Selective CNS Targeting and Distribution with a Refined Region-Specific Intranasal Delivery Technique via the Olfactory Mucosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Manufacturing of the Antibodies 11C7, P3X (Isotype Control) and 11C7 scFv
2.2. Animal Housing and Ethics
2.3. Study 1: Refined Region-Specific Catheter-Based Intranasal Administration of Sodium Fluorescein
2.4. Study 2: Region-Specific Administration of Insulin Detemir (Levemir®)
2.5. Study 3: CNS Distribution of Monoclonal Antibodies and a scFv after Region-Specific Administration
2.6. Staining Procedures and Microscopy
2.7. Statistics
3. Results
3.1. Refinement of Intranasal Delivery and Establishing a Region-Specific Administration at the Olfactory Region (Study 1)
3.2. Peripheral Bioactivity after Region-Specific Intranasal Administration of Insulin Detemir (Study 2)
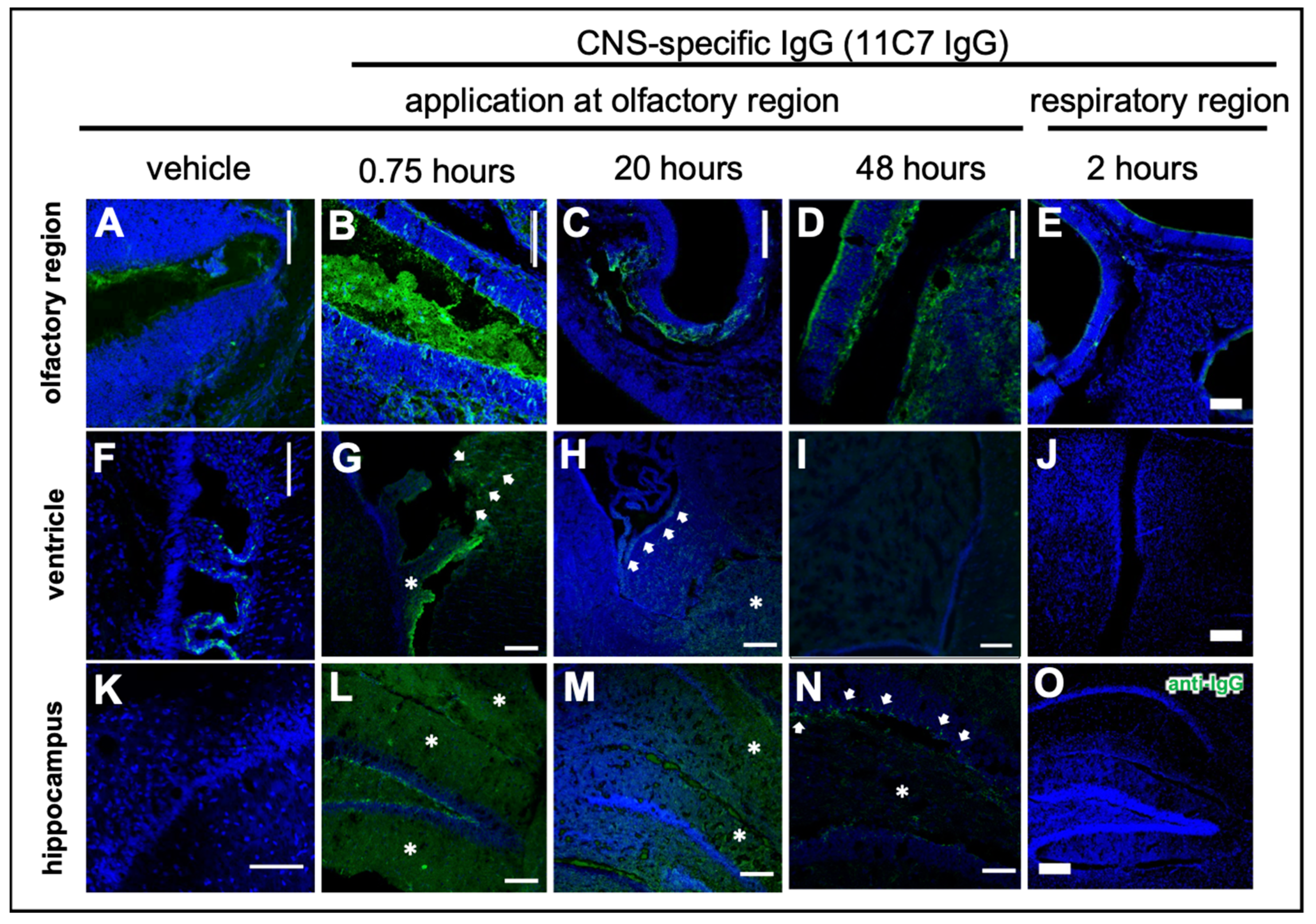
3.3. Antibody CNS Distribution after Region-Specific Intranasal Administration (Study 3)
3.4. Fc-Dependent Uptake into Olfactory Mucosa and Transport along Neuronal Bundles to the CNS
3.5. Reduced Elimination from CNS of Nogo-A-Binding IgG
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Xiong, G.; Tsang, W.C.; Schätzlein, A.G.; Uchegbu, I.F. Nose-to-brain delivery. J. Pharmacol. Exp. Ther. 2019, 370, 593–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Chan, H.F.; Leong, K.W. Advanced materials and processing for drug delivery: The past and the future. Adv. Drug Deliv. Rev. 2013, 65, 104–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuwelt, E.; Abbott, N.J.; Abrey, L.; Banks, W.A.; Blakley, B.; Davis, T.; Engelhardt, B.; Grammas, P.; Nedergaard, M.; Nutt, J. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008, 7, 84–96. [Google Scholar] [CrossRef]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef]
- Illum, L. Transport of drugs from the nasal cavity to the central nervous system. Eur. J. Pharm. Sci. 2000, 11, 1–18. [Google Scholar] [CrossRef]
- Gänger, S.; Schindowski, K. Tailoring Formulations for Intranasal Nose-to-Brain Delivery: A Review on Architecture, Physico-Chemical Characteristics and Mucociliary Clearance of the Nasal Olfactory Mucosa. Pharmaceutics 2018, 10, 116. [Google Scholar] [CrossRef] [Green Version]
- Keller, L.A.; Merkel, O.; Popp, A. Intranasal drug delivery: Opportunities and toxicologic challenges during drug development. Drug Deliv. Transl. Res. 2021, 1–23. [Google Scholar] [CrossRef]
- Thorne, R.G.; Emory, C.R.; Ala, T.A.; Frey, W.H., II. Quantitative analysis of the olfactory pathway for drug delivery to the brain. Brain Res. 1995, 692, 278–282. [Google Scholar] [CrossRef]
- Doty, R.L. Handbook of Olfaction and Gustation, 3rd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015; ISBN 9781118139226. [Google Scholar]
- Pabst, R. Mucosal vaccination by the intranasal route. Nose-associated lymphoid tissue (NALT)—Structure, function and species differences. Vaccine 2015, 33, 4406–4413. [Google Scholar] [CrossRef]
- Costantino, H.R.; Illum, L.; Brandt, G.; Johnson, P.H.; Quay, S.C. Intranasal delivery: Physicochemical and therapeutic aspects. Int. J. Pharm. 2007, 337, 1–24. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Schipper, N.G.M.; Verhoef, J.C.; Merkus, F.W.H.M. The Nasal Mucociliary Clearance: Relevance to Nasal Drug Delivery. Pharm. Res. An Off. J. Am. Assoc. Pharm. Sci. 1991, 8, 807–814. [Google Scholar]
- Hüttenbrink, K.-B.; Wrede, H.; Lagemann, S.; Schleicher, E.; Hummel, T. Endonasal measurement of mucociliary clearance at various locations in the nose: A new diagnostic tool for nasal function? Laryngorhinootologie 2006, 85, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L. Handbook of Olfaction and Gustation, 2nd ed.; Marcel Dekker: New York, NY, USA, 2003; ISBN 0824707192. [Google Scholar]
- Mori, E.; Merkonidis, C.; Cuevas, M.; Gudziol, V.; Matsuwaki, Y.; Hummel, T. The administration of nasal drops in the ‘Kaiteki’ position allows for delivery of the drug to the olfactory cleft: A pilot study in healthy subjects. Eur. Arch. Otorhinolaryngol. 2015, 273, 939–943. [Google Scholar] [CrossRef]
- Harkema, J.R.; Carey, S.; Wagner, J.G. The nose revisited: A brief review of the comparative structure, function, and toxicologic pathology of the nasal epithelium. Toxicol. Pathol. 2006, 34, 252–269. [Google Scholar] [CrossRef]
- Nazareth, L.; Shelper, T.B.; Chacko, A.; Basu, S.; Delbaz, A.; Lee, J.Y.P.; Chen, M.; St John, J.A.; Ekberg, J.A.K. Key differences between olfactory ensheathing cells and Schwann cells regarding phagocytosis of necrotic cells: Implications for transplantation therapies. Sci. Rep. 2020, 10, 18936. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Chen, J.; Qiu, Y.; Yuan, Y.; Zhu, F.; Zhu, Y.; Liu, X.; Pu, Y.; He, C. Olfactory ensheathing cells: The primary innate immunocytes in the olfactory pathway to engulf apoptotic olfactory nerve debris. Glia 2013, 61. [Google Scholar] [CrossRef]
- Carr, V.M.M.; Farbman, A.I. The dynamics of cell death in the olfactory epithelium? Exp. Neurol. 1993, 124. [Google Scholar] [CrossRef]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Wolak, D.J.; Pizzo, M.E.; Thorne, R.G. Rapid transport within cerebral perivascular spaces underlies widespread tracer distribution in the brain after intranasal administration. J. Cereb. Blood Flow Metab. 2015, 35, 371–381. [Google Scholar] [CrossRef]
- Johnston, M.; Zakharov, A.; Papaiconomou, C.; Salmasi, G.; Armstrong, D. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cereb. Fluid. Res. 2004, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Kozlovskaya, L.; Abou-Kaoud, M.; Stepensky, D. Quantitative analysis of drug delivery to the brain via nasal route. J. Control. Release 2014, 189, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Flamm, J.; Boscher, M.; Maigler, F.; Akana, C.; Lindemann, J.; Kleiner, S.; Sommer, F.; Schindowski, K. Standardized refined intranasal administration for region-specific intranasal drug deposition in mice established with 3D rapid prototypes under 3R criteria. Berl. Münch. Tierärztl. Wochenschc. 2018, 131, 408–416. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Kellohen, K.L.; Ronaldson, P.T.; Davis, T.P. Distribution of insulin in trigeminal nerve and brain after intranasal administration. Sci. Rep. 2019, 9, 2621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Röhm, M.; Carle, S.; Maigler, F.; Flamm, J.; Kramer, V.; Mavoungou, C.; Schmid, O.; Schindowski, K. A comprehensive screening platform for aerosolizable protein formulations for intranasal and pulmonary drug delivery. Int. J. Pharm. 2017, 532, 537–546. [Google Scholar] [CrossRef]
- Schindelin, J.; Arg, I.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Kontermann, R.E. Half-life extended biotherapeutics. Expert Opin. Biol. Ther. 2016, 16, 903–915. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood-Brain Barrier and Delivery of Protein and Gene Therapeutics to Brain. Front. Aging Neurosci. 2020, 11, 373. [Google Scholar] [CrossRef]
- Richard, M.; Giannetti, N.; Saucier, D.; Sacquet, J.; Jourdan, F.; Pellier-Monnin, V. Neuronal expression of Nogo-A mRNA and protein during neurite outgrowth in the developing rat olfactory system. Eur. J. Neurosci. 2005, 22, 2145–2158. [Google Scholar] [CrossRef]
- Iketani, M.; Yokoyama, T.; Kurihara, Y.; Strittmatter, S.M.; Goshima, Y.; Kawahara, N.; Takei, K. Axonal branching in lateral olfactory tract is promoted by Nogo signaling. Sci. Rep. 2016, 6, 39586. [Google Scholar] [CrossRef] [Green Version]
- Wahl, A.S.; Correa, D.; Imobersteg, S.; Maurer, M.A.; Kaiser, J.; Augath, M.A.; Schwab, M.E. Targeting Therapeutic Antibodies to the CNS: A Comparative Study of Intrathecal, Intravenous, and Subcutaneous Anti-Nogo A Antibody Treatment after Stroke in Rats. Neurotherapeutics 2020, 17, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Sartori, A.M.; Hofer, A.S.; Schwab, M.E. Recovery after spinal cord injury is enhanced by anti-Nogo-A antibody therapy—From animal models to clinical trials. Curr. Opin. Physiol. 2020, 14, 1–6. [Google Scholar] [CrossRef]
- Schwab, M.E. Functions of Nogo proteins and their receptors in the nervous system. Nat. Rev. Neurosci. 2010, 11, 799–811. [Google Scholar] [CrossRef]
- Schwab, M.E.; Strittmatter, S.M. Nogo limits neural plasticity and recovery from injury. Curr. Opin. Neurobiol. 2014, 27, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Kempf, A.; Schwab, M.E. Nogo-A represses anatomical and synaptic plasticity in the central nervous system. Physiology 2013, 28, 151–163. [Google Scholar] [CrossRef] [Green Version]
- Ladel, S.; Flamm, J.; Zadeh, A.S.; Filzwieser, D.; Walter, J.-C.; Schlossbauer, P.; Kinscherf, R.; Lischka, K.; Luksch, H.; Schindowski, K. Allogenic Fc Domain-Facilitated Uptake of IgG in Nasal Lamina Propria: Friend or Foe for Intranasal CNS Delivery? Pharmaceutics 2018, 10, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ladel, S.; Maigler, F.; Flamm, J.; Schlossbauer, P.; Handl, A.; Hermann, R.; Herzog, H.; Hummel, T.; Mizaikoff, B.; Schindowski, K. Impact of glycosylation and species origin on the uptake and permeation of IgGs through the nasal airway mucosa. Pharmaceutics 2020, 12, 1014. [Google Scholar] [CrossRef] [PubMed]
- Pyzik, M.; Sand, K.M.K.; Hubbard, J.J.; Andersen, J.T.; Sandlie, I.; Blumberg, R.S. The neonatal Fc Receptor (FcRn): A misnomer? Front. Immunol. 2019, 10, 1540. [Google Scholar] [CrossRef]
- Ruano-Salguero, J.S.; Lee, K.H. Antibody transcytosis across brain endothelial-like cells occurs nonspecifically and independent of FcRn. Sci. Rep. 2020, 10, 3685. [Google Scholar] [CrossRef] [Green Version]
- Schlachetzki, F.; Zhu, C.; Pardridge, W.M. Expression of the neonatal Fc receptor (FcRn) at the blood-brain barrier. J. Neurochem. 2002, 81, 203–206. [Google Scholar] [CrossRef]
- Gil, V.; Nicolas, O.; Mingorance, A.; Ureña, J.M.; Tang, B.L.; Hirata, T.; Sáez-Valero, J.; Ferrer, I.; Soriano, E.; Del Río, J.A. Nogo-A expression in the human hippocampus in normal aging and in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2006, 65, 433–444. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.N.; Lochhead, J.J.; Pizzo, M.E.; Nehra, G.; Boroumand, S.; Greene, G.; Thorne, R.G. Delivery of immunoglobulin G antibodies to the rat nervous system following intranasal administration: Distribution, dose-response, and mechanisms of delivery. J. Control. Release 2018, 286, 467–484. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.R.; Ciambrone, G.J.; Kliwinski, C.M.; Maze, E.; Johnson, L.; Li, Q.; Feng, Y.; Hornby, P.J. Efflux of monoclonal antibodies from rat brain by neonatal Fc receptor, FcRn. Brain Res. 2013, 1534, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Boroumand, S.; Kumar, N.; Lochhead, J.; Pizzo, M.; Nehra, G.; Thorne, R.G. Intranasal delivery of antibodies bypasses the blood-brain barrier and results in significantly higher central nervous system levels than systemic administration. FASEB J. 2018, 32, lb617. [Google Scholar] [CrossRef]
- McLean, B.N.; Miller, D.; Thompson, E.J. Oligoclonal banding of IgG in CSF, blood-brain barrier function, and MRI findings in patients with sarcoidosis, systemic lupus erythematosus, and Behcet’s disease involving the nervous system. J. Neurol. Neurosurg. Psychiatry 1995, 58, 548–554. [Google Scholar] [CrossRef] [Green Version]
- Link, H.; Tibbling, G. Principles of albumin and igg analyses in neurological disorders. III. Evaluation of igg synthesis within the central nervous system in multiple sclerosis. Scand. J. Clin. Lab. Investig. 1977, 37, 397–401. [Google Scholar] [CrossRef]
- Lefvert, A.K.; Link, H. IgG production within the central nervous system: A critical review of proposed formulae. Ann. Neurol. 1985, 17, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Inoue, D.; Furubayashi, T.; Tanaka, A.; Sakane, T.; Sugano, K. Effect of Cerebrospinal Fluid Circulation on Nose-to-Brain Direct Delivery and Distribution of Caffeine in Rats. Mol. Pharm. 2020, 17, 4067–4076. [Google Scholar] [CrossRef]
- Sun, B.L.; Wang, L.H.; Yang, T.; Sun, J.Y.; Mao, L.L.; Yang, M.F.; Yuan, H.; Colvin, R.A.; Yang, X.Y. Lymphatic drainage system of the brain: A novel target for intervention of neurological diseases. Prog. Neurobiol. 2018, 163–164, 118–143. [Google Scholar] [CrossRef]
- Shetty, A.K.; Zanirati, G. The interstitial system of the brain in health and disease. Aging Dis. 2020, 11, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, J.K.S.; Arun, P.; Chembukave, B.; Appu, A.P.; Vijayakumar, N.; Moffett, J.R.; Puthillathu, N.; Namboodiri, A.M.A. Effect of administration method, animal weight and age on the intranasal delivery of drugs to the brain. J. Neurosci. Methods 2017, 286, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Stützle, M.; Flamm, J.; Carle, S.; Schindowski, K. Nose-to-Brain delivery of insulin for Alzheimer’s disease. ADMET DMPK 2015, 3, 190–202. [Google Scholar] [CrossRef] [Green Version]
- Thorne, R.G.; Pronk, G.J.; Padmanabhan, V.; Frey, W.H. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 2004, 127, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.J.; Martinez, J.A.; Liu, W.Q.; Xu, K.; Ayer, A.; Fine, J.; Tuor, U.I.; Glazner, G.; Hanson, L.R.; Frey, W.H., 2nd; et al. Intranasal insulin prevents cognitive decline, cerebral atrophy and white matter changes in murine type I diabetic encephalopathy. Brain 2008, 131, 3311–3334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedict, C.; Hallschmid, M.; Hatke, A.; Schultes, B.; Fehm, H.L.; Born, J.; Kern, W. Intranasal insulin improves memory in humans. Psychoneuroendocrinology 2004, 29, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, E.; Rudich, A.; Harman-Boehm, I.; Cukierman-Yaffe, T. Effect of intranasal insulin on cognitive function: A systematic review. J. Clin. Endocrinol. Metab. 2012, 97, 366–376. [Google Scholar] [CrossRef] [Green Version]
- Plum, L.; Schubert, M.; Bruning, J.C. The role of insulin receptor signaling in the brain. Trends Endocrinol. Metab. 2005, 16, 59–65. [Google Scholar] [CrossRef]
- Craft, S.; Watson, G.S. Insulin and neurodegenerative disease: Shared and specific mechanisms. Lancet Neurol. 2004, 3, 169–178. [Google Scholar] [CrossRef]
- Freiherr, J.; Hallschmid, M.; Frey, W.H., 2nd; Brunner, Y.F.; Chapman, C.D.; Holscher, C.; Craft, S.; De Felice, F.G.; Benedict, C. Intranasal insulin as a treatment for Alzheimer’s disease: A review of basic research and clinical evidence. CNS Drugs 2013, 27, 505–514. [Google Scholar] [CrossRef] [Green Version]
- Craft, S. The role of metabolic disorders in Alzheimer disease and vascular dementia: Two roads converged. Arch. Neurol. 2009, 66, 300–305. [Google Scholar] [CrossRef] [Green Version]
- Hong, M.; Lee, V.M. Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J. Biol. Chem. 1997, 272, 19547–19553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marks, D.R.; Tucker, K.; Cavallin, M.A.; Mast, T.G.; Fadool, D.A. Awake intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors. J. Neurosci. 2009, 29, 6734–6751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Born, J.; Lange, T.; Kern, W.; McGregor, G.P.; Bickel, U.; Fehm, H.L. Sniffing neuropeptides: A transnasal approach to the human brain. Nat. Neurosci. 2002, 5, 514–516. [Google Scholar] [CrossRef]
- Bloomberg CPEX Pharmaceuticals Announces Preliminary Results from Its Phase 2a Clinical Trial of Nasulin. Available online: http://www.bloomberg.com/apps/news?pid=newsarchive&sid=aF8dtG4W0xT4 (accessed on 20 September 2021).
- Claxton, A.; Baker, L.D.; Hanson, A.; Trittschuh, E.H.; Cholerton, B.; Morgan, A.; Callaghan, M.; Arbuckle, M.; Behl, C.; Craft, S. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. J. Alzheimers Dis. 2015, 44, 897–906. [Google Scholar] [CrossRef] [Green Version]
- Craft, S.; Claxton, A.; Baker, L.; Cholerton, B.; Hanson, A.; Callaghan, M.; Trittschuh, E.; Arbuckle, M. Therapeutic effects of long-acting intranasal insulin detemir for Alzheimer’s dementia or mild cognitive impairment. Alzheimer’s Dement. 2013, 9, 139–140. [Google Scholar] [CrossRef]
- Craft, S.; Baker, L.D.; Montine, T.J.; Minoshima, S.; Watson, G.S.; Claxton, A.; Arbuckle, M.; Callaghan, M.; Tsai, E.; Plymate, S.R.; et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: A pilot clinical trial. Arch. Neurol. 2012, 69, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, Z.; Jani, P.K.; Szikora, B.; Végh, A.; Kövesdi, D.; Iliás, A.; Cervenak, J.; Balogh, P.; Kurucz, I.; Kacskovics, I. Overexpression of bovine FcRn in mice enhances T-dependent immune responses by amplifying T helper cell frequency and germinal center enlargement in the spleen. Front. Immunol. 2015, 6, 357. [Google Scholar] [CrossRef]
- Stapleton, N.M.; Brinkhaus, M.; Armour, K.L.; Bentlage, A.E.H.; de Taeye, S.W.; Temming, A.R.; Mok, J.Y.; Brasser, G.; Maas, M.; van Esch, W.J.E.; et al. Reduced FcRn-mediated transcytosis of IgG2 due to a missing Glycine in its lower hinge. Sci. Rep. 2019, 9, 7363. [Google Scholar] [CrossRef]
- Tzaban, S.; Massol, R.H.; Yen, E.; Hamman, W.; Frank, S.R.; Lapierre, L.A.; Hansen, S.H.; Goldenring, J.R.; Blumberg, R.S.; Lencer, W.I. The recycling and transcytotic pathways for IgG transport by FcRn are distinct and display an inherent polarity. J. Cell Biol. 2009, 185, 673–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, L.; Zeng, R.; Bai, Y.; Roopenian, D.C.; Zhu, X. Efficient mucosal vaccination mediated by the neonatal Fc receptor. Nat. Biotechnol. 2011, 29, 158–163. [Google Scholar] [CrossRef] [Green Version]
- Roopenian, D.C.; Akilesh, S. FcRn: The neonatal Fc receptor comes of age. Nat. Rev. Immunol. 2007, 7, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, B.L.; Blumberg, R.S.; Wayne, I.; Invest, J.C.; Dickinson, B.L.; Badizadegan, K.; Wu, Z.; Ahouse, J.C.; Zhu, X.; Simister, N.E.; et al. Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line. J. Clin. Investig. 1999, 104, 903–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maigler, F.; Ladel, S.; Flamm, J.; Gänger, S.; Kurpiers, B.; Kiderlen, S.; Völk, R.; Hamp, C.; Hartung, S.; Spiegel, S.; et al. Selective CNS Targeting and Distribution with a Refined Region-Specific Intranasal Delivery Technique via the Olfactory Mucosa. Pharmaceutics 2021, 13, 1904. https://doi.org/10.3390/pharmaceutics13111904
Maigler F, Ladel S, Flamm J, Gänger S, Kurpiers B, Kiderlen S, Völk R, Hamp C, Hartung S, Spiegel S, et al. Selective CNS Targeting and Distribution with a Refined Region-Specific Intranasal Delivery Technique via the Olfactory Mucosa. Pharmaceutics. 2021; 13(11):1904. https://doi.org/10.3390/pharmaceutics13111904
Chicago/Turabian StyleMaigler, Frank, Simone Ladel, Johannes Flamm, Stella Gänger, Barbara Kurpiers, Stefanie Kiderlen, Ronja Völk, Carmen Hamp, Sunniva Hartung, Sebastian Spiegel, and et al. 2021. "Selective CNS Targeting and Distribution with a Refined Region-Specific Intranasal Delivery Technique via the Olfactory Mucosa" Pharmaceutics 13, no. 11: 1904. https://doi.org/10.3390/pharmaceutics13111904





