Optimization of Printing Parameters for Digital Light Processing 3D Printing of Hollow Microneedle Arrays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
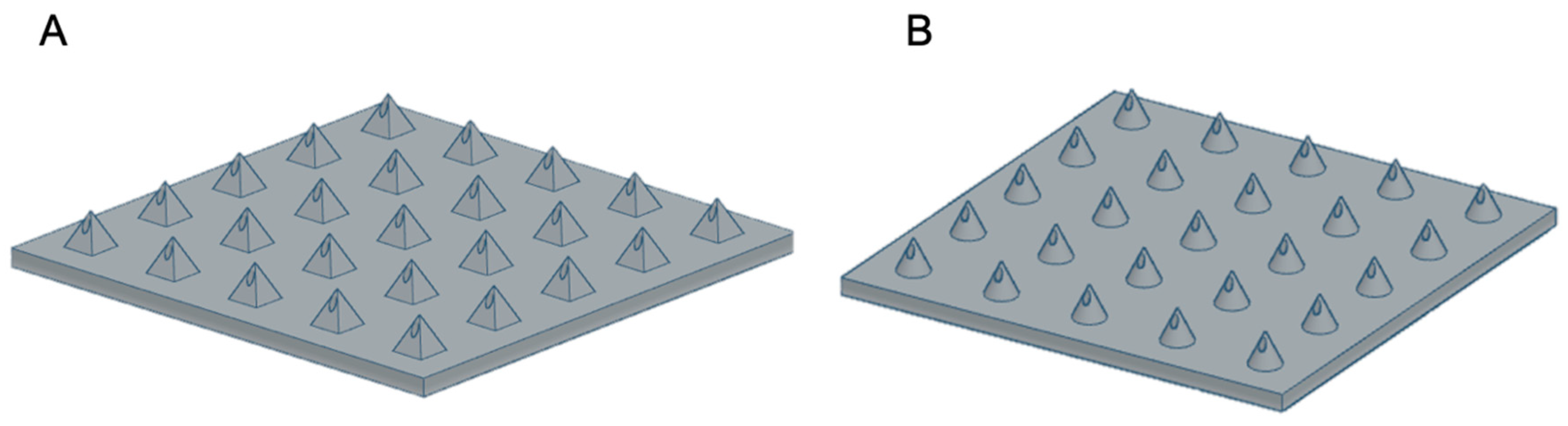
2.2. Design and Manufacture of MNs
2.2.1. DLP Printing
2.2.2. SLA 3D Printing
2.2.3. UV LCD Printing
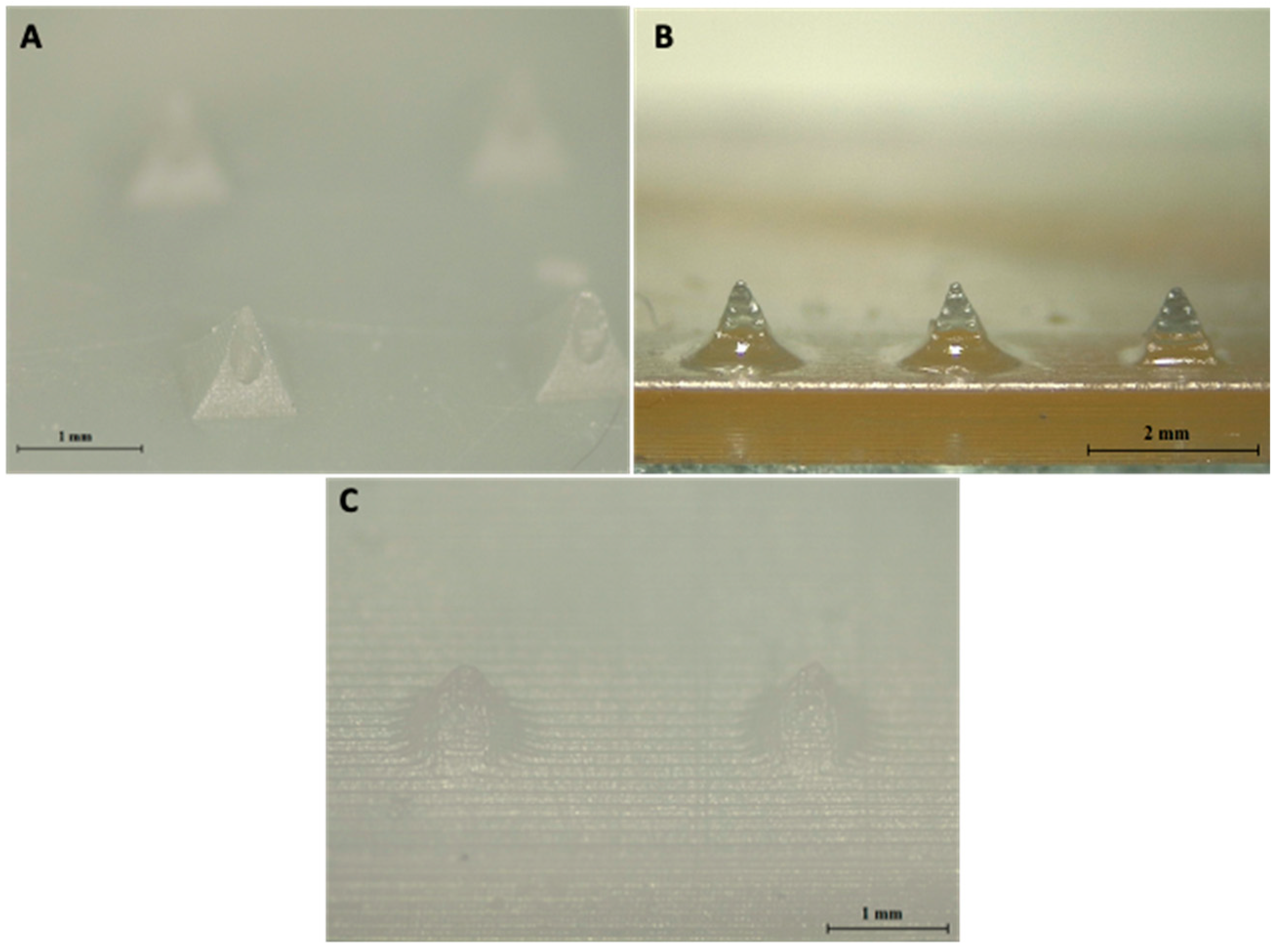
2.3. Imaging of 3D Printed MNs
2.4. Angled Prints for Print Optimisation
2.5. Parafilm Insertion Tests
2.6. Mechanical Testing of MN Arrays
2.7. Statistical Analysis
3. Results and Discussion
3.1. Comparison of Resin-Based Printers
3.2. Printing Capabilities of DLP Printer
3.3. Parafilm Insertion Tests
3.4. Print Angle Optimisation
3.5. Mechanical Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brown, M.B.; Williams, A.C. The Art and Science of Dermal Formulation Development; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Benson, H.A.; Watkinson, A.C. Topical and Transdermal Drug Delivery: Principles and Practice; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Cárcamo-Martínez, Á.; Mallon, B.; Domínguez-Robles, J.; Vora, L.K.; Anjani, Q.K.; Donnelly, R.F. Hollow microneedles: A perspective in biomedical applications. Int. J. Pharm. 2021, 599, 120455. [Google Scholar] [CrossRef]
- Dharadhar, S.; Majumdar, A.; Dhoble, S.; Patravale, V. Microneedles for transdermal drug delivery: A systematic review. Drug Dev. Ind. Pharm. 2019, 45, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Vora, L.K.; Domínguez-Robles, J.; Naser, Y.A.; Li, M.; Larrañeta, E.; Donnelly, R.F. Hydrogel-forming microneedles for rapid and efficient skin deposition of controlled release tip-implants. Mater. Sci. Eng. C 2021, 127, 112226. [Google Scholar] [CrossRef]
- Kochhar, J.S.; Tan, J.J.; Kwang, Y.C.; Kang, L. Microneedles for Transdermal Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Donnelly, R.F.; Majithiya, R.; Singh, T.R.R.; Morrow, D.I.; Garland, M.J.; Demir, Y.K.; Migalska, K.; Ryan, E.; Gillen, D.; Scott, C.J. Design, optimization and characterisation of polymeric microneedle arrays prepared by a novel laser-based micromoulding technique. Pharm. Res. 2011, 28, 41–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, S.P.; Landis, B.J.; Adams, Z.H.; Allen, M.G.; Prausnitz, M.R. Insertion of microneedles into skin: Measurement and prediction of insertion force and needle fracture force. J. Biomech. 2004, 37, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Vinayakumar, K.B.; Hegde, G.M.; Nayak, M.M.; Dinesh, N.S.; Rajanna, K. Fabrication and characterization of gold coated hollow silicon microneedle array for drug delivery. Microelectron. Eng. 2014, 128, 12–18. [Google Scholar] [CrossRef]
- Wang, P.M.; Cornwell, M.; Hill, J.; Prausnitz, M.R. Precise microinjection into skin using hollow microneedles. J. Investig. Dermatol. 2006, 126, 1080–1087. [Google Scholar] [CrossRef] [Green Version]
- Economidou, S.N.; Pere, C.P.P.; Reid, A.; Uddin, M.J.; Windmill, J.F.; Lamprou, D.A.; Douroumis, D. 3D printed microneedle patches using stereolithography (SLA) for intradermal insulin delivery. Mater. Sci. Eng. C 2019, 102, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Li, J.; Wang, Z.; Wang, Q.; Li, Z. Recent Advances on fabrication of microneedles on flexible substrate. J. Micromech. Microeng. 2021, 31, 073001. [Google Scholar] [CrossRef]
- Moussi, K.; Bukhamsin, A.; Hidalgo, T.; Kosel, J. Biocompatible 3D Printed Microneedles for Transdermal, Intradermal, and Percutaneous Applications. Adv. Eng. Mater. 2019, 22, 1901358. [Google Scholar] [CrossRef] [Green Version]
- Mathew, E.; Domínguez-Robles, J.; Stewart, S.A.; Mancuso, E.; O’Donnell, K.; Larraneta, E.; Lamprou, D.A. Fused Deposition Modeling as an Effective Tool for Anti-Infective Dialysis Catheter Fabrication. ACS Biomater. Sci. Eng. 2019, 5, 6300–6310. [Google Scholar] [CrossRef]
- Dabbagh, S.R.; Sarabi, M.R.; Rahbarghazi, R.; Sokullu, E.; Yetisen, A.K.; Tasoglu, S. 3D-printed microneedles in biomedical applications. iScience 2021, 24, 102012. [Google Scholar] [CrossRef] [PubMed]
- Gittard, S.D.; Ovsianikov, A.; Monteiro-Riviere, N.A.; Lusk, J.; Morel, P.; Minghetti, P.; Lenardi, C.; Chichkov, B.N.; Narayan, R.J. Fabrication of polymer microneedles using a two-photon polymerization and micromolding process. J. Diabetes Sci. Technol. 2009, 3, 304–311. [Google Scholar] [CrossRef] [Green Version]
- Ovsianikov, A.; Chichkov, B.; Mente, P.; Monteiro-Riviere, N.; Doraiswamy, A.; Narayan, R. Two photon polymerization of polymer–ceramic hybrid materials for transdermal drug delivery. Int. J. Appl. Ceram. Technol. 2007, 4, 22–29. [Google Scholar] [CrossRef]
- Shewale, J.J.; Bhole, K.S. 3D polymer microneedle array: Fabrication and analysis. In Proceedings of the 2015 International Conference on Nascent Technologies in the Engineering Field (ICNTE), Navi Mumbai, India, 9–10 January 2015; pp. 1–6. [Google Scholar]
- Economidou, S.N.; Uddin, M.J.; Marques, M.J.; Douroumis, D.; Sow, W.T.; Li, H.; Reid, A.; Windmill, J.F.; Podoleanu, A. A novel 3D printed hollow microneedle microelectromechanical system for controlled, personalized transdermal drug delivery. Addit. Manuf. 2021, 38, 101815. [Google Scholar]
- Yeung, C.; Chen, S.; King, B.; Lin, H.; King, K.; Akhtar, F.; Diaz, G.; Wang, B.; Zhu, J.; Sun, W. A 3D-printed microfluidic-enabled hollow microneedle architecture for transdermal drug delivery. Biomicrofluidics 2019, 13, 064125. [Google Scholar] [CrossRef] [Green Version]
- Mathew, E.; Pitzanti, G.; Larrañeta, E.; Lamprou, D.A. 3D Printing of Pharmaceuticals and Drug Delivery Devices; Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2020. [Google Scholar]
- Larrañeta, E.; Moore, J.; Vicente-Pérez, E.M.; González-Vázquez, P.; Lutton, R.; Woolfson, A.D.; Donnelly, R.F. A proposed model membrane and test method for microneedle insertion studies. Int. J. Pharm. 2014, 472, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Khanna, P.; Luongo, K.; Strom, J.A.; Bhansali, S. Sharpening of hollow silicon microneedles to reduce skin penetration force. J. Micromech. Microeng. 2010, 20, 045011. [Google Scholar] [CrossRef]
- Shen, J.; Ricketts, D.S. Additive Manufacturing of Complex Millimeter-Wave Waveguides Structures Using Digital Light Processing. IEEE Trans. Microw. Theory Tech. 2019, 67, 883–895. [Google Scholar] [CrossRef]
- Hanon, M.; Zsidai, L. Sliding Surface Structure Comparison of 3D Printed Polymers Using FDM and DLP Technologies; IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; p. 012015. [Google Scholar]
- Economidou, S.N.; Pissinato Pere, C.P.; Okereke, M.; Douroumis, D. Optimisation of Design and Manufacturing Parameters of 3D Printed Solid Microneedles for Improved Strength, Sharpness, and Drug Delivery. Micromachines 2021, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- Nagarkar, R.; Singh, M.; Nguyen, H.X.; Jonnalagadda, S. A review of recent advances in microneedle technology for transdermal drug delivery. J. Drug Deliv. Sci. Technol. 2020, 59, 101923. [Google Scholar] [CrossRef]







| Features | Advantages | Disadvantages | |
|---|---|---|---|
| DLP | Digital projector flashes single image of each layer over build platform. | Faster than SLA as full layer is projected onto tray each time. Less material waste for small prints. | Smaller resin tank so not suitable for large prints. Vertical voxel lines created. |
| SLA | UV laser combined with galvanometers to direct laser beam across print area. | Suitable for large prints due to presence of larger resin tanks. Wide range of compatible resins available. Smooth print finish. | Slower than DLP and LCD as laser needs to scan through each layer. |
| LCD. | LED array Light source in combination with an LCD photomask. | Fast printing. Cheap. | Lower print quality than SLA and DLP. Vertical voxel lines visible. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathew, E.; Pitzanti, G.; Gomes dos Santos, A.L.; Lamprou, D.A. Optimization of Printing Parameters for Digital Light Processing 3D Printing of Hollow Microneedle Arrays. Pharmaceutics 2021, 13, 1837. https://doi.org/10.3390/pharmaceutics13111837
Mathew E, Pitzanti G, Gomes dos Santos AL, Lamprou DA. Optimization of Printing Parameters for Digital Light Processing 3D Printing of Hollow Microneedle Arrays. Pharmaceutics. 2021; 13(11):1837. https://doi.org/10.3390/pharmaceutics13111837
Chicago/Turabian StyleMathew, Essyrose, Giulia Pitzanti, Ana L. Gomes dos Santos, and Dimitrios A. Lamprou. 2021. "Optimization of Printing Parameters for Digital Light Processing 3D Printing of Hollow Microneedle Arrays" Pharmaceutics 13, no. 11: 1837. https://doi.org/10.3390/pharmaceutics13111837
APA StyleMathew, E., Pitzanti, G., Gomes dos Santos, A. L., & Lamprou, D. A. (2021). Optimization of Printing Parameters for Digital Light Processing 3D Printing of Hollow Microneedle Arrays. Pharmaceutics, 13(11), 1837. https://doi.org/10.3390/pharmaceutics13111837









