Abstract
The unique properties of chitosan make it a useful choice for various nanoparticulate drug delivery applications. Although chitosan is biocompatible and enables cellular uptake, its interactions at cellular and systemic levels need to be studied in more depth. This review focuses on the various physical and chemical properties of chitosan that affect its performance in biological systems. We aim to analyze recent research studying interactions of chitosan nanoparticles (NPs) upon their cellular uptake and their journey through the various compartments of the cell. The positive charge of chitosan enables it to efficiently attach to cells, increasing the probability of cellular uptake. Chitosan NPs are taken up by cells via different pathways and escape endosomal degradation due to the proton sponge effect. Furthermore, we have reviewed the interaction of chitosan NPs upon in vivo administration. Chitosan NPs are immediately surrounded by a serum protein corona in systemic circulation upon intravenous administration, and their biodistribution is mainly to the liver and spleen indicating RES uptake. However, the evasion of RES system as well as the targeting ability and bioavailability of chitosan NPs can be improved by utilizing specific routes of administration and covalent modifications of surface properties. Ongoing clinical trials of chitosan formulations for therapeutic applications are paving the way for the introduction of chitosan into the pharmaceutical market and for their toxicological evaluation. Chitosan provides specific biophysical properties for effective and tunable cellular uptake and systemic delivery for a wide range of applications.
1. Introduction
The nontoxic, biocompatible, and biodegradable properties of chitosan make it a polymer of choice for many biomedical and pharmaceutical applications. Chitosan microspheres and NPs for drug delivery were first reported in the late 1990s [1,2,3]. The chemical versatility of chitosan relies on its ability to form a poly-cationic charged molecule at physiological pH due to the protonation of D-glucosamine in its polymeric structure and its modifiable molecular weight [4]. Chitosan is a product of the deacetylation of chitin, and its chemical and biological properties are dependent on the degree of deacetylation and acetylation along with other factors such as molecular weight and types of surface modifications [5]. Chitosan has a pKa of 6.5, and it is insoluble in water but soluble in acidic solutions. The protonated species is capable of complexing with a diverse range of anionic biomolecules such as DNA, lipids, and proteins in the form of micro and nanoparticles by means of polyelectrolyte interactions leading to self-assembly. Chitosan NPs are used in a range of drug delivery applications from oral drug delivery to systemic cancer therapy for a variety of payloads including insulin, anticancer drugs, and gene delivery [6]. The biological interactions of chitosan NPs and its derivatives are principally governed by its physicochemical properties, such as size and charge [7,8,9]. The cationic nature of chitosan imparts mucoadhesive properties for mucosal drug delivery applications such as ocular and intranasal delivery. Chitosan NPs have improved the cellular uptake of therapeutics such as anticancer drugs and large molecules such as DNA and proteins [10,11,12,13]. Vaccines formulated with chitosan have high mucosal uptake and the activation of macrophages due to the mucoadhesive and adjuvant activity of chitosan [14]. The fate of chitosan at the cellular level determines its effectiveness in delivering these therapeutic molecules. Consequently, the passage of chitosan through the body and its elimination determines the effectiveness and subsequent toxicity of the therapeutic molecules that it is intended to transport.
Hence, it is essential to understand the nature of chitosan interactions at both the cellular and tissue levels in order to design effective delivery systems. There have been many reviews of chitosan NPs preparation and application. However, recent reviews on its biological implications and impact on cellular disposition, which will help in the design of advanced formulations, have been informative. Thus, this review paper presents an insight into the various properties that regulate the interactions of chitosan with the cell membrane, its subsequent uptake and passage through the cell, and finally its exocytosis. In addition, the various aspects of in vivo tissue distribution and bioavailability along with its toxicity are discussed.
2. Chitosan Cell Interactions
The cell membrane is a multifaceted structure composed of lipids and proteins that provides an effective barrier against the majority of substances. It is important that drug molecules are able to pass this barrier in order to achieve therapeutic activity [15]. The plasma membrane of mammalian cells has a net negative charge attributed to the presence of phospholipids having negative head groups [16]. Hence, a cationic polysaccharide, such as chitosan, can easily attach to the surface of cell membranes by electrostatic interactions, which results in enhanced cellular uptake. Early cellular uptake studies of chitosan solutions with varying molecular weight and degree of acetylation were conducted by Schipper et al. They observed the uptake enhancement potential of chitosan in Caco-2 cells and found that a low degree of acetylation up to 35% and/or a high molecular weight resulted in greater epithelial permeability to mannitol [17]. Similarly, Kotzé et al. were one of the first to report the reduction in transepithelial electrical (TEER) activity with the help of chitosan to enable the transport of peptides across Caco-2 cell monolayers. They compared two salts of chitosan polymer—namely, chitosan hydrochloride and chitosan glutamate—and a chitosan derivative, trimethyl chitosan, and they found chitosan hydrochloride to have the greatest effect in reducing the TEER [18]. Recent mechanistic research on how chitosan affects cellular uptake is discussed in depth in the sections below.
2.1. Effect of pH and Zeta Potential on Cell Membrane
The effect of the physicochemical properties of chitosan on the cell membrane has previously been explored by Yue et al. They investigated the effect of surface charge of chitosan NPs on their uptake in eight different cell lines including epithelial cells, endothelial cells, fibroblasts, and blood cells. They observed that positively charged chitosan NPs having a zeta potential of +39.25 ± 2.68 mV had faster cellular internalization kinetics and a greater extent of cellular uptake in all cell lines as compared to neutral (0.51 ± 1.31 mV) and negatively charged (−45.84 ± 2.18 mV) NPs, which were attributed to electrostatic interactions [19]. Introducing surface modification but preserving the positive charge of chitosan NPs retained the cellular uptake-facilitating properties of chitosan. The surface functionalization of gold NPs with chitosan resulted in cationic particles having a zeta potential of +65 ± 1.0 mV. These particles generated rapid internalization within 30 min of treatment of human monocytic THP-1 cells, whereas the negatively charged particles took up to 6 h for apparent cellular uptake [20]. Hu et al. compared the cellular uptake of positively charged chondroitin sulfate chitosan NPs loaded with FITC-BSA having a zeta potential of +16 mV to negatively charged particles with −30 mV. The authors reported that the positively charged particles showed rapid uptake in cells, as demonstrated by a greater fluorescence intensity after 2 h as compared to the negatively charged particles [21]. The surface charge of chitosan NPs is closely associated with the environmental pH. Chitosan has a pKa of 6.5, which allows it to solubilize in acidic pH due to the protonation of amino groups leading to a higher surface charge of the NPs [22,23]. Many NPs have been designed to take advantage of this property of chitosan to selectively release the drug into the desired cell environment, which is especially advantageous for drug delivery to tumors [10,24,25]. For example, pH-responsive NPs using self-assembling polymers made of dimethyl maleic acid–chitosan–urocanic acid with doxorubicin displayed a charge reversal property when transported from a physiological pH of 7.2 to a tumor environment pH of 6.8. The negative zeta potential at physiological pH allowed for better stability during blood circulation with the least non-specific protein adsorption. On the other hand, a switch to a positive zeta potential in the tumor microenvironment allowed enhanced cellular uptake and rapid release of doxorubicin from the NPs [12]. In another research study, chitosan was used as a pH-sensitive stealth coating for the delivery of paclitaxel to tumor cells. NP formulations with PLGA and low molecular weight chitosan (50–190 kDa) showed better association with SKOV-3 and NCI/ADR-RES cells at a pH of 6.2 due to the positive charge of the particles as compared to those at a pH of 7.2 [24]. This pH-sensitive property of chitosan allows it not only to better associate with cells and provide enhanced cellular uptake but also to target specific microenvironments in and around cells.
2.2. Effect of Chitosan on Cell Adhesion
The passive attachment of cells to a static substrate, such as culture flasks and Petri dishes, is attributed to specific membrane integrin binding interactions providing mechanical linkage between the intracellular actin and the extracellular matrix [25]. Chitosan membranes have been used to recover cultured cells from substrate without any enzymatic treatments [26]. They demonstrated that up to 90% of cells detached from a chitosan-coated surface when the medium pH was changed from the pH in which the cells were incubated for 24 h (7.2) to a pH of 7.65 for one hour with up to 95% cell viability. This pH-induced detachment was observed in five different types of cell lines: namely, HeLa, primary corneal fibroblasts, HaCaT, H1299, and NIH-3T3 cell lines. This observation can be explained by the deprotonation of chitosan at higher pH, which also leads to the desorption of fibronectin from the substrate, thus further increasing the detachment of cells [26]. The charge-dependent interaction of chitosan with the cell membrane to control cell adhesion behavior has been exploited recently. Stronger adhesion was demonstrated for the PC-3 prostate cancer cell line to hyaluronic acid–chitosan films from pH 3.0–5.0 [27]. Better adherence of bovine chondrocytes to chitosan films neutralized with sodium hydroxide was observed, which impacted cell proliferation and adhesion on scaffolds [28]. The enhanced absorption of fibronectin on collagen-chitosan film surfaces aided in the better adhesion of PC12 cells [29]. The degree of acetylation of chitosan has been reported to influence its impact on cell adhesion and proliferation properties due to changes in hydrophobicity and the number of protonated groups available for cell adhesion. A greater degree of acetylation of chitosan used in films displayed increased hydrophobicity and a lower surface charge, leading to decreased adhesion of fibroblast and chondrocytic cells [30]. Similarly, olfactory epithelial cells displayed better cell compatibility and proliferation activity at a higher degree of deacetylation of up to 85% in turn, indicating that a lesser acetylation of chitosan was beneficial for cell growth and adhesion [31]. The effect of chitosan on cell adhesion has found various applications in tissue engineering such as scaffolds for bone and cartilage regeneration [32], peripheral nerve regeneration [33], skin grafting [34,35], wound healing [36,37], and 3D cell cultures [38,39].
2.3. Effect on Tight Junctions
Chitosan has been used as a permeation enhancer due to its unique property to transiently open the epithelial tight junction to allow the penetration of hydrophilic molecules such as insulin [40,41]. Epithelial cells form an effective protective barrier with intercellular contacts through tight junctions, adherin junctions, and desmosomes. Tight junctions are composed of a combination of various proteins such as occludens, claudins, and junctional adhesion molecules found between epithelial and endothelial cells, which protect organs from the outside environment and help to maintain homeostasis [42]. Tight junction integrity is typically indicated by changes in the transepithelial electrical resistance. Tight junction proteins have an intracellular and extracellular portion that are responsible for maintaining intercellular communication and the transport of small molecules, ions, and water via active transport [43,44]. The intestinal tight junctions play a pivotal role in protecting the body against stress stimuli such as infections and inflammation [42]. A mixture of carboxymethyl chitosan with chitosan NPs, creating a hydrogel, was found to be effective for the delivery of insulin across the intestinal barriers. The NPs not only had gastric protective ability but were able to selectively release insulin in the intestinal medium in vivo. Similarly, the chitosan-related redistribution of claudin-4 from the cell membrane to the cytosol leading to a reversible opening of tight junctions in Caco-2 cells was also observed [40,45]. Thus, it is clear that the penetration of chitosan via tight junctions has multiple mechanisms.
2.4. Chitosan and Transepithelial Electrical Resistance (TEER)
The effect of chitosan on paracellular permeability has been well established. Transepithelial electrical resistance measurements are a valuable tool that provide an insight into the cell membrane permeability and integrity. TEER is the measurement of electrical resistance across the cell monolayer, which reflects the integrity of the tight junctions and cellular barriers [46]. Chitosan solutions applied to cells induced a dose-dependent decrease in TEER of Caco-2 cell monolayers of up to 83% at 0.5% (w/v) concentration, which was independent of the pH of the medium [47]. In turn, this reduction in electrical resistance is indicative of the simultaneous increase in membrane permeability. Chitosan, either in solution or prepared as NPs, has shown a decrease in TEER and an increase in cell permeability via the tight junction pathways by the displacement of the zonula occludens-1 proteins in Calu-3 cells [48]. TEER measurements can be conducted across a wide range of spectrum frequencies and in real time without causing any damage to live cells. However, variations can arise due to changes in temperature, the passage number of cells, the type of medium, and the degree of cell confluency [46]. The various physical and chemical properties of chitosan affecting its interactions with cells have been summarized in Table 1.

Table 1.
Properties of chitosan and its effect on cell interactions.
3. Pathways of Cellular Uptake
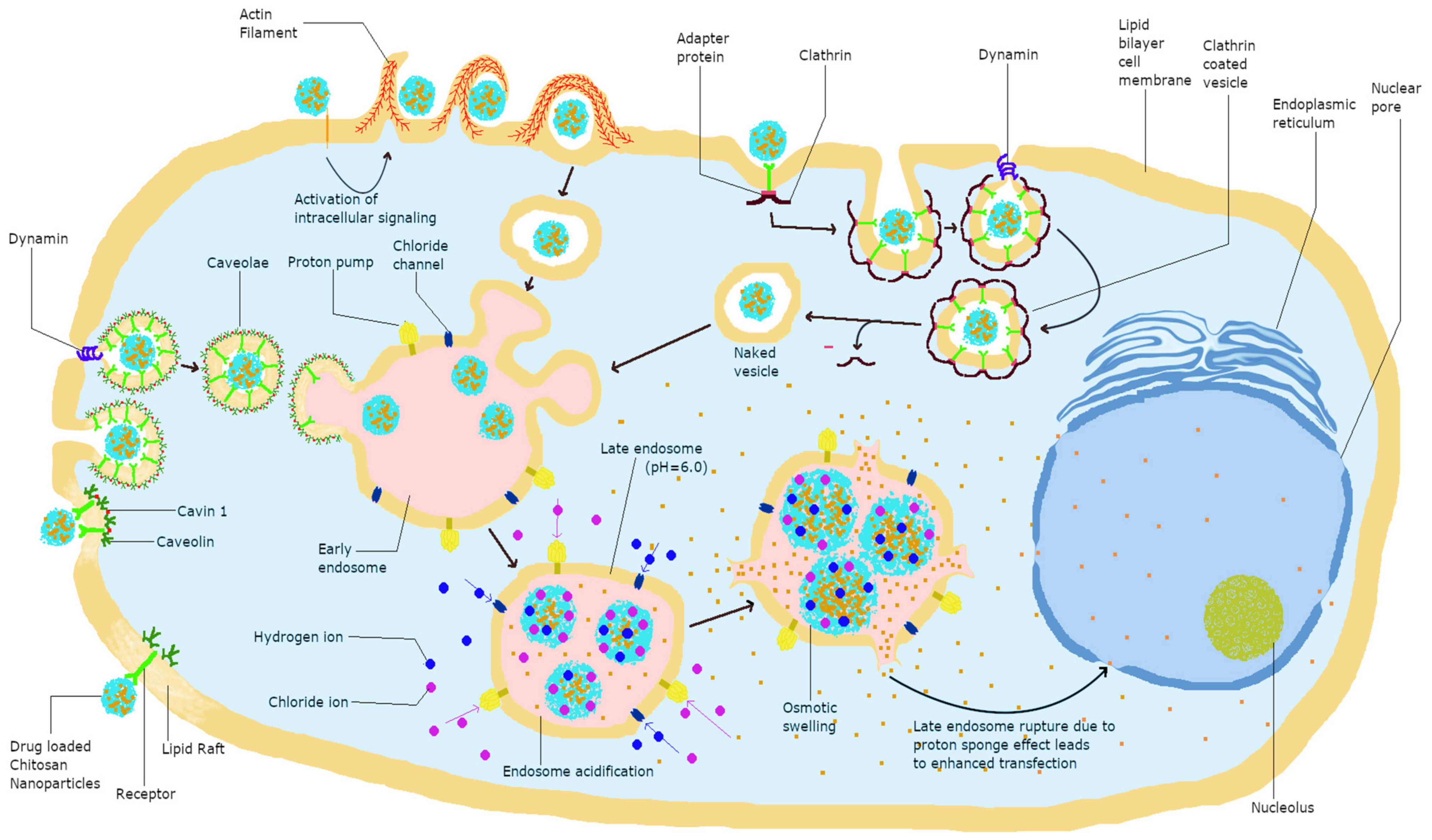
Charged biomolecules are mainly transported across the cell membrane through an active endocytosis transport mechanism [49]. Chitosan is known to be transported mainly via this mechanism and specifically, the endocytosis of chitosan NPs involves the two major pathways of phagocytosis and pinocytosis. Furthermore, chitosan uptake by pinocytosis can be divided into caveolin-mediated, cadherin-mediated, and clathrin-mediated uptake. Phagocytosis is the uptake of particles larger than 250 nm, whereas pinocytosis involves the pathways of cellular uptake of chitosan and is dependent on its size, charge, and surface modification. Clathrin-mediated cellular uptake involves transport via clathrin-coated pits, leading to internalization followed by integration into late endosomes delivering their cargo to lysosomes [50]. Caveolae-mediated endocytosis utilizes cholesterol-rich, flask-shaped invaginations in the plasma membrane, which internalize from the plasma membrane with the help of GTPase dynamin, delivering cargo to lysosomes [50,51]. The different interactions of chitosan with cells are depicted in Figure 1, and further visual representation of chitosan NPs with cells using confocal microscopy can be found in the following references [9,52,53,54].
Figure 1.
Modes of cellular uptake and eventual fate of chitosan in cells.
3.1. Effect of Size and Charge on Uptake Pathway
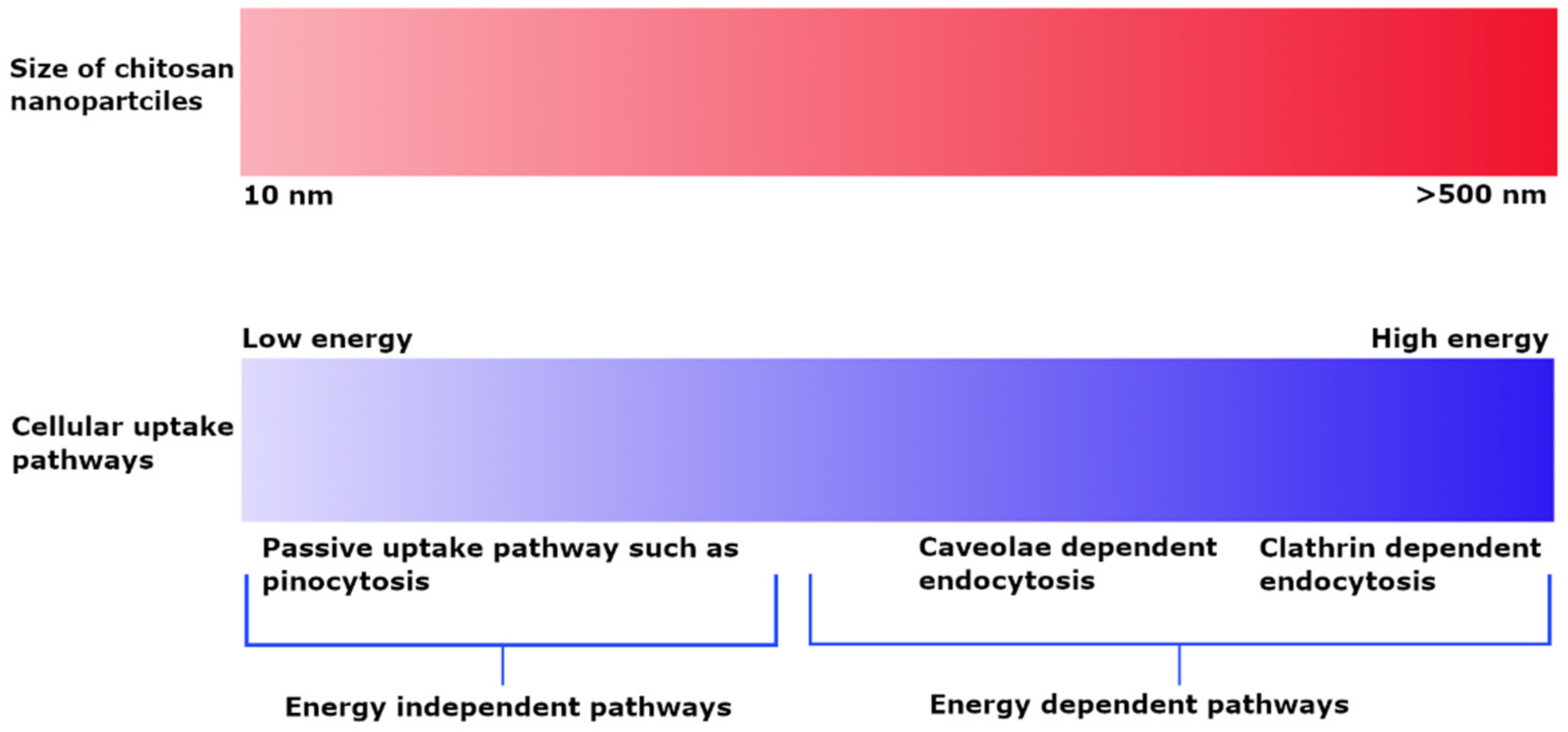
The cellular uptake of chitosan NPs is based on their size and is dependent on the type of cell line and experimental conditions. PLGA NPs coated with chitosan having a particle size of up to 200 nm demonstrated more extensive cellular uptake into A549 cells as compared to 1000 nm particles and suggested an energy-dependent clathrin-mediated endocytic process [55]. The cellular uptake of chitosan particles loaded with ovalbumin and FITC–bovine serum albumin on bone marrow-derived dendritic cells and the RAW 264.7 mouse macrophage cell line indicated that uptake was dependent on size rather than incubation time and concentration. Particles in the 1 µm range were taken up to a greater extent by both macrophage and dendritic cells as compared to smaller (300 nm) and bigger (3 µm) particles [56]. In contrast, FITC-labeled chitosan NPs having a particle size of about 250 nm exhibited clathrin-mediated endocytosis; phagocytosis occurred to a smaller extent in macrophages [9]. The uptake of smaller chitosan NPs in the 25 nm range in L929 fibroblast cells indicated a passive uptake, whereas the uptake of larger NPs of up to 150 nm was more energy-dependent in the presence of sodium azide. Both NP sizes showed caveoli- and lipid raft-dependent endocytic processes [57]. Observation of the dependence of the uptake pathway on charge indicates that non-phagocytic cells preferentially uptake cationic NPs, although charge density and hydrophobicity also have a role. Both anionic and cationic charged chitosan NPs prefer clathrin-mediated endocytosis, although no general rule applies [58]. He et al. investigated the phagocytic and non-phagocytic uptake of rhodamine-labeled negatively charged carboxymethyl chitosan NPs and positively charged chitosan hydrochloride-grafted NPs with particle sizes of 300 nm and 500 nm in murine peritoneal macrophage cell lines. Positively charged particles displayed greater phagocytic uptake as compared to negatively charged particles. Similarly, the non-phagocytic uptake of these particles favored positively charged NPs as compared to negatively charged ones, although the extent of uptake was highly dependent on the type of cell line. Furthermore, the uptake process was highly energy dependent, as demonstrated by the observation of significant inhibition of uptake at 4 °C. Clathrin and caveolae-mediated cell uptake processes were also common [59]. The effect of particle size on uptake pathways is summarized in Figure 2.
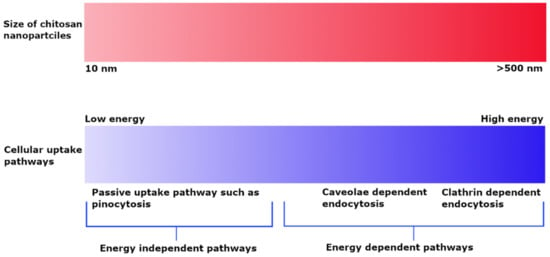
Figure 2.
Effect of particle size on cellular uptake pathways depicting energy dependent uptake of larger sized NPs and passive uptake of small NPs.
3.2. Effect of Hydrophobicity/Hydrophilicity on Uptake Pathway
The incorporation of hydrophobic chains to polymers can enhance cellular uptake for the delivery of genes as well as uptake by tumor cells [60,61]. This enhanced uptake is mainly attributed to the increased hydrophobic interactions with the lipid cell membrane, although cellular uptake still takes places via various mechanisms including clathrin- and caveoli-mediated pathways. Hydrophobic modifications on glycol chitosan were utilized by Nam et al. for targeted delivery to tumors. The uptake in HeLa cells by hydrophobically modified chitosan NPs having an average particle size of 310 nm involved both clathrin- and caveoli-mediated pathways in addition to micropinocytosis, with all pathways displaying an additive effect on the cellular uptake. Therefore, this report suggested that all these pathways were involved [61]. Hydrophobically modified N-palmitoyl chitosan with a particle size of about 200 nm indicated non-clathrin-mediated uptake at lower degrees of hydrophobic substitution (e.g., 5%, 10%, and 15%), whereas macropinocytosis was the preferred route of uptake at higher degrees of substitution of 15% and 20%. The caveolae-mediated route of uptake was the most affected as the degree of hydrophobicity of NPs increased, which indicated that lipid raft-mediated caveoli was the most preferred route at high hydrophobicity [62].
Hexanoic acid and monomethoxy-PEG modifications on chitosan to form pDNA polyplexes were utilized for the efficient delivery of genes to HEK 293 cells. These polyplexes with a diameter of less than 200 nm and positive zeta potential showed predominantly clathrin-mediated cellular uptake [63]. Similarly, both hydrophilic and hydrophobic modifications of chitosan with linoleic acid and poly (β-malic acid) forming a doubly grafted chitosan formic complex with pDNA (mean diameter <200 nm) indicated a process dominated by clathrin-mediated uptake [64].
3.3. Covalent Modifications
Chitosan and its derivatives have been exploited to fabricate targeted drug delivery systems to achieve the desired effect of drug at lower concentrations [65] by attaching various ligands, which include peptides, vitamins, hormones, carbohydrates, and proteins, as shown in Table 2. Receptor-mediated endocytosis is the primary route of uptake for targeted systems functionalized with ligands [66,67,68,69]. Overexpression of the receptors on specific cells in certain disease states facilitates the selective binding of chitosan and its derivatives by targeted drug delivery [11,13,70,71], which leads to enhanced drug delivery to specific target sites. Kai Jiang et al. utilized the pH-sensitive nature of chitosan polymers to deliver a hydrophobic drug, namely ursolic acid, into endosomes (pH < 5.5) and reported the suppression of cancer cell growth [72]. The derivatization of chitosan polymers broadens opportunities such as dual-ligand functionalization [73], co-polymerization to develop amphoteric block co-polymers [11], co-delivery of drug molecules and oligonucleotides [68], and improving solubility [70]. Zhu et al. reported that chitosan derivative N-succinyl-N′-octyl chitosan self-assembles to form micelles due to its amphipathic nature, and conjugation with folic acid imparts a targeting nature to the micelles [74]. Further modifications of chitosan affecting pathways of cellular uptake are summarized in Table 2.

Table 2.
Various modifications of chitosan depicting different types of cellular uptake.
4. Intracellular Disposition of Chitosan
The endocytosis mechanism determines the intracellular fate of NPs. Endocytosis transports the NPs within vesicles where they are released into the subcellular compartments such as lysosomes, mitochondria, or Golgi bodies. Nevertheless, it is important that the NPs are able to release their cargo once transported inside the cells. Once released into the cells, the NPs must be able to travel through the cytoplasm to reach their target organelles, which is dependent on the physicochemical properties of the NPs such as size, charge, and physicochemical properties [84].
4.1. Endosomal Escape
One essential requirement of gene therapy is the efficient escape of NPs from endosomes to deliver drugs into the cytoplasm, which can be later taken up by the nucleus for effective gene transfection. The enzymes within lysosomes can cause significant degradation of the encapsulated molecules, leading to reduced efficiency. Chitosan NPs have shown the potential for enhanced endosomal escape due to the phenomenon well-known as the “proton sponge effect.” The acidic environment within endosomes causes amine groups on chitosan, which have a pka of 6.5, to become increasingly protonated in the endosomes, thus leading to a high influx of water and chloride ions to balance the charges. This influx causes the lysosomes to swell and rupture, releasing their contents into the cytoplasm [85]. However, chitosan on its own has a poor buffering capacity [86], as demonstrated by the uptake of poly (methacrylic acid) surface decorated on 10-hydroxy camptothecin-loaded CTS NPs having a positive charge at pH 6.5. The positive charge of chitosan NPs facilitated protonation at endosomal pH 6.5, leading to endosomal rupture due to the proton sponge effect. This was confirmed by the assessment of the integrity of the endosomal membranes, as indicated by a characteristic shift in the fluorescence of the endosomal marker acridine orange after the rupture of the endosomes [87]. Similarly, various modifications on chitosan for gene delivery have been utilized for efficient endosomal escape: grafting imidazole moieties on the chitosan backbone from DNA–polymer complexes for improved transfection in HEK 293 cells and HepG2 cell lines [88]; the addition of histidine to the chitosan backbone, forming complexes with pDNA in HEK cell lines and enhancing the buffering capacity of chitosan to enhance endosomal escape [89]; and enhancing the buffering capacity of chitosan with the help of alkyl amino acids for delivery of the p53 gene in HEK and A549 lung cells [90].
4.2. Co-Localization with Lysosomes
Molecules endocytosed into cells reach lysosomes for degradation via early and late endosomes. After NPs are taken up by different mechanisms, they reach the lysosomes where their preferential uptake is determined by their size and surface properties [8]. Lysosomes contain various degradative enzymes and are the most acidic organelles, with a pH ranging from 4.5 to 5.5, which causes enzymatic degradation of NPs. NPs are designed to target lysosomes in order to treat lysosome-related diseases or to escape lysosomal degradation to deliver materials into areas of the cell [91,92]. Chitosan causes the rupture of lysosomes by a process similar to that for endosomes through the proton sponge effect, as shown by Fong et al., where they studied the effect of acetylation of chitosan to produce lysosomal rupture and subsequent inflammatory response. They found that the lysosomal disruption was dose dependent with lower doses producing a mild disruption, whereas higher doses of chitosan produced higher levels of lysosome disruption in U937 cell lines differentiated to macrophages [93]. In another study, the cationic charge of chitosan enabled efficient lysosomal escape in HEK cells to increase the chitosan–pDNA polyplex transfection efficiency. Free chitosan, when pre-incubated in cells before allowing transfection, led to chitosan localizing in the lysosomes, which increased the transfection of DNA polyplexes previously having a low transfection efficiency [94]. In contrast, hyaluronic acid-conjugated chitosan NPs have been shown to entirely escape lysosomal uptake in corneal and conjunctival cell lines [95].
4.3. Nuclear and Perinuclear Localization
Drug delivery to the nucleus involves overcoming the nuclear envelope barrier and transport through the nuclear pore complexes, which are perforations in the nuclear envelope. Transport through the nucleus is mainly dependent on size, where smaller molecules are transported across by passive diffusion and larger molecules are sorted via oligopeptide receptor-mediated active transport using nuclear localization signals (NLS) [96]. Nuclear localization is essential for drugs exhibiting their main mechanism of action at this site [97]. Tammam et al. have indicated that chitosan NPs having a smaller size of up to 25 nm displayed up to a five-fold increase in nuclear localization rates of albumin–FITC-loaded NPs as determined by FRET microscopy, whereas larger NPs of up to 150 nm required modification with an octapeptide nuclear localization sequence to produce nuclear localization of up to 3.7-fold improvement in murine fibroblast L929 cells [57]. Furthermore, an NLS peptide sequence associated with a chitosan–DNA complex improved the transfection efficiency up to 74-fold as compared to a plain chitosan–DNA complex in HeLa cells. These ternary complexes had a particle size of up to 500 nm and zeta potential of about +12 mV [98]. The perinuclear localization of NPs provides proof of endosomal/lysosomal escape and potential uptake by the nucleus. Additionally, chitosan NPs persist in cells for a long time and can become entrapped inside the nucleus subsequent to nuclear envelope reassembly at the end of mitosis; this temporal advantage also helps to target drugs into the nucleus, irrespective of size of the NPs and nuclear localization signal [52]. Additional examples include various other covalent modifications on chitosan: alkyl glyceryl chitosan NPs in mouse brain capillary endothelial cells [99]; tamoxifen-loaded lecithin/chitosan NPs in Caco-2 cells [100]; and glycol chitosan-β cholanic acid NPs loaded with protoporphyrin IX for targeted photosensitizer delivery in squamous cell carcinomas [101]. These approaches have been investigated to localize chitosan NPs into the perinuclear region, therefore enhancing their uptake into the nucleus.
4.4. Mitochondrial Metabolism
Chitosan NPs have been employed for the mitochondrial targeting of drugs; however, their mechanism of targeting this intracellular site is unclear. Targeted delivery to the mitochondria has been employed in the treatment of several tumors. NPs can take advantage of the difference in mitochondrial membrane potential and release of reactive oxygen species (ROS), causing rupture and the release of mitochondrial DNA and ultimately causing cell death. The mitochondrial membrane potential and ROS generation have an exponential correlation; hence, even a small difference in membrane potential can lead to a considerable increase in ROS [102]. Chitosan NPs having a size of about 65 nm and zeta potential of +52 mV caused a dose-dependent decrease in mitochondrial membrane potential in human gastric carcinoma MGC803 cells causing disruption of the mitochondrial membrane and subsequent necrosis [103]. Similarly, chitosan NPs having an average particle size of 84 nm and charge of +17 mV caused a decrease in mitochondrial membrane potential and generation of ROS to induce cell death in hepatocellular carcinoma SMMC-7721 cells [104]. Covalent modifications of chitosan to aid in its mitochondrial targeting have also been investigated, such as N-glycyrrhetinic acid-PEG-chitosan and N-quaternary ammonium chitosan NQC loaded with brucine, which indicated mitochondrial targeting in HepG2 cells [105]. Triphenyl phosphine-conjugated chitosan NPs, hyaluronic acid-coated chitosan NPs, and glycol chitosan polymerized with dequalinium have all been used; however, in these cases, chitosan was utilized as an aid for efficient cellular uptake, whereas the mitochondrial targeting moieties were triphenyl phosphine, hyaluronic acid, and dequalinium, respectively [106,107,108]. Conversely, carboxymethylated chitosan and chitosan-coated iron oxide NPs have been used to prevent mitochondrial stress and hydrogen peroxide-induced cell death in Schwan cells in addition to mitochondrial membrane protection with reduced ROS generation in HeLa, A549, and HEK 293 cell lines, respectively [109,110].
4.5. Exocytosis of Chitosan Nanoparticles
Although chitosan is considered relatively safe, its exocytosis from cells determines its therapeutic toxicity and biosafety [111,112]. The exocytosis of NPs from cells is affected by the various physicochemical properties of NPs such as size, shape, surface modification, concentration, and incubation time with cells [111,112]. As demonstrated by Park et al., the exocytosis of N-acetyl histidine chitosan NPs was dependent on the pre-incubation time with nanoparticles before the removal of free nanoparticles from the media. With short incubation times, the self-assembled N-acetyl histidine chitosan NPs dissociated in the acidic endosomes. However, with longer incubation times of 6 h, the exocytosis of NPs was observed from HeLa and A549 cells [113]. Chitosan-coated PLGA NPs again showed an incubation time-dependent exocytosis of NPs from mast cells with plain PLGA NPs displaying higher exocytosis as compared to chitosan PLGA NPs [114]. This strategy may thereby promote entry of the payload in the cytosol. On the other hand, chitosan-coated ultra-small superparamagnetic iron oxide NPs have been designed for long-term retention in a variety of cell lines with NPs observed in cells for up to 10 days [115]. Doxorubicin-loaded deoxycholic acid-modified carboxymethyl chitosan NPs indicated time-dependent accumulation in MCF-7 cells with faster uptake within 6 h of incubation and longer retention time in cells after 24 h as compared to free doxorubicin [116]. Thus, it can be concluded that chitosan helps prolong the retention time of these types of particles within cells.
4.6. Cytotoxicity upon Cell Internalization
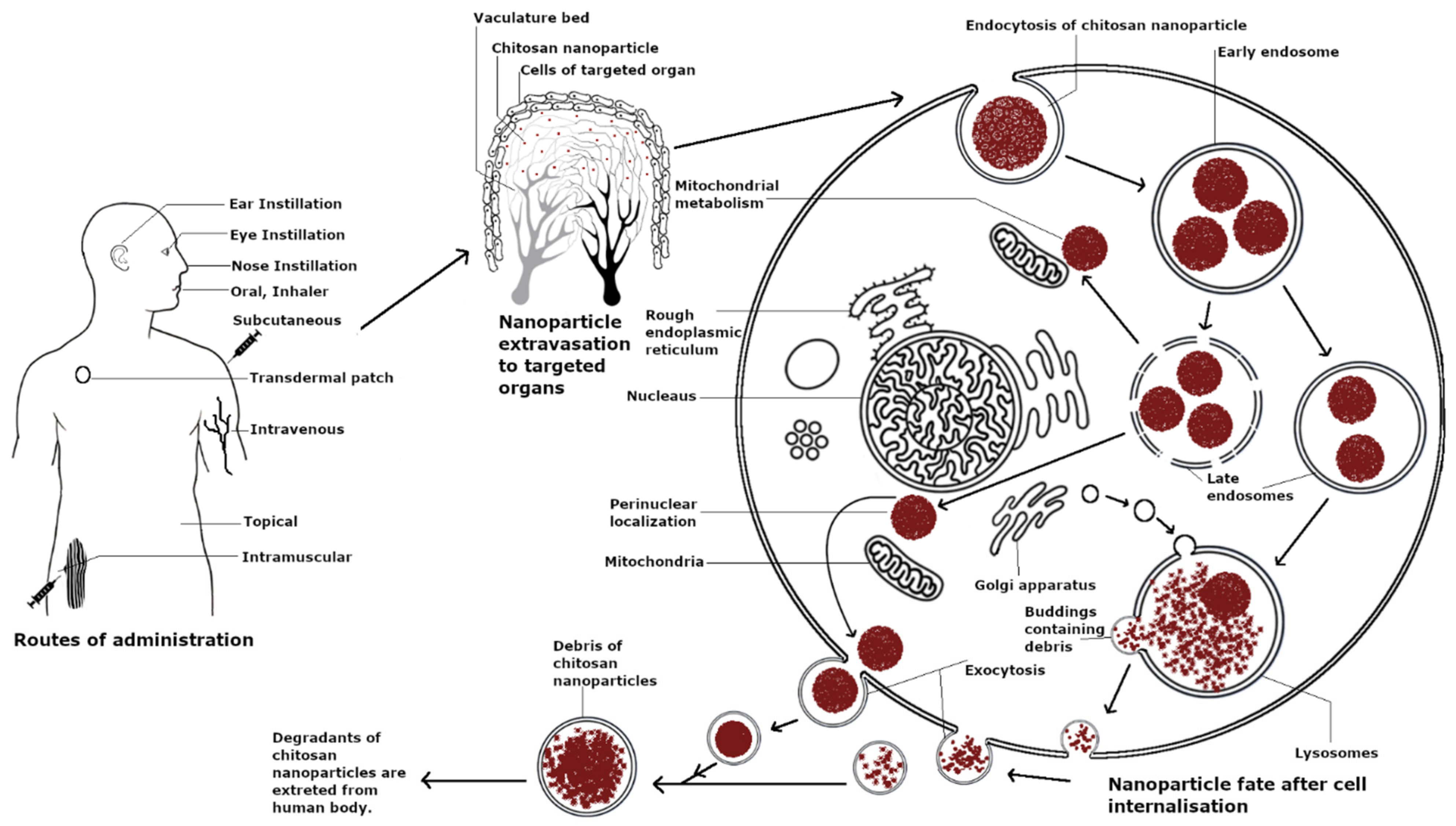
Chitosan is considered a biocompatible material. Chitosan and chitosan-coated NPs have displayed no toxicity in a variety of cell lines such as MDBK cells, Colo 205 cells, human antigen-presenting cells, respiratory epithelial cells Calu-3 and A549, and 3T3 fibroblast cells [117,118,119,120]. Similarly, amino acid modification on glutaraldehyde cross-linked chitosan NPs, such as lysine and glutamic acid modifications, induced no cytotoxicity even at concentrations up to 250 µg mL−1 and suppressed the toxicity of copper oxide-loaded NPs in HepG2, A549, and RAW264.7 cells [121]. However, chitosan cytotoxicity has been shown to be dependent on the cell line type, particle size, and concentration and particularly favors cancerous cells as compared to non-cancerous cells. Fast-growing cancer cells, namely COS-1, showed higher sensitivity to chitosan-coated PLGA NPs as compared to epithelial A549 and Calu-3 cells in terms of concentration and incubation times; however, the charge of particles did not affect the toxicity in COS-1 cells [122]. Similarly, chitosan NPs displayed an IC50 of 10 µg/mL after 24 h in human T lymphocyte acute leukemia cells and were significantly cytotoxic at 50 µg/mL but displayed no cytotoxicity at the same concentration in human embryonic kidney HEK 293 cells. The higher toxicity of chitosan NPs in tumor cells as compared to non-cancerous cells can be attributed to various factors such as differences in the mitochondrial membrane potential, increase in mitochondrial dehydrogenase activity, the generation of ROS, and enhanced uptake in tumor cells [123]. Chitosan NPs (50–100 nm) were recently used for immune cell anti-tumor therapy in γδ T cells, which are innate-like T lymphocytes and are an early source of IFN-γ. The NPs functionally upregulated Vγ9Vδ2 T cells, induced their activation, and enhanced tumor cytotoxicity through α-tubulin cytoskeleton rearrangement and polarization [124]. The cellular lifespan of chitosan is summarized in Figure 3.
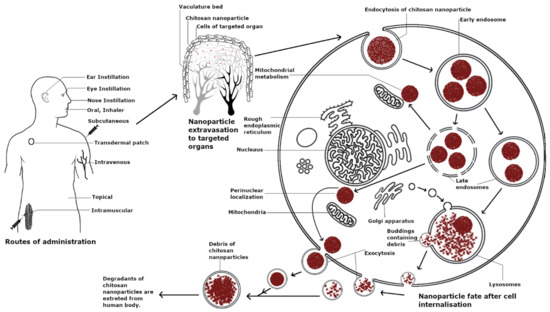
Figure 3.
Lifespan of chitosan nanoparticles.
5. In Vivo Tissue Distribution and Bioavailability of Chitosan Nanoparticles
The effect of chitosan at the biological interface involves not only its effect on cells but also behavior upon intake through various routes. Early pioneering research on chitosan particles biodistribution was reported by Richardson, Suzuki, Onishi, and Banerjee et al. [125,126,127,128] and extensively reviewed by Thanou et al. [129]. They found that following administration into the body, chitosan particles rapidly distribute and are quickly cleared, especially after intravenous administration. However, the size and charge of chitosan NPs, its MW, degree of acetylation, covalent modifications, and the extent of protein corona formation all play a major role in its ability to achieve long circulation times in blood to prevent premature elimination of drugs from the body. The significance of chitosan NPs in preventing the premature elimination of encapsulated drugs from the body has been summarized in Table 3. In turn, these properties affect the safety of these NPs for clinical applications [129].

Table 3.
Significance of chitosan nanoparticles in prevention of premature elimination of loaded cargo.
5.1. Effect of Protein Corona
The fate of NPs upon entering systemic circulation is governed by the protein corona surrounding it. Protein coronas are characterized by a dynamic “soft corona” and a long lasting “hard corona”, which remains strongly adsorbed onto the nanoparticle surface. The composition of the protein corona affects how NPs interact with platelets and blood cells in addition to how they influence various aspects of nanoparticle design such as drug release, active targeting, and surface functionalization [7,138,139]. Negatively charged serum proteins are preferentially adsorbed onto the surface of positively charged NPs due to electrostatic interactions [140]. Comparably, positively charged chitosan NPs have been demonstrated to attract large quantities of protein on their surface, as evidenced by Almalik et al. In addition, they demonstrated that decreasing the zeta potential from cationic to anionic, by coating the chitosan nanoparticle surface with negatively charged hyaluronic acid, significantly decreased the non-specific protein interactions of the NPs [141,142]. Furthermore, chitosan-coated PLGA NPs displayed higher affinity to serum immunoglobulins due to the innate immunogenic response of positively charged chitosan as compared to heparin-coated PLGA NPs, which displayed higher affinity to serum albumin [143]. Abouelmagd et al. maintained that despite the high serum protein adsorption on the surface, chitosan NPs maintained the pH sensitivity necessary for efficient drug delivery. They prepared PLGA NPs coated with low MW chitosan for the delivery of paclitaxel in SKOV-3 human ovarian cancer cells. PLGA NPs were produced either from PLGA pre-conjugated to LMW chitosan, or the PLGA NPs were formed first, which was followed by surface conjugation with polymerized dopamine and subsequent incubation with LMW chitosan, effectively spacing the chitosan away from the surface. This reduced the hydrophilicity of the NP to better enable hydrophobic drug loading. The LMW chitosan-modified NPs had a protein corona increasingly enriched with immunoglobulins in addition to serum albumins. They were more effective for enhancing cell uptake under acidic compared to neutral pH conditions, thus demonstrating pH sensitivity. When formulated as microparticles (1–3 μm), this approach demonstrated that the surface modification with LMW chitosan reduced phagocytic uptake by J774A.1 mouse macrophages, which was possibly due to the hydrophilicity imparted by the chitosan coating [144]. In another strategy to reduce RES uptake, a recent study of the protein corona formation and biodistribution of chitosan and its polyelectrolyte complex with carboxymethyl dextran and thiolated dextran indicated that dextran modification of chitosan resulted in a reduced protein corona and low liver uptake. A net negative surface charge was associated with lower protein binding, and the protein corona was dominated by protein C, hemoglobin subunits, and apolipoproteins, which play a role in the induction of uptake of NPs by macrophages as well as their eventual phagocytosis and biodistribution [145].
5.2. Effect of Mucosal Routes of Administration
The cationic surface properties of chitosan NPs allow it to bind strongly to negatively charged mucosal surfaces, making it an excellent candidate for mucosal routes of administration such as oral, intranasal, ophthalmic, and vaginal routes. Chen et al. prepared chitosan NPs for the oral delivery of heparin and followed the biodistribution of 99mTc-labeled chitosan NPs upon oral administration. They found that the mucoadhesive property of chitosan allowed these NPs to predominantly accumulate in the intestinal mucosa with a gastric retention time of 8 h and the majority of radioactivity observed in the colon after 24 h. There was minimal systemic absorption and accumulation in internal organs [146]. Similarly, colon-targeted curcumin-loaded chitosan NPs coated with Eudragit FS 30D were found to retain highly in the colon with little accumulation in other organs [147]. Navarro et al. reported less than 1% accumulation of chitosan NPs in the spleen, liver, kidneys, heart, lungs, and brain after oral administration of fluorescent tagged FITC–PLGA–chitosan NPs daily for 7 days and attributed these findings to chitosan’s mucoadhesive properties and the rapid elimination from the systemic circulation [148]. Similarly, the mucoadhesiveness of chitosan leads to longer retention time in the nasal cavity. Gartziandia et al. reported NPs accumulation in the brain after 30 min of intranasal administration, which was observed by fluorescence imaging of DIR-labeled chitosan–lipid nanocarrriers, which were retained after 24 h. Additionally, fluorescent NPs were found to concentrate mainly in the lungs and to a smaller extent (less than 1%) in liver, spleen, and kidneys due to non-specific mononuclear phagocyte capture. Nanoparticles were present in the nasal cavity for up to 24 h, allowing additional time for them to reach the brain [149]. Similarly, chitosan–lecithin NPs for brain targeting loaded with phenytoin demonstrated relatively high accumulation in brain and low phenytoin levels in plasma after intranasal delivery as compared to intraperitoneal injection, which produced initial high plasma levels before crossing the blood–brain barrier [150]. The intraocular delivery of chitosan inserts radiolabeled with Tc-99m resulted in the retention of NPs for up to 6–8 h at the site of instillation in the eye, after which the formulation accumulated in the gastrointestinal tract, mainly the large intestine, via nasolacrimal clearance [151,152].
5.3. Effect of Surface Modifications
The surface modification of chitosan NPs allows them to target the desired site of action and has been utilized for various applications depending on the type of modification such as tumor targeting, oral delivery for GIT uptake, ocular delivery, lymphatic system targeting, and prolonged systemic circulation. Kamiyama et al. first reported the biodistribution of modified chitosan to form water-soluble chitosan derivatives N-succinyl chitosan and glycol chitosan for tumor sarcomas targeting. The fluorescent isothiocyanate FITC-modified glycol chitosan solutions displayed higher partition into the tumors as compared to other organs, whereas FITC-labeled succinyl chitosan solutions displayed longer blood residence time and a greater accumulation rate into the tumor [153]. Li et al. prepared dual ligand-decorated galactosylated chitosan with glycyrrhetinic acid for hepatocellular carcinoma targeting. The NPs specifically accumulated at the tumor site followed by liver, spleen, and kidneys [154]. The modification of chitosan NPs with the thermos-sensitive polymer poly (N-vinylcaprolactam) and a cell-penetrating peptide (RLYMRYYSPTTRRYG) for targeting triple negative breast cancer with doxorubicin collected extensively in the tumor region and also to a lesser extent in the liver, kidneys, and spleen. The modified chitosan polymer had a phase transition temperature that was clinically achievable for tumor heating (<40 °C), enhancing local drug release. Furthermore, the slightly acidic tumor environment promoted dissolution of the chitosan NPs and thus triggered release from these dual pH/thermo-responsive NPs [155]. Carboxymethyl-β-glucan/chitosan NPs have been recently used to target vaccines to the lymphatic system. These NPs having a size of about 150 nm and charge +30 mV were loaded with ovalbumin and showed significant lymphatic accumulation due to the size and charge of the NPs and the ability of carboxymethyl-β-glucan to interact with antigen-presenting cells [156]. Surface-modified negatively charged poly(methacrylic acid) conjugated chitosan NPs loaded with 10-hydroxy camptothecin (HCPT) significantly increased blood circulation time from 12 h to 24 h and reduced blood clearance (CI) from 30 to 6 mL/h as compared to the non-surface modified NPs. Additionally, the t1/2 of the surface-modified NPs was longer by 4.37-fold and that of the non-surface modified NPs was longer by 2.48-fold compared to free drug [87]. Other examples of chitosan modifications for various targeting applications are summarized in Table 4. These examples provide evidence of the versatile nature of chitosan modified to suit multiple targeting applications.

Table 4.
Various modifications of chitosan depicting enhanced absorption and bioavailability.
5.4. Effect of Physical Properties on Biodistribution
So far, we have discussed the effect of particle size and charge and hydrophobicity on chitosan NP cellular uptake and toxicity and given examples of modifying the surface properties. These attributes also impact systemic biodistribution. Onishi et al. studied the biodegradation and distribution of solutions of 50% deacetylated chitosan labeled with FITC upon intraperitoneal administration. The FITC–chitosan solution was found to accumulate in the kidneys and rapidly eliminate in urine after 14 h of administration with little accumulation in liver and spleen [127]. Furthermore, Banerjee et al. reported the biodistribution of 100 nm chitosan NPs which were 100% cross-linked with glutaraldehyde labeled with 99mTc. They observed a rapid and maximum accumulation of the NPs in the liver within 30 min of intravenous administration followed by other organs, which subsequently declined over 4 h. They also observed increased radioactivity in the stomach after 4 h, indicating dissociation of the radioactive complex. They attribute this effect to the small particle size of chitosan NPs and hydrophilicity of the polymer [128]. Zhang et al. analyzed the biodistribution of N-octyl-O-sulfate chitosan micelles with 65 kDa chitosan loaded with paclitaxel having a size of about 200 nm and negative surface charge of −28.8 mV, following intravenous administration. They observed rapid and wide distribution to all organs of the body with maximum accumulation in spleen followed by liver after 8 h of administration, with the longest retention time in the lungs [166]. Similarly, Bachir et al. compared chitosan (MW 11 kDa) NPs conjugated with PEG at various degrees of substitution loaded with methotrexate having a particle size of about 110–171 nm and zeta potential +7–35 mV found that non-PEGylated chitosan NPs accumulated significantly in the liver and spleen at 24 h following intravenous administration and to a lower extent in lungs, kidneys, and heart. In contrast, PEGylated chitosan NPs distributed primarily to the kidneys [167]. Intraperitoneal injection of FITC-labeled carboxymethyl chitosan revealed similar high uptake in the liver of 300 kDa molecular weight chitosan, whereas unspecified chitosan degradation products of about 45 kDa were found in the urine [168]. Feng et al. prepared pH-responsive NPs of chitosan and O-carboxymethyl chitosan of MW 10–12 kDa having a size of about 270 nm and negative zeta potential (−33 mV) loaded with doxorubicin for oral administration. These NPs were capable of selectively releasing doxorubicin into the intestinal environment and enhanced transport across the epithelial tight junctions. They achieved prolonged accumulation and retention in the liver, spleen, and lungs. This controlled release property of the NPs reduced the cardiac and renal toxicity of doxorubicin. Thus, chitosan NPs have the liver and spleen as the significant sites of accumulation, similarly to other types of NPs, suggesting that their reticuloendothelial uptake dominates biodistribution [162].
6. Systemic Toxicity and Elimination of Chitosan Nanoparticles
Designing a drug delivery system requires in vivo toxicity studies to assess their applicability, especially for nanoparticulate systems. Chitosan is widely considered a nontoxic and biologically compatible polymer; however, chitosan NPs need to be scrutinized for their safety potential. A review of chitosan’s in vivo toxicity by Thanou et al. previously found chitosan to be relatively nontoxic at doses needed for therapeutic delivery. Some toxicity was observed at high doses, but they generally concluded chitosan to be safe for in vivo applications [129]. Sonin et al. recently assessed various factors such as the hemolytic activity and acute and sub-acute toxicity of chitosan NPs over a 14-day period. Chitosan MW 138 kDa NPs of about 100 nm in size having a weak positive charge of +10 mV were administered as a single intravenous dose of 1, 2, and 4 mg/kg of chitosan in mice and observed over 2 weeks. The NPs displayed negligible in vivo antiplatelet activity and ex vivo anticoagulant activity with the least toxicity in cardiomyocyte cell cultures. Upon IV administration, about 93% of the NPs accumulated in the liver with about 6% accumulation in lungs within 30 min of administration with no organ cytolysis nor necrosis observed after 14 days. They found small granulomas in the lungs consistent with the physiological macrophage containment of foreign bodies and by which the authors suggest a slow degradation process. Overall, these results suggest that chitosan NPs and chitosan degradation products are well tolerated and naturally eliminated at least for those NP with near-neutral surface charge [169]. Nadesh et al. established that chitosan NPs having a high surface charge of more than +30 mV produced hematotoxicity, whereas NPs having a highly negative charge of about −40 mV resulted in their phagocytosis and rapid elimination. Hence, the biocompatible range of charge for chitosan NPs is +15 to −30 mV [59,170]. This also highlights the need to conduct hemolysis assays for novel chitosan NPs intended for IV administration. Sonaje et al. studied the toxicity of pH-sensitive chitosan NPs conjugated with poly-γ-glutamic acid in the presence of MgSO4 and TPP for the oral delivery of insulin (size ≈ 200 nm, charge +25 mV). Blank chitosan NPs (100 mg/kg) upon daily administration over 14 days displayed no signs of toxicity such as diarrhea or fever and no mortality at the end of the study, indicating no oral toxicity [171]. Shan et al. studied the acute and chronic toxicity of angiopoietin-2 with small interfering RNA plasmid chitosan magnetic NPs after intravenous administration of different doses and observed them over 14 days. Upon observation of acute toxicity, the mice in the middle and high dosage groups (254.6, 424.2, and 707.0 mg.kg−1.d−1) exhibited short-term reduced activities and heavy breathing attributed to partial lung congestion due to lung phagocytosis as compared to the low-dosage groups (91.6, 152.8 mg.kg−1.d−1) and control group. Chronic toxicity studies revealed no significant impact on the well-being of the animals and no deaths. However, chronic lung congestion was found upon morphological observation of lungs after 14 days of daily NP administration with a high number of white blood cells in the middle and high-dose groups (70.70 and 353.50 mg.kg−1.d−1) as compared to the control and low-dose group (35.35 mg.kg−1.d−1) indicative of the lung being the target organ for the elimination of these magnetic NPs upon IV injection [172]. However, no other studies reporting lung congestion were found within the scope of the literature search for this review. Hence, most studies have demonstrated chitosan as minimally toxic except for pulmonary congestion on IV administration of very high doses, justifying its selection as a safe material in drug delivery. The high pulmonary accumulation of chitosan is likely due to its degradation primarily by the enzyme lysozyme, which is abundant in lungs and also highly expressed in hematopoietic cells, granulocytes, monocytes, and macrophages. However, for novel chitosan nanoparticles, it would be prudent to conduct lung histopathology studies in the course of early toxicological evaluations as a part of dose–response studies. Although there are no conclusive in vivo studies on the complete degradation and elimination profiles of chitosan, most studies conclude that chitosan is biodegradable. In vitro degradation studies of chitosan suggest that the rate and degree of degradation depends on various factors such as the concentration of enzymes, molecular weight of chitosan, degree of acetylation, type and concentration of cross-linking agents, and pH of the medium [30,173].
7. Current Clinical Investigations and Challenges
Chitosan has been used in various marketed products in the food and beverage industry. The chief markets for nutraceutical formulations of chitosan are Asia including Japan, Korea, and China with the USA and Europe quickly expanding as well. Chitosan products include ChitoClear®, MicroChitosan NutriCology®, and Chitoseen™-F, which are marketed as fat reducers and cholesterol-reducing agents for obesity and weight management and Epakitin™, Nutri + Gen®, which are marketed as nutritional supplements and used to treat chronic kidney disease in dogs and cats. There are a large number of applications of chitosan in beverages, cosmetics, agriculture, and the paper industry; the biomedical applications of chitosan are mainly in the area of wound healing. Marketed wound dressings using chitosan include HemCon® Bandage, TraumaStat®, ChitoGauze® PRO, ChitoFlex® PRO, Svek-Patch®, Chitodine®, Celox™, etc. ChitoSeat™ is a hemostatic sealant used as a surgical hemorrhage sealant for hard and soft tissues [174]. In addition to these formulations, there are various other chitosan-based formulations for drug or vaccine delivery currently under different stages of clinical investigations, as summarized in Table 5. The main challenge in the clinical applications of chitosan is the origin from natural sources; hence, its characterization and standardization are challenges. The structural characteristics of chitosan are dependent on the source of chitin, the extraction process, and the method and degree of deacetylation. Additionally, the formulation of chitosan into NPs introduces the need for additional controls related to nanoparticle size for long-term circulation and elimination [175]. Recently, chitosan has demonstrated immunostimulatory and adjuvant activity, and it is being explored as a delivery vehicle for vaccines, especially through the intranasal route [176,177]. However, in some cases, these immunostimulatory effects of chitosan can be undesirable if an excessive inflammatory response is induced. Although there are some obstacles involved in the clinical translation of chitosan NPs, there have been many formulations in different stages of clinical trials in the past 10 years. With various evolving standardization techniques, chitosan has the potential to become the delivery vehicle of choice for a number of clinical therapeutic applications.

Table 5.
Chitosan-based formulations currently in clinical trials (Source: Clinical trials.gov identifier, accessed on 21 March 2020).
8. Conclusions
Although chitosan is considered to be biodegradable and non-toxic and has been utilized in various marketed formulations in different countries for various dietary applications, the only FDA-approved pharmaceutical application of chitosan is for wound dressings. Chitosan has been the subject of a vast number of publications exploring its suitability in various forms for therapeutic drug delivery and for optimizing NP properties for drug loading and desired release profiles. However, the modifications of chitosan can alter its biological and toxicological profile, and we have many examples now of achieving a targeted and triggered release from chitosan-based NPs. Chitosan has been established as suitable for enhancing cellular uptake and improving bioavailability for various applications mainly due to its cationic nature and pH sensitivity; thus, chitosan provides an advantage for cellular targeting and controlled drug release at the site of action. The biological activity of chitosan in cells and in vivo is chiefly dependent on its size and charge and is boosted by its mucoadhesive properties. Longer-term toxicological studies of chitosan formulations for therapeutic applications need to be established for their successful clinical translation, particularly to ensure that intravenous chitosan formulations do not show evidence of rarely observed hematological toxicity, adverse effects upon pulmonary accumulation, or immune responses. These have not been commonly reported in animal models but should be considered. The versatility of chitosan to be modified physically and chemically to suit various applications provides a challenge for their regulatory approval, as the safety of all modifications and formulations needs to be considered on a case-by-case basis. Additionally, the immunological activation of chitosan and its ability to enhance penetration across the blood–brain barrier can prove to be undesirable in certain cases [4]. The origin of chitosan from natural sources further adds to unforeseeable challenges in their clinical translation. The standardization of extraction methods and analytical techniques for chitosan polymers would help enable faster translation to clinical applications. Despite the challenges, various formulations of chitosan are now in clinical trials. Chitosan nanoparticle formulations have great potential to achieve highly efficient, effective drug delivery by overcoming barriers to tissue and cellular uptake as well as drug release.
Author Contributions
Conceptualization, editing and review: E.K.W.; Writing and original draft preparation: N.A. and R.R.; Tables and Figures: P.P.; Current clinical trials and review: G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by NMIN (NanoMedicines Innovation Network), a member of the Networks of Centres of Excellence Canada program, grant number 2019-T1-09 to E.K.W. N. Aibani is funded by a postdoctoral fellowship award from NMIN.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Novel Hydrophilic Chitosan-Polyethylene Oxide Nanoparticles as Protein Carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Calvo, P.; Vila-Jato, J.L.; Alonso, M.J. Evaluation of Cationic Polymer-Coated Nanocapsules as Ocular Drug Carriers. Int. J. Pharm. 1997, 153, 41–50. [Google Scholar] [CrossRef]
- Berthold, A.; Cremer, K.; Kreuter, J. Preparation and Characterization of Chitosan Microspheres as Drug Carrier for Prednisolone Sodium Phosphate as Model for Anti-Inflammatory Drugs. J. Control Release 1996, 39, 17–25. [Google Scholar] [CrossRef]
- Ojeda-Hernández, D.D.; Canales-Aguirre, A.A.; Matias-Guiu, J.; Gomez-Pinedo, U.; Mateos-Díaz, J.C. Potential of Chitosan and Its Derivatives for Biomedical Applications in the Central Nervous System. Front. Bioeng. Biotechnol. 2020, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, J.; Raichura, Z.; Khan, T.; Momin, M.; Omri, A. Chitosan Nanoparticles-Insight into Properties, Functionalization and Applications in Drug Delivery and Theranostics. Molecules 2021, 26, 272. [Google Scholar] [CrossRef] [PubMed]
- Moraru, C.; Mincea, M.; Menghiu, G.; Ostafe, V. Understanding the Factors Influencing Chitosan-Based Nanoparticles-Protein Corona Interaction and Drug Delivery Applications. Molecules 2020, 25, 4758. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E. Cellular Screening Methods for the Study of Nanoparticle- Induced Lysosomal Damage. In Lysosomes—Associated Diseases and Methods to Study Their Function; Sharma, P.D., Ed.; InTech: London, UK, 2017; ISBN 978-953-51-3507-4. [Google Scholar]
- Jiang, L.Q.; Wang, T.Y.; Webster, T.J.; Duan, H.-J.; Qiu, J.Y.; Zhao, Z.M.; Yin, X.-X.; Zheng, C.L. Intracellular Disposition of Chitosan Nanoparticles in Macrophages: Intracellular Uptake, Exocytosis, and Intercellular Transport. Int. J. Nanomed. 2017, 12, 6383–6398. [Google Scholar] [CrossRef]
- Pilipenko, I.; Korzhikov-Vlakh, V.; Sharoyko, V.; Zhang, N.; Schäfer-Korting, M.; Rühl, E.; Zoschke, C.; Tennikova, T. PH-Sensitive Chitosan–Heparin Nanoparticles for Effective Delivery of Genetic Drugs into Epithelial Cells. Pharmaceutics 2019, 11, 317. [Google Scholar] [CrossRef]
- Nam, J.-P.; Nah, J.-W. Target Gene Delivery from Targeting Ligand Conjugated Chitosan–PEI Copolymer for Cancer Therapy. Carbohydr. Polym. 2016, 135, 153–161. [Google Scholar] [CrossRef]
- Chen, W.; Li, F.; Tang, Y.; Yang, S.; Li, J.; Yuan, Z.; Liu, Y.; Zhou, X.; Liu, C.; Zhang, X. Stepwise PH-Responsive Nanoparticles for Enhanced Cellular Uptake and on-Demand Intracellular Release of Doxorubicin. Int. J. Nanomed. 2017, 12, 4241–4256. [Google Scholar] [CrossRef]
- Chen, G.; Svirskis, D.; Lu, W.; Ying, M.; Huang, Y.; Wen, J. N-Trimethyl Chitosan Nanoparticles and CSKSSDYQC Peptide: N-Trimethyl Chitosan Conjugates Enhance the Oral Bioavailability of Gemcitabine to Treat Breast Cancer. J. Control Release 2018, 277, 142–153. [Google Scholar] [CrossRef]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef]
- Yang, N.J.; Hinner, M.J. Getting Across the Cell Membrane: An Overview for Small Molecules, Peptides, and Proteins. Site-Specif. Protein Labeling 2015, 1266, 29–53. [Google Scholar] [CrossRef]
- Magalhaes, M.A.O.; Glogauer, M. Pivotal Advance: Phospholipids Determine Net Membrane Surface Charge Resulting in Differential Localization of Active Rac1 and Rac2. J. Leukoc. Biol. 2010, 87, 545–555. [Google Scholar] [CrossRef]
- Schipper, N.G.M.; Vårum, K.M.; Artursson, P. Chitosans as Absorption Enhancers for Poorly Absorbable Drugs. 1: Influence of Molecular Weight and Degree of Acetylation on Drug Transport Across Human Intestinal Epithelial (Caco-2) Cells. Pharm. Res. 1996, 13, 1686–1692. [Google Scholar] [CrossRef]
- Kotzé, A.F.; de Leeuw, B.J.; Lueßen, H.L.; de Boer, A.G.; Verhoef, J.C.; Junginger, H.E. Chitosans for Enhanced Delivery of Therapeutic Peptides across Intestinal Epithelia: In Vitro Evaluation in Caco-2 Cell Monolayers. Int. J. Pharm. 1997, 159, 243–253. [Google Scholar] [CrossRef]
- Yue, Z.-G.; Wei, W.; Lv, P.-P.; Yue, H.; Wang, L.-Y.; Su, Z.-G.; Ma, G.-H. Surface Charge Affects Cellular Uptake and Intracellular Trafficking of Chitosan-Based Nanoparticles. Biomacromolecules 2011, 12, 2440–2446. [Google Scholar] [CrossRef]
- Boyles, M.S.P.; Kristl, T.; Andosch, A.; Zimmermann, M.; Tran, N.; Casals, E.; Himly, M.; Puntes, V.; Huber, C.G.; Lütz-Meindl, U.; et al. Chitosan Functionalisation of Gold Nanoparticles Encourages Particle Uptake and Induces Cytotoxicity and Pro-Inflammatory Conditions in Phagocytic Cells, as Well as Enhancing Particle Interactions with Serum Components. J. Nanobiotechnol. 2015, 13, 84. [Google Scholar] [CrossRef]
- Hu, C.; Chiang, C.; Hong, P.; Yeh, M. Influence of Charge on FITC-BSA-Loaded Chondroitin Sulfate-Chitosan Nanoparticles upon Cell Uptake in Human Caco-2 Cell Monolayers. Int. J. Nanomed. 2012, 7, 4861–4872. [Google Scholar] [CrossRef][Green Version]
- Kalliola, S.; Repo, E.; Srivastava, V.; Zhao, F.; Heiskanen, J.P.; Sirviö, J.A.; Liimatainen, H.; Sillanpää, M. Carboxymethyl Chitosan and Its Hydrophobically Modified Derivative as PH-Switchable Emulsifiers. Langmuir 2018, 34, 2800–2806. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, D.R.; Tavano, L.; Mitjans, M.; Pérez, L.; Infante, M.R.; Vinardell, M.P. In Vitro Antitumor Activity of Methotrexate via PH-Sensitive Chitosan Nanoparticles. Biomaterials 2013, 34, 2758–2772. [Google Scholar] [CrossRef] [PubMed]
- Amoozgar, Z.; Park, J.; Lin, Q.; Yeo, Y. Low Molecular-Weight Chitosan as a PH-Sensitive Stealth Coating for Tumor-Specific Drug Delivery. Mol. Pharm. 2012, 9, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Khalili, A.; Ahmad, M. A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Chung, Y.-C.; Wang, I.-J.; Young, T.-H. Control of Cell Attachment on PH-Responsive Chitosan Surface by Precise Adjustment of Medium PH. Biomaterials 2012, 33, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Rocha Neto, J.B.M.; Taketa, T.B.; Bataglioli, R.A.; Pimentel, S.B.; Santos, D.M.; Fiamingo, A.; Costa, C.A.R.; Campana-Filho, S.P.; Carvalho, H.F.; Beppu, M.M. Tailored Chitosan/Hyaluronan Coatings for Tumor Cell Adhesion: Effects of Topography, Charge Density and Surface Composition. Appl. Surf. Sci. 2019, 486, 508–518. [Google Scholar] [CrossRef]
- Noriega, S.E.; Subramanian, A. Consequences of Neutralization on the Proliferation and Cytoskeletal Organization of Chondrocytes on Chitosan-Based Matrices. Int. J. Carbohydr. Chem. 2011, 2011, 1–13. [Google Scholar] [CrossRef]
- Lü, X.; Zhang, H.; Huang, Y.; Zhang, Y. A Proteomics Study to Explore the Role of Adsorbed Serum Proteins for PC12 Cell Adhesion and Growth on Chitosan and Collagen/Chitosan Surfaces. Regen. Biomater. 2018, 5, 261–273. [Google Scholar] [CrossRef]
- Lim, S.M.; Song, D.K.; Cho, K.J.; Oh, S.H.; Lee-Yoon, D.S.; Bae, E.H.; Lee, J.H. Cell Adhesion and Degradation Behaviors of Acetylated Chitosan Films. In Proceedings of the 3rd Kuala Lumpur International Conference on Biomedical Engineering 2006, Kuala Lumpur, Malaysia, 11–14 December 2006; Ibrahim, F., Osman, N.A.A., Usman, J., Kadri, N.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 94–97. [Google Scholar]
- Foster, L.J.R.; Ho, S.; Hook, J.; Basuki, M.; Marçal, H. Chitosan as a Biomaterial: Influence of Degree of Deacetylation on Its Physiochemical, Material and Biological Properties. PLoS ONE 2015, 10, e0135153. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. BioMed Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef]
- Boecker, A.; Daeschler, S.C.; Kneser, U.; Harhaus, L. Relevance and Recent Developments of Chitosan in Peripheral Nerve Surgery. Front. Cell. Neurosci. 2019, 13, 104. [Google Scholar] [CrossRef]
- Dixit, S.; Baganizi, D.R.; Sahu, R.; Dosunmu, E.; Chaudhari, A.; Vig, K.; Pillai, S.R.; Singh, S.R.; Dennis, V.A. Immunological Challenges Associated with Artificial Skin Grafts: Available Solutions and Stem Cells in Future Design of Synthetic Skin. J. Biol. Eng. 2017, 11, 49. [Google Scholar] [CrossRef]
- Salati, A.; Keshvari, H.; Karkhaneh, A.; Taranejoo, S. Design and Fabrication of Artificial Skin: Chitosan and Gelatin Immobilization on Silicone by Poly Acrylic Acid Graft Using a Plasma Surface Modification Method. J. Macromol. Sci. Part B 2011, 50, 1972–1982. [Google Scholar] [CrossRef]
- Vijayan, A.; Sabareeswaran, A.; Kumar, G.S.V. PEG Grafted Chitosan Scaffold for Dual Growth Factor Delivery for Enhanced Wound Healing. Sci. Rep. 2019, 9, 19165. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A Functional Chitosan-Based Hydrogel as a Wound Dressing and Drug Delivery System in the Treatment of Wound Healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef]
- Cho, M.O.; Li, Z.; Shim, H.-E.; Cho, I.-S.; Nurunnabi, M.; Park, H.; Lee, K.Y.; Moon, S.-H.; Kim, K.-S.; Kang, S.-W.; et al. Bioinspired Tuning of Glycol Chitosan for 3D Cell Culture. NPG Asia Mater. 2016, 8, e309. [Google Scholar] [CrossRef]
- Kafi, M.A.; Aktar, K.; Todo, M.; Dahiya, R. Engineered Chitosan for Improved 3D Tissue Growth through Paxillin-FAK-ERK Activation. Regen. Biomater. 2020, 7, 141–151. [Google Scholar] [CrossRef]
- Yeh, T.-H.; Hsu, L.-W.; Tseng, M.T.; Lee, P.-L.; Sonjae, K.; Ho, Y.-C.; Sung, H.-W. Mechanism and Consequence of Chitosan-Mediated Reversible Epithelial Tight Junction Opening. Biomaterials 2011, 32, 6164–6173. [Google Scholar] [CrossRef]
- Sonaje, K.; Lin, K.-J.; Tseng, M.T.; Wey, S.-P.; Su, F.-Y.; Chuang, E.-Y.; Hsu, C.-W.; Chen, C.-T.; Sung, H.-W. Effects of Chitosan-Nanoparticle-Mediated Tight Junction Opening on the Oral Absorption of Endotoxins. Biomaterials 2011, 32, 8712–8721. [Google Scholar] [CrossRef]
- Lee, B.; Moon, K.M.; Kim, C.Y. Tight Junction in the Intestinal Epithelium: Its Association with Diseases and Regulation by Phytochemicals. J. Immunol. Res. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Bhat, A.A.; Uppada, S.; Achkar, I.W.; Hashem, S.; Yadav, S.K.; Shanmugakonar, M.; Al-Naemi, H.A.; Haris, M.; Uddin, S. Tight Junction Proteins and Signaling Pathways in Cancer and Inflammation: A Functional Crosstalk. Front. Physiol. 2019, 9, 1942. [Google Scholar] [CrossRef] [PubMed]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight Junctions: From Simple Barriers to Multifunctional Molecular Gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kong, M.; Zhou, Z.; Yan, D.; Yu, X.; Cheng, X.; Feng, C.; Liu, Y.; Chen, X. Mechanism of Surface Charge Triggered Intestinal Epithelial Tight Junction Opening upon Chitosan Nanoparticles for Insulin Oral Delivery. Carbohydr. Polym. 2017, 157, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for In Vitro Barrier Model Systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Wood, E.; Dornish, M. Effect of Chitosan on Epithelial Cell Tight Junctions. Pharm. Res. 2004, 21, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Vllasaliu, D.; Exposito-Harris, R.; Heras, A.; Casettari, L.; Garnett, M.; Illum, L.; Stolnik, S. Tight Junction Modulation by Chitosan Nanoparticles: Comparison with Chitosan Solution. Int. J. Pharm. 2010, 400, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Zhu, D.; Li, S.; Yu, X.; Zhao, Y.; Ouyang, X.; Xie, Z.; Li, L. Transporting Carriers for Intracellular Targeting Delivery via Non-Endocytic Uptake Pathways. Drug Deliv. 2017, 24, 45–55. [Google Scholar] [CrossRef]
- Juliano, R.L.; Carver, K. Cellular Uptake and Intracellular Trafficking of Oligonucleotides. Adv. Drug Deliv. Rev. 2015, 87, 35–45. [Google Scholar] [CrossRef]
- Malatesta, M.; Grecchi, S.; Chiesa, E.; Cisterna, B.; Costanzo, M.; Zancanaro, C. Internalized Chitosan Nanoparticles Persist for Long Time in Cultured Cells. Eur. J. Histochem. 2015, 59, 2492. [Google Scholar] [CrossRef]
- Huang, M.; Ma, Z.; Khor, E.; Lim, L.-Y. Uptake of FITC-Chitosan Nanoparticles by A549 Cells. Pharm. Res. 2002, 19, 1488–1494. [Google Scholar] [CrossRef]
- Park, J.S.; Cho, Y.W. In Vitro Cellular Uptake and Cytotoxicity of Paclitaxel-Loaded Glycol Chitosan Self-Assembled Nanoparticles. Macromol. Res. 2007, 15, 513–519. [Google Scholar] [CrossRef]
- Tahara, K.; Sakai, T.; Yamamoto, H.; Takeuchi, H.; Hirashima, N.; Kawashima, Y. Improved Cellular Uptake of Chitosan-Modified PLGA Nanospheres by A549 Cells. Int. J. Pharm. 2009, 382, 198–204. [Google Scholar] [CrossRef]
- Koppolu, B.; Zaharoff, D.A. The Effect of Antigen Encapsulation in Chitosan Particles on Uptake, Activation and Presentation by Antigen Presenting Cells. Biomaterials 2013, 34, 2359–2369. [Google Scholar] [CrossRef]
- Tammam, S.N.; Azzazy, H.M.E.; Breitinger, H.G.; Lamprecht, A. Chitosan Nanoparticles for Nuclear Targeting: The Effect of Nanoparticle Size and Nuclear Localization Sequence Density. Mol. Pharm. 2015, 12, 4277–4289. [Google Scholar] [CrossRef]
- Fröhlich, E. The Role of Surface Charge in Cellular Uptake and Cytotoxicity of Medical Nanoparticles. Int. J. Nanomed. 2012, 7, 5577. [Google Scholar] [CrossRef]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of Particle Size and Surface Charge on Cellular Uptake and Biodistribution of Polymeric Nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Zhou, C.; Jiao, Y. Hydrophobic Modifications of Cationic Polymers for Gene Delivery. Prog. Polym. Sci. 2010, 35, 1144–1162. [Google Scholar] [CrossRef]
- Nam, H.Y.; Kwon, S.M.; Chung, H.; Lee, S.-Y.; Kwon, S.-H.; Jeon, H.; Kim, Y.; Park, J.H.; Kim, J.; Her, S.; et al. Cellular Uptake Mechanism and Intracellular Fate of Hydrophobically Modified Glycol Chitosan Nanoparticles. J. Control. Release 2009, 135, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-L.; Ho, Y.-C.; Chen, Y.-M.; Peng, S.-F.; Ke, C.-J.; Chen, K.-J.; Mi, F.-L.; Sung, H.-W. The Characteristics, Cellular Uptake and Intracellular Trafficking of Nanoparticles Made of Hydrophobically-Modified Chitosan. J. Control. Release 2010, 146, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Layek, B.; Haldar, M.K.; Sharma, G.; Lipp, L.; Mallik, S.; Singh, J. Hexanoic Acid and Polyethylene Glycol Double Grafted Amphiphilic Chitosan for Enhanced Gene Delivery: Influence of Hydrophobic and Hydrophilic Substitution Degree. Mol. Pharm. 2014, 11, 982–994. [Google Scholar] [CrossRef]
- Wang, B.; He, C.; Tang, C.; Yin, C. Effects of Hydrophobic and Hydrophilic Modifications on Gene Delivery of Amphiphilic Chitosan Based Nanocarriers. Biomaterials 2011, 32, 4630–4638. [Google Scholar] [CrossRef]
- Zhan, J.; Ting, X.L.; Zhu, J. The Research Progress of Targeted Drug Delivery Systems. IOP Conf. Ser. Mater. Sci. Eng. 2017, 207, 012017. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Hu, W.; Hu, Z.-H.; Bei, Y.-Y.; Xu, J.-Y.; Wang, W.-J.; Zhang, X.-N.; Zhang, Q. Norcantharidin-Associated Galactosylated Chitosan Nanoparticles for Hepatocyte-Targeted Delivery. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 371–381. [Google Scholar] [CrossRef]
- Liu, L.; Dong, X.; Zhu, D.; Song, L.; Zhang, H.; Leng, X.G. TAT-LHRH Conjugated Low Molecular Weight Chitosan as a Gene Carrier Specific for Hepatocellular Carcinoma Cells. Int. J. Nanomed. 2014, 9, 2879–2889. [Google Scholar] [CrossRef]
- Han, L.; Tang, C.; Yin, C. Dual-Targeting and PH/Redox-Responsive Multi-Layered Nanocomplexes for Smart Co-Delivery of Doxorubicin and SiRNA. Biomaterials 2015, 60, 42–52. [Google Scholar] [CrossRef]
- Shi, G.-N.; Zhang, C.-N.; Xu, R.; Niu, J.-F.; Song, H.-J.; Zhang, X.-Y.; Wang, W.-W.; Wang, Y.-M.; Li, C.; Wei, X.-Q.; et al. Enhanced Antitumor Immunity by Targeting Dendritic Cells with Tumor Cell Lysate-Loaded Chitosan Nanoparticles Vaccine. Biomaterials 2017, 113, 191–202. [Google Scholar] [CrossRef]
- Nascimento, A.V.; Singh, A.; Bousbaa, H.; Ferreira, D.; Sarmento, B.; Amiji, M.M. Overcoming Cisplatin Resistance in Non-Small Cell Lung Cancer with Mad2 Silencing SiRNA Delivered Systemically Using EGFR-Targeted Chitosan Nanoparticles. Acta Biomater. 2017, 47, 71–80. [Google Scholar] [CrossRef]
- Yang, H.; Tang, C.; Yin, C. Estrone-Modified PH-Sensitive Glycol Chitosan Nanoparticles for Drug Delivery in Breast Cancer. Acta Biomater. 2018, 73, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Chi, T.; Li, T.; Zheng, G.; Fan, L.; Liu, Y.; Chen, X.; Chen, S.; Jia, L.; Shao, J. A Smart PH-Responsive Nano-Carrier as a Drug Delivery System for the Targeted Delivery of Ursolic Acid: Suppresses Cancer Growth and Metastasis by Modulating P53/MMP-9/PTEN/CD44 Mediated Multiple Signaling Pathways. Nanoscale 2017, 9, 9428–9439. [Google Scholar] [CrossRef] [PubMed]
- Hefnawy, A.; Khalil, I.H.; Arafa, K.; Emara, M.; El-Sherbiny, I.M. Dual-Ligand Functionalized Core-Shell Chitosan-Based Nanocarrier for Hepatocellular Carcinoma-Targeted Drug Delivery. Int. J. Nanomed. 2020, 15, 821–837. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liu, F.; Guo, J.; Xue, J.; Qian, Z.; Gu, Y. Folate-Modified Chitosan Micelles with Enhanced Tumor Targeting Evaluated by near Infrared Imaging System. Carbohydr. Polym. 2011, 86, 1118–1129. [Google Scholar] [CrossRef]
- Ramya, A.N.; Joseph, M.M.; Maniganda, S.; Karunakaran, V.; Sreelekha, T.T.; Maiti, K.K. Emergence of Gold-Mesoporous Silica Hybrid Nanotheranostics: Dox-Encoded, Folate Targeted Chemotherapy with Modulation of SERS Fingerprinting for Apoptosis Toward Tumor Eradication. Small 2017, 13, 1700819. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Sharma, S.; Gupta, P.K.; Singh, A.; Teja, B.V.; Dwivedi, P.; Gupta, G.K.; Trivedi, R.; Mishra, P.R. Vitamin B12 Functionalized Layer by Layer Calcium Phosphate Nanoparticles: A Mucoadhesive and PH Responsive Carrier for Improved Oral Delivery of Insulin. Acta Biomater. 2016, 31, 288–300. [Google Scholar] [CrossRef]
- Rekha, M.R.; Sharma, C.P. Synthesis and Evaluation of Lauryl Succinyl Chitosan Particles towards Oral Insulin Delivery and Absorption. J. Control. Release 2009, 135, 144–151. [Google Scholar] [CrossRef]
- Zhao, R.; Li, T.; Zheng, G.; Jiang, K.; Fan, L.; Shao, J. Simultaneous Inhibition of Growth and Metastasis of Hepatocellular Carcinoma by Co-Delivery of Ursolic Acid and Sorafenib Using Lactobionic Acid Modified and PH-Sensitive Chitosan-Conjugated Mesoporous Silica Nanocomplex. Biomaterials 2017, 143, 1–16. [Google Scholar] [CrossRef]
- Zhu, R.; Zhang, C.; Liu, Y.; Yuan, Z.; Chen, W.; Yang, S.; Li, J.; Zhu, W.; Zhou, X.; You, B.; et al. CD147 Monoclonal Antibody Mediated by Chitosan Nanoparticles Loaded with α-Hederin Enhances Antineoplastic Activity and Cellular Uptake in Liver Cancer Cells. Sci. Rep. 2015, 5, 17904. [Google Scholar] [CrossRef]
- Shen, J.-M.; Tang, W.-J.; Zhang, X.-L.; Chen, T.; Zhang, H.-X. A Novel Carboxymethyl Chitosan-Based Folate/Fe3O4/CdTe Nanoparticle for Targeted Drug Delivery and Cell Imaging. Carbohydr. Polym. 2012, 88, 239–249. [Google Scholar] [CrossRef]
- Mathew, M.E.; Mohan, J.C.; Manzoor, K.; Nair, S.V.; Tamura, H.; Jayakumar, R. Folate Conjugated Carboxymethyl Chitosan–Manganese Doped Zinc Sulphide Nanoparticles for Targeted Drug Delivery and Imaging of Cancer Cells. Carbohydr. Polym. 2010, 80, 442–448. [Google Scholar] [CrossRef]
- Guan, M.; Zhou, Y.; Zhu, Q.-L.; Liu, Y.; Bei, Y.-Y.; Zhang, X.-N.; Zhang, Q. N-Trimethyl Chitosan Nanoparticle-Encapsulated Lactosyl-Norcantharidin for Liver Cancer Therapy with High Targeting Efficacy. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1172–1181. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhou, Y.; Guan, M.; Zhou, X.; Yang, S.; Liu, Y.; Chen, W.; Zhang, C.; Yuan, Z.; Liu, C.; et al. Low-Density Lipoprotein-Coupled N-Succinyl Chitosan Nanoparticles Co-Delivering SiRNA and Doxorubicin for Hepatocyte-Targeted Therapy. Biomaterials 2014, 35, 5965–5976. [Google Scholar] [CrossRef]
- Chou, L.Y.T.; Ming, K.; Chan, W.C.W. Strategies for the Intracellular Delivery of Nanoparticles. Chem. Soc. Rev. 2011, 40, 233–245. [Google Scholar] [CrossRef]
- Cao, Y.; Tan, Y.F.; Wong, Y.S.; Liew, M.W.J.; Venkatraman, S. Recent Advances in Chitosan-Based Carriers for Gene Delivery. Mar. Drugs 2019, 17, 381. [Google Scholar] [CrossRef]
- Lai, W.-F.; Wong, W.-T. Design of Polymeric Gene Carriers for Effective Intracellular Delivery. Trends Biotechnol. 2018, 36, 713–728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Ma, J.; Wang, X.; Yuan, Z.; Wang, W. Convenient Preparation of Charge-Adaptive Chitosan Nanomedicines for Extended Blood Circulation and Accelerated Endosomal Escape. Nano Res. 2018, 11, 4278–4292. [Google Scholar] [CrossRef]
- Moreira, C.; Oliveira, H.; Pires, L.R.; Simões, S.; Barbosa, M.A.; Pêgo, A.P. Improving Chitosan-Mediated Gene Transfer by the Introduction of Intracellular Buffering Moieties into the Chitosan Backbone. Acta Biomater. 2009, 5, 2995–3006. [Google Scholar] [CrossRef]
- Chang, K.-L.; Higuchi, Y.; Kawakami, S.; Yamashita, F.; Hashida, M. Efficient Gene Transfection by Histidine-Modified Chitosan through Enhancement of Endosomal Escape. Bioconj. Chem. 2010, 21, 1087–1095. [Google Scholar] [CrossRef]
- Huang, G.; Chen, Q.; Wu, W.; Wang, J.; Chu, P.K.; Bai, H.; Tang, G. Reconstructed Chitosan with Alkylamine for Enhanced Gene Delivery by Promoting Endosomal Escape. Carbohydr. Polym. 2020, 227, 115339. [Google Scholar] [CrossRef]
- Rathore, B.; Sunwoo, K.; Jangili, P.; Kim, J.; Kim, J.H.; Huang, M.; Xiong, J.; Sharma, A.; Yang, Z.; Qu, J.; et al. Nanomaterial Designing Strategies Related to Cell Lysosome and Their Biomedical Applications: A Review. Biomaterials 2019, 211, 25–47. [Google Scholar] [CrossRef]
- Manshian, B.B.; Pokhrel, S.; Mädler, L.; Soenen, S.J. The Impact of Nanoparticle-Driven Lysosomal Alkalinization on Cellular Functionality. J. Nanobiotechnol. 2018, 16, 85. [Google Scholar] [CrossRef]
- Fong, D.; Grégoire-Gélinas, P.; Cheng, A.P.; Mezheritsky, T.; Lavertu, M.; Sato, S.; Hoemann, C.D. Lysosomal Rupture Induced by Structurally Distinct Chitosans Either Promotes a Type 1 IFN Response or Activates the Inflammasome in Macrophages. Biomaterials 2017, 129, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Thibault, M.; Astolfi, M.; Tran-Khanh, N.; Lavertu, M.; Darras, V.; Merzouki, A.; Buschmann, M.D. Excess Polycation Mediates Efficient Chitosan-Based Gene Transfer by Promoting Lysosomal Release of the Polyplexes. Biomaterials 2011, 32, 4639–4646. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Ruiz, L.; de la Fuente, M.; Párraga, J.E.; López-García, A.; Fernández, I.; Seijo, B.; Sánchez, A.; Calonge, M.; Diebold, Y. Intracellular Trafficking of Hyaluronic Acid-Chitosan Oligomer-Based Nanoparticles in Cultured Human Ocular Surface Cells. Mol. Vis. 2011, 17, 279–290. [Google Scholar] [PubMed]
- Yameen, B.; Choi, W.I.; Vilos, C.; Swami, A.; Shi, J.; Farokhzad, O.C. Insight into Nanoparticle Cellular Uptake and Intracellular Targeting. J. Control Release 2014, 190, 485–499. [Google Scholar] [CrossRef]
- Tammam, S.N.; Azzazy, H.M.E.; Lamprecht, A. How Successful Is Nuclear Targeting by Nanocarriers? J. Control Release 2016, 229, 140–153. [Google Scholar] [CrossRef]
- Opanasopit, P.; Rojanarata, T.; Apirakaramwong, A.; Ngawhirunpat, T.; Ruktanonchai, U. Nuclear Localization Signal Peptides Enhance Transfection Efficiency of Chitosan/DNA Complexes. Int. J. Pharm. 2009, 382, 291–295. [Google Scholar] [CrossRef]
- Lien, C.-F.; Molnár, É.; Toman, P.; Tsibouklis, J.; Pilkington, G.J.; Górecki, D.C.; Barbu, E. In Vitro Assessment of Alkylglyceryl-Functionalized Chitosan Nanoparticles as Permeating Vectors for the Blood–Brain Barrier. Biomacromolecules 2012, 13, 1067–1073. [Google Scholar] [CrossRef]
- Barbieri, S.; Sonvico, F.; Como, C.; Colombo, G.; Zani, F.; Buttini, F.; Bettini, R.; Rossi, A.; Colombo, P. Lecithin/Chitosan Controlled Release Nanopreparations of Tamoxifen Citrate: Loading, Enzyme-Trigger Release and Cell Uptake. J. Control Release 2013, 167, 276–283. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, K.; Oh, Y.-K.; Kwon, S.-H.; Her, S.; Kim, I.-S.; Choi, K.; Lee, S.J.; Kim, H.; Lee, S.G. Tumor Specificity and Therapeutic Efficacy of Photosensitizer-Encapsulated Glycol Chitosan-Based Nanoparticles in Tumor-Bearing Mice. Biomaterials 2009, 30, 2929–2939. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.J.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Zorov, S.D.; Babenko, V.A.; Zorov, D.B. Functional Significance of the Mitochondrial Membrane Potential. Biochem. Mosc. Suppl. Ser. Membr. Cell Biol. 2018, 12, 20–26. [Google Scholar] [CrossRef]
- Qi, L.-F.; Xu, Z.-R.; Li, Y.; Jiang, X.; Han, X.-Y. In Vitro Effects of Chitosan Nanoparticles on Proliferation of Human Gastric Carcinoma Cell Line MGC803 Cells. World J. Gastroenterol. 2005, 11, 5136–5141. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, X.; Su, C.; Zhao, L.; Shi, Y. Chitosan Nanoparticles Induced the Antitumor Effect in Hepatocellular Carcinoma Cells by Regulating ROS-Mediated Mitochondrial Damage and Endoplasmic Reticulum Stress. Artif. Cells Nanomed. Biotechnol. 2019, 47, 747–756. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, L.; Song, Y.; He, J.; Wu, L.; Zhao, C.; Xiao, Y.; Li, W.; Cai, B.; Cheng, H.; et al. Hierarchical Targeted Hepatocyte Mitochondrial Multifunctional Chitosan Nanoparticles for Anticancer Drug Delivery. Biomaterials 2015, 52, 240–250. [Google Scholar] [CrossRef]
- Hou, J.; Yu, X.; Shen, Y.; Shi, Y.; Su, C.; Zhao, L. Triphenyl Phosphine-Functionalized Chitosan Nanoparticles Enhanced Antitumor Efficiency Through Targeted Delivery of Doxorubicin to Mitochondria. Nanoscale Res. Lett. 2017, 12, 158. [Google Scholar] [CrossRef]
- Wang, T.; Hou, J.; Su, C.; Zhao, L.; Shi, Y. Hyaluronic Acid-Coated Chitosan Nanoparticles Induce ROS-Mediated Tumor Cell Apoptosis and Enhance Antitumor Efficiency by Targeted Drug Delivery via CD44. J. Nanobiotechnol. 2017, 15, 7. [Google Scholar] [CrossRef]
- Mallick, S.; Song, S.J.; Bae, Y.; Choi, J.S. Self-Assembled Nanoparticles Composed of Glycol Chitosan-Dequalinium for Mitochondria-Targeted Drug Delivery. Int. J. Biol. Macromol. 2019, 132, 451–460. [Google Scholar] [CrossRef]
- He, B.; Wu, F.; Fan, L.; Li, X.-H.; Liu, Y.; Liu, Y.-J.; Ding, W.-J.; Deng, M.; Zhou, Y. Carboxymethylated Chitosan Protects Schwann Cells against Hydrogen Peroxide-Induced Apoptosis by Inhibiting Oxidative Stress and Mitochondria Dependent Pathway. Eur. J. Pharmacol. 2018, 825, 48–56. [Google Scholar] [CrossRef]
- Shukla, S.; Jadaun, A.; Arora, V.; Sinha, R.K.; Biyani, N.; Jain, V.K. In Vitro Toxicity Assessment of Chitosan Oligosaccharide Coated Iron Oxide Nanoparticles. Toxicol. Rep. 2015, 2, 27–39. [Google Scholar] [CrossRef]
- Park, J.H.; Oh, N. Endocytosis and Exocytosis of Nanoparticles in Mammalian Cells. Int. J. Nanomed. 2014, 9, 51. [Google Scholar] [CrossRef]
- Sakhtianchi, R.; Minchin, R.F.; Lee, K.-B.; Alkilany, A.M.; Serpooshan, V.; Mahmoudi, M. Exocytosis of Nanoparticles from Cells: Role in Cellular Retention and Toxicity. Adv. Colloid Interface Sci. 2013, 201–202, 18–29. [Google Scholar] [CrossRef]
- Park, J.S.; Han, T.H.; Lee, K.Y.; Han, S.S.; Hwang, J.J.; Moon, D.H.; Kim, S.Y.; Cho, Y.W. N-Acetyl Histidine-Conjugated Glycol Chitosan Self-Assembled Nanoparticles for Intracytoplasmic Delivery of Drugs: Endocytosis, Exocytosis and Drug Release. J. Control Release 2006, 115, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Tahara, K.; Tadokoro, S.; Yamamoto, H.; Kawashima, Y.; Hirashima, N. The Suppression of IgE-Mediated Histamine Release from Mast Cells Following Exocytic Exclusion of Biodegradable Polymeric Nanoparticles. Biomaterials 2012, 33, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Bakhru, S.H.; Altiok, E.; Highley, C.; Delubac, D.; Suhan, J.; Hitchens, T.K.; Ho, C.; Zappe, S. Enhanced Cellular Uptake and Long-Term Retention of Chitosan-Modified Iron-Oxide Nanoparticles for MRI-Based Cell Tracking. Int. J. Nanomed. 2012, 7, 4613. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-H.; Hu, H.-Y.; Qiao, M.-X.; Zhu, J.; Qi, J.-W.; Hu, C.-J.; Zhang, Q.; Chen, D.-W. PH-Sensitive Chitosan-Derived Nanoparticles as Doxorubicin Carriers for Effective Anti-Tumor Activity: Preparation and in Vitro Evaluation. Colloids Surf. B Biointerfaces 2012, 94, 184–191. [Google Scholar] [CrossRef]
- Trif, M.; Florian, P.E.; Roseanu, A.; Moisei, M.; Craciunescu, O.; Astete, C.E.; Sabliov, C.M. Cytotoxicity and Intracellular Fate of PLGA and Chitosan-Coated PLGA Nanoparticles in Madin-Darby Bovine Kidney (MDBK) and Human Colorectal Adenocarcinoma (Colo 205) Cells. J. Biomed. Mater. Res. A 2015, 103, 3599–3611. [Google Scholar] [CrossRef]
- Durán, V.; Yasar, H.; Becker, J.; Thiyagarajan, D.; Loretz, B.; Kalinke, U.; Lehr, C.-M. Preferential Uptake of Chitosan-Coated PLGA Nanoparticles by Primary Human Antigen Presenting Cells. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102073. [Google Scholar] [CrossRef]
- Grenha, A.; Grainger, C.I.; Dailey, L.A.; Seijo, B.; Martin, G.P.; Remuñán-López, C.; Forbes, B. Chitosan Nanoparticles Are Compatible with Respiratory Epithelial Cells in Vitro. Eur. J. Pharm. Sci. 2007, 31, 73–84. [Google Scholar] [CrossRef]
- Rajam, M.; Pulavendran, S.; Rose, C.; Mandal, A.B. Chitosan Nanoparticles as a Dual Growth Factor Delivery System for Tissue Engineering Applications. Int. J. Pharm. 2011, 410, 145–152. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Xi, X.; Shrestha, S.; Jiang, P.; Zhang, W.; Gao, C. Amino Acid-Modified Chitosan Nanoparticles for Cu2+ Chelation to Suppress CuO Nanoparticle Cytotoxicity. J. Mater. Chem. B 2017, 5, 3521–3530. [Google Scholar] [CrossRef]
- Nafee, N.; Schneider, M.; Schaefer, U.F.; Lehr, C.-M. Relevance of the Colloidal Stability of Chitosan/PLGA Nanoparticles on Their Cytotoxicity Profile. Int. J. Pharm. 2009, 381, 130–139. [Google Scholar] [CrossRef]
- Sarangapani, S.; Patil, A.; Ngeow, Y.K.; Mohan, R.E.; Asundi, A.; Lang, M.J. Chitosan Nanoparticles’ Functionality as Redox Active Drugs through Cytotoxicity, Radical Scavenging and Cellular Behaviour. Integr. Biol. 2018, 10, 313–324. [Google Scholar] [CrossRef]
- Lin, L.; He, J.; Li, J.; Xu, Y.; Li, J.; Wu, Y. Chitosan Nanoparticles Strengthen Vγ9Vδ2 T-Cell Cytotoxicity through Upregulation of Killing Molecules and Cytoskeleton Polarization. Int. J. Nanomed. 2019, 14, 9325–9336. [Google Scholar] [CrossRef]
- Richardson, S.C.W.; Kolbe, H.V.J.; Duncan, R. Potential of Low Molecular Mass Chitosan as a DNA Delivery System: Biocompatibility, Body Distribution and Ability to Complex and Protect DNA. Int. J. Pharm. 1999, 178, 231–243. [Google Scholar] [CrossRef]
- Suzuki, Y.S.; Momose, Y.; Higashi, N.; Shigematsu, A.; Park, K.B.; Kim, Y.M.; Kim, J.R.; Ryu, J.M. Biodistribution and Kinetics of Holmium-166-Chitosan Complex (DW-166HC) in Rats and Mice. J. Nucl. Med. 1998, 39, 2161–2166. [Google Scholar]
- Onishi, H.; Machida, Y. Biodegradation and Distribution of Water-Soluble Chitosan in Mice. Biomaterials 1999, 20, 175–182. [Google Scholar] [CrossRef]
- Banerjee, T.; Mitra, S.; Kumar Singh, A.; Kumar Sharma, R.; Maitra, A. Preparation, Characterization and Biodistribution of Ultrafine Chitosan Nanoparticles. Int. J. Pharm. 2002, 243, 93–105. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, Biodistribution and Toxicity of Chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Kadam, R.S.; Bourne, D.W.A.; Kompella, U.B. Nano-Advantage in Enhanced Drug Delivery with Biodegradable Nanoparticles: Contribution of Reduced Clearance. Drug Metab. Dispos. 2012, 40, 1380–1388. [Google Scholar] [CrossRef]
- Termsarasab, U.; Yoon, I.-S.; Park, J.-H.; Moon, H.T.; Cho, H.-J.; Kim, D.-D. Polyethylene Glycol-Modified Arachidyl Chitosan-Based Nanoparticles for Prolonged Blood Circulation of Doxorubicin. Int. J. Pharm. 2014, 464, 127–134. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, J.; Cao, J.; Zhang, Y.; Xu, Z.; Qin, X.; Wang, W.; Yuan, Z. Facile Fabrication of Poly(Acrylic Acid) Coated Chitosan Nanoparticles with Improved Stability in Biological Environments. Eur. J. Pharm. Biopharm. 2017, 112, 148–154. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, P.; Zhao, X.; Wang, J.; Wang, J.; Wang, C.; Ren, L.; Zhang, Q. O-Carboxymethyl-Chitosan/Organosilica Hybrid Nanoparticles as Non-Viral Vectors for Gene Delivery. Mater. Sci. Eng. C 2009, 29, 2045–2049. [Google Scholar] [CrossRef]
- Katas, H.; Alpar, H.O. Development and Characterisation of Chitosan Nanoparticles for SiRNA Delivery. J. Control Release 2006, 115, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhang, Z.; Luo, Z.; Yu, J.; Liang, R.; Li, X.; Chen, H. Chitosan Grafted Methoxy Poly(Ethylene Glycol)-Poly(ε-Caprolactone) Nanosuspension for Ocular Delivery of Hydrophobic Diclofenac. Sci. Rep. 2015, 5, 11337. [Google Scholar] [CrossRef] [PubMed]
- Sun, I.-C.; Na, J.H.; Jeong, S.Y.; Kim, D.-E.; Kwon, I.C.; Choi, K.; Ahn, C.-H.; Kim, K. Biocompatible Glycol Chitosan-Coated Gold Nanoparticles for Tumor-Targeting CT Imaging. Pharm. Res. 2014, 31, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Duan, R.; Liu, R.; Guan, M.; Chen, W.; Ma, J.; Chen, M.; Du, B.; Zhang, Q. Chitosan-Stabilized Self-Assembled Fluorescent Gold Nanoclusters for Cell Imaging and Biodistribution In Vivo. ACS Biomater. Sci. Eng. 2018, 4, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Grenier, P.; Mahmoudi, M.; Lima, E.M.; Appel, E.A.; Dormont, F.; Lim, J.-M.; Karnik, R.; Langer, R.; Farokhzad, O.C. Mechanistic Understanding of In Vivo Protein Corona Formation on Polymeric Nanoparticles and Impact on Pharmacokinetics. Nat. Commun. 2017, 8, 777. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Bertrand, N.; Zope, H.; Farokhzad, O.C. Emerging Understanding of the Protein Corona at the Nano-Bio Interfaces. Nano Today 2016, 11, 817–832. [Google Scholar] [CrossRef]
- Forest, V.; Pourchez, J. Preferential Binding of Positive Nanoparticles on Cell Membranes Is Due to Electrostatic Interactions: A Too Simplistic Explanation That Does Not Take into Account the Nanoparticle Protein Corona. Mater. Sci. Eng. C 2017, 70, 889–896. [Google Scholar] [CrossRef]
- Almalik, A.; Donno, R.; Cadman, C.J.; Cellesi, F.; Day, P.J.; Tirelli, N. Hyaluronic Acid-Coated Chitosan Nanoparticles: Molecular Weight-Dependent Effects on Morphology and Hyaluronic Acid Presentation. J. Control Release 2013, 172, 1142–1150. [Google Scholar] [CrossRef]
- Almalik, A.; Benabdelkamel, H.; Masood, A.; Alanazi, I.O.; Alradwan, I.; Majrashi, M.A.; Alfadda, A.A.; Alghamdi, W.M.; Alrabiah, H.; Tirelli, N.; et al. Hyaluronic Acid Coated Chitosan Nanoparticles Reduced the Immunogenicity of the Formed Protein Corona. Sci. Rep. 2017, 7, 10542. [Google Scholar] [CrossRef]
- Basu, A.; Kundu, S.; Basu, C.; Ghosh, S.K.; Sur, R.; Mukherjee, A. Biopolymer Nanoparticle Surface Chemistry Dictates the Nature and Extent of Protein Hard Corona. J. Mol. Liq. 2019, 282, 169–176. [Google Scholar] [CrossRef]
- Abouelmagd, S.A.; Ku, Y.J.; Yeo, Y. Low Molecular Weight Chitosan-Coated Polymeric Nanoparticles for Sustained and PH-Sensitive Delivery of Paclitaxel. J. Drug Target. 2015, 23, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Tekie, F.S.M.; Hajiramezanali, M.; Geramifar, P.; Raoufi, M.; Dinarvand, R.; Soleimani, M.; Atyabi, F. Controlling Evolution of Protein Corona: A Prosperous Approach to Improve Chitosan-Based Nanoparticle Biodistribution and Half-Life. Sci. Rep. 2020, 10, 9664. [Google Scholar] [CrossRef]
- Chen, M.-C.; Wong, H.-S.; Lin, K.-J.; Chen, H.-L.; Wey, S.-P.; Sonaje, K.; Lin, Y.-H.; Chu, C.-Y.; Sung, H.-W. The Characteristics, Biodistribution and Bioavailability of a Chitosan-Based Nanoparticulate System for the Oral Delivery of Heparin. Biomaterials 2009, 30, 6629–6637. [Google Scholar] [CrossRef]
- Raj, P.M.; Raj, R.; Kaul, A.; Mishra, A.K.; Ram, A. Biodistribution and Targeting Potential Assessment of Mucoadhesive Chitosan Nanoparticles Designed for Ulcerative Colitis via Scintigraphy. RSC Adv. 2018, 8, 20809–20821. [Google Scholar] [CrossRef]
- Navarro, S.M.; Darensbourg, C.; Cross, L.; Stout, R.; Coulon, D.; Astete, C.E.; Morgan, T.; Sabliov, C.M. Biodistribution of PLGA and PLGA/Chitosan Nanoparticles after Repeat-Dose Oral Delivery in F344 Rats for 7 Days. Ther. Deliv. 2014, 5, 1191–1201. [Google Scholar] [CrossRef]
- Gartziandia, O.; Herran, E.; Pedraz, J.L.; Carro, E.; Igartua, M.; Hernandez, R.M. Chitosan Coated Nanostructured Lipid Carriers for Brain Delivery of Proteins by Intranasal Administration. Colloids Surf. B Biointerfaces 2015, 134, 304–313. [Google Scholar] [CrossRef]
- Yousfan, A.; Rubio, N.; Hakim Natouf, A.; Daher, A.; Al-Kafry, N.; Venner, K.; Kafa, H. Preparation and Characterisation of PHT-Loaded Chitosan Lecithin Nanoparticles for Intranasal Drug Delivery to the Brain. RSC Adv. 2020, 10, 28992–29009. [Google Scholar] [CrossRef]
- Franca, J.R.; Foureaux, G.; Fuscaldi, L.L.; Ribeiro, T.G.; Rodrigues, L.B.; Bravo, R.; Castilho, R.O.; Yoshida, M.I.; Cardoso, V.N.; Fernandes, S.O.; et al. Bimatoprost-Loaded Ocular Inserts as Sustained Release Drug Delivery Systems for Glaucoma Treatment: In Vitro and In Vivo Evaluation. PLoS ONE 2014, 9, e95461. [Google Scholar] [CrossRef]
- Franca, J.R.; Fuscaldi, L.L.; Ribeiro, T.G.; Foureaux, G.; Cesar, A.L.A.; Castilho, R.O.; Cronemberger, S.; Ferreira, A.J.; Fernandes, S.O.A.; Cardoso, V.N.; et al. Use of Chitosan as Pharmaceutical Excipient in Ocular Drug Delivery Systems: Sterilization and Pharmacokinetics. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2227–2237. [Google Scholar] [CrossRef]
- Kamiyama, K.; Onishi, H.; Machida, Y. Biodisposition Characteristics of N-Succinyl-Chitosan and Glycol-Chitosan in Normal and Tumor-Bearing Mice. Biol. Pharm. Bull. 1999, 22, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Y.; Jiang, S.; Gao, Y.; Zhang, W.; Hu, S.; Cheng, X.; Zhang, C.; Sun, P.; Ke, W.; et al. Biodistribution and Biocompatibility of Glycyrrhetinic Acid and Galactose-Modified Chitosan Nanoparticles as a Novel Targeting Vehicle for Hepatocellular Carcinoma. Nanomedicine 2020, 15, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Williams, G.R.; Wu, J.; Wu, J.; Zhang, X.; Chen, X.; Li, S.; Jiao, J.; Zhu, L.-M. A Chitosan-Based Cascade-Responsive Drug Delivery System for Triple-Negative Breast Cancer Therapy. J. Nanobiotechnol. 2019, 17, 95. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, A.S.; Farsakoglu, Y.; Crecente-Campo, J.; de la Fuente, M.; González, S.F.; Alonso, M.J. Carboxymethyl-β-Glucan/Chitosan Nanoparticles: New Thermostable and Efficient Carriers for Antigen Delivery. Drug Deliv. Transl. Res. 2021, 11, 1689–1702. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Wu, Z.; Wang, Z.; Niu, H.; Li, C. Nasal Absorption Enhancement of Insulin Using PEG-Grafted Chitosan Nanoparticles. Eur. J. Pharm. Biopharm. 2008, 68, 526–534. [Google Scholar] [CrossRef]
- Zu, Y.; Zhang, Y.; Wang, W.; Zhao, X.; Han, X.; Wang, K.; Ge, Y. Preparation and In Vitro/In Vivo Evaluation of Resveratrol-Loaded Carboxymethyl Chitosan Nanoparticles. Drug Deliv. 2016, 23, 971–981. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.; Sun, Y.; Ding, J.; Li, J.; Duan, X.; Li, Y.; Junyaprasert, V.B.; Mao, S. In Vitro and In Vivo Evaluation of Chitosan Graft Glyceryl Monooleate as Peroral Delivery Carrier of Enoxaparin. Int. J. Pharm. 2014, 471, 391–399. [Google Scholar] [CrossRef]
- Asasutjarit, R.; Theerachayanan, T.; Kewsuwan, P.; Veeranodha, S.; Fuongfuchat, A.; Ritthidej, G.C. Development and Evaluation of Diclofenac Sodium Loaded-N-Trimethyl Chitosan Nanoparticles for Ophthalmic Use. AAPS PharmSciTech 2015, 16, 1013–1024. [Google Scholar] [CrossRef]
- Narayanan, D.; Anitha, A.; Jayakumar, R.; Chennazhi, K.P. In Vitro and In Vivo Evaluation of Osteoporosis Therapeutic Peptide PTH 1–34 Loaded PEGylated Chitosan Nanoparticles. Mol. Pharm. 2013, 10, 4159–4167. [Google Scholar] [CrossRef]
- Feng, C.; Wang, Z.; Jiang, C.; Kong, M.; Zhou, X.; Li, Y.; Cheng, X.; Chen, X. Chitosan/o-Carboxymethyl Chitosan Nanoparticles for Efficient and Safe Oral Anticancer Drug Delivery: In Vitro and In Vivo Evaluation. Int. J. Pharm. 2013, 457, 158–167. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.; Chen, D.; Jin, X.; Zhao, X.; Zhang, C.; Ping, Q. In Vivo Evaluation of Novel Chitosan Graft Polymeric Micelles for Delivery of Paclitaxel. Drug Deliv. 2011, 18, 181–189. [Google Scholar] [CrossRef]
- Braz, L.; Grenha, A.; Ferreira, D.; Rosa da Costa, A.M.; Gamazo, C.; Sarmento, B. Chitosan/Sulfated Locust Bean Gum Nanoparticles: In Vitro and In Vivo Evaluation towards an Application in Oral Immunization. Int. J. Biol. Macromol. 2017, 96, 786–797. [Google Scholar] [CrossRef]
- Yu, J.-M.; Li, Y.-J.; Qiu, L.-Y.; Jin, Y. Polymeric Nanoparticles of Cholesterol-Modified Glycol Chitosan for Doxorubicin Delivery: Preparation and in-Vitro and in-Vivo Characterization. J. Pharm. Pharmacol. 2009, 61, 713–719. [Google Scholar] [CrossRef]
- Zhang, C.; Qu, G.; Sun, Y.; Wu, X.; Yao, Z.; Guo, Q.; Ding, Q.; Yuan, S.; Shen, Z.; Ping, Q.; et al. Pharmacokinetics, Biodistribution, Efficacy and Safety of N-Octyl-O-Sulfate Chitosan Micelles Loaded with Paclitaxel. Biomaterials 2008, 29, 1233–1241. [Google Scholar] [CrossRef]
- Bachir, Z.A.; Huang, Y.; He, M.; Huang, L.; Hou, X.; Chen, R.; Gao, F. Effects of PEG Surface Density and Chain Length on the Pharmacokinetics and Biodistribution of Methotrexate-Loaded Chitosan Nanoparticles. Int. J. Nanomed. 2018, 13, 5657–5671. [Google Scholar] [CrossRef]
- Dong, W.; Han, B.; Feng, Y.; Song, F.; Chang, J.; Jiang, H.; Tang, Y.; Liu, W. Pharmacokinetics and Biodegradation Mechanisms of a Versatile Carboxymethyl Derivative of Chitosan in Rats: In Vivo and In Vitro Evaluation. Biomacromolecules 2010, 11, 1527–1533. [Google Scholar] [CrossRef]
- Sonin, D.; Pochkaeva, E.; Zhuravskii, S.; Postnov, V.; Korolev, D.; Vasina, L.; Kostina, D.; Mukhametdinova, D.; Zelinskaya, I.; Skorik, Y.; et al. Biological Safety and Biodistribution of Chitosan Nanoparticles. Nanomaterials 2020, 10, 810. [Google Scholar] [CrossRef]
- Nadesh, R.; Narayanan, D.; Sreerekha, P.R.; Vadakumpully, S.; Mony, U.; Koyakkutty, M.; Nair, S.V.; Menon, D. Hematotoxicological Analysis of Surface-Modified and -Unmodified Chitosan Nanoparticles. J. Biomed. Mater. Res. A 2013, 101, 2957–2966. [Google Scholar] [CrossRef]
- Sonaje, K.; Lin, Y.-H.; Juang, J.-H.; Wey, S.-P.; Chen, C.-T.; Sung, H.-W. In Vivo Evaluation of Safety and Efficacy of Self-Assembled Nanoparticles for Oral Insulin Delivery. Biomaterials 2009, 30, 2329–2339. [Google Scholar] [CrossRef]
- Shan, X.; Xu, T.; Liu, Z.; Hu, X.; Zhang, Y.-D.; Wang, B. Safety and Toxicology of the Intravenous Administration of Ang2-SiRNA Plasmid Chitosan Magnetic Nanoparticles. Mol. Med. Rep. 2017, 15, 736–742. [Google Scholar] [CrossRef]
- Islam, N.; Dmour, I.; Taha, M.O. Degradability of Chitosan Micro/Nanoparticles for Pulmonary Drug Delivery. Heliyon 2019, 5, e01684. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of Chitosan in Food, Pharmaceuticals, Medicine, Cosmetics, Agriculture, Textiles, Pulp and Paper, Biotechnology, and Environmental Chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef]
- Marques, C.; Som, C.; Schmutz, M.; Borges, O.; Borchard, G. How the Lack of Chitosan Characterization Precludes Implementation of the Safe-by-Design Concept. Front. Bioeng. Biotechnol. 2020, 8, 165. [Google Scholar] [CrossRef]
- El-Kamary, S.S.; Pasetti, M.F.; Mendelman, P.M.; Frey, S.E.; Bernstein, D.I.; Treanor, J.J.; Ferreira, J.; Chen, W.H.; Sublett, R.; Richardson, C.; et al. Adjuvanted Intranasal Norwalk Virus-Like Particle Vaccine Elicits Antibodies and Antibody-Secreting Cells That Express Homing Receptors for Mucosal and Peripheral Lymphoid Tissues. J. Infect. Dis. 2010, 202, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.J.; Noulin, N.; Catchpole, A.; Stittelaar, K.J.; de Waal, L.; Kroeze, E.J.B.V.; Hinchcliffe, M.; Smith, A.; Montomoli, E.; Piccirella, S.; et al. Intranasal H5N1 Vaccines, Adjuvanted with Chitosan Derivatives, Protect Ferrets against Highly Pathogenic Influenza Intranasal and Intratracheal Challenge. PLoS ONE 2014, 9, e93761. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).