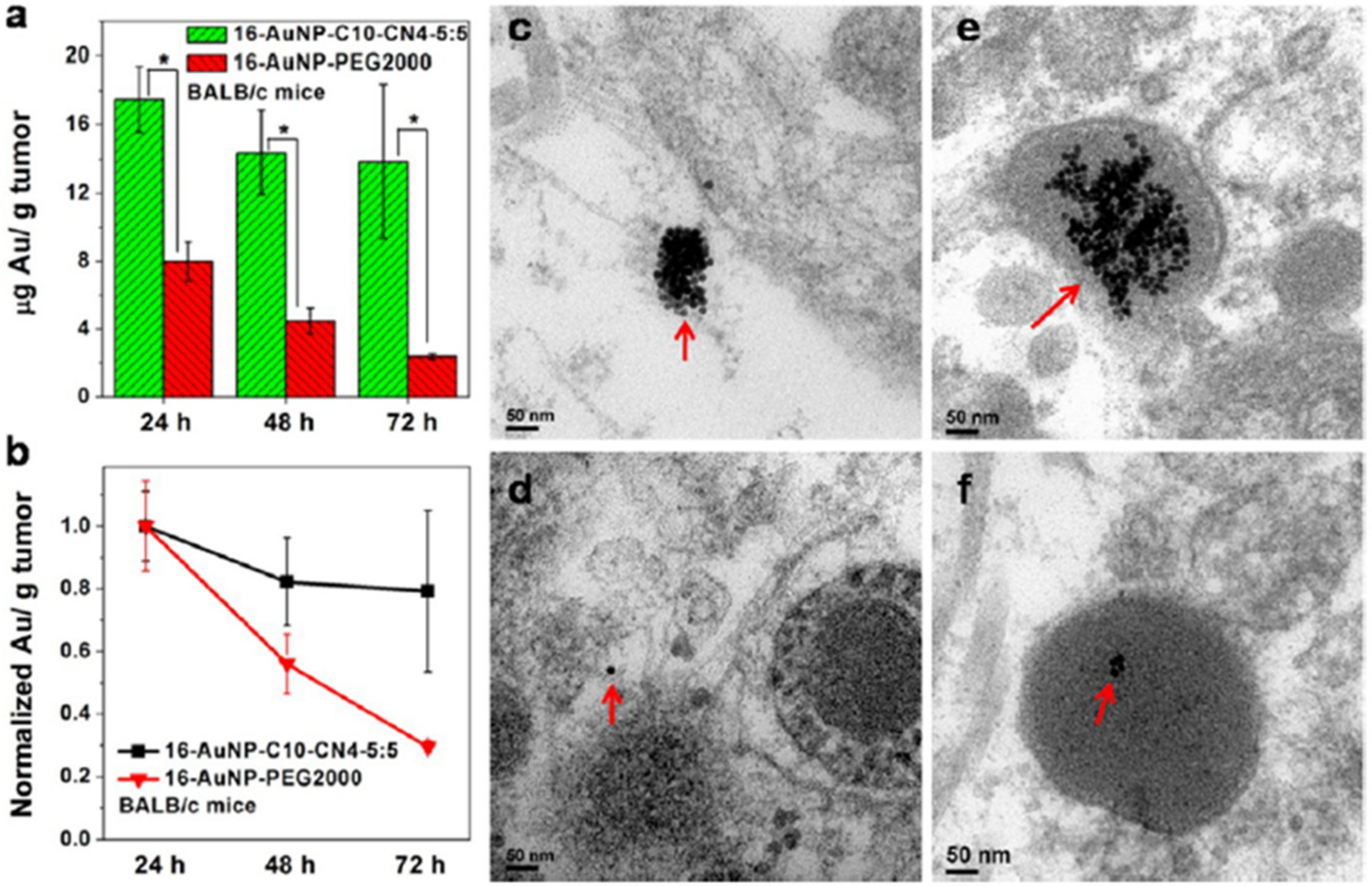
| Process | NPs | Application | Remarks | Ref. |
|---|---|---|---|---|
| Absorption | NCs | Insulin delivery | NPs possessed high epithelial absorption mediated by CPP and excellent permeation in mucus. The absorption of NPs on mucus-secreting epithelium cells was 20-fold greater than free insulin and improved the concentration of insulin in diabetic rats. | [108] |
| Au | Cardiovascular disease | Inhaled AuNPs translocated from the lung to the circulation and selectively accumulated at vascular inflammation sites in human and animal models. | [109] | |
| P-R8-Pho | Insulin delivery | P-R8-Pho NPs improved absorption in the intestine in vivo and oral administration of insulin-loaded P-R8-Pho NPs exhibited 1.9-fold higher oral bioavailability, as well as better hypoglycemic effects on diabetic rats. | [110] | |
| Distribution | Au | Cancer immunotherapy | Cellular distribution of 50-nm PEG coated AuNPs in the spleen, exhibiting the presence of NPs in B cells, T cells, granulocytes, and dendritic cells. | [111] |
| PLGA | Drug delivery | PLGA-PEG blends caused quick diffusion in human CVM and increased vaginal distribution in mice in vivo. | [112] | |
| Qds | Biomedicine | In vivo biodistribution of indium-based water-soluble QDs in rats was measured up to 90 days and indicated the accumulation of QDs largely in the liver and spleen. | [113] | |
| Metabolism | LPSi | Drug delivery | LPSi NPs were degraded in vivo over a period of 4 weeks and NIR imaging confirmed the elimination of NPs into the bladder at 1 h post-injection. | [114] |
| Iron oxide | MRI | Magnetic nanocrystals were metabolized via the loss of their peculiar superparamagnetic properties using ferromagnetic resonance and SQUID measurements. | [115] | |
| PLGA | Drug delivery | Quantitative measurement of 200 nm and 500 nm PLGA degradation in liver and spleen after intravenous administration in mice. | [116] | |
| C3 | Photothermal/photodynamic therapies | Lower toxicity and faster metabolic rate than Au nanorods. | [117] | |
| Elimination | BPQds | Photodynamic therapy for cancer | BPQDs revealed proper stability, with no observable toxicity after PEG conjugation. They also eliminated rapidly through renal clearance due to their small size (5.4 nm) and showed great antitumor efficiency. | [118] |
| Au | X-ray imaging of kidney dysfunction | X-ray imaging of renal-clearable AuNPs in nephropathic and normal kidneys. The ureteral obstruction inhibited the clearance of NPs via the ureter and slowed down the transportation of NPs from the medulla to the pelvis with improved cellular uptake. | [119] | |
| Cu2ZnSnS4 | Tumor photothermal therapy | Peroxidase-like catalytic activity of nanocrystals could lead to oxidative lesions and kill cancerous cells. The rapid in vivo clearance of nanocrystals was confirmed via MRI, PA, and ICP-MS. | [120] | |
| Polycation | Metastatic breast cancer | Fluorination slowed the rate of renal clearance of NPs and helped to improve accumulations in tumor sites. | [121] | |
| Gd@GCNPs | Photodynamic therapy | Compared with C-dots, Gd@GC NPs indicated better T1 relaxivity, strong red fluorescence, high crystallinity, and suitable Gd encapsulation. These 50nm Gd@GC NPs accumulated in tumors via the EPR effect and were efficiently removed through renal clearance, resulting in no long-term toxicity. | [122] |
| Highlighted Factor | NPs | NP Features | Application | Remarks | Ref. |
|---|---|---|---|---|---|
| Size | Silica | 20–80 nm | BBB | 40-nm DOX-MSNPs showed higher permeability across the BBB, inhibited the undesired toxic side effects to normal brain tissue, and exhibited good anti-glioblastoma efficacy. | [199] |
| PLGA | 50 and 150 nm | Psoriasis | NPs of 50 nm enhanced sustained release, accumulation, and penetration, with deeper penetration into the psoriatic skin. | [200] | |
| Gold | 1.5, 4, and 14 nm | Stem cell | AuNP of 1.5 nm was highly toxic, and AuNPs had size-dependent impacts on the pluripotency, viability, and neuronal differentiation potentials of stem cells. | [201] | |
| Nanodrug | 10–90 nm | Cancer theranostics | A size-reducible self-assembled nanodrug significantly increased photodynamic effects, and deep tumor penetration was observed for smaller particles. | [202] | |
| Micelle | 40, 90, 130, and 180 nm | siRNA delivery | The highest tumor retention and the best balance of cellular uptake and prolonged circulation were observed for 90 nm. | [203] | |
| PEG-PLGA | 50, 100, and 150 nm | Chemoradiotherapy | Smaller NPs (sub 50 nm) penetrated the tumor more homogeneously, whereas they induced greater toxicity than larger particles. | [204] | |
| Gold | 3.8 and 22.1 nm | Multidrug resistance cancer | TAT-modified AuNPs improved anticancer activity, and the larger NP was desirable to overcome multidrug resistance. | [205] | |
| Shape | Carbon | SWCNTs and fullerenes | Angiogenesis | SWCNTs enhanced endothelial tubulogenesis, triggered integrin clustering, and activated focal adhesion PI3K signaling in endothelial cells, whereas the spherical NPs of fullerenols or fullerenols-DOX exerted a highly different antiangiogenic activity in zebrafish and murine tumor angiogenesis. | [206] |
| Qds | Sphere and rod shapes | Tumor penetration | Nanorods showed faster tumor penetration compared to spherical NPs. Flexible nanorods possessed better circulation times than rigid rods. | [207] | |
| Hydroxyapatite | Sphere and rod shapes | Stem cell | Although no important impact on cell proliferation or migration was observed for both nanorods and nanospheres, NPs of spherical shape highly improved the osteoblastic differentiation of rat mesenchymal stem cells compared to nanorods. | [208] | |
| Quinoline–malononitrile | Sphere and rod shapes | Tumor targeting | The sphere-shaped NPs showed great tumor-targeted bioimaging ability than their rod counterparts. | [209] | |
| Polymer | Sphere and non-sphere | Drug delivery | Spherical NPs displayed higher cellular uptake than non-spherical particles. | [210] | |
| Silica | Short rod and long rod | In vivo areas | Long rod MSNPs were trapped in the spleen, whereas short rod particles were distributed in the liver and showed faster clearance rate. | [211] | |
| Gold | Sphere and star shapes | siRNA delivery | Spheres of 50 nm and 40-nm star NPs had much greater uptake efficiency than 13-nm sphere particles. | [212] | |
| Surface charge | Silica | Positive charge | Drug delivery | Highly charged moieties were removed rapidly from the liver to GIT, whereas less charged moieties remained sequestered within the liver. | [213] |
| Cellulose | Positive and negative | Bioimaging and drug delivery | The cationic cellulose nanocrystal (CNC)-rhodamine B isothiocyanate (RBITC) was uptaken via human embryonic kidney 293 and Spodoptera frugiperda cells without affecting the cell membrane integrity. At physiological pH, no significant internalization was observed for negatively charged CNC-fluorescein isothiocyanate (FITC). | [214] | |
| Fe3O4 | Positive and negative | Biomedical applications | The cellular uptake of negative poly(acrylic acid) (PAA-MNPs) was under 50%, as opposed to almost 100% for cationic PEI-MNPs. | [215] | |
| Silicon | Positive and negative | Drug delivery | Cationic amine modified hydrophilic silicon NPs induced higher ATP depletion and genotoxicity than negatively charged hydrophilic and hydrophobic silicon NPs. | [216] | |
| Lipid | Positive | Cancer therapy | Decreasing the PEG density and increasing the surface charge could notably enhance the toxic role of cationic lipid NPs in systemic platelet activation and aggregation in vivo. Pretreatment of the mice with heparin enhanced the circulation time, were very efficient in preventing toxicity, and increased the anti-cancer drug delivery. | [217] | |
| Phospholipids | Positive, neutral, and negative | Ear drug delivery | Cationic PEG delivered DEX to inner ear hair cells with higher efficiency than Dex-sodium phosphate. | [218] | |
| Functionality | Polystyrene | Amino groups | Leukemia | Amino-functionalized polystyrene NPs avoided proliferation in three leukemia cells and G2 cell cycle arrest. | [219] |
| Polystyrene | Biotin | Drug delivery | Pretreated biotin-functionalized NPs showed 6- to 18-fold greater uptake and fusion protein control. | [220] | |
| Silver | Peptide | Antibacterial | The modified NPs indicated antibacterial activity against both Gram-negative (P. aeruginosa) and Gram-positive (S. aureus) bacteria, and eliminated antibiotic-resistant bacteria without toxicity to mammalian cells. | [221] | |
| CNT | Carboxyl groups | Biomacromolecules | Ideal photothermal encapsulation efficiency was observed for fibers constructed from 0.4 mg mL−1 of CNTs. | [222] | |
| Zirconium-metal–organic frameworks | DNA | Biosensor/bioimaging | DNA-functionalized NPs not only provided targeted imaging of cancer cells, facilitated the therapeutic delivery, and improved immunostimulatory effects, but also exhibited no toxicity. | [223] | |
| PLGA | Albumin | Tumor drug delivery | Only with a native conformation could albumin act as an ideal modifier for the tumor drug delivery of NPs. | [224] | |
| Coordination polymer | Bisphosphonates | Breast cancer | The modified NPs exhibited much greater affinity for bone in vivo and hydroxyapatite in vitro, decreased the osteocalastic bone destruction, and avoided the tumor growth effectively with notably reduced toxicity of cisplatin. | [225] | |
| Gadofullerene | Amino acid | Tumor vascular targeting | Excellent antitumor activity in hepatoma H22 models was reported for functionalized NPs. The use of amino acids enhanced the tumor inhibition rate up to 76.85%, and increased blood circulation time. | [226] | |
| MWCNT | Hydroxyl or carboxyl groups | Nanomedicine | Compared with pristine NPs, surface functionalization reduced the toxicity of MWCNTs and resulted in the intracellular uptake of particles. | [227] | |
| Silica | Amine, sulfonate, PEG, and PEI | Targeted drug delivery | In the presence of serum, sulfonate-functionalized dox-loaded MSNPs were effectively taken up into the cells and caused the highest cytotoxic and anti-proliferative effects in osteosarcoma. | [228] | |
| Hydrophobicity | Amphiphilic | Hydrophobic | Immunoengineering | The hydrophobicity of amphiphilic poly(γ-glutamic acid)-graft-L-phenylalanine ethyl ester (γ-PGA-Phe) NPs influenced the uptake of encapsulated antigens by DCs. Compared to the conventional vaccine, the activation of DCs could be manipulated about 5- to 30-fold through adjusting the hydrophobicity of encapsulated antigens, and the effect on cellular immunity was reported to be almost 10- to 40-fold. | [229] |
| Gold | Hydrophobic | Nanomedicine | AuNP surface hydrophobicity did not notably influence cellular uptake, whereas increasing NP surface hydrophobicity caused membrane damage and autophagy. | [230] | |
| Silver | Hydrophobic | Nanomedicine | The increase in hydrophobicity of Ag NPs in the presence of serum proteins resulted in higher cytotoxicity, cell uptake, and ROS production. | [231] | |
| Aggregation | Maghemite | Cell therapy | The aggregation of NPs not only confounded global iron quantification and MRI cell detection but also displayed adverse impacts on chondrogenetic differentiation. | [232] | |
| Magnetic | Hyperthermia | The aggregation of magnetic NPs was independent of NP coating, cell type, and incubation time. One of the reasons for various magnetic behaviors in cell-induced aggregation may be attributed to the aggregation of magnetic NPs in a non-controlled way. | [233] | ||
| Gold | Nanotechnology | Nanosized liposomes enhanced steric hindrance, which avoided the aggregation of AuNPs on a lipid membrane in a fluidic liquid-crystalline phase. | [234] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domb, A.J.; Sharifzadeh, G.; Nahum, V.; Hosseinkhani, H. Safety Evaluation of Nanotechnology Products. Pharmaceutics 2021, 13, 1615. https://doi.org/10.3390/pharmaceutics13101615
Domb AJ, Sharifzadeh G, Nahum V, Hosseinkhani H. Safety Evaluation of Nanotechnology Products. Pharmaceutics. 2021; 13(10):1615. https://doi.org/10.3390/pharmaceutics13101615
Chicago/Turabian StyleDomb, Abraham J., Ghorbanali Sharifzadeh, Victoria Nahum, and Hossein Hosseinkhani. 2021. "Safety Evaluation of Nanotechnology Products" Pharmaceutics 13, no. 10: 1615. https://doi.org/10.3390/pharmaceutics13101615







