Targeting Engineered Nanoparticles for Breast Cancer Therapy
Abstract
1. Introduction
2. Properties of BC Drugs
| Trade Name | Therapeutic BC Drugs | Chemical Structure | References |
|---|---|---|---|
| Nonpolar/Hydrophobic drugs | |||
| Platinol® | Cisplatin | 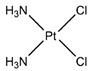 | [22,33] |
| Taxotere | Docetaxel | 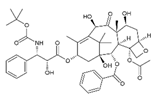 | [21,31,32] |
| Adriamycin® | Doxorubicin | 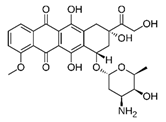 | [26,27,28,29] |
| VP–16 | Etoposide | 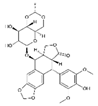 | [34,35] |
| Otrexup™, Rasuvo®, Rheumatrex® andTrexall™ | Methotrexate | 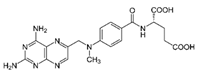 | [36,37] |
| Taxol | Paclitaxel |  | [38,39,40,41] |
| Polar/Hydrophilic drugs | |||
| Avastin | Bevacizumab | Monoclonal antibody | [24,42] |
| Erbitux® | Cetuximab | Monoclonal antibody | [43,44] |
| Cytoxan or Neosar | Cyclophosphamide | 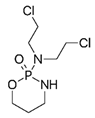 | [23,24,25] |
| Gemzar | Gemcitabine | 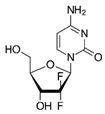 | [38,40] |
| Adrucil® | 5-Fluorouracil | 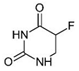 | [33,45] |
| Zevalin | Ibritumomab | 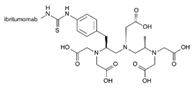 | [46] |
| Elspar | L-asparaginase | Monoclonal antibody | [47,48] |
| Vectibix | Panitumumab | Monoclonal antibody | [49,50] |
| Rituxan | Rituximab | Monoclonal antibody | [51,52] |
| Bexxar | Tositumomab | Monoclonal antibody | [46,53] |
| Herceptin | Trastuzumab | Monoclonal antibody | [25,41] |
| Zolinza | Vorinostat | 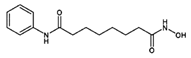 | [54] |
| Highly charged/Neutral drugs | |||
| - | siRNA/miRNA |  | [55,56] |
2.1. Polar/Hydrophilic Drugs
2.2. Non-Polar/Hydrophobic Drugs
2.3. Neutral/Charged Drugs
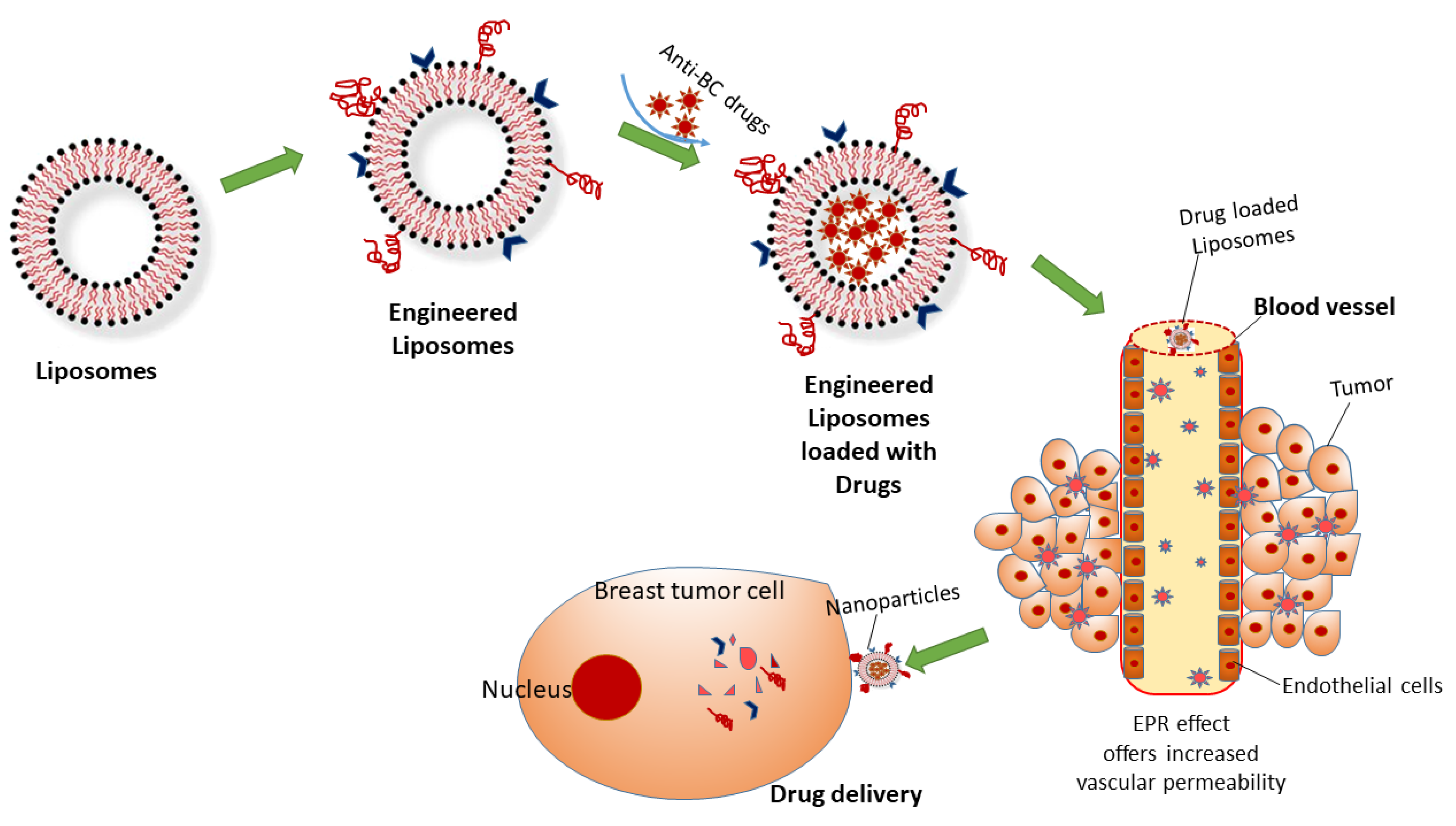
3. NPs for DDS
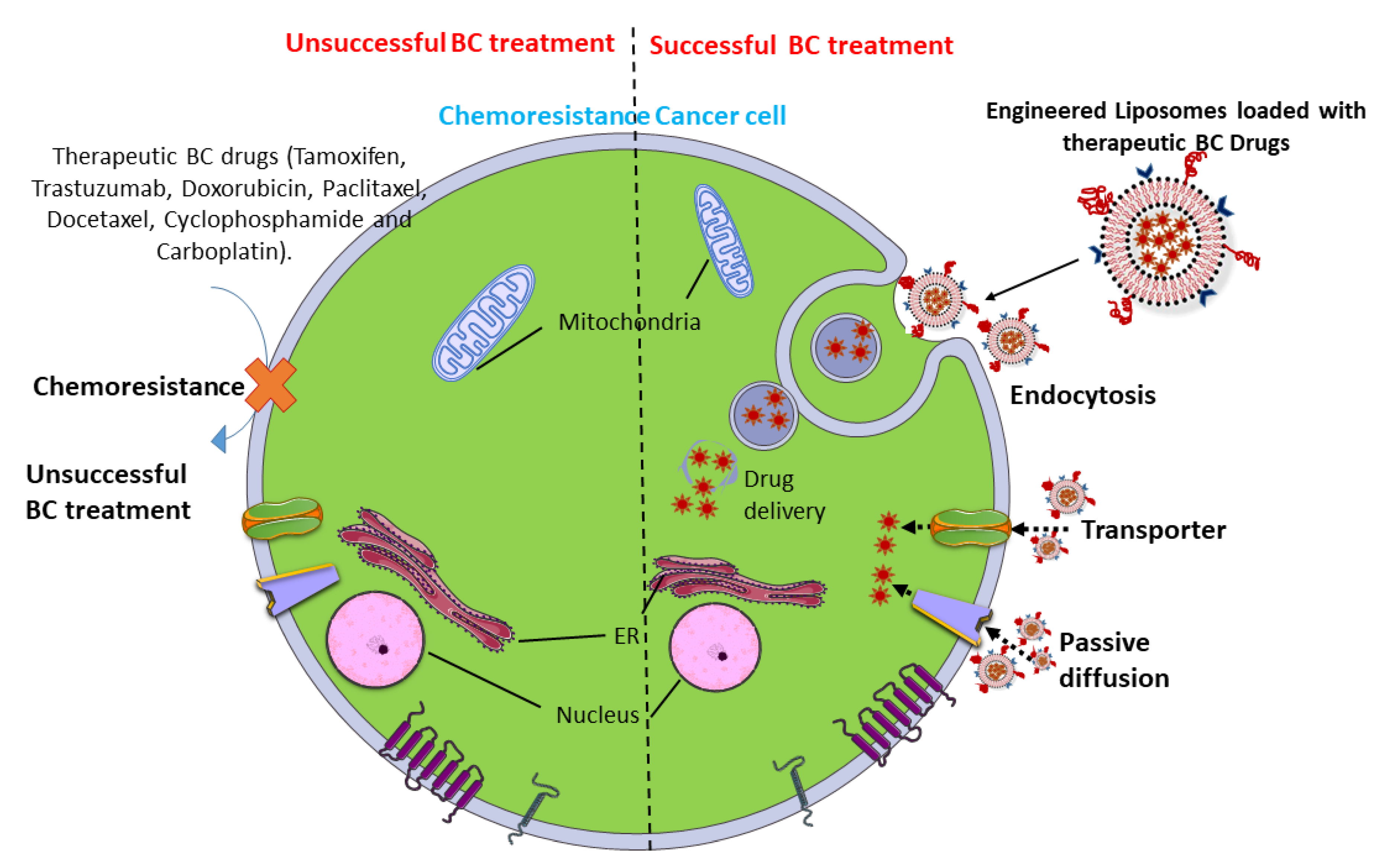
3.1. In Vitro DDS
| Drug | Drug Uptake Pathway | Chemoresistance Mechanisms | Treatment with Nps | References |
|---|---|---|---|---|
| Anthracyclines | Passive diffusion | Doxorubicin-resistant MCF7 cells are more condensed, with low permeability on the plasma membrane. The overexpression of fatty acid synthase limits doxorubicin uptake through the high amount of palmitic acid in MCF7 cells. Statins reduce the lipid content and membrane rigidity | Photosensitizer nanoparticles, polyhydroxybutyrate-coated magnetic nanoparticles, and 3-aminopropoxy-linked quercetin loaded with doxorubicin have synergistic effects on a doxorubicin-resistant MCF-7 cell line | [80,81,82] |
| Transporters | Overexpression of organic cation transporter 6 leads to greater resistance to doxorubicin | The loading of colchicine and coumarin-6 in oil-core carriers protects doxorubicin-resistant BC cells | [83,84] | |
| Endocytosis | Non-specific, adsorptive pinocytosis is increased in BC cell lines which are resistant to doxorubicin | Encapsulation of polymeric prodrug containing hyaluronic acid reduces the resistance to doxorubicin | [85] | |
| Taxanes | Passive diffusion | Extracellular pH triggers a high migratory capacity and chemoresistance to paclitaxel and doxorubicin in MCF7 cells. The addition of cholesterol to a plasma membrane reduces paclitaxel entry into BC cells | Polymer NPs containing poly(γ-glutamic acid)-g-poly(lactic-co-glycolic acid) (γ-PGA-g-PLGA) loaded with doxorubicin and cholesterol-PEG form a type of combination therapy against MDR BC cells | [86] |
| Endocytosis | Down-regulation of Plastin 3 increases the sensitivity of MDA-MB-231 cells to paclitaxel by an endocytosis mechanism | Surfactin loaded with doxorubicin reverses MDR BC cells | [87] | |
| Platinum-based drugs | Passive diffusion | Levels of lipid bilayer constituents such as cholesterol, sphingomyelin, phosphatidylglycerol, and phosphatidylserine are elevated and those of phosphatidylcholine and phosphatidylethanolamines are decreased in cisplatin-resistant BC cells. Based on the membrane molecular dynamics, lipid content and cholesterol levels reduce diffusion and permeability. | Fucoidan and mesoporous platinum NPs and photothermal nanocarriers can be promising drugs for treating MDR BC cells | [88,89] |
3.2. In Vivo DDS
| Therapeutic BC Drug | Nanocarriers | Dose and Duration | Phase of Development | BC Types | References |
|---|---|---|---|---|---|
| Paclitaxel | Albumin-bound NPs | 300 mg/m2 for 3 weeks | Phase II | Metastatic BC | [121] |
| Paclitaxel | Albumin-bound NPs | 100 or 125 mg/m2 for 1 week | Phase II | Metastatic BC | [122] |
| Paclitaxel | Albumin-bound NPs | 260 mg/m2 for 3 weeks | Phase III | Metastatic BC | [117] |
| Paclitaxel | Albumin-bound NPs | 300 mg/m2 for 3 weeks or 100–150 mg/m2 for 1 week | Phase IIb | Metastatic BC | [31] |
| Docetaxel | Albumin-bound NPs | 100 mg/m2 for 1 week | Phase IIb | Metastatic BC | [31] |
| Paclitaxel with cyclophosphamide and trastuzumab | Albumin-bound NPs | 100 mg/m2 for 1, 8, and 15 days | Phase II | HER2-positive BC | [23] |
| Paclitaxel with gemcitabine, and trastuzumab | Albumin-bound NPs | 100 mg/m2 for 1 and 8, every 3 weeks for 6 cycles | Phase II | HER2-positive BC | [38] |
| Paclitaxel withpegfilgrastim | Albumin-bound NPs | 260 mg/m2 for 3 weeks | Phase I | Metastatic BC | [39] |
| Paclitaxel with bevacizumab and gemcitabine | Albumin-bound NPs | 150 mg/m2 on days 1 and 15 of a 28-day cycle | Phase II | HER2-negative metastatic BC | [40] |
| Paclitaxel with or without trastuzumab | Albumin-bound NPs | 125 mg/m2 infusion weekly for 3 of 4 weeks | Phase II | HER2-positive metastatic BC | [41] |
| Paclitaxel with doxorubicin and atezolizumab | Albumin-bound NPs | 125 mg/m2 for 12 weeks | Phase I | TNBC | [27] |
| Paclitaxel with durvalumab | Albumin-bound NPs | 125 mg/m2 for 4 weeks | Phase II | TNBC | [123] |
| Paclitaxel with ipatasertib | Albumin-bound NPs | 80 mg/m2 for 12 weeks | Phase II | TNBC | [124] |
| Paclitaxel with bevacizumab | Albumin-bound NPs | 100 mg/m2 for 28 days | Phase II | TNBC | [20] |
| Paclitaxel with carboplatin and bevacizumab | Albumin-bound NPs | 100 mg/m2 for 28 days | Phase III | TNBC | [20] |
| Paclitaxel with bevacizumab, erlotinib | Albumin-bound NPs | 150 mg/m2 for 21 days | Phase II | TNBC | [20] |
| Paclitaxel with capecitabine | Albumin-bound NPs | 260 mg/m2 for 28 days | Phase II | Locally advanced BC | [20] |
| Paclitaxel with grastuzumab, vinorelbine | Albumin-bound NPs | 80 mg/m2 for 4 weeks | Phase II | Locally advanced, HER2-positive BC | [20] |
| Paclitaxel with carboplatin, bevacizumab, doxorubicin, cyclophosphamide | Albumin-bound NPs | 150 mg/m2 for 4 weeks | Phase II | Locally advanced, HER2-negative BC | [20] |
| Paclitaxel with trastuzumab | Albumin-bound NPs | 100 mg/m2 for 4 weeks | Phase II | Locally advanced, low HER2 BC | [20] |
| Paclitaxel with bevacizumab, doxorubicin, and cyclophosphamide | Albumin-bound NPs | 80 mg/m2 for 4 weeks | Phase II | HER2-negative locally advanced BC or inflammatory BC | [24] |
| Doxorubicin with cyclophosphamide and mangiferin | Gold NPs | 60 mg/m2 for 4 weeks | Phase III | Metastatic BC | [11] |
| Paclitaxel | Liposome | 75 mg/m2 for 21 days | Phase III | Metastatic BC | [125] |
| Paclitaxel with cyclophosphamade | Liposome | 60 mg/m2 for 21 days | Phase III | Metastatic BC | [126] |
| Doxorubicin with cyclophosphamide, paclitaxel, and bevacizumab | Liposome | 30 mg/m2 for 28 days | Phase II | TNBC and ER/PR + BC | [3] |
| Paclitaxel | Micellar NPs | 150 mg/m2 for 21 days | Phase II | Metastatic BC | [127] |
| Paclitaxel | Micellar NPs or albumin-bound NPs | 260 mg/m2 for 3 weeks | Phase II | Metastatic BC | [128] |
| Doxorubicin with carboplatin | Non-PEGylated liposome | 20 mg/mg/m2 infusion twice weekly for 3 weeks | Phase III | TNBC, HER2-positive, luminal B subtypes | [129] |
| Doxorubicin with cisplatin, 5-fluorouracil and trastuzumab | Non-PEGylated liposome | 60 mg/m2 for 21 days | Phase II | ER-positive and HER2-positive BC | [33] |
| Doxorubicin with cyclophosphamide, docetaxel, and trastuzumab | Non-PEGylated liposome | 60 mg/m2 for or 28 days | Phase II | ER-positive and HER2-positive BC | [25] |
| Cytocidal cyclin G1 construct | Pathotropic NPs | 80 mg/m2 for 4 weeks | Phase I/II | Metastatic BC | [130] |
| Doxorubicin | PEGylated liposome | 50 mg/m2 for 4 weeks | Approved | Metastatic BC | [18] |
| Doxorubicin | PEGylated liposome | 25 mg/m2 for 28 days | Phase II | Metastatic BC | [131] |
| Doxorubicin with vinorelbine | PEGylated liposome | 40 mg/m2 for 28 days | Phase II | Metastatic BC | [29] |
| Doxorubicin with gemcitabine | PEGylated liposome | 25 mg/m2 for 3 weeks | Phase III | Metastatic BC | [132] |
| Doxorubicin with capecitabine | PEGylated liposome | 45 mg/m2 for 4 weeks | Phase II | Metastatic BC | [133] |
| Doxorubicin with bevacizumab | PEGylated liposome | 50 mg/m2 for 3 weeks | Phase I | Metastatic TNBC | [42] |
| Doxorubicin | PEGylated liposome | 50 mg/m2 for 4 weeks | Phase II | Metastatic TNBC | [112] |
| Doxorubicin | PEGylated liposome | 25 mg/m2 for 21 days | Phase I-III | Metastatic TNBC | [134] |
| Doxorubicin | PEGylated liposome | 25 mg/m2 for 28 days | Phase I-III | HER2-positive BC | [135] |
| Paclitaxel with doxorubicin | PEGylated liposome | 30 mg/m2 for 21 days | Phase III | Metastatic BC | [109] |
| Doxorubicin with trastuzumab | PEGylated liposome | 40 mg/m2 for 28 days | Phase II | metastatic BC patients with HER2/neu over-expressing BC | [118] |
| Paclitaxel | Polymeric micellar NPs | 300 mg/m2 for 4 weeks | Phase II | Metastatic BC | [136] |
| Paclitaxel | Polymeric micellar NPs | 135–390 mg/m2 for 3 weeks | Phase I | Metastatic BC | [137] |
| Docetaxel | Polymeric NPs | 20–75 mg/m2 for 21 days | Phase I | Metastatic BC | [32] |
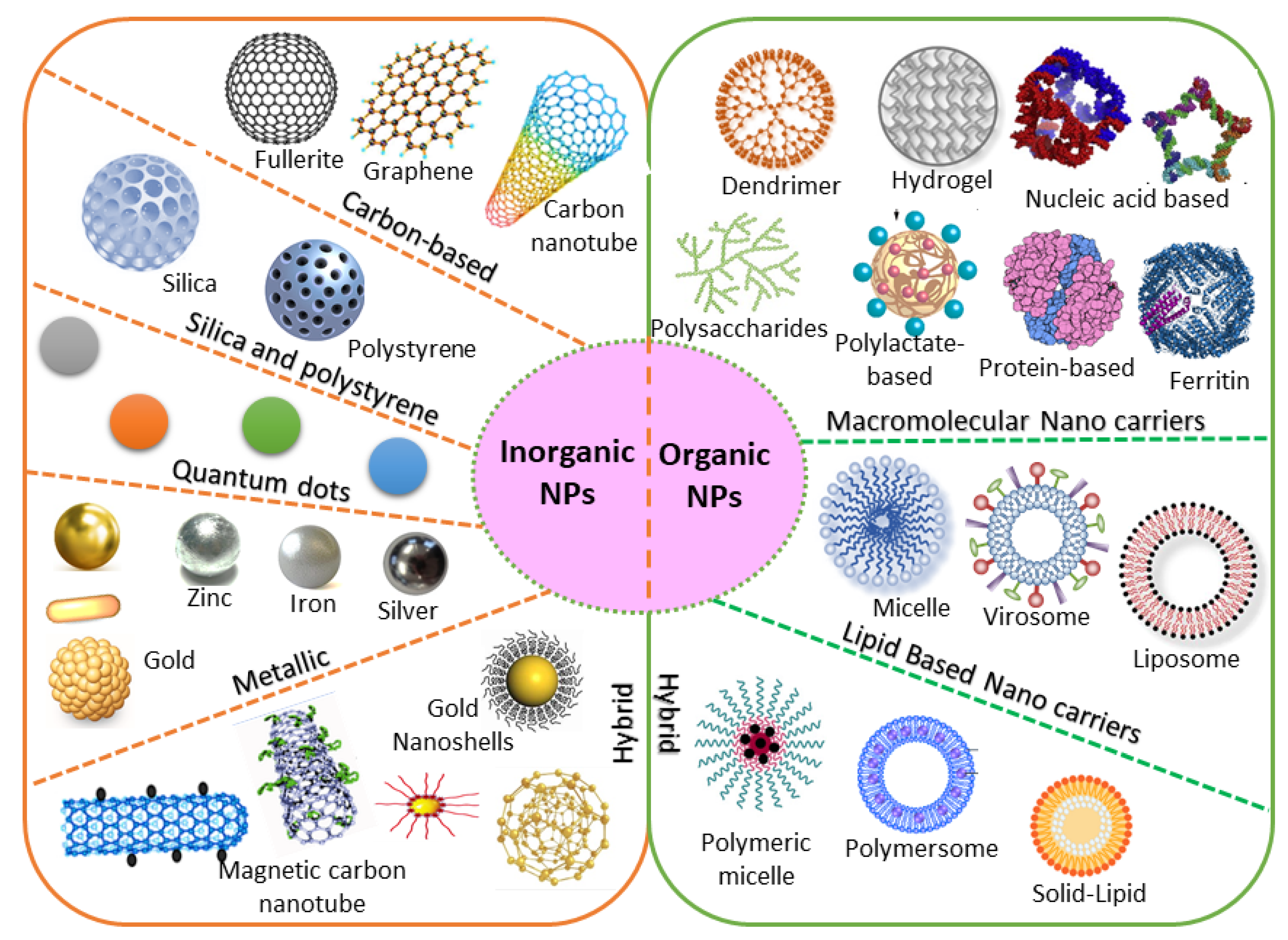
4. Designing of Engineered NP Carriers
4.1. Organic/Inorganic Nanocarriers
4.2. Natural/Synthetic Nanocarriers
| Drug | Nanocarriers | Natural Compound | Size | The Outcome of the Study | BC Types | References |
|---|---|---|---|---|---|---|
| Doxorubicin | Poly-glycerol-malic acid-dodecanedioic acid | Curcumin | ~110–218 nm | Significantly increased cytotoxicity, apoptotic cell death, and cellular intake compared to free drug in MCF-7 and MDA-MB-231 | Luminal BC and TNBC | [164] |
| Doxorubicin | Silver NPs | Andrographolide | ~450 nm | Significantly increased cytotoxicity, apoptotic cell death, and cellular intake compared to free drug in MDA-MB-453 | TNBC | [158] |
| Adriamycin | Silver NPs | Camellia sinensis | ~220 nm | Significantly increased cytotoxicity, apoptotic cell death, and cellular intake compared to free drug in MCF-7 | Luminal BC | [165] |
| Doxorubicin | Folate and chitosan | Ursolic acid | ~420 nm | Anticancer effects in an MCF-7 xenograft mouse model | Luminal BC | [160] |
| Doxorubicin | Lipid carriers (precirol® ATO 5, vitamin E, poloxamer 188, Tween 80) | Sulforaphane/Isothiocyanate | 145 nm | Anticancer effects in an MCF-7 xenograft mouse model | Luminal BC | [159] |
| Doxorubicin | Hydrophobically modified glycol chitosan with 5 beta-cholanic acid | Camptothecin | 280–330 nm | Anticancer effects in an MDA-MB-231 xenograft mousemodel | TNBC | [166] |
| Doxorubicin | Phytosome | Quercetin | ~85 nm | Anticancer effects in an MCF-7 xenograft mouse model | Luminal BC | [161] |
| Doxorubicin | PEGylated liposome | Gambogic acid | ~107 nm | Anticancer effects in an MDA-MB-231 orthotopic xenograft mouse model | TNBC | [167] |
4.3. Geometric Morphometry
4.4. Surface Properties
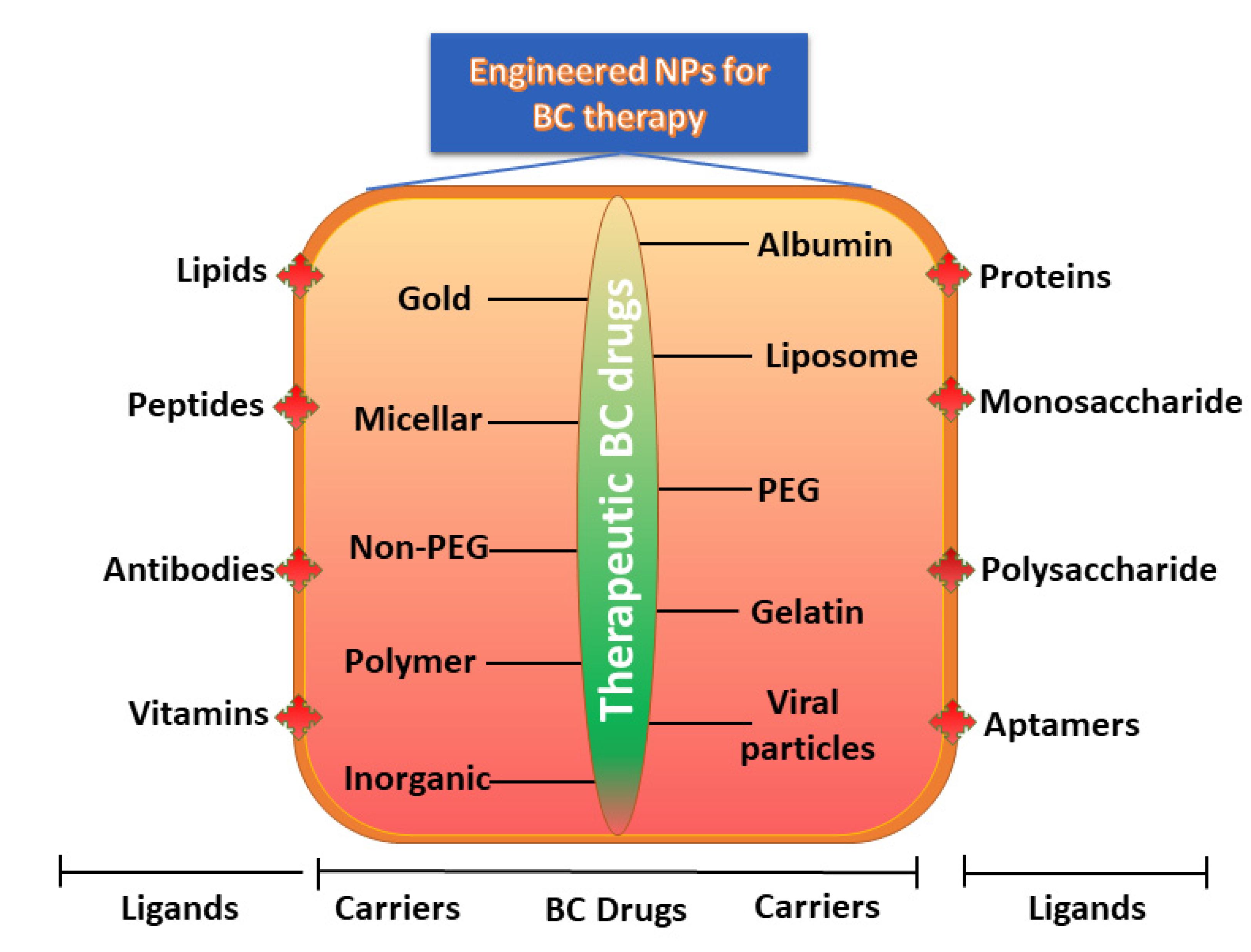
4.5. Ligands
| Type of Nps | Therapeutic BC Drug | Size of the Nps | Ligands Used for Engineering | The Outcome of the Study | BC Types | Reference |
|---|---|---|---|---|---|---|
| Albumin-bound NPs | 2-methoxy-estradiol | ~240 nm | Bovine serum albumin | Significantly enhanced cytotoxicity and cellular uptake when compared with the free drug examined in the SK-BR-3 and MCF-7 cell lines and tumor-bearing mice | HER2 + BC | [182] |
| Chitosan | Doxorubicin | ~50 nm | Anti-HER2 peptide (5–10%) and O-succinyl chitosan graft Pluronic® F127 | Significantly enhanced cytotoxicity and cellular uptake when compared with the free drug in the MCF-7 cell line | HER2 + BC | [187] |
| Iron oxide | siRNA | 130 nm | Caffeic acid, calcium phosphate, iron oxide, PEG-polyanion block copolymer | Significantly enhanced cytotoxicity and cellular uptake when compared with free drug on HCC1954. mRNA expression was decreased by 38% when compared with naked siRNA | HER2 + BC | [188] |
| Iron oxide | Baicalein | 100 nm | PEG-coated iron oxide magnetic NPs | Significantly increased anti-apoptotic activity | TNBC | [189] |
| Liposome | Doxorubicin | ~80 nm | 1,2-Distearoyl-sn-glycero-3-phosphorylethanolamine, Distearoylphosphatidylcholine, HER2pep-K3-palmitic acid conjugate, mPEG2000 | Significantly enhanced cytotoxicity and cellular uptake and reduced systemic toxicity when compared with the free drug in BT-474, SK-BR-3, and MCF-7 cell lines. | HER2 + BC | [178] |
| Liposome | Anti-IL6R antibody, doxorubicin | ~100 nm | 1,2-dioleoyl-sn-glycero-3-phosphocholine, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine, cholesterol | Significantly increased tumor-targeting efficacy with anti-tumor metastasis effects in BALB/c mice bearing 4T1 cells | Luminal BC | [190] |
| Liposome | Doxorubicin | 194 nm | 1,2-distearoyl-sn-glycero-3-phosphoryl ethanolamine, estrone conjugated dipalmitoyl phosphatidylcholine- PEG2000-NH2 liposomes | Significantly increased uptake in MCF-7 BC cell lines and decreased uptake in MDA-MB-231 BC cell lines | Luminal BC | [191] |
| PolymericNPs | Curcumin | ~10 nm | Chitosan NPs with an apoptosis-inducing ligand (TRAIL) | Significantly reduced tumor volume when compared to control when tested in BALB/c mice | TNBC | [192] |
| Polymeric NPs | Trastuzumab | ~125 nm | Antigen-binding fragments cut from trastuzumab)-modified NPs (Fab’-NPs) with curcumin | Significantly increased cytotoxicity and cellular uptake when compared with the free drug in the MDA-MB-453 cell lines and a xenograft mice model. | HER2 + BC | [193] |
| Polymeric NPs | Paclitaxel | ~225 nm | Poly(lactic-co-glycolic acid) NP coated with hyaluronic acid | Significantly increased cytotoxicity and cellular uptake when compared with the free drug in MDA-MB-231. | TNBC | [194] |
| Polymeric NPs | Paclitaxel | 131.7 nm | Hyaluronic acid-coated polyethylenimine-poly(d,l-lactide-co-glycolide) NPs with miR-542-3p | Significantly increased cytotoxicity and cellular uptake when compared with the free drug in MDA-MB-231. | TNBC | [195] |
| Polymeric NPs | Gambogic acid | 121.5 nm | Hyaluronic acid-coated polyethylenimine-poly(d,l-lactide-co-glycolide) NPs with RAIL plasmid (pTRAIL) and gambogic acid | Significantly increased cytotoxicity, apoptotic cell death, and cellular uptake when compared with the free drug in MDA-MB-231. | TNBC | [196] |
| Polymeric NPs | Thymoquinone | ~22 nm | Pluronic® F127 NPs, hyaluronic acid-conjugated Pluronic® P123. | Significantly reduced cell growth and migration of MDA-MB-231 cell lines and xenograft Balb/c mice | TNBC | [197] |
| Solid–lipid NPs | Di-allyl-disulfide | ~116 nm | Pluronic F-68, solid–lipid NPs engineered with palmitic acid and soya lecithin and surface-modified with glycation end product antibodies | Significantly enhanced cytotoxicity and cellular uptake, augmented activity at the tumor site, and reduced systemic toxicity when compared with the free drug in MDA-MB231 | TNBC | [198] |
4.6. Polymeric Nanocarriers
4.6.1. Conjugation with Polymeric Protein
4.6.2. Liposomes
4.6.3. Lipid–Hybrid Polymer
4.6.4. Dendrimers
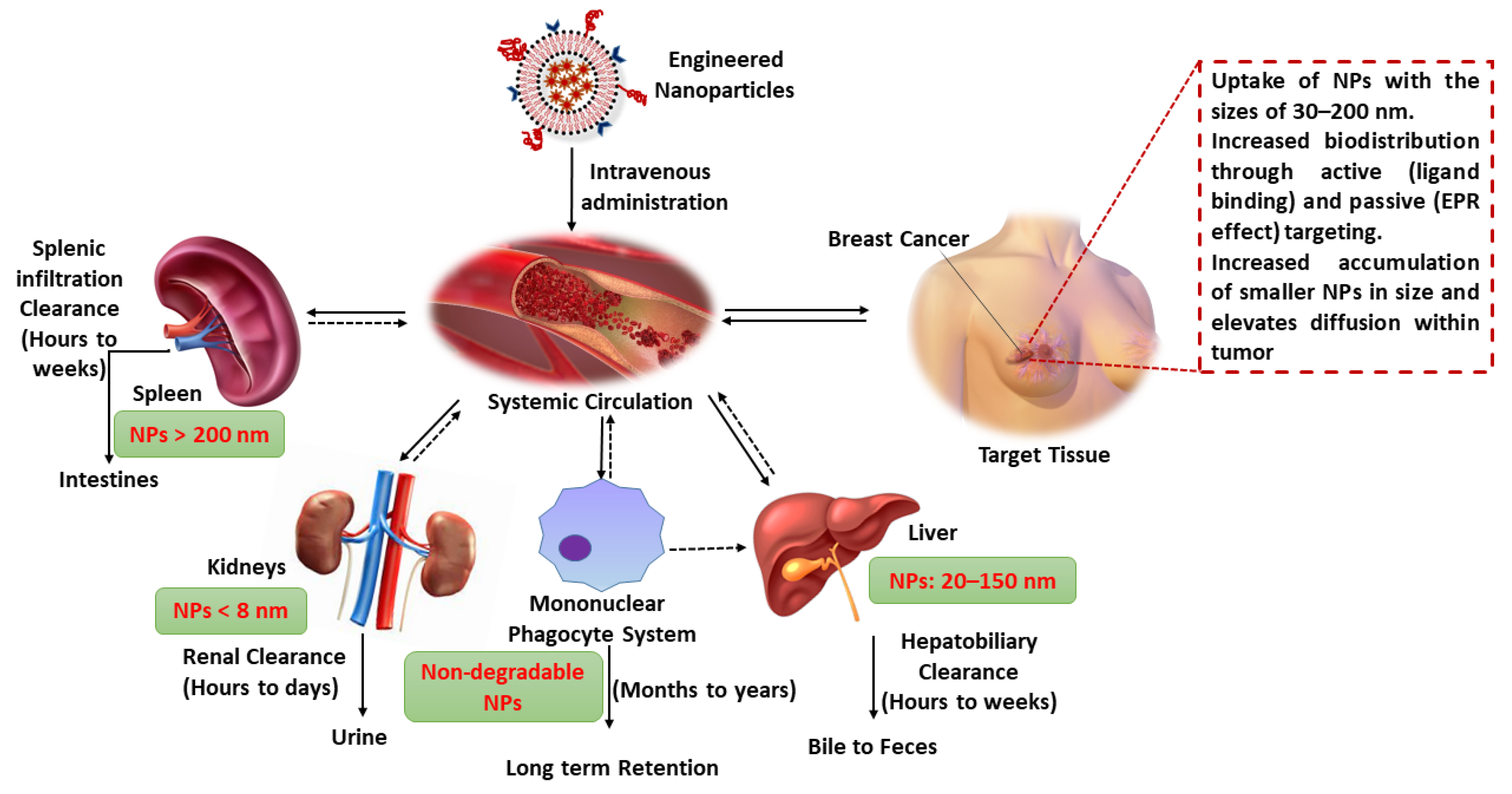
5. Engineered NPs Increases the Circulation Half-Life
6. Toxicity of NPs
7. Future Prospective
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5fu | 5-Fluorouracil |
| AI | Artificial intelligence |
| AIDS | Acquired immunodeficiency syndrome |
| BC | Breast cancer |
| DDS | Drug delivery system |
| DNA | Deoxyribonucleic acids |
| EPR | Effect enhanced retention andpermeability effect |
| FDA | Food and drug administration |
| HER2 | Human epidermal growth factor receptor 2 |
| MCF7 cells | Michigan cancer foundation-7 breastcancer cells |
| MDR | Multidrug resistance |
| miRNA | Micro ribonucleic acids |
| NPS | Nanoparticles |
| PEG | Polyethylene glycol |
| siRNA | Small interfering ribonucleic acids |
| TNBC | Triple-negative breast cancer |
References
- Ganesan, K.; Xu, B. Deep frying cooking oils promote the high risk of metastases in the breast—A critical review. Food Chem. Toxicol. 2020, 144, 111648. [Google Scholar] [CrossRef]
- Fisusi, F.A.; Akala, E.O. Drug Combinations in Breast Cancer Therapy. Pharm. Nanotechnol. 2019, 7, 3–23. [Google Scholar] [CrossRef]
- Tampaki, E.C.; Tampakis, A.; Alifieris, C.E.; Krikelis, D.; Pazaiti, A.; Kontos, M.; Trafalis, D.T. Efficacy and Safety of Neoadjuvant Treatment with Bevacizumab, Liposomal Doxorubicin, Cyclophosphamide and Paclitaxel Combination in Locally/Regionally Advanced, HER2-Negative, Grade III at Premenopausal Status Breast Cancer: A Phase II Study. Clin. Drug Investig. 2018, 38, 639–648. [Google Scholar] [CrossRef]
- Taylor, C.W.; Kirby, A.M. Cardiac Side-effects From Breast Cancer Radiotherapy. Clin. Oncol. 2015, 27, 621–629. [Google Scholar] [CrossRef]
- Zhu, H.; You, J.; Wen, Y.; Jia, L.; Gao, F.; Ganesan, K.; Chen, J. Tumorigenic risk of Angelica sinensis on ER-positive breast cancer growth through ER-induced stemness in vitro and in vivo. J. Ethnopharmacol. 2021, 280, 114415. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. Molecular targets of vitexin and isovitexin in cancer therapy: A critical review. Ann. N. Y. Acad. Sci. 2017, 1401, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Si, M.; Xue, H.-Y.; Wong, H.-L. Nanomedicine applications in the treatment of breast cancer: Current state of the art. Int. J. Nanomed. 2017, 12, 5879–5892. [Google Scholar] [CrossRef]
- Sinn, H.P.; Kreipe, H. A Brief Overview of the WHO Classification of Breast Tumors, 4th Edition, Focusing on Issues and Updates from the 3rd Edition. Breast Care 2013, 8, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Ganesan, K.; Sukalingam, K.; Xu, B. Impact of consumption of repeatedly heated cooking oils on the incidence of various cancers—A critical review. Crit. Rev. Food Sci. Nutr. 2019, 59, 488–505. [Google Scholar] [CrossRef] [PubMed]
- Khoobchandani, M.; Katti, K.K.; Karikachery, A.R.; Thipe, V.C.; Srisrimal, D.; Dhurvas Mohandoss, D.K.; Darshakumar, R.D.; Joshi, C.M.; Katti, K.V. New Approaches in Breast Cancer Therapy Through Green Nanotechnology and Nano-Ayurvedic Medicine—Pre-Clinical and Pilot Human Clinical Investigations. Int. J. Nanomed. 2020, 15, 181–197. [Google Scholar] [CrossRef]
- Xiao, K.; Liu, Q.; Suby, N.; Xiao, W.; Agrawal, R.; Vu, M.; Zhang, H.; Luo, Y.; Li, Y.; Lam, K.S. LHRH-Targeted Redox-Responsive Crosslinked Micelles Impart Selective Drug Delivery and Effective Chemotherapy in Triple-Negative Breast Cancer. Adv. Healthc. Mater. 2021, 10, e2001196. [Google Scholar] [CrossRef] [PubMed]
- Mickymaray, S. One-step Synthesis of Silver Nanoparticles Using Saudi Arabian Desert Seasonal Plant Sisymbrium irio and Antibacterial Activity Against Multidrug-Resistant Bacterial Strains. Biomolecules 2019, 9, 662. [Google Scholar] [CrossRef]
- Ke, Y.; Al Aboody, M.S.; Alturaiki, W.; Alsagaby, S.A.; Alfaiz, F.A.; Veeraraghavan, V.P.; Mickymaray, S. Photosynthesized gold nanoparticles from Catharanthus roseus induces caspase-mediated apoptosis in cervical cancer cells (HeLa). Artif. Cells Nanomed. Biotechnol. 2019, 47, 1938–1946. [Google Scholar] [CrossRef]
- Alsagaby, S.A.; Vijayakumar, R.; Premanathan, M.; Mickymaray, S.; Alturaiki, W.; Al-Baradie, R.S.; AlGhamdi, S.; Aziz, M.A.; Alhumaydhi, F.A.; Alzahrani, F.A.; et al. Transcriptomics-Based Characterization of the Toxicity of ZnO Nanoparticles Against Chronic Myeloid Leukemia Cells. Int. J. Nanomed. 2020, 15, 7901–7921. [Google Scholar] [CrossRef]
- Mamnoon, B.; Loganathan, J.; Confeld, M.I.; De Fonseka, N.; Feng, L.; Froberg, J.; Choi, Y.; Tuvin, D.M.; Sathish, V.; Mallik, S. Targeted polymeric nanoparticles for drug delivery to hypoxic, triple-negative breast tumors. ACS Appl. Bio. Mater. 2021, 4, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Feng, X.; Gao, L.; Mickymaray, S.; Paramasivam, A.; Abdulaziz Alfaiz, F.; Almasmoum, H.A.; Ghaith, M.M.; Almaimani, R.A.; Aziz Ibrahim, I.A. Inhibiting the PI3K/AKT/mTOR signalling pathway with copper oxide nanoparticles from Houttuynia cordata plant: Attenuating the proliferation of cervical cancer cells. Artif. Cells Nanomed. Biotechnol. 2021, 49, 240–249. [Google Scholar] [CrossRef]
- O’Brien, M.E.; Wigler, N.; Inbar, M.; Rosso, R.; Grischke, E.; Santoro, A.; Catane, R.; Kieback, D.G.; Tomczak, P.; Ackland, S.P.; et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann. Oncol. 2004, 15, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Montero, A.J.; Adams, B.; Diaz-Montero, C.M.; Glück, S. Nab-paclitaxel in the treatment of metastatic breast cancer: A comprehensive review. Expert Rev. Clin. Pharmacol. 2011, 4, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Vishnu, P.; Roy, V. Nab-Paclitaxel: A Novel Formulation of Taxane for Treatment of Breast Cancer. Women’s Health 2010, 6, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Astruc, D. Docetaxel nanotechnology in anticancer therapy. ChemMedChem 2012, 7, 952–972. [Google Scholar] [CrossRef] [PubMed]
- Ghafari, M.; Haghiralsadat, F.; Khanamani Falahati-pour, S.; Zavar Reza, J. Development of a novel liposomal nanoparticle formulation of cisplatin to breast cancer therapy. J. Cell. Biochem. 2020, 121, 3584–3592. [Google Scholar] [CrossRef] [PubMed]
- Yardley, D.; Burris, H., 3rd; Peacock, N.; Raefsky, E.; Melnik, M.; Inhorn, R.; Shipley, D.; Hainsworth, J. A pilot study of adjuvant nanoparticle albumin-bound (nab) paclitaxel and cyclophosphamide, with trastuzumab in HER2-positive patients, in the treatment of early-stage breast cancer. Breast Cancer Res. Treat. 2010, 123, 471–475. [Google Scholar] [CrossRef]
- Nahleh, Z.A.; Barlow, W.E.; Hayes, D.F.; Schott, A.F.; Gralow, J.R.; Sikov, W.M.; Perez, E.A.; Chennuru, S.; Mirshahidi, H.R.; Corso, S.W.; et al. SWOG S0800 (NCI CDR0000636131): Addition of bevacizumab to neoadjuvant nab-paclitaxel with dose-dense doxorubicin and cyclophosphamide improves pathologic complete response (pCR) rates in inflammatory or locally advanced breast cancer. Breast Cancer Res. Treat. 2016, 158, 485–495. [Google Scholar] [CrossRef]
- Saracchini, S.; Foltran, L.; Tuccia, F.; Bassini, A.; Sulfaro, S.; Micheli, E.; Del Conte, A.; Bertola, M.; Gion, M.; Lorenzon, M.; et al. Phase II study of liposome-encapsulated doxorubicin plus cyclophosphamide, followed by sequential trastuzumab plus docetaxel as primary systemic therapy for breast cancer patients with HER2 overexpression or amplification. Breast 2013, 22, 1101–1107. [Google Scholar] [CrossRef]
- Manatunga, D.C.; de Silva, R.M.; de Silva, K.M.N.; Malavige, G.N.; Wijeratne, D.T.; Williams, G.R.; Jayasinghe, C.D.; Udagama, P.V. Effective delivery of hydrophobic drugs to breast and liver cancer cells using a hybrid inorganic nanocarrier: A detailed investigation using cytotoxicity assays, fluorescence imaging and flow cytometry. Eur. J. Pharm. Biopharm. 2018, 128, 18–26. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef]
- Shrestha, B.; Wang, L.; Zhang, H.; Hung, C.Y.; Tang, L. Gold Nanoparticles Mediated Drug-Gene Combinational Therapy for Breast Cancer Treatment. Int. J. Nanomed. 2020, 15, 8109–8119. [Google Scholar] [CrossRef] [PubMed]
- Ardavanis, A.; Mavroudis, D.; Kalbakis, K.; Malamos, N.; Syrigos, K.; Vamvakas, L.; Kotsakis, A.; Kentepozidis, N.; Kouroussis, C.; Agelaki, S.; et al. Pegylated liposomal doxorubicin in combination with vinorelbine as salvage treatment in pretreated patients with advanced breast cancer: A multicentre phase II study. Cancer Chemother. Pharmacol. 2006, 58, 742–748. [Google Scholar] [CrossRef]
- Megerdichian, C.; Olimpiadi, Y.; Hurvitz, S.A. nab-Paclitaxel in combination with biologically targeted agents for early and metastatic breast cancer. Cancer Treat. Rev. 2014, 40, 614–625. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Krasnojon, D.; Cheporov, S.; Makhson, A.N.; Manikhas, G.M.; Clawson, A.; Bhar, P. Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J. Clin. Oncol. 2009, 27, 3611–3619. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Kim, K.-P.; Yoon, D.H.; Hong, Y.S.; Choi, C.-M.; Ahn, J.-H.; Lee, D.H.; Lee, J.-L.; Ryu, M.-H.; Ryoo, B.-Y.; et al. A phase I trial to determine the maximum tolerated dose and evaluate the safety and pharmacokinetics (PK) of docetaxel-PNP, polymeric nanoparticle formulation of docetaxel, in subjects with advanced solid malignancies. J. Clin. Oncol. 2012, 30, e13104. [Google Scholar] [CrossRef]
- Torrisi, R.; Cardillo, A.; Cancello, G.; Dellapasqua, S.; Balduzzi, A.; Ghisini, R.; Luini, A.; Veronesi, P.; Viale, G.; Goldhirsch, A.; et al. Phase II trial of combination of pegylated liposomal doxorubicin, cisplatin, and infusional 5-fluorouracil (CCF) plus trastuzumab as preoperative treatment for locally advanced and inflammatory breast cancer. Clin. Breast Cancer 2010, 10, 483–488. [Google Scholar] [CrossRef]
- Sledge, G.W., Jr. Etoposide in the management of metastatic breast cancer. Cancer 1991, 67, 266–270. [Google Scholar] [CrossRef]
- Icli, F.; Akbulut, H.; Onur, H.; Yalcin, B.; Demirkazık, A.; Senler, F. Adjuvant oral etoposide plus cisplatin (EoP) following sequential doxorubicin/cyclophosphamide (AC) and docetaxel (T) in early breast cancer patients with 4 or more positive lymph nodes (10 years follow-up). Breast 2011, 20, 155–157. [Google Scholar] [CrossRef]
- Kapke, J.T.; Schneidewend, R.J.; Jawa, Z.A.; Huang, C.C.; Connelly, J.M.; Chitambar, C.R. High-dose intravenous methotrexate in the management of breast cancer with leptomeningeal disease: Case series and review of the literature. Hematol. Oncol. Stem Cell Ther. 2019, 12, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Niwińska, A.; Rudnicka, H.; Murawska, M. Breast cancer leptomeningeal metastasis: The results of combined treatment and the comparison of methotrexate and liposomal cytarabine as intra-cerebrospinal fluid chemotherapy. Clin. Breast Cancer 2015, 15, 66–72. [Google Scholar] [CrossRef]
- Im, S.A.; Lee, K.S.; Ro, J.; Lee, E.S.; Kwon, Y.; Ahn, J.H.; Ahn, J.S.; Kim, J.H.; Kang, H.S.; Shin, K.H.; et al. Phase II trial of preoperative paclitaxel, gemcitabine, and trastuzumab combination therapy in HER2 positive stage II/III breast cancer: The Korean Cancer Study Group BR 07-01. Breast Cancer Res. Treat. 2012, 132, 589–600. [Google Scholar] [CrossRef]
- Robert, N.; Krekow, L.; Stokoe, C.; Clawson, A.; Iglesias, J.; O’Shaughnessy, J. Adjuvant dose-dense doxorubicin plus cyclophosphamide followed by dose-dense nab-paclitaxel is safe in women with early-stage breast cancer: A pilot study. Breast Cancer Res. Treat. 2011, 125, 115–120. [Google Scholar] [CrossRef]
- Lobo, C.; Lopes, G.; Baez, O.; Castrellon, A.; Ferrell, A.; Higgins, C.; Hurley, E.; Hurley, J.; Reis, I.; Richman, S.; et al. Final results of a phase II study of nab-paclitaxel, bevacizumab, and gemcitabine as first-line therapy for patients with HER2-negative metastatic breast cancer. Breast Cancer Res. Treat. 2010, 123, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Mirtsching, B.; Cosgriff, T.; Harker, G.; Keaton, M.; Chidiac, T.; Min, M. A phase II study of weekly nanoparticle albumin-bound paclitaxel with or without trastuzumab in metastatic breast cancer. Clin. Breast Cancer 2011, 11, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Basho, R.K.; Gilcrease, M.; Murthy, R.K.; Helgason, T.; Karp, D.D.; Meric-Bernstam, F.; Hess, K.R.; Herbrich, S.M.; Valero, V.; Albarracin, C.; et al. Targeting the PI3K/AKT/mTOR Pathway for the Treatment of Mesenchymal Triple-Negative Breast Cancer: Evidence From a Phase 1 Trial of mTOR Inhibition in Combination With Liposomal Doxorubicin and Bevacizumab. JAMA Oncol. 2017, 3, 509–515. [Google Scholar] [CrossRef]
- Liao, W.S.; Ho, Y.; Lin, Y.W.; Naveen Raj, E.; Liu, K.K.; Chen, C.; Zhou, X.Z.; Lu, K.P.; Chao, J.I. Targeting EGFR of triple-negative breast cancer enhances the therapeutic efficacy of paclitaxel- and cetuximab-conjugated nanodiamond nanocomposite. Acta Biomater. 2019, 86, 395–405. [Google Scholar] [CrossRef]
- Yin, L.; Qi, X.W.; Liu, X.Z.; Yang, Z.Y.; Cai, R.L.; Cui, H.J.; Chen, L.; Yu, S.C. Icaritin enhances the efficacy of cetuximab against triple-negative breast cancer cells. Oncol. Lett. 2020, 19, 3950–3958. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, A.; Scagnoli, S.; Roberto, M.; Lionetto, L.; Cerbelli, B.; Simmaco, M.; Marchetti, P. 5-Fluorouracil degradation rate as a predictive biomarker of toxicity in breast cancer patients treated with capecitabine. J. Oncol. Pharm. Pract. 2020, 26, 1836–1842. [Google Scholar] [CrossRef] [PubMed]
- Dillman, R.O. Cancer immunotherapy. Cancer Biother. Radiopharm. 2011, 26, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Mazloum-Ravasan, S.; Madadi, E.; Mohammadi, A.; Mansoori, B.; Amini, M.; Mokhtarzadeh, A.; Baradaran, B.; Darvishi, F. Yarrowia lipolytica L-asparaginase inhibits the growth and migration of lung (A549) and breast (MCF7) cancer cells. Int. J. Biol. Macromol. 2021, 170, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Plyasova, A.A.; Pokrovskaya, M.V.; Lisitsyna, O.M.; Pokrovsky, V.S.; Alexandrova, S.S.; Hilal, A.; Sokolov, N.N.; Zhdanov, D.D. Penetration into Cancer Cells via Clathrin-Dependent Mechanism Allows L-Asparaginase from Rhodospirillum rubrum to Inhibit Telomerase. Pharmaceuticals 2020, 13, 286. [Google Scholar] [CrossRef]
- Matsuda, N.; Wang, X.; Lim, B.; Krishnamurthy, S.; Alvarez, R.H.; Willey, J.S.; Parker, C.A.; Song, J.; Shen, Y.; Hu, J.; et al. Safety and Efficacy of Panitumumab Plus Neoadjuvant Chemotherapy in Patients With Primary HER2-Negative Inflammatory Breast Cancer. JAMA Oncol. 2018, 4, 1207–1213. [Google Scholar] [CrossRef]
- Yook, S.; Cai, Z.; Lu, Y.; Winnik, M.A.; Pignol, J.P.; Reilly, R.M. Radiation Nanomedicine for EGFR-Positive Breast Cancer: Panitumumab-Modified Gold Nanoparticles Complexed to the β-Particle-Emitter, (177)Lu. Mol. Pharm. 2015, 12, 3963–3972. [Google Scholar] [CrossRef]
- Li, N.; Wang, X.; Lin, B.; Zhu, H.; Liu, C.; Xu, X.; Zhang, Y.; Zhai, S.; OuYang, T.; Li, J.; et al. Clinical Evaluation of 99mTc-Rituximab for Sentinel Lymph Node Mapping in Breast Cancer Patients. J. Nucl. Med. 2016, 57, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Zhang, W.C.; An, C.X.; Li, X.M.; Ma, L. Comparative research on (99 m)Tc-Rituximab and (99 m)Tc-sulfur colloid in sentinel lymph node imaging of breast cancer. BMC Cancer 2019, 19, 956. [Google Scholar] [CrossRef]
- Cersosimo, R.J. Monoclonal antibodies in the treatment of cancer, Part 1. Am. J. Health Syst. Pharm. 2003, 60, 1531–1548. [Google Scholar] [CrossRef]
- Shamsian, A.; Sepand, M.R.; Javaheri Kachousangi, M.; Dara, T.; Ostad, S.N.; Atyabi, F.; Ghahremani, M.H. Targeting Tumorigenicity of Breast Cancer Stem Cells Using SAHA/Wnt-b Catenin Antagonist Loaded Onto Protein Corona of Gold Nanoparticles. Int. J. Nanomed. 2020, 15, 4063–4078. [Google Scholar] [CrossRef] [PubMed]
- Gote, V.; Pal, D. Octreotide-Targeted Lcn2 siRNA PEGylated Liposomes as a Treatment for Metastatic Breast Cancer. Bioengineering 2021, 8, 44. [Google Scholar] [CrossRef]
- Song, Y.; Tang, C.; Yin, C. Combination antitumor immunotherapy with VEGF and PIGF siRNA via systemic delivery of multi-functionalized nanoparticles to tumor-associated macrophages and breast cancer cells. Biomaterials 2018, 185, 117–132. [Google Scholar] [CrossRef]
- Torchilin, V.P.; Lukyanov, A.N.; Gao, Z.; Papahadjopoulos-Sternberg, B. Immunomicelles: Targeted pharmaceutical carriers for poorly soluble drugs. Proc. Natl. Acad. Sci. USA 2003, 100, 6039–6044. [Google Scholar] [CrossRef]
- de Martimprey, H.; Vauthier, C.; Malvy, C.; Couvreur, P. Polymer nanocarriers for the delivery of small fragments of nucleic acids: Oligonucleotides and siRNA. Eur. J. Pharm. Biopharm. 2009, 71, 490–504. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. Engl. 2014, 53, 12320–12364. [Google Scholar] [CrossRef]
- Jahangirian, H.; Kalantari, K.; Izadiyan, Z.; Rafiee-Moghaddam, R.; Shameli, K.; Webster, T.J. A review of small molecules and drug delivery applications using gold and iron nanoparticles. Int. J. Nanomed. 2019, 14, 1633–1657. [Google Scholar] [CrossRef]
- McCarron, P.A.; Hall, M. Incorporation of novel 1-alkylcarbonyloxymethyl prodrugs of 5-fluorouracil into poly(lactide-co-glycolide) nanoparticles. Int. J. Pharm. 2008, 348, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Leo, E.; Pecquet, S.; Rojas, J.; Couvreur, P.; Fattal, E. Changing the pH of the external aqueous phase may modulate protein entrapment and delivery from poly(lactide-co-glycolide) microspheres prepared by a w/o/w solvent evaporation method. J. Microencapsul. 1998, 15, 421–430. [Google Scholar] [CrossRef]
- Wang, M.; Alberti, K.; Sun, S.; Arellano, C.L.; Xu, Q. Combinatorially designed lipid-like nanoparticles for intracellular delivery of cytotoxic protein for cancer therapy. Angew. Chem. Int. Ed. Engl. 2014, 53, 2893–2898. [Google Scholar] [CrossRef] [PubMed]
- Juan, A.; Cimas, F.J.; Bravo, I.; Pandiella, A.; Ocaña, A.; Alonso-Moreno, C. Antibody Conjugation of Nanoparticles as Therapeutics for Breast Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 6018. [Google Scholar] [CrossRef]
- Falagan-Lotsch, P.; Grzincic, E.M.; Murphy, C.J. New Advances in Nanotechnology-Based Diagnosis and Therapeutics for Breast Cancer: An Assessment of Active-Targeting Inorganic Nanoplatforms. Bioconjug. Chem. 2017, 28, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.C.; Doak, A.K.; Wassam, P.; Shoichet, M.S.; Shoichet, B.K. Colloidal aggregation affects the efficacy of anticancer drugs in cell culture. ACS Chem. Biol. 2012, 7, 1429–1435. [Google Scholar] [CrossRef]
- Xu, J.Z.; Shao, C.C.; Wang, X.J.; Zhao, X.; Chen, J.Q.; Ouyang, Y.X.; Feng, J.; Zhang, F.; Huang, W.H.; Ying, Q.; et al. circTADA2As suppress breast cancer progression and metastasis via targeting miR-203a-3p/SOCS3 axis. Cell Death Dis. 2019, 10, 175. [Google Scholar] [CrossRef]
- Wang, H.; Tan, Z.; Hu, H.; Liu, H.; Wu, T.; Zheng, C.; Wang, X.; Luo, Z.; Wang, J.; Liu, S.; et al. microRNA-21 promotes breast cancer proliferation and metastasis by targeting LZTFL1. BMC Cancer 2019, 19, 738. [Google Scholar] [CrossRef]
- Akinc, A.; Zumbuehl, A.; Goldberg, M.; Leshchiner, E.S.; Busini, V.; Hossain, N.; Bacallado, S.A.; Nguyen, D.N.; Fuller, J.; Alvarez, R.; et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat. Biotechnol. 2008, 26, 561–569. [Google Scholar] [CrossRef]
- Xue, H.Y.; Guo, P.; Wen, W.C.; Wong, H.L. Lipid-Based Nanocarriers for RNA Delivery. Curr. Pharm. Des. 2015, 21, 3140–3147. [Google Scholar] [CrossRef]
- Altınoglu, S.; Wang, M.; Xu, Q. Combinatorial library strategies for synthesis of cationic lipid-like nanoparticles and their potential medical applications. Nanomedicine 2015, 10, 643–657. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles:Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Vasilyeva, E.; Lam, B.; Fang, Z.; Minden, M.D.; Sargent, E.H.; Kelley, S.O. Direct genetic analysis of ten cancer cells: Tuning sensor structure and molecular probe design for efficient mRNA capture. Angew. Chem. Int. Ed. Engl. 2011, 50, 4137–4141. [Google Scholar] [CrossRef]
- Chan, T.G.; Morse, S.V.; Copping, M.J.; Choi, J.J.; Vilar, R. Targeted Delivery of DNA-Au Nanoparticles across the Blood-Brain Barrier Using Focused Ultrasound. ChemMedChem 2018, 13, 1311–1314. [Google Scholar] [CrossRef]
- Schwendener, R.A.; Schott, H. Liposome formulations of hydrophobic drugs. Methods Mol. Biol. 2010, 605, 129–138. [Google Scholar] [CrossRef]
- Chowdhury, N.; Chaudhry, S.; Hall, N.; Olverson, G.; Zhang, Q.-J.; Mandal, T.; Dash, S.; Kundu, A. Targeted Delivery of Doxorubicin Liposomes for Her-2+ Breast Cancer Treatment. AAPS PharmSciTech 2020, 21, 202. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi. Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- Duggan, S.T.; Keating, G.M. Pegylated liposomal doxorubicin: A review of its use in metastatic breast cancer, ovarian cancer, multiple myeloma and AIDS-related Kaposi’s sarcoma. Drugs 2011, 71, 2531–2558. [Google Scholar] [CrossRef] [PubMed]
- Sajid, A.; Lusvarghi, S.; Murakami, M.; Chufan, E.E.; Abel, B.; Gottesman, M.M.; Durell, S.R.; Ambudkar, S.V. Reversing the direction of drug transport mediated by the human multidrug transporter P-glycoprotein. Proc. Natl. Acad. Sci. USA 2020, 117, 29609. [Google Scholar] [CrossRef]
- Shieh, M.J.; Hsu, C.Y.; Huang, L.Y.; Chen, H.Y.; Huang, F.H.; Lai, P.S. Reversal of doxorubicin-resistance by multifunctional nanoparticles in MCF-7/ADR cells. J. Control. Release 2011, 152, 418–425. [Google Scholar] [CrossRef]
- Liu, Z.; Balasubramanian, V.; Bhat, C.; Vahermo, M.; Mäkilä, E.; Kemell, M.; Fontana, F.; Janoniene, A.; Petrikaite, V.; Salonen, J.; et al. Quercetin-Based Modified Porous Silicon Nanoparticles for Enhanced Inhibition of Doxorubicin-Resistant Cancer Cells. Adv. Healthc. Mater. 2017, 6, 1601009. [Google Scholar] [CrossRef]
- Yalcin, S.; Unsoy, G.; Mutlu, P.; Khodadust, R.; Gunduz, U. Polyhydroxybutyrate-coated magnetic nanoparticles for doxorubicin delivery: Cytotoxic effect against doxorubicin-resistant breast cancer cell line. Am. J. Ther. 2014, 21, 453–461. [Google Scholar] [CrossRef]
- Ota, K.; Ito, K.; Akahira, J.; Sato, N.; Onogawa, T.; Moriya, T.; Unno, M.; Abe, T.; Niikura, H.; Takano, T.; et al. Expression of organic cation transporter SLC22A16 in human epithelial ovarian cancer: A possible role of the adriamycin importer. Int. J. Gynecol. Pathol. 2007, 26, 334–340. [Google Scholar] [CrossRef]
- Bazylińska, U.; Zieliński, W.; Kulbacka, J.; Samoć, M.; Wilk, K.A. New diamidequat-type surfactants in fabrication of long-sustained theranostic nanocapsules: Colloidal stability, drug delivery and bioimaging. Colloids Surf. B Biointerfaces 2016, 137, 121–132. [Google Scholar] [CrossRef]
- Liu, Y.; Qiao, L.; Zhang, S.; Wan, G.; Chen, B.; Zhou, P.; Zhang, N.; Wang, Y. Dual pH-responsive multifunctional nanoparticles for targeted treatment of breast cancer by combining immunotherapy and chemotherapy. Acta. Biomater. 2018, 66, 310–324. [Google Scholar] [CrossRef]
- Chen, H.H.; Lu, I.L.; Liu, T.I.; Tsai, Y.C.; Chiang, W.H.; Lin, S.C.; Chiu, H.C. Indocyanine green/doxorubicin-encapsulated functionalized nanoparticles for effective combination therapy against human MDR breast cancer. Colloids Surf. B Biointerfaces 2019, 177, 294–305. [Google Scholar] [CrossRef]
- Huang, W.; Lang, Y.; Hakeem, A.; Lei, Y.; Gan, L.; Yang, X. Surfactin-based nanoparticles loaded with doxorubicin to overcome multidrug resistance in cancers. Int. J. Nanomed. 2018, 13, 1723–1736. [Google Scholar] [CrossRef]
- Kang, S.; Kang, K.; Chae, A.; Kim, Y.K.; Jang, H.; Min, D.H. Fucoidan-coated coral-like Pt nanoparticles for computed tomography-guided highly enhanced synergistic anticancer effect against drug-resistant breast cancer cells. Nanoscale 2019, 11, 15173–15183. [Google Scholar] [CrossRef]
- Fu, B.; Dang, M.; Tao, J.; Li, Y.; Tang, Y. Mesoporous platinum nanoparticle-based nanoplatforms for combined chemo-photothermal breast cancer therapy. J. Colloid Interface Sci. 2020, 570, 197–204. [Google Scholar] [CrossRef]
- Muley, H.; Fadó, R.; Rodríguez-Rodríguez, R.; Casals, N. Drug uptake-based chemoresistance in breast cancer treatment. Biochem. Pharmacol. 2020, 177, 113959. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; Dai, D.D.; Lu, C.T.; Chen, L.J.; Lin, M.; Shen, X.T.; Li, X.K.; Zhang, M.; Jiang, X.; Jin, R.R.; et al. Epirubicin loaded with propylene glycol liposomes significantly overcomes multidrug resistance in breast cancer. Cancer Lett. 2013, 330, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Tang, W.; Liu, J.J.; Gong, X.Q.; Kong, L.; Yao, X.M.; Jing, M.; Cai, F.Y.; Li, X.T.; Ju, R.J. Combination of targeted daunorubicin liposomes and targeted emodin liposomes for treatment of invasive breast cancer. J. Drug Target. 2020, 28, 245–258. [Google Scholar] [CrossRef]
- Batra, H.; Pawar, S.; Bahl, D. Curcumin in combination with anti-cancer drugs: A nanomedicine review. Pharmacol. Res. 2019, 139, 91–105. [Google Scholar] [CrossRef]
- Nie, S. Understanding and overcoming major barriers in cancer nanomedicine. Nanomedicine 2010, 5, 523–528. [Google Scholar] [CrossRef]
- Sun, B.; Hyun, H.; Li, L.T.; Wang, A.Z. Harnessing nanomedicine to overcome the immunosuppressive tumor microenvironment. Acta. Pharmacol. Sin. 2020, 41, 970–985. [Google Scholar] [CrossRef]
- Dusinska, M.; Magdolenova, Z.; Fjellsbø, L.M. Toxicological aspects for nanomaterial in humans. Methods Mol. Biol. 2013, 948, 1–12. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Yaremenko, A.V.; Yuryev, M.V.; Mirkasymov, A.B.; Sokolov, I.L.; Deyev, S.M.; Nikitin, P.I.; Nikitin, M.P. Fast processes of nanoparticle blood clearance: Comprehensive study. J. Control. Release 2020, 326, 181–191. [Google Scholar] [CrossRef]
- Boraschi, D.; Italiani, P.; Palomba, R.; Decuzzi, P.; Duschl, A.; Fadeel, B.; Moghimi, S.M. Nanoparticles and innate immunity: New perspectives on host defence. Semin. Immunol. 2017, 34, 33–51. [Google Scholar] [CrossRef]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef]
- Sabourian, P.; Yazdani, G.; Ashraf, S.S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of Physico-Chemical Properties of Nanoparticles on Their Intracellular Uptake. Int. J. Mol. Sci. 2020, 21, 8019. [Google Scholar] [CrossRef]
- Schmid, R.; Schmidt, S.K.; Hazur, J.; Detsch, R.; Maurer, E.; Boccaccini, A.R.; Hauptstein, J.; Teßmar, J.; Blunk, T.; Schrüfer, S.; et al. Comparison of Hydrogels for the Development of Well-Defined 3D Cancer Models of Breast Cancer and Melanoma. Cancers 2020, 12, 2320. [Google Scholar] [CrossRef]
- Cassano, R.; Mellace, S.; Pellegrino, M.; Ricchio, E.; Mauro, L.; Andò, S.; Picci, N.; Trombino, S. Biocompatible Targeting Hydrogels for Breast Cancer Treatment. Mini Rev. Med. Chem. 2016, 16, 651–657. [Google Scholar] [CrossRef]
- Pradhan, S.; Clary, J.M.; Seliktar, D.; Lipke, E.A. A three-dimensional spheroidal cancer model based on PEG-fibrinogen hydrogel microspheres. Biomaterials 2017, 115, 141–154. [Google Scholar] [CrossRef]
- Pradhan, S.; Hassani, I.; Seeto, W.J.; Lipke, E.A. PEG-fibrinogen hydrogels for three-dimensional breast cancer cell culture. J. Biomed. Mater. Res. A 2017, 105, 236–252. [Google Scholar] [CrossRef]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef]
- Nakamura, H.; Fang, J.; Maeda, H. Development of next-generation macromolecular drugs based on the EPR effect: Challenges and pitfalls. Expert Opin. Drug Deliv. 2015, 12, 53–64. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Chan, S.; Davidson, N.; Juozaityte, E.; Erdkamp, F.; Pluzanska, A.; Azarnia, N.; Lee, L.W. Phase III trial of liposomal doxorubicin and cyclophosphamide compared with epirubicin and cyclophosphamide as first-line therapy for metastatic breast cancer. Ann. Oncol. 2004, 15, 1527–1534. [Google Scholar] [CrossRef]
- Chen, Z.G. Small-molecule delivery by nanoparticles for anticancer therapy. Trends Mol. Med. 2010, 16, 594–602. [Google Scholar] [CrossRef]
- Oerlemans, C.; Bult, W.; Bos, M.; Storm, G.; Nijsen, J.F.; Hennink, W.E. Polymeric micelles in anticancer therapy: Targeting, imaging and triggered release. Pharm. Res. 2010, 27, 2569–2589. [Google Scholar] [CrossRef]
- Khallaf, S.M.; Roshdy, J.; Ibrahim, A. Pegylated liposomal doxorubicin in patients with metastatic triple-negative breast cancer: 8-year experience of a single center. J. Egypt. Natl. Cancer Inst. 2020, 32, 20. [Google Scholar] [CrossRef]
- Wu, H.; Ramanathan, R.K.; Zamboni, B.A.; Strychor, S.; Ramalingam, S.; Edwards, R.P.; Friedland, D.M.; Stoller, R.G.; Belani, C.P.; Maruca, L.J.; et al. Population pharmacokinetics of pegylated liposomal CKD-602 (S-CKD602) in patients with advanced malignancies. J. Clin. Pharmacol. 2012, 52, 180–194. [Google Scholar] [CrossRef]
- Zamboni, W.C.; Strychor, S.; Maruca, L.; Ramalingam, S.; Zamboni, B.A.; Wu, H.; Friedland, D.M.; Edwards, R.P.; Stoller, R.G.; Belani, C.P.; et al. Pharmacokinetic study of pegylated liposomal CKD-602 (S-CKD602) in patients with advanced malignancies. Clin. Pharmacol. Ther. 2009, 86, 519–526. [Google Scholar] [CrossRef]
- Naskar, S.; Koutsu, K.; Sharma, S. Chitosan-based nanoparticles as drug delivery systems: A review on two decades of research. J. Drug Target. 2019, 27, 379–393. [Google Scholar] [CrossRef]
- Elzoghby, A.O. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. J. Control. Release 2013, 172, 1075–1091. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Tjulandin, S.; Davidson, N.; Shaw, H.; Desai, N.; Bhar, P.; Hawkins, M.; O’Shaughnessy, J. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J. Clin. Oncol. 2005, 23, 7794–7803. [Google Scholar] [CrossRef]
- Stickeler, E.; Klar, M.; Watermann, D.; Geibel, A.; Földi, M.; Hasenburg, A.; Gitsch, G. Pegylated liposomal doxorubicin and trastuzumab as 1st and 2nd line therapy in her2/neu positive metastatic breast cancer: A multicenter phase II trial. Breast Cancer Res. Treat. 2009, 117, 591–598. [Google Scholar] [CrossRef][Green Version]
- Savage, D.T.; Hilt, J.Z.; Dziubla, T.D. In Vitro Methods for Assessing Nanoparticle Toxicity. Methods Mol. Biol. 2019, 1894, 1–29. [Google Scholar] [CrossRef]
- Mahapatro, A.; Singh, D.K. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J. Nanobiotechnol. 2011, 9, 55. [Google Scholar] [CrossRef]
- Ibrahim, N.K.; Samuels, B.; Page, R.; Doval, D.; Patel, K.M.; Rao, S.C.; Nair, M.K.; Bhar, P.; Desai, N.; Hortobagyi, G.N. Multicenter phase II trial of ABI-007, an albumin-bound paclitaxel, in women with metastatic breast cancer. J. Clin. Oncol. 2005, 23, 6019–6026. [Google Scholar] [CrossRef]
- Blum, J.L.; Savin, M.A.; Edelman, G.; Pippen, J.E.; Robert, N.J.; Geister, B.V.; Kirby, R.L.; Clawson, A.; O’Shaughnessy, J.A. Phase II study of weekly albumin-bound paclitaxel for patients with metastatic breast cancer heavily pretreated with taxanes. Clin. Breast Cancer 2007, 7, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Untch, M.; Burchardi, N.; Huober, J.; Sinn, B.V.; Blohmer, J.U.; Grischke, E.M.; Furlanetto, J.; Tesch, H.; Hanusch, C.; et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: Clinical results and biomarker analysis of GeparNuevo study. Ann. Oncol. 2019, 30, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Saura, C.; Nuciforo, P.; Calvo, I.; Andersen, J.; Passos-Coelho, J.L.; Gil, M.G.; Bermejo, B.; Patt, D.A.; Ciruelos, E.; et al. FAIRLANE, a double-blind placebo-controlled randomized phase II trial of neoadjuvant ipatasertib plus paclitaxel for early triple-negative breast cancer. Ann. Oncol. 2019, 30, 1289–1297. [Google Scholar] [CrossRef]
- Harris, L.; Batist, G.; Belt, R.; Rovira, D.; Navari, R.; Azarnia, N.; Welles, L.; Winer, E. Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer 2002, 94, 25–36. [Google Scholar] [CrossRef]
- Batist, G.; Ramakrishnan, G.; Rao, C.S.; Chandrasekharan, A.; Gutheil, J.; Guthrie, T.; Shah, P.; Khojasteh, A.; Nair, M.K.; Hoelzer, K.; et al. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. J. Clin. Oncol. 2001, 19, 1444–1454. [Google Scholar] [CrossRef]
- Kato, K.; Chin, K.; Yoshikawa, T.; Yamaguchi, K.; Tsuji, Y.; Esaki, T.; Sakai, K.; Kimura, M.; Hamaguchi, T.; Shimada, Y.; et al. Phase II study of NK105, a paclitaxel-incorporating micellar nanoparticle, for previously treated advanced or recurrent gastric cancer. Investig. New Drugs 2012, 30, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Borgå, O.; Lilienberg, E.; Bjermo, H.; Hansson, F.; Heldring, N.; Dediu, R. Pharmacokinetics of Total and Unbound Paclitaxel After Administration of Paclitaxel Micellar or Nab-Paclitaxel: An Open, Randomized, Cross-Over, Explorative Study in Breast Cancer Patients. Adv. Ther. 2019, 36, 2825–2837. [Google Scholar] [CrossRef]
- Schneeweiss, A.; Möbus, V.; Tesch, H.; Hanusch, C.; Denkert, C.; Lübbe, K.; Huober, J.; Klare, P.; Kümmel, S.; Untch, M.; et al. Intense dose-dense epirubicin, paclitaxel, cyclophosphamide versus weekly paclitaxel, liposomal doxorubicin (plus carboplatin in triple-negative breast cancer) for neoadjuvant treatment of high-risk early breast cancer (GeparOcto-GBG 84): A randomised phase III trial. Eur. J. Cancer 2019, 106, 181–192. [Google Scholar] [CrossRef]
- Chawla, S.P.; Chua, V.S.; Fernandez, L.; Quon, D.; Blackwelder, W.C.; Gordon, E.M.; Hall, F.L. Advanced phase I/II studies of targeted gene delivery in vivo: Intravenous Rexin-G for gemcitabine-resistant metastatic pancreatic cancer. Mol. Ther. 2010, 18, 435–441. [Google Scholar] [CrossRef]
- Jehn, C.F.; Hemmati, P.; Lehenbauer-Dehm, S.; Kümmel, S.; Flath, B.; Schmid, P. Biweekly Pegylated Liposomal Doxorubicin (Caelyx) in Heavily Pretreated Metastatic Breast Cancer: A Phase 2 Study. Clin. Breast Cancer 2016, 16, 514–519. [Google Scholar] [CrossRef]
- Martin-Romano, P.; Baraibar, I.; Espinós, J.; Legaspi, J.; López-Picazo, J.M.; Aramendía, J.M.; Fernández, O.A.; Santisteban, M. Combination of pegylated liposomal doxorubicin plus gemcitabine in heavily pretreated metastatic breast cancer patients: Long-term results from a single institution experience. Breast J. 2018, 24, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Smorenburg, C.H.; de Groot, S.M.; van Leeuwen-Stok, A.E.; Hamaker, M.E.; Wymenga, A.N.; de Graaf, H.; de Jongh, F.E.; Braun, J.J.; Los, M.; Maartense, E.; et al. A randomized phase III study comparing pegylated liposomal doxorubicin with capecitabine as first-line chemotherapy in elderly patients with metastatic breast cancer: Results of the OMEGA study of the Dutch Breast Cancer Research Group BOOG. Ann. Oncol. 2014, 25, 599–605. [Google Scholar] [CrossRef]
- Lien, M.Y.; Liu, L.C.; Wang, H.C.; Yeh, M.H.; Chen, C.J.; Yeh, S.P.; Bai, L.Y.; Liao, Y.M.; Lin, C.Y.; Hsieh, C.Y.; et al. Safety and efficacy of pegylated liposomal doxorubicin-based adjuvant chemotherapy in patients with stage I–III triple-negative breast cancer. Anticancer Res. 2014, 34, 7319–7326. [Google Scholar] [PubMed]
- Yang, F.O.; Hsu, N.C.; Moi, S.H.; Lu, Y.C.; Hsieh, C.M.; Chang, K.J.; Chen, D.R.; Tu, C.W.; Wang, H.C.; Hou, M.F. Efficacy and toxicity of pegylated liposomal doxorubicin-based chemotherapy in early-stage breast cancer: A multicenter retrospective case-control study. Asia Pac. J. Clin. Oncol. 2018, 14, 198–203. [Google Scholar] [CrossRef]
- Lee, K.S.; Chung, H.C.; Im, S.A.; Park, Y.H.; Kim, C.S.; Kim, S.B.; Rha, S.Y.; Lee, M.Y.; Ro, J. Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res. Treat. 2008, 108, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Kim, D.W.; Chung, J.Y.; Shin, S.G.; Kim, S.C.; Heo, D.S.; Kim, N.K.; Bang, Y.J. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin. Cancer Res. 2004, 10, 3708–3716. [Google Scholar] [CrossRef]
- Hedrich, W.D.; Fandy, T.E.; Ashour, H.M.; Wang, H.; Hassan, H.E. Antibody-Drug Conjugates: Pharmacokinetic/Pharmacodynamic Modeling, Preclinical Characterization, Clinical Studies, and Lessons Learned. Clin. Pharm. 2018, 57, 687–703. [Google Scholar] [CrossRef]
- Tang, X.; Loc, W.S.; Dong, C.; Matters, G.L.; Butler, P.J.; Kester, M.; Meyers, C.; Jiang, Y.; Adair, J.H. The use of nanoparticulates to treat breast cancer. Nanomedicine 2017, 12, 2367–2388. [Google Scholar] [CrossRef]
- Shah, D.K.; Haddish-Berhane, N.; Betts, A. Bench to bedside translation of antibody drug conjugates using a multiscale mechanistic PK/PD model: A case study with brentuximab-vedotin. J. Pharm. Pharm. 2012, 39, 643–659. [Google Scholar] [CrossRef]
- Shah, N.; Mohammad, A.S.; Saralkar, P.; Sprowls, S.A.; Vickers, S.D.; John, D.; Tallman, R.M.; Lucke-Wold, B.P.; Jarrell, K.E.; Pinti, M.; et al. Investigational chemotherapy and novel pharmacokinetic mechanisms for the treatment of breast cancer brain metastases. Pharmacol. Res. 2018, 132, 47–68. [Google Scholar] [CrossRef] [PubMed]
- Okeley, N.M.; Miyamoto, J.B.; Zhang, X.; Sanderson, R.J.; Benjamin, D.R.; Sievers, E.L.; Senter, P.D.; Alley, S.C. Intracellular activation of SGN-35, a potent anti-CD30 antibody-drug conjugate. Clin. Cancer Res. 2010, 16, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Fouliard, S.; Chenel, M.; Marcucci, F. Influence of the duration of intravenous drug administration on tumor uptake. Front. Oncol. 2013, 3, 192. [Google Scholar] [CrossRef]
- Fanale, M.A.; Forero-Torres, A.; Rosenblatt, J.D.; Advani, R.H.; Franklin, A.R.; Kennedy, D.A.; Han, T.H.; Sievers, E.L.; Bartlett, N.L. A phase I weekly dosing study of brentuximab vedotin in patients with relapsed/refractory CD30-positive hematologic malignancies. Clin. Cancer Res. 2012, 18, 248–255. [Google Scholar] [CrossRef]
- Li, C.; Wang, B.; Lu, D.; Jin, J.Y.; Gao, Y.; Matsunaga, K.; Igawa, Y.; Nijem, I.; Lu, M.; Strasak, A.; et al. Ethnic sensitivity assessment of the antibody-drug conjugate trastuzumab emtansine (T-DM1) in patients with HER2-positive locally advanced or metastatic breast cancer. Cancer Chemother Pharm. 2016, 78, 547–558. [Google Scholar] [CrossRef]
- Betts, A.M.; Haddish-Berhane, N.; Tolsma, J.; Jasper, P.; King, L.E.; Sun, Y.; Chakrapani, S.; Shor, B.; Boni, J.; Johnson, T.R. Preclinical to Clinical Translation of Antibody-Drug Conjugates Using PK/PD Modeling: A Retrospective Analysis of Inotuzumab Ozogamicin. AAPS J. 2016, 18, 1101–1116. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Ghosh, B.; Biswas, S. Nanocarriers for cancer-targeted drug delivery. J. Drug Target. 2016, 24, 179–191. [Google Scholar] [CrossRef]
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Bayda, S.; Hadla, M.; Palazzolo, S.; Riello, P.; Corona, G.; Toffoli, G.; Rizzolio, F. Inorganic Nanoparticles for Cancer Therapy: A Transition from Lab to Clinic. Curr. Med. Chem. 2018, 25, 4269–4303. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, C. Advances in silica based nanoparticles for targeted cancer therapy. Nanomedicine 2016, 12, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.L.; Tang, W.H.; Li, S.D. Cancer theranostic applications of lipid-based nanoparticles. Drug Discov. Today 2018, 23, 1159–1166. [Google Scholar] [CrossRef]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef]
- Date, T.; Nimbalkar, V.; Kamat, J.; Mittal, A.; Mahato, R.I.; Chitkara, D. Lipid-polymer hybrid nanocarriers for delivering cancer therapeutics. J. Control. Release 2018, 271, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, L. Membrane-core nanoparticles for cancer nanomedicine. Adv. Drug Deliv. Rev. 2020, 156, 23–39. [Google Scholar] [CrossRef]
- Ombredane, A.S.; Silva, V.R.P.; Andrade, L.R.; Pinheiro, W.O.; Simonelly, M.; Oliveira, J.V.; Pinheiro, A.C.; Gonçalves, G.F.; Felice, G.J.; Garcia, M.P.; et al. In Vivo Efficacy and Toxicity of Curcumin Nanoparticles in Breast Cancer Treatment: A Systematic Review. Front. Oncol. 2021, 11, 612903. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Tuli, H.S.; Yerer, M.B.; Sharma, A.; Sak, K.; Srivastava, S.; Pandey, A.; Garg, V.K.; Sethi, G.; Bishayee, A. Natural product-based nanoformulations for cancer therapy: Opportunities and challenges. Semin. Cancer Biol. 2021, 69, 5–23. [Google Scholar] [CrossRef]
- Karuppiah, A.; Rajan, R.; Ramanathan, M.; Nagarajan, A. Cytotoxicity and Synergistic Effect of Biogenically Synthesized Ternary Therapeutic Nano Conjugates Comprising Plant Active Principle, Silver and Anticancer Drug on MDA-MB-453 Breast Cancer Cell Line. Asian Pac. J. Cancer Prev. 2020, 21, 195–204. [Google Scholar] [CrossRef]
- Soni, K.; Rizwanullah, M.; Kohli, K. Development and optimization of sulforaphane-loaded nanostructured lipid carriers by the Box-Behnken design for improved oral efficacy against cancer: In vitro, ex vivo and in vivo assessments. Artif. Cells Nanomed. Biotechnol. 2018, 46, 15–31. [Google Scholar] [CrossRef]
- Jin, H.; Pi, J.; Yang, F.; Jiang, J.; Wang, X.; Bai, H.; Shao, M.; Huang, L.; Zhu, H.; Yang, P.; et al. Folate-Chitosan Nanoparticles Loaded with Ursolic Acid Confer Anti-Breast Cancer Activities in vitro and in vivo. Sci. Rep. 2016, 6, 30782. [Google Scholar] [CrossRef] [PubMed]
- Minaei, A.; Sabzichi, M.; Ramezani, F.; Hamishehkar, H.; Samadi, N. Co-delivery with nano-quercetin enhances doxorubicin-mediated cytotoxicity against MCF-7 cells. Mol. Biol. Rep. 2016, 43, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggård, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; McNeil, S.E. Immunological properties of engineered nanomaterials. Nat. Nanotechnol. 2007, 2, 469–478. [Google Scholar] [CrossRef]
- Kumari, M.; Sharma, N.; Manchanda, R.; Gupta, N.; Syed, A.; Bahkali, A.H.; Nimesh, S. PGMD/curcumin nanoparticles for the treatment of breast cancer. Sci. Rep. 2021, 11, 3824. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Mendhulkar, V.D. Antiproliferative activity of Camellia sinensis mediated silver nanoparticles on three different human cancer cell lines. J. Cancer Res. Ther. 2018, 14, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Min, K.H.; Park, K.; Kim, Y.S.; Bae, S.M.; Lee, S.; Jo, H.G.; Park, R.W.; Kim, I.S.; Jeong, S.Y.; Kim, K.; et al. Hydrophobically modified glycol chitosan nanoparticles-encapsulated camptothecin enhance the drug stability and tumor targeting in cancer therapy. J. Control. Release 2008, 127, 208–218. [Google Scholar] [CrossRef]
- Doddapaneni, R.; Patel, K.; Owaid, I.H.; Singh, M. Tumor neovasculature-targeted cationic PEGylated liposomes of gambogic acid for the treatment of triple-negative breast cancer. Drug Deliv. 2016, 23, 1232–1241. [Google Scholar] [CrossRef]
- Toy, R.; Hayden, E.; Shoup, C.; Baskaran, H.; Karathanasis, E. The effects of particle size, density and shape on margination of nanoparticles in microcirculation. Nanotechnology 2011, 22, 115101. [Google Scholar] [CrossRef]
- Toy, R.; Peiris, P.M.; Ghaghada, K.B.; Karathanasis, E. Shaping cancer nanomedicine: The effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine 2014, 9, 121–134. [Google Scholar] [CrossRef]
- White, B.E.; White, M.K.; Adhvaryu, H.; Makhoul, I.; Nima, Z.A.; Biris, A.S.; Ali, N. Nanotechnology approaches to addressing HER2-positive breast cancer. Cancer Nanotechnol. 2020, 11, 12. [Google Scholar] [CrossRef]
- Shen, Z.; Ye, H.; Yi, X.; Li, Y. Membrane Wrapping Efficiency of Elastic Nanoparticles during Endocytosis: Size and Shape Matter. ACS Nano 2019, 13, 215–228. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, H.; Ye, H.; Zheng, Y. Receptor-Mediated Endocytosis of Nanoparticles: Roles of Shapes, Orientations, and Rotations of Nanoparticles. J. Phys. Chem. B 2018, 122, 171–180. [Google Scholar] [CrossRef]
- Kim, S.; Seo, J.; Park, H.H.; Kim, N.; Oh, J.W.; Nam, J.M. Plasmonic Nanoparticle-Interfaced Lipid Bilayer Membranes. Acc. Chem. Res. 2019, 52, 2793–2805. [Google Scholar] [CrossRef] [PubMed]
- Khor, S.Y.; Vu, M.N.; Pilkington, E.H.; Johnston, A.P.R.; Whittaker, M.R.; Quinn, J.F.; Truong, N.P.; Davis, T.P. Elucidating the Influences of Size, Surface Chemistry, and Dynamic Flow on Cellular Association of Nanoparticles Made by Polymerization-Induced Self-Assembly. Small 2018, 14, e1801702. [Google Scholar] [CrossRef]
- Jafari, M.; Sriram, V.; Xu, Z.; Harris, G.M.; Lee, J.Y. Fucoidan-Doxorubicin Nanoparticles Targeting P-Selectin for Effective Breast Cancer Therapy. Carbohydr. Polym. 2020, 249, 116837. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; He, M.; Yang, Y.; Ma, M.; Guo, Z.; Zhao, M.; Liang, J.; Sun, Y.; Fan, Y.; Zhang, X. Reversing P-Glycoprotein-Associated Multidrug Resistance of Breast Cancer by Targeted Acid-Cleavable Polysaccharide Nanoparticles with Lapatinib Sensitization. ACS Appl. Mater. Interfaces 2020, 12, 51198–51211. [Google Scholar] [CrossRef]
- Kostryukova, L.V.; Tereshkina, Y.A.; Korotkevich, E.I.; Prozorovsky, V.N.; Torkhovskaya, T.I.; Morozevich, G.E.; Toropygin, I.Y.; Konstantinov, M.A.; Tikhonova, E.G. Targeted drug delivery system for doxorubicin based on a specific peptide and phospholipid nanoparticles. Biomed. Khim. 2020, 66, 464–468. [Google Scholar] [CrossRef]
- Kim, B.; Shin, J.; Wu, J.; Omstead, D.T.; Kiziltepe, T.; Littlepage, L.E.; Bilgicer, B. Engineering peptide-targeted liposomal nanoparticles optimized for improved selectivity for HER2-positive breast cancer cells to achieve enhanced in vivo efficacy. J. Control. Release 2020, 322, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Bano, K.; Bajwa, S.Z.; Ihsan, A.; Hussain, I.; Jameel, N.; Rehman, A.; Taj, A.; Younus, S.; Zubair Iqbal, M.; Butt, F.K.; et al. Synthesis of SPIONs-CNT Based Novel Nanocomposite for Effective Amperometric Sensing of First-Line Antituberculosis Drug Rifampicin. J. Nanosci. Nanotechnol. 2020, 20, 2130–2137. [Google Scholar] [CrossRef] [PubMed]
- Tade, R.S.; Patil, P.O. Theranostic Prospects of Graphene Quantum Dots in Breast Cancer. ACS Biomater. Sci. Eng. 2020, 6, 5987–6008. [Google Scholar] [CrossRef]
- Nakajima, M.; Sakoda, Y.; Adachi, K.; Nagano, H.; Tamada, K. Improved survival of chimeric antigen receptor-engineered T (CAR-T) and tumor-specific T cells caused by anti-programmed cell death protein 1 single-chain variable fragment-producing CAR-T cells. Cancer Sci. 2019, 110, 3079–3088. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, J.; Wang, P.; Liu, X.; Huo, P.; Xu, Y.; Chen, W.; Xu, H.; Tian, Q. Investigation of an antitumor drug-delivery system based on anti-HER2 antibody-conjugated BSA nanoparticles. Anticancer Drugs 2018, 29, 307–322. [Google Scholar] [CrossRef]
- Mohammadinejad, A.; Taghdisi, S.M.; Es’haghi, Z.; Abnous, K.; Mohajeri, S.A. Targeted imaging of breast cancer cells using two different kinds of aptamers-functionalized nanoparticles. Eur. J. Pharm. Sci. 2019, 134, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Narmani, A.; Rezvani, M.; Farhood, B.; Darkhor, P.; Mohammadnejad, J.; Amini, B.; Refahi, S.; Abdi Goushbolagh, N. Folic acid functionalized nanoparticles as pharmaceutical carriers in drug delivery systems. Drug Dev. Res. 2019, 80, 404–424. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Qi, Y.; Zhang, Y.; Wang, Y.; Zhao, X.; Min, H.; Han, X.; Lang, J.; Qin, H.; Shi, Q.; et al. Epidermal Growth Factor Receptor-Targeting Peptide Nanoparticles Simultaneously Deliver Gemcitabine and Olaparib To Treat Pancreatic Cancer with Breast Cancer 2 ( BRCA2) Mutation. ACS Nano 2018, 12, 10785–10796. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, G.S.; Athawale, R.B.; Gude, R.P.; Md, S.; Alhakamy, N.A.; Fahmy, U.A.; Kesharwani, P. Formulation and Development of Transferrin Targeted Solid Lipid Nanoparticles for Breast Cancer Therapy. Front. Pharmacol. 2020, 11, 614290. [Google Scholar] [CrossRef]
- Naruphontjirakul, P.; Viravaidya-Pasuwat, K. Development of anti-HER2-targeted doxorubicin-core-shell chitosan nanoparticles for the treatment of human breast cancer. Int. J. Nanomed. 2019, 14, 4105–4121. [Google Scholar] [CrossRef] [PubMed]
- Cristofolini, T.; Dalmina, M.; Sierra, J.A.; Silva, A.H.; Pasa, A.A.; Pittella, F.; Creczynski-Pasa, T.B. Multifunctional hybrid nanoparticles as magnetic delivery systems for siRNA targeting the HER2 gene in breast cancer cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110555. [Google Scholar] [CrossRef]
- Kavithaa, K.; Paulpandi, M.; Padma, P.R.; Sumathi, S. Induction of intrinsic apoptotic pathway and cell cycle arrest via baicalein loaded iron oxide nanoparticles as a competent nano-mediated system for triple negative breast cancer therapy. RSC Adv. 2016, 6, 64531–64543. [Google Scholar] [CrossRef]
- Guo, C.; Chen, Y.; Gao, W.; Chang, A.; Ye, Y.; Shen, W.; Luo, Y.; Yang, S.; Sun, P.; Xiang, R.; et al. Liposomal Nanoparticles Carrying anti-IL6R Antibody to the Tumour Microenvironment Inhibit Metastasis in Two Molecular Subtypes of Breast Cancer Mouse Models. Theranostics 2017, 7, 775–788. [Google Scholar] [CrossRef]
- Salkho, N.M.; Paul, V.; Kawak, P.; Vitor, R.F.; Martins, A.M.; Al Sayah, M.; Husseini, G.A. Ultrasonically controlled estrone-modified liposomes for estrogen-positive breast cancer therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 462–472. [Google Scholar] [CrossRef]
- Kamalabadi-Farahani, M.; Vasei, M.; Ahmadbeigi, N.; Ebrahimi-Barough, S.; Soleimani, M.; Roozafzoon, R. Anti-tumour effects of TRAIL-expressing human placental derived mesenchymal stem cells with curcumin-loaded chitosan nanoparticles in a mice model of triple negative breast cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, S1011–S1021. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Wang, A.; Ni, L.; Zhang, L.; Yan, X.; Jiang, Y.; Mu, H.; Wu, Z.; Sun, K.; Li, Y. Trastuzumab-and Fab’ fragment-modified curcumin PEG-PLGA nanoparticles: Preparation and evaluation in vitro and in vivo. Int. J. Nanomed. 2018, 13, 1831–1840. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, B.B.S.; Lasham, A.; Shelling, A.N.; Al-Kassas, R. Development of biodegradable PLGA nanoparticles surface engineered with hyaluronic acid for targeted delivery of paclitaxel to triple negative breast cancer cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, J.; Wang, Y.; Chen, M. Hyaluronic acid-coated PEI-PLGA nanoparticles mediated co-delivery of doxorubicin and miR-542-3p for triple negative breast cancer therapy. Nanomedicine 2016, 12, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shao, M.; Zhong, Z.; Wang, A.; Cao, J.; Lu, Y.; Wang, Y.; Zhang, J. Co-delivery of gambogic acid and TRAIL plasmid by hyaluronic acid grafted PEI-PLGA nanoparticles for the treatment of triple negative breast cancer. Drug Deliv. 2017, 24, 1791–1800. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Ghosh, A.; Maiti, S.; Ahir, M.; Debnath, G.H.; Gupta, P.; Bhattacharjee, M.; Ghosh, S.; Chattopadhyay, S.; Mukherjee, P.; et al. Delivery of thymoquinone through hyaluronic acid-decorated mixed Pluronic® nanoparticles to attenuate angiogenesis and metastasis of triple-negative breast cancer. J. Control. Release 2020, 322, 357–374. [Google Scholar] [CrossRef]
- Siddhartha, V.T.; Pindiprolu, S.; Chintamaneni, P.K.; Tummala, S.; Nandha Kumar, S. RAGE receptor targeted bioconjuguate lipid nanoparticles of diallyl disulfide for improved apoptotic activity in triple negative breast cancer: In vitro studies. Artif. Cells Nanomed. Biotechnol. 2018, 46, 387–397. [Google Scholar] [CrossRef]
- Okines, A.F.C.; Ulrich, L. Investigational antibody-drug conjugates in clinical trials for the treatment of breast cancer. Expert Opin. Investig. Drugs 2021, 30, 789–795. [Google Scholar] [CrossRef]
- Bardia, A.; Mayer, I.A.; Diamond, J.R.; Moroose, R.L.; Isakoff, S.J.; Starodub, A.N.; Shah, N.C.; O’Shaughnessy, J.; Kalinsky, K.; Guarino, M.; et al. Efficacy and Safety of Anti-Trop-2 Antibody Drug Conjugate Sacituzumab Govitecan (IMMU-132) in Heavily Pretreated Patients With Metastatic Triple-Negative Breast Cancer. J. Clin. Oncol. 2017, 35, 2141–2148. [Google Scholar] [CrossRef]
- Bardia, A.; Mayer, I.A.; Vahdat, L.T.; Tolaney, S.M.; Isakoff, S.J.; Diamond, J.R.; O’Shaughnessy, J.; Moroose, R.L.; Santin, A.D.; Abramson, V.G.; et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2019, 380, 741–751. [Google Scholar] [CrossRef]
- Dai, W.; Yang, F.; Ma, L.; Fan, Y.; He, B.; He, Q.; Wang, X.; Zhang, H.; Zhang, Q. Combined mTOR inhibitor rapamycin and doxorubicin-loaded cyclic octapeptide modified liposomes for targeting integrin α3 in triple-negative breast cancer. Biomaterials 2014, 35, 5347–5358. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Ahn, R.W.; Chen, F.; Fought, A.J.; O’Halloran, T.V.; Cryns, V.L.; Nguyen, S.T. Biological evaluation of pH-responsive polymer-caged nanobins for breast cancer therapy. ACS Nano 2010, 4, 4971–4978. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.C.; Kulak, N.; Pridgen, E.M.; Farokhzad, O.C.; Langer, R.; Lippard, S.J. Nanoparticle encapsulation of mitaplatin and the effect thereof on in vivo properties. ACS Nano 2013, 7, 5675–5683. [Google Scholar] [CrossRef] [PubMed]
- Massadeh, S.; Omer, M.E.; Alterawi, A.; Ali, R.; Alanazi, F.H.; Almutairi, F.; Almotairi, W.; Alobaidi, F.F.; Alhelal, K.; Almutairi, M.S.; et al. Optimized Polyethylene Glycolylated Polymer-Lipid Hybrid Nanoparticles as a Potential Breast Cancer Treatment. Pharmaceutics 2020, 12, 666. [Google Scholar] [CrossRef]
- Du, M.; Ouyang, Y.; Meng, F.; Zhang, X.; Ma, Q.; Zhuang, Y.; Liu, H.; Pang, M.; Cai, T.; Cai, Y. Polymer-lipid hybrid nanoparticles: A novel drug delivery system for enhancing the activity of Psoralen against breast cancer. Int. J. Pharm. 2019, 561, 274–282. [Google Scholar] [CrossRef]
- Li, J.; Xu, W.; Yuan, X.; Chen, H.; Song, H.; Wang, B.; Han, J. Polymer-lipid hybrid anti-HER2 nanoparticles for targeted salinomycin delivery to HER2-positive breast cancer stem cells and cancer cells. Int. J. Nanomed. 2017, 12, 6909–6921. [Google Scholar] [CrossRef]
- Tambe, V.; Thakkar, S.; Raval, N.; Sharma, D.; Kalia, K.; Tekade, R.K. Surface Engineered Dendrimers in siRNA Delivery and Gene Silencing. Curr. Pharm. Des. 2017, 23, 2952–2975. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, X.H.; Wang, Z.Y.; Meng, M.; Li, X.; Ning, Q. Generation 4 polyamidoamine dendrimers is a novel candidate of nano-carrier for gene delivery agents in breast cancer treatment. Cancer Lett. 2010, 298, 34–49. [Google Scholar] [CrossRef]
- Zhang, L.; Varma, N.R.; Gang, Z.Z.; Ewing, J.R.; Arbab, A.S.; Ali, M.M. Targeting Triple Negative Breast Cancer with a Small-sized Paramagnetic Nanoparticle. J. Nanomed. Nanotechnol. 2016, 7, 404. [Google Scholar] [CrossRef]
- Jin-Wook, Y.; Elizabeth, C.; Samir, M. Factors that Control the Circulation Time of Nanoparticles in Blood: Challenges, Solutions and Future Prospects. Curr. Pharm. Des. 2010, 16, 2298–2307. [Google Scholar]
- Zu, M.; Ma, Y.; Cannup, B.; Xie, D.; Jung, Y.; Zhang, J.; Yang, C.; Gao, F.; Merlin, D.; Xiao, B. Oral delivery of natural active small molecules by polymeric nanoparticles for the treatment of inflammatory bowel diseases. Adv. Drug Deliv. Rev. 2021, 176, 113887. [Google Scholar] [CrossRef]
- Zaman, R.; Islam, R.A.; Ibnat, N.; Othman, I.; Zaini, A.; Lee, C.Y.; Chowdhury, E.H. Current strategies in extending half-lives of therapeutic proteins. J. Control. Release 2019, 301, 176–189. [Google Scholar] [CrossRef]
- Baharifar, H.; Khoobi, M.; Arbabi Bidgoli, S.; Amani, A. Preparation of PEG-grafted chitosan/streptokinase nanoparticles to improve biological half-life and reduce immunogenicity of the enzyme. Int. J. Biol Macromol. 2020, 143, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Biswas, A.; Shukla, A.; Maiti, P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduct. Target. Ther. 2019, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.W.; Cambre, M.; Lee, H.J. The Toxicity of Nanoparticles Depends on Multiple Molecular and Physicochemical Mechanisms. Int. J. Mol. Sci. 2017, 18, 2702. [Google Scholar] [CrossRef] [PubMed]
- Shakibaie, M.; Abharian, A.; Forootanfar, H.; Ameri, A.; Jafari, M.; Reza Rahimi, H. Cytotoxicity investigations of biogenic tellurium nanorods towards PC12 cell line. IET Nanobiotechnol. 2018, 12, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.; Kim, M.K.; Cho, H.J.; Lee, J.A.; Yu, J.; Chung, H.E.; Choi, S.J. Factors influencing the cytotoxicity of zinc oxide nanoparticles: Particle size and surface charge. J. Phys. Conf. Ser. 2011, 304, 012044. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Tagliafico, A.S.; Piana, M.; Schenone, D.; Lai, R.; Massone, A.M.; Houssami, N. Overview of radiomics in breast cancer diagnosis and prognostication. Breast 2020, 49, 74–80. [Google Scholar] [CrossRef]
- Sheth, D.; Giger, M.L. Artificial intelligence in the interpretation of breast cancer on MRI. J. Magn. Reson. Imaging 2020, 51, 1310–1324. [Google Scholar] [CrossRef] [PubMed]
- Manigandan, S.; Praveenkumar, T.R.; Brindhadevi, K. A review on role of nitrous oxide nanoparticles, potential vaccine targets, drug, health care and artificial intelligence to combat COVID-19. Appl. Nanosci. 2021, 1–8. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganesan, K.; Wang, Y.; Gao, F.; Liu, Q.; Zhang, C.; Li, P.; Zhang, J.; Chen, J. Targeting Engineered Nanoparticles for Breast Cancer Therapy. Pharmaceutics 2021, 13, 1829. https://doi.org/10.3390/pharmaceutics13111829
Ganesan K, Wang Y, Gao F, Liu Q, Zhang C, Li P, Zhang J, Chen J. Targeting Engineered Nanoparticles for Breast Cancer Therapy. Pharmaceutics. 2021; 13(11):1829. https://doi.org/10.3390/pharmaceutics13111829
Chicago/Turabian StyleGanesan, Kumar, Yan Wang, Fei Gao, Qingqing Liu, Chen Zhang, Peng Li, Jinming Zhang, and Jianping Chen. 2021. "Targeting Engineered Nanoparticles for Breast Cancer Therapy" Pharmaceutics 13, no. 11: 1829. https://doi.org/10.3390/pharmaceutics13111829
APA StyleGanesan, K., Wang, Y., Gao, F., Liu, Q., Zhang, C., Li, P., Zhang, J., & Chen, J. (2021). Targeting Engineered Nanoparticles for Breast Cancer Therapy. Pharmaceutics, 13(11), 1829. https://doi.org/10.3390/pharmaceutics13111829







