In Vitro Ciliotoxicity and Cytotoxicity Testing of Repeated Chronic Exposure to Topical Nasal Formulations for Safety Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Air-Liquid Culturing of the 3D Human Nasal MucilAir™ Epithelium
2.2. Transmission Electron Microscopy (TEM)
2.3. Scanning Electron Microscopy (SEM)
2.4. Treatment with Nasal Formulations
2.5. CBF Measurement
2.6. LDH Cytotoxicity Assay
2.7. Data Presentation and Statistical Analysis
3. Results
3.1. CBF Measurements in the Nasal MucilAir™ In Vitro Model
3.2. Repeated Exposure to Undiluted Nasal Sprays
3.3. Repeated Exposure to Clinically Relevant Dilutions of Nasal Sprays
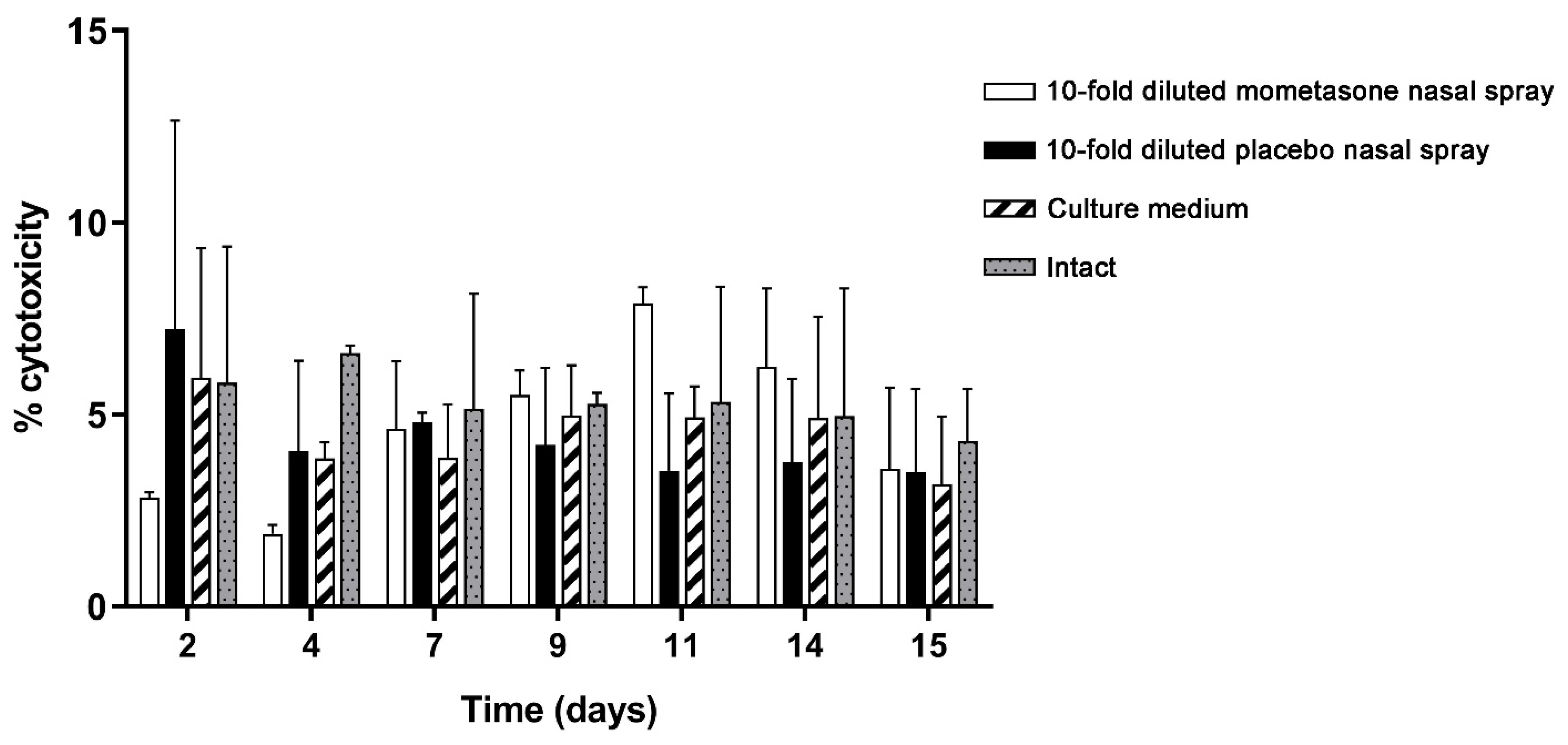
3.4. LDH Cytotoxicity Analysis
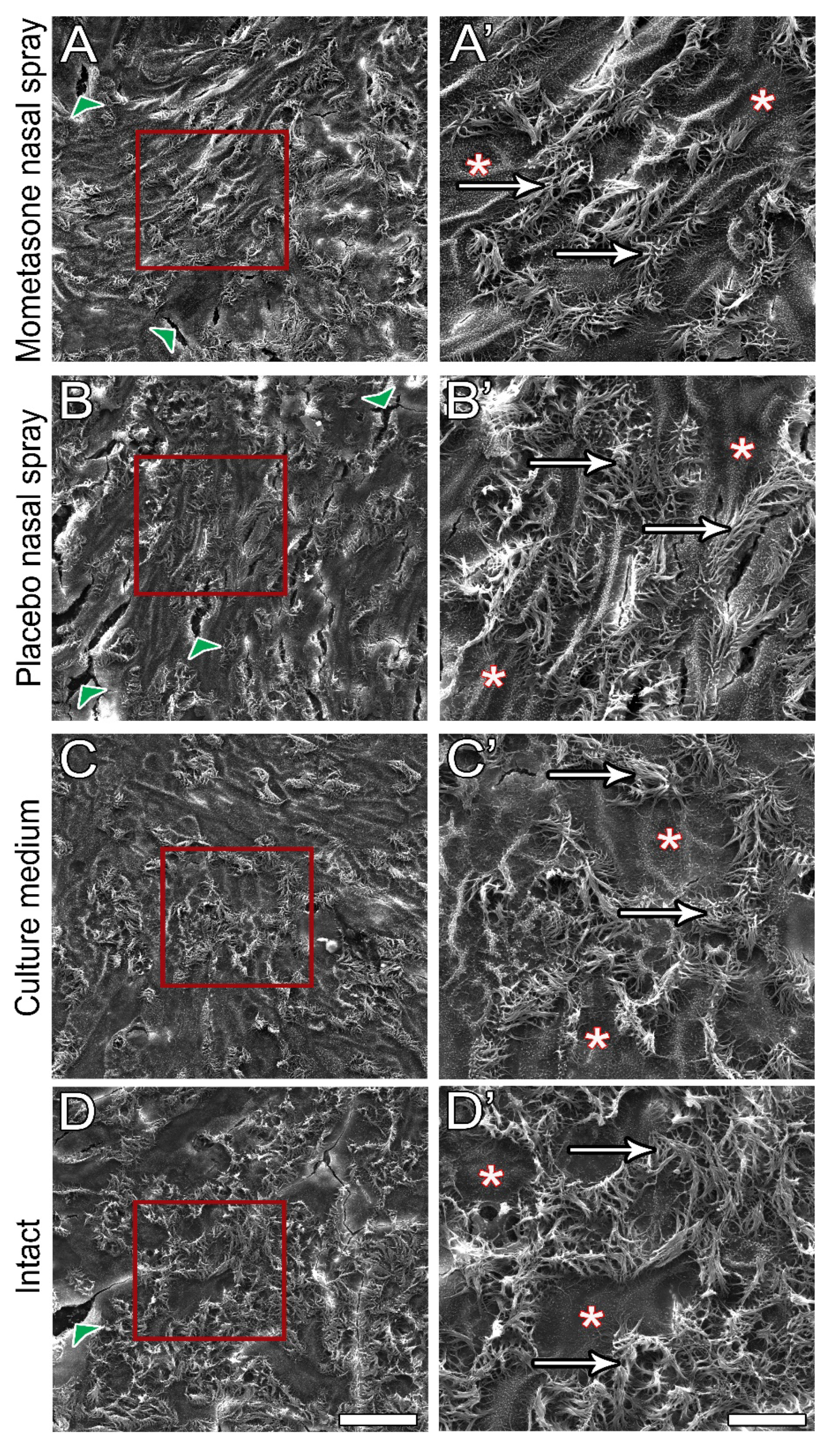
3.5. Post-Treatment Ultrastructural Analysis
4. Discussion
4.1. Applicability of the Nasal MucilAir™ In Vitro Model for the Analysis of Repeated Doses Ciliotoxicity and Cytotoxicity of Topical Nasal Formulations
4.2. The Importance of In Vitro Assessment of the Effect of Chronic Topical Nasal Formulation Exposure on CBF
4.3. The Observed Effects of Repeated Exposure to Clinically Relevant Doses of Mometasone Nasal Spray on CBF Are in Good Agreement with Those of In Vivo Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watts, A.M.; Cripps, A.W.; West, N.P.; Cox, A.J. Modulation of Allergic Inflammation in the Nasal Mucosa of Allergic Rhinitis Sufferers with Topical Pharmaceutical Agents. Front. Pharm. 2019, 10, 294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitter, C.; Suter-Zimmermann, K.; Surber, C. Nasal drug delivery in humans. Curr. Probl. Derm. 2011, 40, 20–35. [Google Scholar]
- Jiao, J.; Zhang, L. Influence of Intranasal Drugs on Human Nasal Mucociliary Clearance and Ciliary Beat Frequency. Allergy Asthma Immunol. Res. 2019, 11, 306–319. [Google Scholar] [CrossRef]
- Seidman, M.D.; Gurgel, R.K.; Lin, S.Y.; Schwartz, S.R.; Baroody, F.M.; Bonner, J.R.; Dawson, D.E.; Dykewicz, M.S.; Hackell, J.M.; Han, J.K.; et al. Clinical practice guideline: Allergic rhinitis. Otolaryngol. Head Neck Surg. 2015, 152 (Suppl. S1), S1–S43. [Google Scholar] [CrossRef]
- Weaver, J.M.J.; Ross-Innes, C.S.; Shannon, N.; Lynch, A.G.; Forshew, T.; Barbera, M.; Murtaza, M.; Ong, C.J.; Lao-Sirieix, P.; Dunning, M.J.; et al. Ordering of mutations in preinvasive disease stages of esophageal carcinogenesis. Nat. Genet. 2014, 46, 837–843. [Google Scholar] [CrossRef]
- Rosenfeld, R.M.; Piccirillo, J.F.; Chandrasekhar, S.S.; Brook, I.; Ashok Kumar, K.; Kramper, M.; Orlandi, R.R.; Palmer, J.N.; Patel, Z.M.; Peters, A.; et al. Clinical practice guideline (update): Adult sinusitis. Otolaryngol Head Neck Surg. 2015, 152 (Suppl. S2), S1–S39. [Google Scholar] [CrossRef]
- Homer, J.J.; Aggarwal, R.; Cordoza, A. Delivery of topical nasal drugs. Am. J. Drug Deliv. 2003, 1, 125–131. [Google Scholar] [CrossRef]
- Kern, R.C.; Decker, J.R. Functional Defense Mechanisms of the Nasal Respiratory Epithelium. In Nasal Physiology and Pathophysiology of Nasal Disorders; Önerci, T.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 27–45. [Google Scholar]
- Inoue, D.; Furubayashi, T.; Ogawara, K.; Kimura, T.; Higaki, K.; Shingaki, T.; Kimura, S.; Tanaka, A.; Katsumi, H.; Sakane, T.; et al. In vitro evaluation of the ciliary beat frequency of the rat nasal epithelium using a high-speed digital imaging system. Biol. Pharm. Bull. 2013, 36, 966–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gizurarson, S. The effect of cilia and the mucociliary clearance on successful drug delivery. Biol. Pharm. Bull. 2015, 38, 497–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lale, A.M.; Mason, J.D.; Jones, N.S. Mucociliary transport and its assessment: A review. Clin. Otolaryngol Allied Sci. 1998, 23, 388–396. [Google Scholar] [CrossRef]
- Rogers, D.F. Physiology of airway mucus secretion and pathophysiology of hypersecretion. Respir. Care 2007, 52, 1134–1146. [Google Scholar]
- Chen, J.; Jing, J.; Su, E.; Badger, C.; Coughlan, C.; Chen, Z.; Wong, B. Measurement of Ciliary Beat Frequency Using Ultra-High. Resolution Optical Coherence Tomography; SPIE: San Francisco, CA, USA, 2016; Volume 9689. [Google Scholar]
- Klocker, N.; Verse, T.; Rudolph, P. The protective effect of dexpanthenol in nasal sprays. First results of cytotoxic and ciliary-toxic studies in vitro. Laryngorhinootologie 2003, 82, 177–182. [Google Scholar]
- Hofmann, T.; Gugatschga, M.; Koidl, B.; Wolf, G. Influence of preservatives and topical steroids on ciliary beat frequency in vitro. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 440–445. [Google Scholar] [CrossRef] [Green Version]
- Merkus, P.; Romeijn, S.G.; Verhoef, J.C.; Merkus, F.W.; Schouwenburg, P.F. Classification of cilio-inhibiting effects of nasal drugs. Laryngoscope 2001, 111, 595–602. [Google Scholar] [CrossRef]
- Dimova, S.; Maes, F.; Brewster, M.E.; Jorissen, M.; Noppe, M.; Augustijns, P. High-speed digital imaging method for ciliary beat frequency measurement. J. Pharm Pharm. 2005, 57, 521–526. [Google Scholar] [CrossRef]
- Palmberger, T.F.; Augustijns, P.; Vetter, A.; Bernkop-Schnürch, A. Safety assessment of thiolated polymers: Effect on ciliary beat frequency in human nasal epithelial cells. Drug Dev. Ind. Pharm. 2011, 37, 1455–1462. [Google Scholar] [CrossRef]
- Vetter, A.; Augustijns, P.; Bernkop-Schnürch, A. Solubilizing agents in nasal formulations and their effect on ciliary beat frequency. Toxicol. Vitr. 2012, 26, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Piqué, N.; De Servi, B. Rhinosectan® spray (containing xyloglucan) on the ciliary function of the nasal respiratory epithelium; results of an in vitro study. Allergy Asthma Clin. Immunol. 2018, 14, 41. [Google Scholar] [CrossRef]
- Kreft, M.E.; Jerman, U.D.; Lasič, E.; Lanišnik Rižner, T.; Hevir-Kene, N.; Peternel, L.; Kristan, K. The characterization of the human nasal epithelial cell line RPMI 2650 under different culture conditions and their optimization for an appropriate in vitro nasal model. Pharm. Res. 2015, 32, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Kreft, M.E.; Tratnjek, L.; Lasič, E.; Hevir, N.; Rižner, T.L.; Kristan, K. Different Culture Conditions Affect Drug Transporter Gene Expression, Ultrastructure, and Permeability of Primary Human Nasal Epithelial Cells. Pharm. Res. 2020, 37, 170. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wiszniewski, L.; Constant, S. The Use of In Vitro 3D Cell Models in Drug Development for Respiratory Diseases. In Drug Discovery and Development—Present and Future; Izet, K., Ed.; InTech: London, UK, 2011. [Google Scholar]
- Mercier, C.; Jacqueroux, E.; He, Z.; Hodin, S.; Constant, S.; Perek, N.; Boudard, D.; Delavenne, X. Pharmacological characterization of the 3D MucilAir™ nasal model. Eur J. Pharm Biopharm 2019, 139, 186–196. [Google Scholar] [CrossRef]
- Ehrhardt, C.; Kim, K.-J. Drug Absorption Studies: In Situ, in Vitro and in Silico Models; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Salade, L.; Wauthoz, N.; Goole, J.; Amighi, K. How to characterize a nasal product. The state of the art of in vitro and ex vivo specific methods. Int. J. Pharm. 2019, 561, 47–65. [Google Scholar]
- Fogel, D.B. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: A review. Contemp. Clin. Trials Commun. 2018, 11, 156–164. [Google Scholar] [CrossRef]
- Klöcker, N.; Rudolph, P.; Verse, T. Evaluation of protective and therapeutic effects of dexpanthenol on nasal decongestants and preservatives: Results of cytotoxic studies in vitro. Am. J. Rhinol. 2004, 18, 315–320. [Google Scholar] [CrossRef]
- Verse, T.; Sikora, C.; Rudolph, P.; Klöcker, N. The tolerability of nasal drugs with special regard to preservatives and physico-chemical parameters. Laryngorhinootologie 2003, 82, 782–789. [Google Scholar] [PubMed]
- Workman, A.D.; Cohen, N.A. The effect of drugs and other compounds on the ciliary beat frequency of human respiratory epithelium. Am. J. Rhinol. Allergy 2014, 28, 454–464. [Google Scholar] [CrossRef]
- Hofmann, T.; Wolf, G.; Koidl, B. Effect of topical corticosteroids and topical antihistaminics on ciliary epithelium of human nasal mucosa in vitro. HNO 1998, 46, 146–151. [Google Scholar] [PubMed]
- Joki, S.; Saano, V.; Nuutinen, J.; Virta, P.; Karttunen, P.; Silvasti, M.; Toskala, E. Effects of Some Preservative Agents on Rat and Guinea Pig Tracheal and Human Nasal Ciliary Beat Frequency. Am. J. Rhinol. 1996, 10, 181–186. [Google Scholar] [CrossRef]
- Riechelmann, H.; Deutschle, T.; Stuhlmiller, A.; Gronau, S.; Bürner, H. Nasal toxicity of benzalkonium chloride. Am. J. Rhinol. 2004, 18, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Tratnjek, L.; Kreft, M.; Kristan, K.; Kreft, M.E. Ciliary beat frequency of in vitro human nasal epithelium measured with the simple high-speed microscopy is applicable for safety studies of nasal drug formulations. Toxicol. Vitr. 2020, 66, 104865. [Google Scholar] [CrossRef] [PubMed]
- Mallants, R.; Jorissen, M.; Augustijns, P. Beneficial effect of antibiotics on ciliary beat frequency of human nasal epithelial cells exposed to bacterial toxins. J. Pharm. Pharm. 2008, 60, 437–443. [Google Scholar] [CrossRef]
- Berg, O.H.; Lie, K.; Steinsvåg, S.K. The effects of topical nasal steroids on rat respiratory mucosa in vivo, with special reference to benzalkonium chloride. Allergy 1997, 52, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Kwun, Y.S.; Jang, H.S.; Kang, J.M.; Won, Y.S.; Yoon, H.R. Long-term use of preservatives on rat nasal respiratory mucosa: Effects of benzalkonium chloride and potassium sorbate. Laryngoscope 2000, 110, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Lebe, E.; Baka, M.; Yavaşoğlu, A.; Aktuğ, H.; Ateş, U.; Uyanikgil, Y. Effects of preservatives in nasal formulations on the mucosal integrity: An electron microscopic study. Pharmacology 2004, 72, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Cüreoğlu, S.; Akkuş, M.; Osma, Ü.; Yaldiz, M.; Oktay, F.; Can, B.; Güven, C.; Tekın, M.; Merıç, F. The effect of benzalkonium chloride on rabbit nasal mucosa in vivo: An electron microscopy study. Eur. Arch. Otorhinolaryngol. 2002, 259, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Kuboyama, Y.; Suzuki, K.; Hara, T. Nasal lesions induced by intranasal administration of benzaikonium chloride in rats. J. Toxicol. Sci. 1997, 22, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Graf, P. Adverse effects of benzalkonium chloride on the nasal mucosa: Allergic rhinitis and rhinitis medicamentosa. Clin. Ther. 1999, 21, 1749–1755. [Google Scholar] [CrossRef]
- Bernstein, I.L. Is the use of benzalkonium chloride as a preservative for nasal formulations a safety concern? A cautionary note based on compromised mucociliary transport. J. Allergy Clin. Immunol. 2000, 105, 39–44. [Google Scholar] [CrossRef]
- Mösges, R.; Shah-Hosseini, K.; Hucke, H.P.; Joisten, M.J. Dexpanthenol: An Overview of its Contribution to Symptom Relief in Acute Rhinitis Treated with Decongestant Nasal Sprays. Adv. Ther. 2017, 34, 1850–1858. [Google Scholar] [CrossRef] [Green Version]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, J.; Meng, N.; Zhang, L. The effect of topical corticosteroids, topical antihistamines, and preservatives on human ciliary beat frequency. ORL J. Otorhinolaryngol. Relat. Spec. 2014, 76, 127–136. [Google Scholar] [CrossRef]
- Djupesland, P.G. Nasal drug delivery devices: Characteristics and performance in a clinical perspective-a review. Drug Deliv. Transl. Res. 2013, 3, 42–62. [Google Scholar] [CrossRef] [Green Version]
- Iskandar, A.R.; Mathis, C.; Martin, F.; Leroy, P.; Sewer, A.; Majeed, S.; Kuehn, D.; Trivedi, K.; Grandolfo, D.; Cabanski, M.; et al. 3-D nasal cultures: Systems toxicological assessment of a candidate modified-risk tobacco product. ALTEX 2017, 34, 23–48. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Wiszniewski, L.; Constant, S.; Roggen, E. Potential of in vitro reconstituted 3D human airway epithelia (MucilAir™) to assess respiratory sensitizers. Toxicol. Vitr. 2013, 27, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, A.R.; Martin, F.; Talikka, M.; Schlage, W.K.; Kostadinova, R.; Mathis, C.; Hoeng, J.; Peitsch, M.C. Systems approaches evaluating the perturbation of xenobiotic metabolism in response to cigarette smoke exposure in nasal and bronchial tissues. Biomed. Res. Int. 2013, 2013, 512086. [Google Scholar] [CrossRef]
- Talikka, M.; Kostadinova, R.; Xiang, Y.; Mathis, C.; Sewer, A.; Majeed, S.; Kuehn, D.; Frentzel, S.; Merg, C.; Geertz, M.; et al. The response of human nasal and bronchial organotypic tissue cultures to repeated whole cigarette smoke exposure. Int. J. Toxicol. 2014, 33, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Czekala, L.; Wieczorek, R.; Simms, L.; Yu, F.; Budde, J.; Trelles Sticken, E.; Rudd, K.; Verron, T.; Brinster, O.; Stevenson, M.; et al. Multi-endpoint analysis of human 3D airway epithelium following repeated exposure to whole electronic vapor product aerosol or cigarette smoke. Curr. Res. Toxicol. 2021, 2, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Rossner, P., Jr.; Cervena, T.; Vojtisek-Lom, M.; Vrbova, K.; Ambroz, A.; Novakova, Z.; Elzeinova, F.; Margaryan, H.; Beranek, V.; Pechout, M.; et al. The Biological Effects of Complete Gasoline Engine Emissions Exposure in a 3D Human Airway Model (MucilAir(TM)) and in Human Bronchial Epithelial Cells (BEAS-2B). Int. J. Mol. Sci. 2019, 20, 5710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constant, S.; Wiszniewski, L.; Huang, S. The use of in vitro 3D cell model of human airway epithelia (MucilAir™) in inhalation toxicity. In Cellular In Vitro Testing: Methods and Protocols; Haycock, J., Ahluwalia, A., Malcol, J., Eds.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Huang, S.; Constant, S.; De Servi, B.; Meloni, M.; Culig, J.; Bertini, M.; Saaid, A. In vitro safety and performance evaluation of a seawater solution enriched with copper, hyaluronic acid, and eucalyptus for nasal lavage. Med. Devices 2019, 12, 399–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balogh Sivars, K.; Sivars, U.; Hornberg, E.; Zhang, H.; Brändén, L.; Bonfante, R.; Huang, S.; Constant, S.; Robinson, I.; Betts, C.J.; et al. A 3D Human Airway Model Enables Prediction of Respiratory Toxicity of Inhaled Drugs In Vitro. Toxicol. Sci. 2018, 162, 301–308. [Google Scholar] [CrossRef]
- Marttin, E.; Schipper, N.G.M.; Verhoef, J.C.; Merkus, F.W.H.M. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv. Drug Deliv. Rev. 1998, 29, 13–38. [Google Scholar] [CrossRef]
- Sun, S.S.; Hsieh, J.F.; Tsai, S.C.; Ho, Y.J.; Kao, C.H. Evaluation of nasal mucociliary clearance function in allergic rhinitis patients with technetium 99m-labeled macroaggregated albumin rhinoscintigraphy. Ann. Otol. Rhinol. Laryngol. 2002, 111, 77–79. [Google Scholar] [CrossRef]
- Antunes, M.B.; Gudis, D.A.; Cohen, N.A. Epithelium, cilia, and mucus: Their importance in chronic rhinosinusitis. Immunol. Allergy Clin. North. Am. 2009, 29, 631–643. [Google Scholar] [CrossRef]
- Ingels, K.J.; Kortmann, M.J.; Nijziel, M.R.; Graamans, K.; Huizing, E.H. Factors influencing ciliary beat measurements. Rhinology 1991, 29, 17–26. [Google Scholar] [PubMed]
- Clary-Meinesz, C.F.; Cosson, J.; Huitorel, P.; Blaive, B. Temperature effect on the ciliary beat frequency of human nasal and tracheal ciliated cells. Biol. Cell 1992, 76, 335–338. [Google Scholar] [CrossRef]
- Salathe, M. Regulation of mammalian ciliary beating. Annu. Rev. Physiol. 2007, 69, 401–422. [Google Scholar] [CrossRef] [PubMed]
- Small, C.B.; Hernandez, J.; Reyes, A.; Schenkel, E.; Damiano, A.; Stryszak, P.; Staudinger, H.; Danzig, M. Efficacy and safety of mometasone furoate nasal spray in nasal polyposis. J. Allergy Clin. Immunol. 2005, 116, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Kuna, P.; Wasiak, W.; Jones, S.; Kreft, K.Z. Comparative safety and efficacy of two formulations of mometasone nasal spray in adult seasonal allergic rhinitis. Allergy Asthma Proc. 2014, 35, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, E.O.; Jalowayski, A.A.; Orgel, H.A.; Harris, A.G. Subjective and objective assessments in patients with seasonal allergic rhinitis: Effects of therapy with mometasone furoate nasal spray. J. Allergy Clin. Immunol 1998, 102, 39–49. [Google Scholar] [CrossRef]
- Zitt, M.; Kosoglou, T.; Hubbell, J. Mometasone furoate nasal spray: A review of safety and systemic effects. Drug Saf. 2007, 30, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Naclerio, R.M.; Baroody, F.M.; Bidani, N.; De Tineo, M.; Penney, B.C. A comparison of nasal clearance after treatment of perennial allergic rhinitis with budesonide and mometasone. Otolaryngol. Head Neck Surg. 2003, 128, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Pata, Y.S.; Akbaş, Y.; Unal, M.; Görür, K.; Ozcan, C.; Vayisoğlu, Y. The effect of mometasone furoate on mucociliary clearance in patients with perennial allergic rhinitis. Kulak Burun Bogaz Ihtis. Derg. 2003, 11, 97–99. [Google Scholar] [PubMed]
- Minshall, E.; Ghaffar, O.; Cameron, L.; O’Brien, F.; Quinn, H.; Rowe-Jones, J.; Davies, R.J.; Prior, A.; Lund, V.J.; Mackay, I.S.; et al. Assessment by nasal biopsy of long-term use of mometasone furoate aqueous nasal spray (Nasonex) in the treatment of perennial rhinitis. Otolaryngol. Head Neck Surg. 1998, 118, 648–654. [Google Scholar]
- Ho, C.Y.; Wu, M.C.; Lan, M.Y.; Tan, C.T.; Yang, A.H. In vitro effects of preservatives in nasal sprays on human nasal epithelial cells. Am. J. Rhinol. 2008, 22, 125–129. [Google Scholar] [CrossRef]
- Berg, O.H.; Henriksen, R.N.; Steinsvåg, S.K. The effect of a benzalkonium chloride-containing nasal spray on human respiratory mucosa in vitro as a function of concentration and time of action. Pharm. Toxicol. 1995, 76, 245–249. [Google Scholar] [CrossRef]
- Richards, D.H. Preservation of nasal sprays. J. Allergy Clin. Immunol. 2000, 106, 595–596. [Google Scholar] [CrossRef]
- Hauptman, G.; Ryan, M.W. The effect of saline solutions on nasal patency and mucociliary clearance in rhinosinusitis patients. Otolaryngol. Head Neck Surg. 2007, 137, 815–821. [Google Scholar] [CrossRef]
- Ural, A.; Oktemer, T.K.; Kizil, Y.; Ileri, F.; Uslu, S. Impact of isotonic and hypertonic saline solutions on mucociliary activity in various nasal pathologies: Clinical study. J. Laryngol. Otol. 2009, 123, 517–521. [Google Scholar] [CrossRef]
- Unal, M.; Görür, K.; Ozcan, C. Ringer-Lactate solution versus isotonic saline solution on mucociliary function after nasal septal surgery. J. Laryngol. Otol. 2001, 115, 796–797. [Google Scholar] [CrossRef]
- Bonnomet, A.; Luczka, E.; Coraux, C.; de Gabory, L. Non-diluted seawater enhances nasal ciliary beat frequency and wound repair speed compared to diluted seawater and normal saline. Int. Forum. Allergy Rhinol. 2016, 6, 1062–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, P.; Johnson, P.; Ramaswamy, P.; Ramadoss, S.; Geetha, B.; Subhashini, A. The effect of ageing on nasal mucociliary clearance in women: A pilot study. Int. Sch. Res. Not. 2013, 2013, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Koparal, M.; Kurt, E.; Altuntas, E.E.; Dogan, F. Assessment of mucociliary clearance as an indicator of nasal function in patients with COVID-19: A cross-sectional study. Eur Arch. Otorhinolaryngol. 2021, 278, 1863–1868. [Google Scholar] [CrossRef]
- Deborah, S.; Prathibha, K. Measurement of nasal mucociliary clearance. Clin. Res. Pulmonol. 2014, 2, 1019. [Google Scholar]
- Sakakura, Y.; Majima, Y.; Harada, T.; Hattori, M.; Ukai, K. Nasal Mucociliary Transport of Chronic Sinusitis in Children. Arch. Otolaryngol. Head Neck Surg. 1992, 118, 1234–1237. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tratnjek, L.; Sibinovska, N.; Kristan, K.; Kreft, M.E. In Vitro Ciliotoxicity and Cytotoxicity Testing of Repeated Chronic Exposure to Topical Nasal Formulations for Safety Studies. Pharmaceutics 2021, 13, 1750. https://doi.org/10.3390/pharmaceutics13111750
Tratnjek L, Sibinovska N, Kristan K, Kreft ME. In Vitro Ciliotoxicity and Cytotoxicity Testing of Repeated Chronic Exposure to Topical Nasal Formulations for Safety Studies. Pharmaceutics. 2021; 13(11):1750. https://doi.org/10.3390/pharmaceutics13111750
Chicago/Turabian StyleTratnjek, Larisa, Nadica Sibinovska, Katja Kristan, and Mateja Erdani Kreft. 2021. "In Vitro Ciliotoxicity and Cytotoxicity Testing of Repeated Chronic Exposure to Topical Nasal Formulations for Safety Studies" Pharmaceutics 13, no. 11: 1750. https://doi.org/10.3390/pharmaceutics13111750
APA StyleTratnjek, L., Sibinovska, N., Kristan, K., & Kreft, M. E. (2021). In Vitro Ciliotoxicity and Cytotoxicity Testing of Repeated Chronic Exposure to Topical Nasal Formulations for Safety Studies. Pharmaceutics, 13(11), 1750. https://doi.org/10.3390/pharmaceutics13111750





