Experimental Evaluation of 65Zn Decorporation Kinetics Following Rapid and Delayed Zn-DTPA Interventions in Rats. Biphasic Compartmental and Square-Root Law Mathematical Modeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Equipment
2.3. Animals Studies
2.4. Data Analysis
3. Results and Discussion
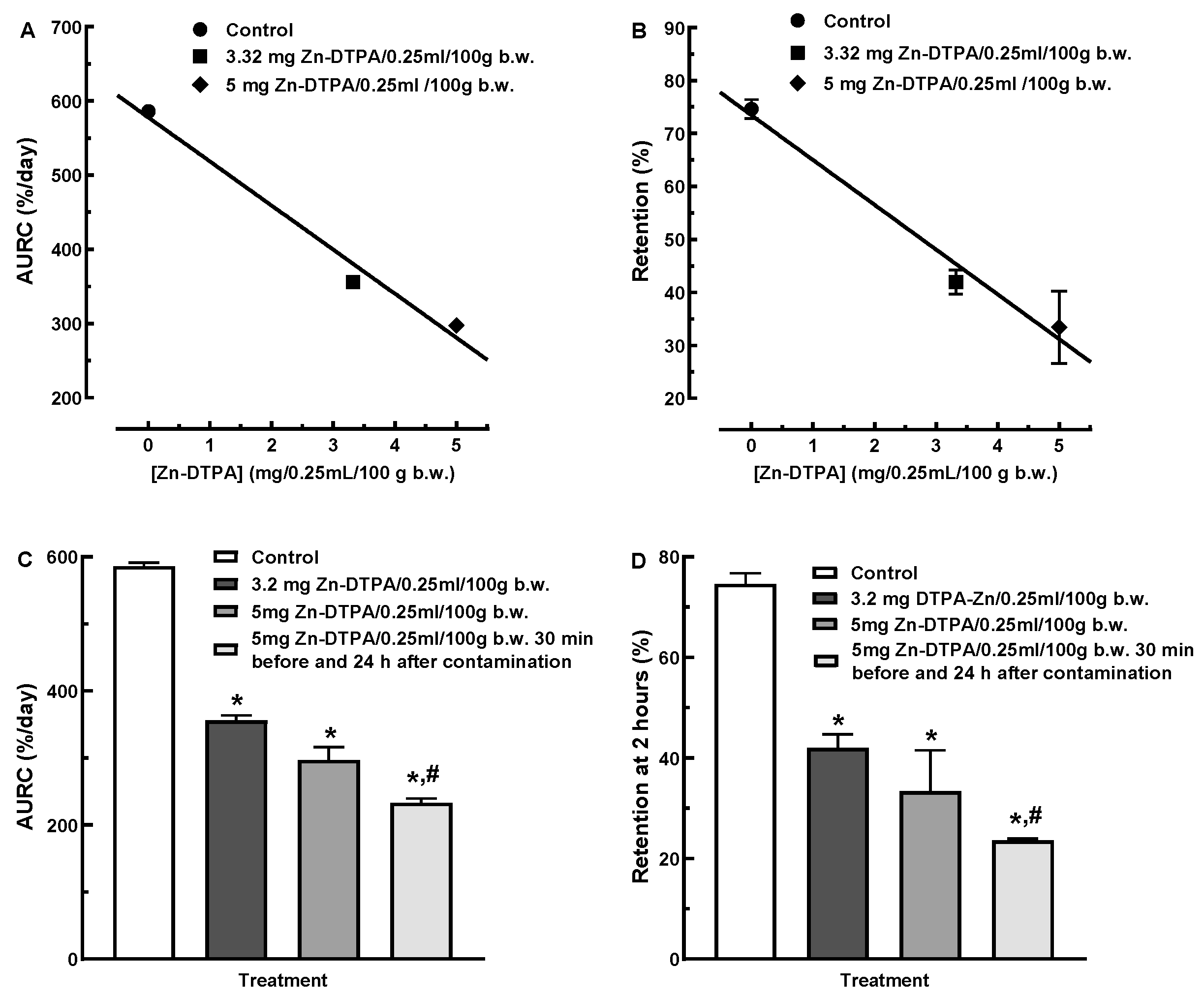
3.1. Dose Dependence of 65Zn Decorporation
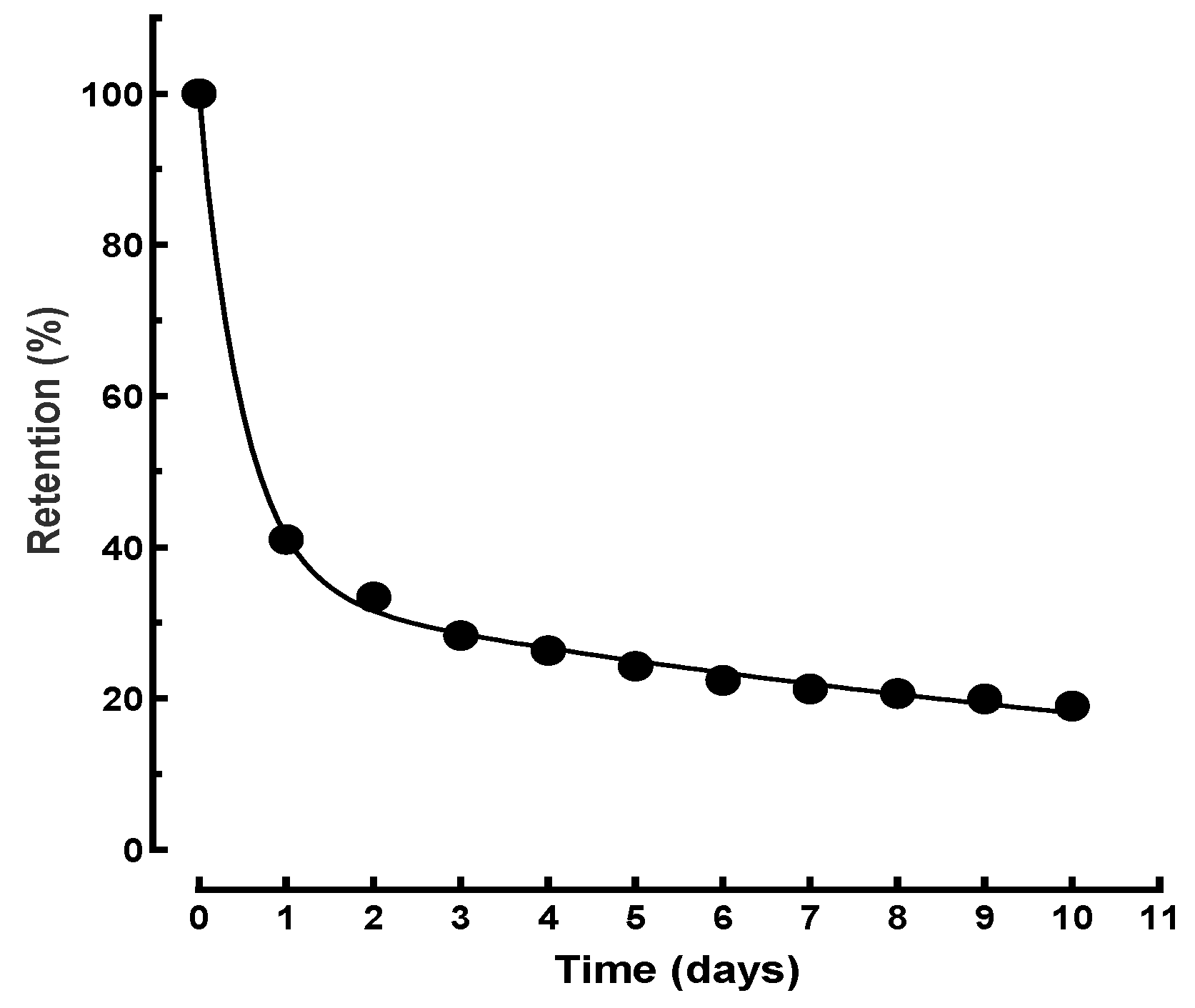
3.2. Mono-Exponential Kinetics Modeling of 65ZnCl2 Decorporation Following Rapid Intervention with Zn-DTPA
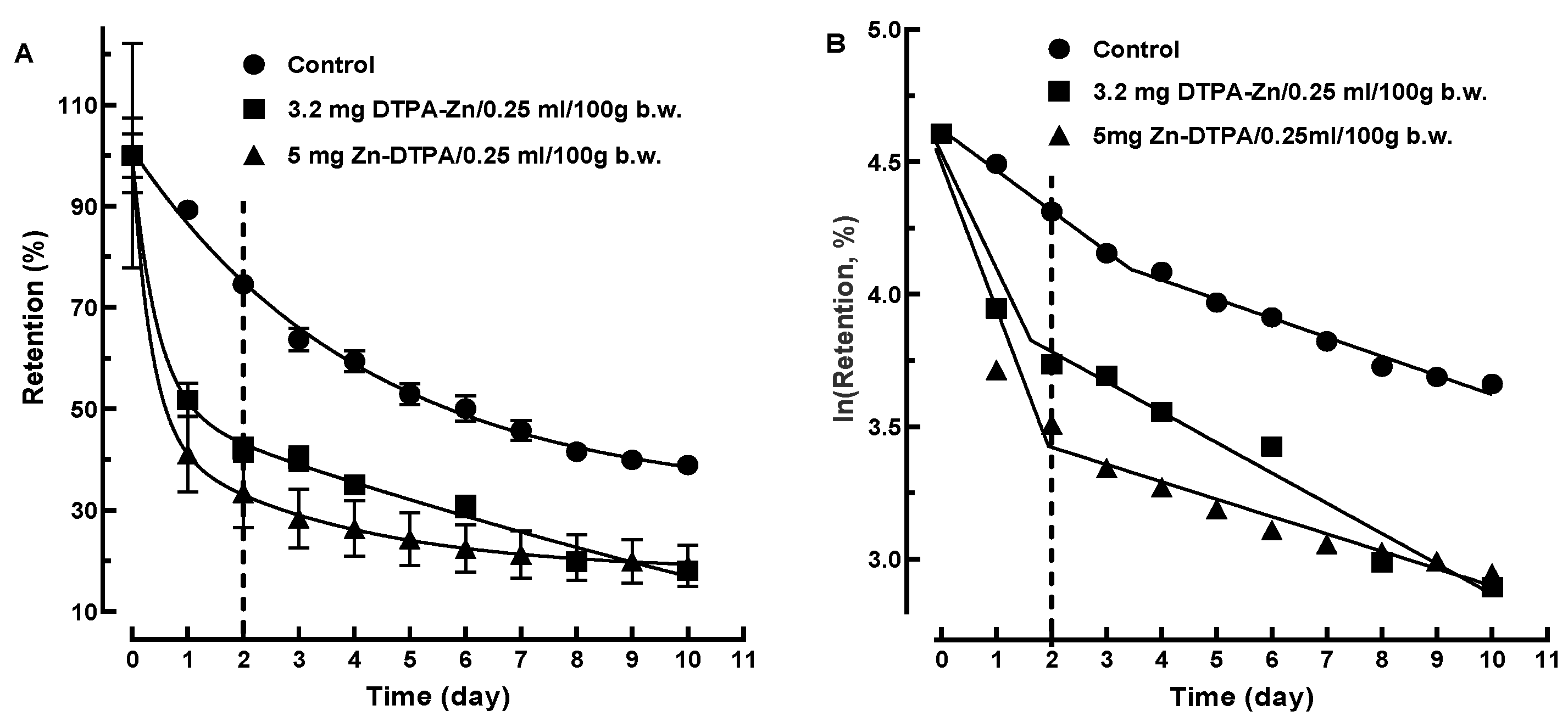
3.3. Two Mono-Exponential Steps Kinetics Model
3.3.1. 65Zn Decorporation Modeling Following Rapid Intervention with Zn-DTPA
3.3.2. 65Zn Decorporation Modeling Following Delayed Intervention with Zn-DTPA
3.4. Compartmental Mathematical Modeling
3.4.1. The Bi-Compartmental Model
3.4.2. Degeneration of the Bi-Compartmental Model
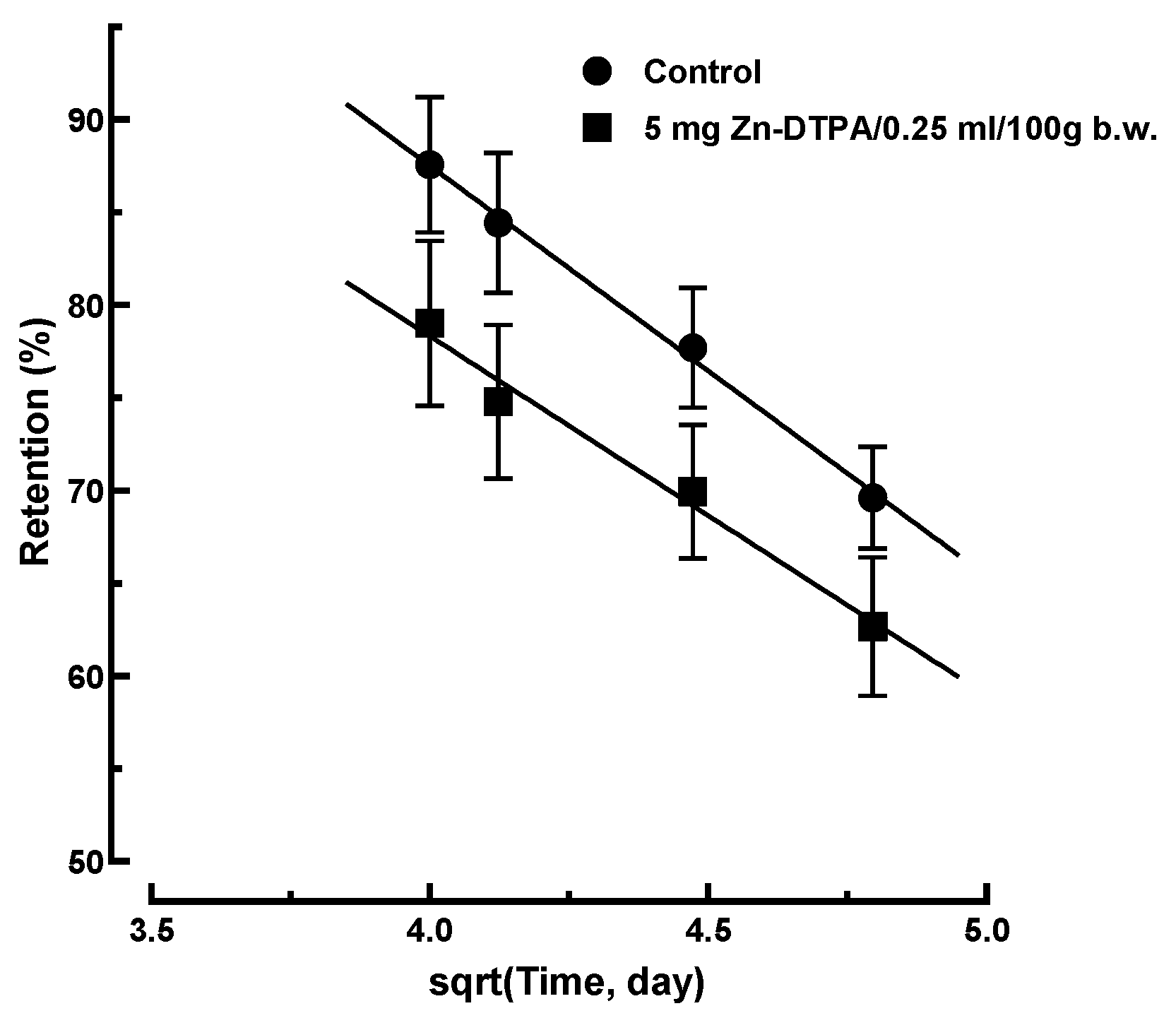
3.5. The Diffusion Model. The Square-Root Law
3.5.1. Transfer Modeling Following Rapid Intervention (Days 2 to 10)
3.5.2. Long-Term 65Zn Decorporation Modeling Following Delayed Intervention with Zn-DTPA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Högberg, L. Root Causes and Impacts of Severe Accidents at Large Nuclear Power Plants. Ambio 2013, 42, 267–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fushiki, S. Radiation hazards in children–Lessons from Chernobyl, Three Mile Island and Fukushima. Brain Dev. 2013, 35, 220–227. [Google Scholar] [CrossRef]
- Cassatt, D.R.; Kaminski, J.M.; Hatchett, R.J.; DiCarlo, A.L.; Benjamin, J.M.; Maidment, B.W. Medical Countermeasures against Nuclear Threats: Radionuclide Decorporation Agents. Radiat. Res. 2008, 170, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Waller, E.; Wilkinson, D. MEDECOR—A medical decorporation tool to assist first responders, receivers, and medical reach-back personnel in triage, treatment, and risk assessment after internalization of radionuclides. Health Phys. 2010, 99, 581–590. [Google Scholar] [CrossRef]
- Grappin, L.; Berard, P. Autorisation de mise sur le marché du Ca-DTPA. Radioprotection 2008, 43, 465–466. [Google Scholar] [CrossRef]
- Fukuda, S. Assessment of Toxicity on Chelating Agent DTPA Diethylenetriaminepentaacetic Acid. Jpn. J. Health Phys. 1989, 24, 201–210. [Google Scholar] [CrossRef]
- Chen, S.; Ko, R.; Lai, E.P.C.; Wyatt, H.; Abergel, R.J.; Li, C. Encapsulated 3,4,3-Li(1,2-Hopo) in Chitosan Nanoparticles for Decorporation Via Inhalation. Radiat. Prot. Dosim. 2018, 182, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Dumit, S.; Avtandilashvili, M.; Strom, D.J.; McComish, S.L.; Tabatadze, G.; Tolmachev, S.Y. Improved Modeling of Plutonium-DTPA Decorporation. Radiat. Res. 2018, 191, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Gervelas, C.; Serandour, A.-L.; Geiger, S.; Grillon, G.; Fritsch, P.; Taulelle, C.; Le Gall, B.; Benech, H.; Deverre, J.-R.; Fattal, E.; et al. Direct lung delivery of a dry powder formulation of DTPA with improved aerosolization properties: Effect on lung and systemic decorporation of plutonium. J. Control. Release 2007, 118, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Grémy, O.; Miccoli, L.; Lelan, F.; Bohand, S.; Chérel, M.; Mougin-Degraef, M. Delivery of DTPA through Liposomes as a Good Strategy for Enhancing Plutonium Decorporation Regardless of Treatment Regimen. Radiat. Res. 2018, 189, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Breustedt, B.; Blanchardon, E.; Bérard, P.; Fritsch, P.; Giussani, A.; Lopez, M.A.; Luciani, A.; Nosske, D.; Piechowski, J.; Schimmelpfeng, J.; et al. The CONRAD approach to biokinetic modeling of DTPA decorporation therapy. Health Phys. 2010, 99, 547–552. [Google Scholar] [CrossRef]
- Mircioiu, C.; Voicu, V.; Anuta, V.; Tudose, A.; Celia, C.; Paolino, D.; Fresta, M.; Sandulovici, R.; Mircioiu, I. Mathematical Modeling of Release Kinetics from Supramolecular Drug Delivery Systems. Pharmaceutics 2019, 11, 140. [Google Scholar] [CrossRef] [Green Version]
- Amiard, J.-C. Military Nuclear Accidents. Environmental, Ecological, Health and Socio-Economic Consequences, 1st ed.; J. Wiley & Son: Hoboken, NJ, USA, 2018; p. 45. [Google Scholar]
- Wastney, M.E.; Aamodt, R.L.; Rumble, W.F.; Henkin, R.I. Kinetic analysis of zinc metabolism and its regulation in normal humans. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1986, 251, R398–R408. [Google Scholar] [CrossRef] [PubMed]
- Llobet, J.M.; Domingo, J.L.; Colomina, M.T.; Mayayo, E.; Corbella, J. Subchronic oral toxicity of zinc in rats. Bull. Environ. Contam. Toxicol. 1988, 41, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-E.; Lomeda, R.-A.R.; Ryu, S.-H.; Lee, J.-H.; Beattie, J.H.; Kwun, I.-S. Cellular Zn depletion by metal ion chelators (TPEN, DTPA and chelex resin) and its application to osteoblastic MC3T3-E1 cells. Nutr. Res. Pract. 2007, 1, 29–35. [Google Scholar] [CrossRef]
- Council of the European Union. Council Directive 86/609/EEC of 24 November 1986 on the approximation of laws, regulations and administrative provisions of the Member States regarding the protection of animals used for experimental and other scientific purposes. O. J. 1986, 358, 1–28. [Google Scholar]
- Enache, F.; Mircioiu, I.; Corlan, G.; Sandulovici, R.; Mircioiu, C. Estimation of therapeutic equivalence using bioequivalence statistical methods for Algopirin tablets versus Excedrin analgesic formulations. Farmacia 2012, 60, 227–239. [Google Scholar]
- Stather, J.W.; Smith, H.; Bailey, M.R.; Birchall, A.; Bulman, R.A.; Crawley, F.E.H. The Retention of 14C-DTPA in Human Volunteers after Inhalation or Intravenous Injection. Health Phys. 1983, 44, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Mircioiu, I.; Anuta, V.; Ibrahim, N.; Mircioiu, C. Dissolution of tamoxifen in biorelevant media. A two phase release model. Farmacia 2012, 6, 315–324. [Google Scholar]
- Preda, I.A.; Mircioiu, I.; Mircioiu, C.; Corlan, G.; Pahomi, G.; Prasacu, I.; Anuta, V. Research concerning the development of a biorelevant dissolution test for formulations containing norfloxacin. I. Modelling of in vitro release kinetics. Farmacia 2012, 60, 675–687. [Google Scholar]
- Johnson, J.R.; Jiang, H.; Smith, B.D. Zinc(II)-Coordinated Oligotyrosine: A New Class of Cell Penetrating Peptide. Bioconjug. Chem. 2008, 19, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Phan, G.; Le Gall, B.; Deverre, J.-R.; Fattal, E.; Bénech, H. Predicting Plutonium Decorporation Efficacy after Intravenous Administration of DTPA Formulations: Study of Pharmacokinetic–Pharmacodynamic Relationships in Rats. Pharm. Res. 2006, 23, 2030–2035. [Google Scholar] [CrossRef] [PubMed]
- Guillard, O.; Courtois, P.; Murai, P.; Ducassou, D.; Reiss, D. Comparative Pharmacokinetics of [65Zn]Zinc Sulfate and [65Zn]Zinc Pantothenate Injected Intravenously in Rabbits. J. Pharm. Sci. 1984, 73, 1642–1643. [Google Scholar] [CrossRef] [PubMed]
- ICRP. Human respiratory tract model for radiological protection. ICRP Publication 66. Ann. ICRP 1994, 24, 1–3. [Google Scholar]
- ICRP. Occupational intakes of radionuclides: Part 1. ICRP Publication 130. Ann. ICRP 2015, 44. [Google Scholar] [CrossRef]
- Henkin, R.I. Metal-albumin-amino acid interactions: Chemical and physiological interrelationships. In Chemical and Physiological Interrelationships in Protein-Metal Interactions; Friedman, M., Ed.; Plenum Press: New York, NY, USA, 1974; pp. 299–328. [Google Scholar]
- Mircioiu, C.; Voicu, V.A.; Ionescu, M.; Miron, D.S.; Radulescu, F.S.; Nicolescu, A.C. Evaluation of in vitro absorption, decontamination and desorption of organophosphorous compounds from skin and synthetic membranes. Toxicol. Lett. 2013, 219, 99–106. [Google Scholar] [CrossRef]
- Anuta, V.; Mircioiu, C.; Voicu, V.; Mircioiu, I.; Sandulovici, R. Square root law model for the delivery and intestinal absorption of drugs: A case of hydrophilic captopril. Drug Deliv. 2021, 28, 1685–1694. [Google Scholar] [CrossRef]
- Carslaw, H.S.; Jaeger, J.C. Conduction of Heat in Solids, 2nd ed.; Clarendon Press: Oxford, UK, 1959. [Google Scholar]
- ICRP. Occupational Intakes of Radionuclides: Part 2. ICRP Publication 134. Ann. ICRP 2016, 45, 1–352. [Google Scholar]
- Nosske, D.; Berkovski, V.; Birchall, A.; Blanchardon, E.; Cantone, M.C.; Davis, K.; Giussani, A.; Luciani, A.; Marsh, J.; Oeh, U.; et al. The work of the CONRAD task group 5.2: Research studies on biokinetic models. Radiat. Prot. Dosim. 2007, 127, 93–96. [Google Scholar] [CrossRef]
- Leggett, R.W. Reliability of the ICRP’s dose coefficients for members of the public. III. Plutonium as a case study of uncertainties in the systemic biokinetics of radionuclides. Radiat. Prot. Dosim. 2003, 106, 103–120. [Google Scholar] [CrossRef]
- Semelka, R.C.; Ramalho, M.; Jay, M.; Hickey, L.; Hickey, J. Intravenous Calcium-/Zinc-Diethylene Triamine Penta-Acetic Acid in Patients with Presumed Gadolinium Deposition Disease: A Preliminary Report on 25 Patients. Investig. Radiol. 2018, 53, 373–379. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voicu, V.; Jiquidi, M.; Mircioiu, C.; Sandulovici, R.; Nicolescu, A. Experimental Evaluation of 65Zn Decorporation Kinetics Following Rapid and Delayed Zn-DTPA Interventions in Rats. Biphasic Compartmental and Square-Root Law Mathematical Modeling. Pharmaceutics 2021, 13, 1830. https://doi.org/10.3390/pharmaceutics13111830
Voicu V, Jiquidi M, Mircioiu C, Sandulovici R, Nicolescu A. Experimental Evaluation of 65Zn Decorporation Kinetics Following Rapid and Delayed Zn-DTPA Interventions in Rats. Biphasic Compartmental and Square-Root Law Mathematical Modeling. Pharmaceutics. 2021; 13(11):1830. https://doi.org/10.3390/pharmaceutics13111830
Chicago/Turabian StyleVoicu, Victor, Marilena Jiquidi, Constantin Mircioiu, Roxana Sandulovici, and Adrian Nicolescu. 2021. "Experimental Evaluation of 65Zn Decorporation Kinetics Following Rapid and Delayed Zn-DTPA Interventions in Rats. Biphasic Compartmental and Square-Root Law Mathematical Modeling" Pharmaceutics 13, no. 11: 1830. https://doi.org/10.3390/pharmaceutics13111830





