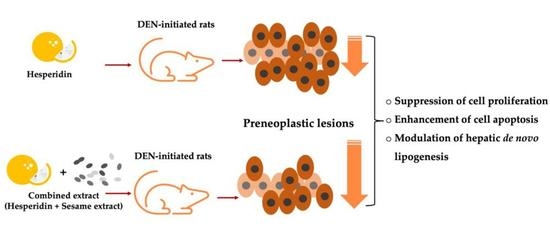
Sesame Extract Promotes Chemopreventive Effect of Hesperidin on Early Phase of Diethylnitrosamine-Initiated Hepatocarcinogenesis in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Test Compounds
2.3. Chemical Analysis
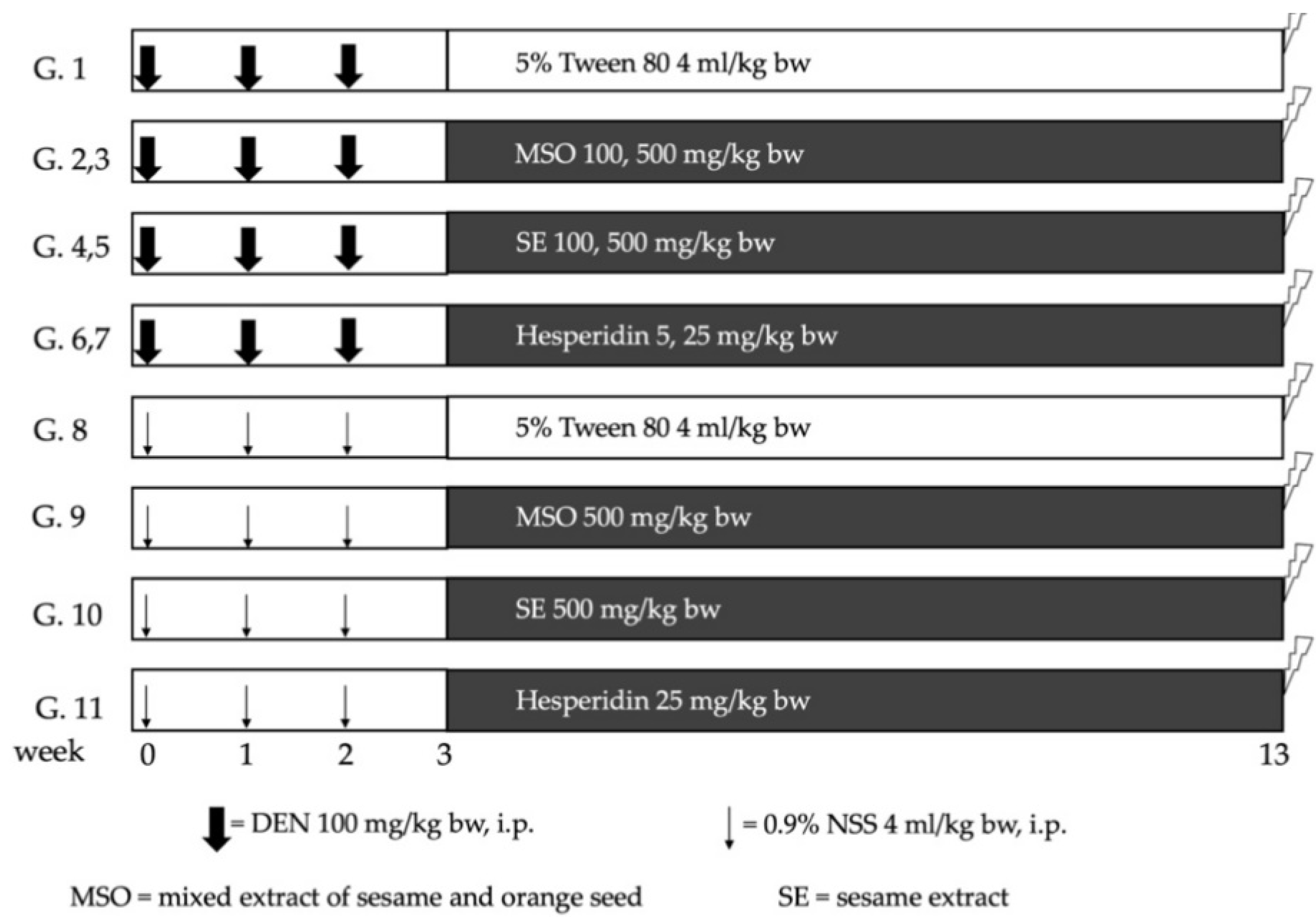
2.4. Animals
2.5. Effect of MSO and Its Compositions on Early Stages of Hepatocarcinogenesis in Rats
2.6. Evaluation of Glutathione S-Transferase Placental form Positive Foci in Liver Tissues
2.7. Determination of Cell Proliferation, Apoptosis and Fatty Acid Synthase Expression in Liver Tissues
2.8. Determination of Hepatic Triglyceride Content
2.9. Determination of Phases I and Phases II Xenobiotic Metabolizing Enzyme Activities
2.10. Statistical Analysis
3. Results
3.1. Phytochemical Constituents of Mixed Extract of Sesame and Orange Seed and Sesame Extract
3.2. Effect of MSO and Its Various Compositions on Preneoplastic Lesion Formation in the Livers of Rats
3.3. Effect of MSO and Its Compositions on Cell Proliferation and Apoptosis in the Livers of Rats
3.4. Effect of MSO and Its Compositions on Lipid Metabolism in the Livers of Rats
3.5. Effect of MSO and Its Compositions on the Activity of Xenobiotic Metabolizing Enzymes in the Livers of Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Llovet, J.M.; Zucman-Rossi, J.; Pikarsky, E.; Sangro, B.; Schwartz, M.; Sherman, M.; Gores, G. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2016, 2, 16018. [Google Scholar] [CrossRef]
- Raza, A.; Sood, G.K. Hepatocellular carcinoma review: Current treatment, and evidence-based medicine. World J. Gastroenterol. 2014, 20, 4115–4127. [Google Scholar] [CrossRef]
- Carr, C.; Ng, J.; Wigmore, T. The side effects of chemotherapeutic agents. Curr. Anaesth. Crit. Care 2008, 19, 70–79. [Google Scholar] [CrossRef]
- Darvesh, A.S.; Aggarwal, B.B.; Bishayee, A. Curcumin and liver cancer: A review. Curr. Pharm. Biotechnol. 2012, 13, 218–228. [Google Scholar] [CrossRef]
- Bishayee, A.; Mbimba, T.; Thoppil, R.J.; Haznagy-Radnai, E.; Sipos, P.; Darvesh, A.S.; Folkesson, H.G.; Hohmann, J. Anthocyanin-rich black currant (Ribes nigrum L.) extract affords chemoprevention against diethylnitrosamine-induced hepatocellular carcinogenesis in rats. J. Nutr. Biochem. 2011, 22, 1035–1046. [Google Scholar] [CrossRef]
- Bishayee, A.; Thoppil, R.J.; Mandal, A.; Darvesh, A.S.; Ohanyan, V.; Meszaros, J.G.; Haznagy-Radnai, E.; Hohmann, J.; Bhatia, D. Black currant phytoconstituents exert chemoprevention of diethylnitrosamine-initiated hepatocarcinogenesis by suppression of the inflammatory response. Mol. Carcinog. 2013, 52, 304–317. [Google Scholar] [CrossRef]
- Mansour, M.A.; Bekheet, S.A.; Al-Rejaie, S.S.; Al-Shabanah, O.A.; Al-Howiriny, T.A.; Al-Rikabi, A.C.; Abdo, A.A. Ginger ingredients inhibit the development of diethylnitrosoamine induced premalignant phenotype in rat chemical hepatocarcinogenesis model. Biofactors 2010, 36, 483–490. [Google Scholar] [CrossRef]
- Yadav, A.S.; Bhatnagar, D. Chemo-preventive effect of Star anise in N-nitrosodiethylamine initiated and phenobarbital promoted hepato-carcinogenesis. Chem. Biol. Interact. 2007, 169, 207–214. [Google Scholar] [CrossRef]
- Phua, D.H.; Zosel, A.; Heard, K. Dietary supplements and herbal medicine toxicities-when to anticipate them and how to manage them. Int. J. Emerg. Med. 2009, 2, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Teo, D.C.; Ng, P.S.; Tan, S.H.; Lim, A.T.; Toh, D.S.; Chan, S.Y.; Cheong, H.H. Drug-induced liver injury associated with complementary and alternative medicine: A review of adverse event reports in an Asian community from 2009 to 2014. BMC Complement. Altern. Med. 2016, 16, 192. [Google Scholar] [CrossRef] [Green Version]
- Rui, W.; Xie, L.; Liu, X.; He, S.; Wu, C.; Zhang, X.; Zhang, L.; Yang, Y. Compound Astragalus and Salvia miltiorrhiza extract suppresses hepatocellular carcinoma progression by inhibiting fibrosis and PAI-1 mRNA transcription. J. Ethnopharmacol. 2014, 151, 198–209. [Google Scholar] [CrossRef]
- Boye, A.; Wu, C.; Jiang, Y.; Wang, J.; Wu, J.; Yang, X.; Yang, Y. Compound Astragalus and Salvia miltiorrhiza extracts modulate MAPK-regulated TGF-beta/Smad signaling in hepatocellular carcinoma by multi-target mechanism. J. Ethnopharmacol. 2015, 169, 219–228. [Google Scholar] [CrossRef]
- Wu, C.; Kan, H.; Hu, M.; Liu, X.; Boye, A.; Jiang, Y.; Wu, J.; Wang, J.; Yang, X.; Yang, Y. Compound Astragalus and Salvia miltiorrhiza extract inhibits hepatocarcinogenesis via modulating TGF-beta/TbetaR and Imp7/8. Exp. Ther. Med. 2018, 16, 1052–1060. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.-B.; Meng, X.-T.; Jia, Q.-A.; Bu, Y.; Ren, Z.-G.; Zhang, B.-H.; Tang, Z.-Y. Herbal compound Songyou Yin and moderate swimming suppress growth and metastasis of liver cancer by enhancing immune function. Integr. Cancer Ther. 2016, 15, 368–375. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Zhong, Z.; Tan, H.Y.; Guo, W.; Zhang, C.; Tan, C.-w.; Li, S.; Wang, N.; Feng, Y. Uncovering the anticancer mechanisms of Chinese herbal medicine formulas: Therapeutic alternatives for liver cancer. Front. Pharmacol. 2020, 11, 293. [Google Scholar] [CrossRef] [Green Version]
- Pari, L.; Karthikeyan, A.; Karthika, P.; Rathinam, A. Protective effects of hesperidin on oxidative stress, dyslipidaemia and histological changes in iron-induced hepatic and renal toxicity in rats. Toxicol. Rep. 2015, 2, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Li, J.; Cai, L.; Hu, C.M.; Zhang, L. Suppression of adjuvant arthritis by hesperidin in rats and its mechanisms. J. Pharm. Pharmacol. 2008, 60, 221–228. [Google Scholar] [CrossRef]
- Morand, C.; Dubray, C.; Milenkovic, D.; Lioger, D.; Martin, J.F.; Scalbert, A.; Mazur, A. Hesperidin contributes to the vascular protective effects of orange juice: A randomized crossover study in healthy volunteers. Am. J. Clin. Nutr. 2011, 93, 73–80. [Google Scholar] [CrossRef]
- Roza, J.M.; Xian-Liu, Z.; Guthrie, N. Effect of citrus flavonoids and tocotrienols on serum cholesterol levels in hypercholesterolemic subjects. Altern. Ther. Health Med. 2007, 13, 44–48. [Google Scholar]
- Kamaraj, S.; Ramakrishnan, G.; Anandakumar, P.; Jagan, S.; Devaki, T. Antioxidant and anticancer efficacy of hesperidin in benzo(a)pyrene induced lung carcinogenesis in mice. Investig. New Drugs 2009, 27, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Kongtawelert, P.; Wudtiwai, B.; Shwe, T.H.; Pothacharoen, P.; Phitak, T. Inhibitory effect of hesperidin on the expression of programmed death ligand (PD-L1) in breast cancer. Molecules 2020, 25, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dar, A.A.; Arumugam, N. Lignans of sesame: Purification methods, biological activities and biosynthesis—A review. Bioorg. Chem. 2013, 50, 1–10. [Google Scholar] [CrossRef]
- Harikumar, K.B.; Sung, B.; Tharakan, S.T.; Pandey, M.K.; Joy, B.; Guha, S.; Krishnan, S.; Aggarwal, B.B. Sesamin manifests chemopreventive effects through the suppression of NF-kappa B-regulated cell survival, proliferation, invasion, and angiogenic gene products. Mol. Cancer Res. 2010, 8, 751–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, B.W.; Bray, F.; Forman, D.; Ohgaki, H.; Straif, K.; Ullrich, A.; Wild, C.P. Cancer prevention as part of precision medicine: ‘plenty to be done’. Carcinogenesis 2016, 37, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant activity of apple peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef]
- Chariyakornkul, A.; Punvittayagul, C.; Taya, S.; Wongpoomchai, R. Inhibitory effect of purple rice husk extract on AFB1-induced micronucleus formation in rat liver through modulation of xenobiotic metabolizing enzymes. BMC Complement. Altern. Med. 2019, 19, 237. [Google Scholar] [CrossRef] [Green Version]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Thumvijit, T.; Taya, S.; Punvittayagul, C.; Peerapornpisal, Y.; Wongpoomchai, R. Cancer chemopreventive effect of Spirogyra neglecta (Hassall) Kützing on diethylnitrosamine-induced hepatocarcinogenesis in rats. Asian Pac. J. Cancer Prev. 2014, 15, 1611–1616. [Google Scholar] [CrossRef] [Green Version]
- Suwannakul, N.; Punvittayagul, C.; Jarukamjorn, K.; Wongpoomchai, R. Purple rice bran extract attenuates the aflatoxin B1-induced initiation stage of hepatocarcinogenesis by alteration of xenobiotic metabolizing enzymes. Asian Pac. J. Cancer Prev. 2015, 16, 3371–3376. [Google Scholar] [CrossRef] [Green Version]
- Muramatsu, M.; Sakai, M. Mechanisms of a tumor marker, glutathione transferase P, expression during hepatocarcinogenesis of the rat. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2006, 82, 339–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Wang, G.-J.; Wang, Z.; Gao, N.; Li, J.; Zhang, Y.-F.; Zhou, J.; Zhang, H.-X.; Wen, Q.; Jin, H.; et al. High CYP2E1 activity correlates with hepatofibrogenesis induced by nitrosamines. Oncotarget 2017, 8, 112199–112210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sever, R.; Brugge, J.S. Signal transduction in cancer. Cold Spring Harb. Perspect. Med. 2015, 5, a006098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howes, M.J.; Simmonds, M.S. The role of phytochemicals as micronutrients in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, R.T.; Takami, A.; Espinoza, J.L. Dietary phytochemicals and cancer chemoprevention: A review of the clinical evidence. Oncotarget 2016, 7, 52517–52529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasanin, A.H.; Matboli, M.; Seleem, H.S. Hesperidin suppressed hepatic precancerous lesions via modulation of exophagy in rats. J. Cell Biochem. 2020, 121, 1295–1306. [Google Scholar] [CrossRef]
- Zaghloul, R.A.; Elsherbiny, N.M.; Kenawy, H.I.; El-Karef, A.; Eissa, L.A.; El-Shishtawy, M.M. Hepatoprotective effect of hesperidin in hepatocellular carcinoma: Involvement of Wnt signaling pathways. Life Sci. 2017, 185, 114–125. [Google Scholar] [CrossRef]
- Mo’men, Y.S.; Hussein, R.M.; Kandeil, M.A. Involvement of PI3K/Akt pathway in the protective effect of hesperidin against a chemically induced liver cancer in rats. J. Biochem. Mol. Toxicol. 2019, 33, e22305. [Google Scholar] [CrossRef]
- Wu, M.S.; Aquino, L.B.B.; Barbaza, M.Y.U.; Hsieh, C.L.; Castro-Cruz, K.A.; Yang, L.L.; Tsai, P.W. Anti-inflammatory and anticancer properties of bioactive compounds from Sesamum indicum L.—A review. Molecules 2019, 24, 4426. [Google Scholar] [CrossRef] [Green Version]
- Andargie, M.; Vinas, M.; Rathgeb, A.; Möller, E.; Karlovsky, P. Lignans of sesame (Sesamum indicum L.): A comprehensive review. Molecules 2021, 26, 883. [Google Scholar] [CrossRef]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef] [Green Version]
- Berndt, N.; Eckstein, J.; Heucke, N.; Gajowski, R.; Stockmann, M.; Meierhofer, D.; Holzhutter, H.G. Characterization of lipid and lipid droplet metabolism in human HCC. Cells 2019, 8, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Che, L.; Pilo, M.G.; Cigliano, A.; Latte, G.; Simile, M.M.; Ribback, S.; Dombrowski, F.; Evert, M.; Chen, X.; Calvisi, D.F. Oncogene dependent requirement of fatty acid synthase in hepatocellular carcinoma. Cell Cycle 2017, 16, 499–507. [Google Scholar] [CrossRef]
- Che, L.; Chi, W.; Qiao, Y.; Zhang, J.; Song, X.; Liu, Y.; Li, L.; Jia, J.; Pilo, M.G.; Wang, J.; et al. Cholesterol biosynthesis supports the growth of hepatocarcinoma lesions depleted of fatty acid synthase in mice and humans. Gut 2020, 69, 177–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peck, B.; Schulze, A. Lipid desaturation—The next step in targeting lipogenesis in cancer? Febs J. 2016, 283, 2767–2778. [Google Scholar] [CrossRef]
- Röhrig, F.; Schulze, A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer 2016, 16, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Wanwimolruk, S.; Prachayasittikul, V. Cytochrome P450 enzyme mediated herbal drug interactions (Part 1). EXCLI J. 2014, 13, 347. [Google Scholar] [CrossRef]
- Brewer, C.T.; Chen, T. Hepatotoxicity of herbal supplements mediated by modulation of cytochrome P450. Int. J. Mol. Sci. 2017, 18, 2353. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Group | Treatment (mg/kg bw) | Final Body Weight (g) | Liver Weight (g) | Liver/Body Weight Ratio | GST-P Number/ Liver Area (cm2) | GST-P Area(mm2)/ Liver Area (cm2) | ALT (U/L) |
|---|---|---|---|---|---|---|---|
| 1 | DEN + 5% Tween 80 | 277.2 ± 16.0 | 7.86 ± 0.72 a | 2.84 ± 0.25 a | 12.6 ± 3.5 a | 0.56 ± 0.43 a | 60.1 ± 13.7 a |
| 2 | DEN + MSO 100 | 262.2 ± 27.1 | 7.73 ± 1.07 | 2.96 ± 0.35 | 13.2 ± 4.4 a | 0.31 ± 0.43 a | 50.8 ± 10.5 b |
| 3 | DEN + MSO 500 | 245.7 ± 20.5 b | 7.23 ± 0.70 | 2.95 ± 0.20 | 14.1 ± 6.1 a | 0.53 ± 0.45 a | 53.0 ± 10.5 b |
| 4 | DEN + Hesperidin 5 | 252.5 ± 43.2 | 6.67 ± 2.05 | 2.74 ± 0.78 | 9.2 ± 4.4 a | 0.30 ± 0.32 a | 43.3 ± 9.3 b |
| 5 | DEN + Hesperidin 25 | 263.9 ± 22.0 | 7.49 ± 0.67 | 2.84 ± 0.19 | 14.7 ± 4.4 a | 0.23 ± 0.22 a | 43.2 ± 9.1 b |
| 6 | NSS + 5% Tween 80 | 289.0 ± 19.8 | 10.19 ± 0.92 | 3.52 ± 0.14 | 0 | 0 | 33.0 ± 2.7 |
| 7 | NSS + MSO 100 | 296.0 ± 39.9 | 11.31 ± 1.74 | 3.82 ± 0.26 | 0 | 0 | 29.0 ± 2.6 |
| 8 | NSS + MSO 500 | 279.0 ± 40.4 | 10.11 ± 0.96 | 3.65 ± 0.24 | 0 | 0 | 30.0 ± 2.4 |
| 9 | NSS + Hesperidin 5 | 291.0 ± 42.2 | 10.45 ± 1.96 | 3.58 ± 0.27 | 0 | 0 | 31.6 ± 3.7 |
| 10 | NSS + Hesperidin 25 | 284.0 ± 25.1 | 10.85 ± 0.78 | 3.83 ± 0.27 | 0 | 0 | 32.4 ± 3.6 |
| Group | Chemical | Treatment (mg/kg, bw) | Enzyme Activities | |||
|---|---|---|---|---|---|---|
| CYP1A1 a | CYP1A2 a | CYP3A2 b | GST c | |||
| 6 | NSS | 5% Tween 80 | 4.06 ± 1.09 | 2.34 ± 0.63 | 6.96 ± 1.33 | 48.5 ± 7.49 |
| 7 | NSS | MSO 100 | 6.48 ± 2.26 | 2.63 ± 1.21 | 7.01 ± 0.42 | 45.6 ± 4.24 |
| 8 | NSS | MSO 500 | 4.12 ± 1.93 | 1.61 ± 0.43 | 6.21 ± 0.93 | 52.6 ± 4.49 |
| 9 | NSS | Hesperidin 5 | 5.67 ± 1.51 | 2.68 ± 0.58 | 7.47 ± 1.57 | 52.5 ± 7.49 |
| 10 | NSS | Hesperidin 25 | 6.13 ± 1.40 | 2.70 ± 0.73 | 7.59 ± 1.15 | 51.6 ± 4.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khuanphram, N.; Taya, S.; Kongtawelert, P.; Wongpoomchai, R. Sesame Extract Promotes Chemopreventive Effect of Hesperidin on Early Phase of Diethylnitrosamine-Initiated Hepatocarcinogenesis in Rats. Pharmaceutics 2021, 13, 1687. https://doi.org/10.3390/pharmaceutics13101687
Khuanphram N, Taya S, Kongtawelert P, Wongpoomchai R. Sesame Extract Promotes Chemopreventive Effect of Hesperidin on Early Phase of Diethylnitrosamine-Initiated Hepatocarcinogenesis in Rats. Pharmaceutics. 2021; 13(10):1687. https://doi.org/10.3390/pharmaceutics13101687
Chicago/Turabian StyleKhuanphram, Napaporn, Sirinya Taya, Prachya Kongtawelert, and Rawiwan Wongpoomchai. 2021. "Sesame Extract Promotes Chemopreventive Effect of Hesperidin on Early Phase of Diethylnitrosamine-Initiated Hepatocarcinogenesis in Rats" Pharmaceutics 13, no. 10: 1687. https://doi.org/10.3390/pharmaceutics13101687
APA StyleKhuanphram, N., Taya, S., Kongtawelert, P., & Wongpoomchai, R. (2021). Sesame Extract Promotes Chemopreventive Effect of Hesperidin on Early Phase of Diethylnitrosamine-Initiated Hepatocarcinogenesis in Rats. Pharmaceutics, 13(10), 1687. https://doi.org/10.3390/pharmaceutics13101687