3D Printing and Bioprinting Applications in Pharmaceutics
Share This Topical Collection
Editors
 Dr. Eneko Larrañeta Landa
Dr. Eneko Larrañeta Landa
 Dr. Eneko Larrañeta Landa
Dr. Eneko Larrañeta Landa
E-Mail
Website
Collection Editor
School of Pharmacy, Queen’s University Belfast, Belfast BT9 7BL, UK
Interests: 3D printing; polymeric drug delivery systems; medical nanotechnology; transdermal drug delivery systems; microneedles; medical devices; implantable devices
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
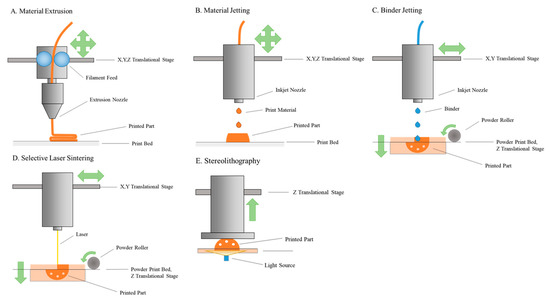
The 3D printing (3DP) process was patented in 1986; however, only in the last decade has it been used for medical applications, as well as being utilised in the fields of bio-fabrication and pharmaceutical printing. 3DP or additive manufacturing (AM) permits the fabrication of high degrees of complexity with great reproducibility, in a fast and cost-effective fashion. The term involves many processes, which use different types of printing technologies, hundreds of materials, and various resolutions and speeds. 3DP techniques can be used in pharmaceutical fields isuch as stereolithography (SLA), selective laser sintering (SLS), inkjet-based 3DP, extrusion-based fused deposition modeling (FDM), and pressure-assisted microsyringe (PAM) printing. 3DP allows the manufacturing of complex drug delivery devices and seems to be a pragmatic approach to realise the quest for a truly customised and personalised drug delivery. 3DP technology, with innovations in pharmaceutical development and an interdisciplinary approach to finding newer drug-delivery systems, can usher in a new era of treatments to various diseases. This collection invites papers in the area of 3DP and bioprinting for pharmaceutical applications, covering recent advantages and future directions of AM for pharmaceutical products, including regulatory considerations and modelling approaches. Original research papers and review articles are welcome.
Dr. Dimitrios A. Lamprou
Dr. Eneko Larrañeta
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Pharmaceutics is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- 3D printing
- 4D printing
- additive manufacturing
- bioprinting
- biomaterials
- controlled drug delivery
- computer-aided design (CAD)
- drug discovery and development
- drug delivery systems
- finite element method (FEM)
- implantable devices
- mehod evaluation and assessment
- organs-on-chips
- personalised medicine
- pharmaceutics
- regulatory
Related Special Issue
Published Papers (30 papers)
Open AccessArticle
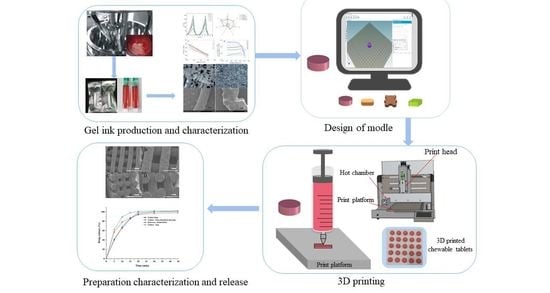
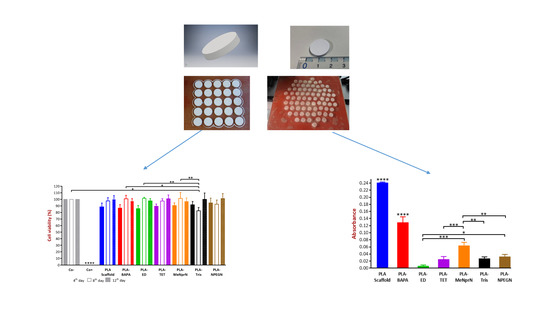
3D Printing Technology Based on Versatile Gelatin-Carrageenan Gel System for Drug Formulations
by
En Liang, Zengming Wang, Xiang Li, Shanshan Wang, Xiaolu Han, Daquan Chen and Aiping Zheng
Cited by 10 | Viewed by 3284
Abstract
Currently, there is a shortage of pediatric medicines on the market, and 3D printing technology can more flexibly produce personalized medicines to meet individual needs. The study developed a child-friendly composite gel ink (carrageenan-gelatin), created 3D models by computer-aided design technology, then produced
[...] Read more.
Currently, there is a shortage of pediatric medicines on the market, and 3D printing technology can more flexibly produce personalized medicines to meet individual needs. The study developed a child-friendly composite gel ink (carrageenan-gelatin), created 3D models by computer-aided design technology, then produced personalized medicines using 3D printing to improve the safety and accuracy of medication for pediatric patients. An in-depth understanding of the printability of different formulations was obtained by analyzing the rheological and textural properties of different gel inks and observing the microstructure of different gel inks, which guided the formulation optimization. Through formulation optimization, the printability and thermal stability of gel ink were improved, and F6 formulation (carrageenan: 0.65%; gelatin: 12%) was selected as the 3D printing inks. Additionally, a personalized dose linear model was established with the F6 formulation for the production of 3D printed personalized tablets. Moreover, the dissolution tests showed that the 3D printed tablets were able to dissolve more than 85% within 30 min and had similar dissolution profiles to the commercially available tablets. This study demonstrates that 3D printing is an effective manufacturing technique that allows for flexible, rapid, and automated production of personalized formulations.
Full article
►▼
Show Figures
Open AccessArticle
Optimization of Printing Parameters for Digital Light Processing 3D Printing of Hollow Microneedle Arrays
by
Essyrose Mathew, Giulia Pitzanti, Ana L. Gomes dos Santos and Dimitrios A. Lamprou
Cited by 58 | Viewed by 6387
Abstract
3D printing is an emerging technology aiming towards personalized drug delivery, among many other applications. Microneedles (MN) are a viable method for transdermal drug delivery that is becoming more popular for delivery through the skin. However, there is a need for a faster
[...] Read more.
3D printing is an emerging technology aiming towards personalized drug delivery, among many other applications. Microneedles (MN) are a viable method for transdermal drug delivery that is becoming more popular for delivery through the skin. However, there is a need for a faster fabrication process with potential for easily exploring different geometries of MNs. In the current study, a digital light processing (DLP) method of 3D printing for fabrication of hollow MN arrays using commercial UV curable resin was proposed. Print quality was optimised by assessing the effect of print angle on needle geometries. Mechanical testing of MN arrays was conducted using a texture analyser. Angled prints were found to produce prints with geometries closer to the CAD designs. Curing times were found to affect the mechanical strength of MNs, with arrays not breaking when subjected to 300 N of force but were bent. Overall, DLP process produced hollow MNs with good mechanical strength and depicts a viable, quick, and efficient method for the fabrication of hollow MN arrays.
Full article
►▼
Show Figures
Open AccessReview
Production of Drug Delivery Systems Using Fused Filament Fabrication: A Systematic Review
by
Bahaa Shaqour, Aseel Samaro, Bart Verleije, Koen Beyers, Chris Vervaet and Paul Cos
Cited by 63 | Viewed by 7014
Abstract
Fused filament fabrication (FFF) 3D printing technology is widely used in many fields. For almost a decade, medical researchers have been exploring the potential use of this technology for improving the healthcare sector. Advances in personalized medicine have been more achievable due to
[...] Read more.
Fused filament fabrication (FFF) 3D printing technology is widely used in many fields. For almost a decade, medical researchers have been exploring the potential use of this technology for improving the healthcare sector. Advances in personalized medicine have been more achievable due to the applicability of producing drug delivery devices, which are explicitly designed based on patients’ needs. For the production of these devices, a filament—which is the feedstock for the FFF 3D printer—consists of a carrier polymer (or polymers) and a loaded active pharmaceutical ingredient (API). This systematic review of the literature investigates the most widely used approaches for producing drug-loaded filaments. It also focusses on several factors, such as the polymeric carrier and the drug, loading capacity and homogeneity, processing conditions, and the intended applications. This review concludes that the filament preparation method has a significant effect on both the drug homogeneity within the polymeric carrier and drug loading efficiency.
Full article
►▼
Show Figures
Open AccessArticle
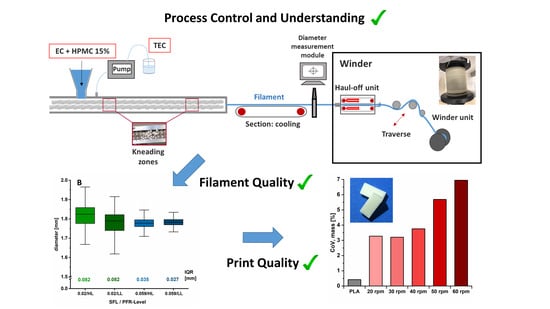
Hot-Melt Extrusion Process Fluctuations and Their Impact on Critical Quality Attributes of Filaments and 3D-Printed Dosage Forms
by
Hanna Ponsar, Raphael Wiedey and Julian Quodbach
Cited by 56 | Viewed by 6008
Abstract
Fused deposition modeling (FDM
TM) is a 3D-printing technology of rising interest for the manufacturing of customizable solid dosage forms. The coupling of hot-melt extrusion with FDM
TM is favored to allow the production of pharma-grade filaments for the printing of medicines.
[...] Read more.
Fused deposition modeling (FDM
TM) is a 3D-printing technology of rising interest for the manufacturing of customizable solid dosage forms. The coupling of hot-melt extrusion with FDM
TM is favored to allow the production of pharma-grade filaments for the printing of medicines. Filament diameter consistency is a quality of great importance to ensure printability and content uniformity of 3D-printed drug delivery systems. A systematical process analysis referring to filament diameter variations has not been described in the literature. The presented study aimed at a process setup optimization and rational process analysis for filament fabrication related to influencing parameters on diameter inhomogeneity. In addition, the impact of diameter variation on the critical quality attributes of filaments (mechanical properties) and uniformity of mass of printed drug-free dosage forms was investigated. Process optimization by implementing a winder with a special haul-off unit was necessary to obtain reliable filament diameters. Subsequently, the optimized setup was used for conduction of rational extrusion analysis. The results revealed that an increased screw speed led to diameter fluctuations with a decisive influence on the mechanical resilience of filaments and mass uniformity of printed dosage forms. The specific feed load was identified as a key parameter for filament diameter consistency.
Full article
►▼
Show Figures
Open AccessArticle
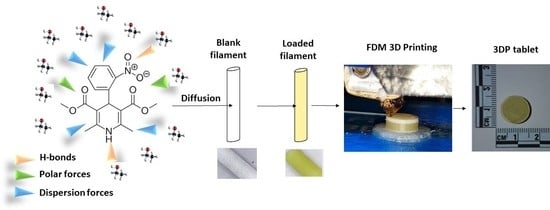
Personalised 3D Printed Medicines: Optimising Material Properties for Successful Passive Diffusion Loading of Filaments for Fused Deposition Modelling of Solid Dosage Forms
by
Jose R. Cerda, Talaya Arifi, Sejad Ayyoubi, Peter Knief, Maria Paloma Ballesteros, William Keeble, Eugen Barbu, Anne Marie Healy, Aikaterini Lalatsa and Dolores R. Serrano
Cited by 68 | Viewed by 7907
Abstract
Although not readily accessible yet to many community and hospital pharmacists, fuse deposition modelling (FDM) is a 3D printing technique that can be used to create a 3D pharmaceutical dosage form by employing drug loaded filaments extruded via a nozzle, melted and deposited
[...] Read more.
Although not readily accessible yet to many community and hospital pharmacists, fuse deposition modelling (FDM) is a 3D printing technique that can be used to create a 3D pharmaceutical dosage form by employing drug loaded filaments extruded via a nozzle, melted and deposited layer by layer. FDM requires printable filaments, which are commonly manufactured by hot melt extrusion, and identifying a suitable extrudable drug-excipient mixture can sometimes be challenging. We propose here the use of passive diffusion as an accessible loading method for filaments that can be printed using FDM technology to allow for the fabrication of oral personalised medicines in clinical settings. Utilising Hansen Solubility Parameters (HSP) and the concept of HSP distances (Ra) between drug, solvent, and filament, we have developed a facile pre-screening tool for the selection of the optimal combination that can provide a high drug loading (a high solvent-drug Ra, >10, and an intermediate solvent–filament Ra value, ~10). We have identified that other parameters such as surface roughness and stiffness also play a key role in enhancing passive diffusion of the drug into the filaments. A predictive model for drug loading was developed based on Support Vector Machine (SVM) regression and indicated a strong correlation between both Ra and filament stiffness and the diffusion capacity of a model BCS Class II drug, nifedipine (NFD), into the filaments. A drug loading, close to 3% w/w, was achieved. 3D printed tablets prepared using a PVA-derived filament (Hydrosupport, 3D Fuel) showed promising characteristics in terms of dissolution (with a sustained release over 24 h) and predicted chemical stability (>3 years at 25 °C/60% relative humidity), similar to commercially available NFD oral dosage forms. We believe FDM coupled with passive diffusion could be implemented easily in clinical settings for the manufacture of tailored personalised medicines, which can be stored over long periods of time (similar to industrially manufactured solid dosage forms).
Full article
►▼
Show Figures
Open AccessEditorial
3D Printing of Pharmaceuticals and Drug Delivery Devices
by
Essyrose Mathew, Giulia Pitzanti, Eneko Larrañeta and Dimitrios A. Lamprou
Cited by 127 | Viewed by 14164
Abstract
The process of 3D printing (3DP) was patented in 1986; however, the research in the field of 3DP did not become popular until the last decade. There has been an increasing research into the areas of 3DP for medical applications for fabricating prosthetics,
[...] Read more.
The process of 3D printing (3DP) was patented in 1986; however, the research in the field of 3DP did not become popular until the last decade. There has been an increasing research into the areas of 3DP for medical applications for fabricating prosthetics, bioprinting and pharmaceutics. This novel method allows the manufacture of dosage forms on demand, with modifications in the geometry and size resulting in changes to the release and dosage behaviour of the product. 3DP will allow wider adoption of personalised medicine due to the diversity and simplicity to change the design and dosage of the products, allowing the devices to be designed specific to the individual with the ability to alternate the drugs added to the product. Personalisation also has the potential to decrease the common side effects associated with generic dosage forms. This Special Issue Editorial outlines the current innovative research surrounding the topic of 3DP, focusing on bioprinting and various types of 3DP on applications for drug delivery as well advantages and future directions in this field of research.
Full article
Open AccessArticle
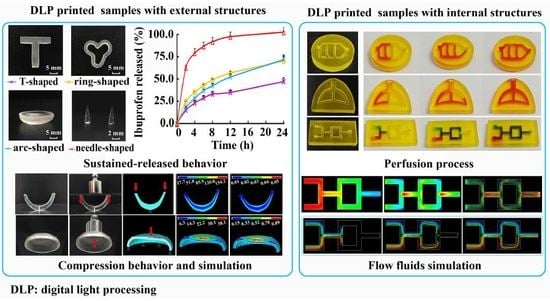
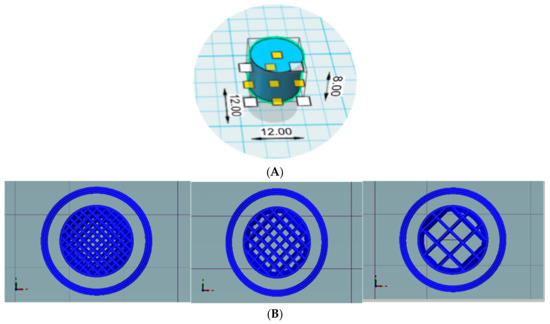
Printability of External and Internal Structures Based on Digital Light Processing 3D Printing Technique
by
Yan Yang, Yanjun Zhou, Xiao Lin, Qingliang Yang and Gengshen Yang
Cited by 78 | Viewed by 7374
Abstract
The high printing efficiency and easy availability of desktop digital light processing (DLP) printers have made DLP 3D printing a promising technique with increasingly broad application prospects, particularly in personalized medicine. The objective of this study was to fabricate and evaluate medical samples
[...] Read more.
The high printing efficiency and easy availability of desktop digital light processing (DLP) printers have made DLP 3D printing a promising technique with increasingly broad application prospects, particularly in personalized medicine. The objective of this study was to fabricate and evaluate medical samples with external and internal structures using the DLP technique. The influence of different additives and printing parameters on the printability and functionality of this technique was thoroughly evaluated. It was observed that the printability and mechanical properties of external structures were affected by the poly(ethylene glycol) diacrylate (PEGDA) concentration, plasticizers, layer height, and exposure time. The optimal printing solutions for 3D external and internal structures were 100% PEGDA and 75% PEGDA with 0.25 mg/mL tartrazine, respectively. And the optimal layer height for 3D external and internal structures were 0.02 mm and 0.05 mm, respectively. The optimal sample with external structures had an adequate drug-loading ability, acceptable sustained-release characteristics, and satisfactory biomechanical properties. In contrast, the printability of internal structures was affected by the photoabsorber, PEGDA concentration, layer height, and exposure time. The optimal samples with internal structures had good morphology, integrity and perfusion behavior. The present study showed that the DLP printing technique was capable of fabricating implants for drug delivery and physiological channels for in vivo evaluation.
Full article
►▼
Show Figures
Open AccessArticle
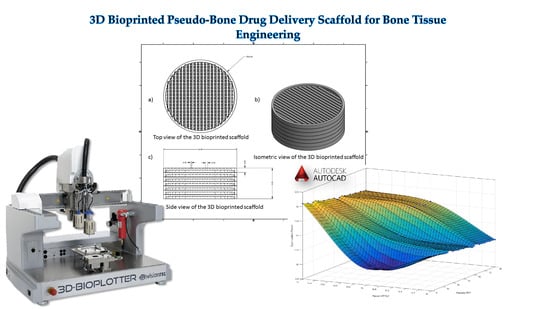
A 3D Bioprinted Pseudo-Bone Drug Delivery Scaffold for Bone Tissue Engineering
by
Pariksha Jolene Kondiah, Pierre P. D. Kondiah, Yahya E. Choonara, Thashree Marimuthu and Viness Pillay
Cited by 63 | Viewed by 7173
Abstract
A 3D bioprinted pseudo-bone drug delivery scaffold was fabricated to display matrix strength, matrix resilience, as well as porous morphology of healthy human bone. Computer-aided design (CAD) software was employed for developing the 3D bioprinted scaffold. Further optimization of the scaffold was undertaken
[...] Read more.
A 3D bioprinted pseudo-bone drug delivery scaffold was fabricated to display matrix strength, matrix resilience, as well as porous morphology of healthy human bone. Computer-aided design (CAD) software was employed for developing the 3D bioprinted scaffold. Further optimization of the scaffold was undertaken using MATLAB
® software and artificial neural networks (ANN). Polymers employed for formulating the 3D scaffold comprised of polypropylene fumarate (PPF), free radical polymerized polyethylene glycol- polycaprolactone (PEG-PCL-PEG), and pluronic (PF127). Simvastatin was incorporated into the 3D bioprinted scaffolds to further promote bone healing and repair properties. The 3D bioprinted scaffold was characterized for its chemical, morphological, mechanical, and in vitro release kinetics for evaluation of its behavior for application as an implantable scaffold at the site of bone fracture. The ANN-optimized 3D bioprinted scaffold displayed significant properties as a controlled release platform, demonstrating drug release over 20 days. The 3D bioprinted scaffold further displayed formation as a pseudo-bone matrix, using a human clavicle bone model, induced with a butterfly fracture. The strength of the pseudo-bone matrix, evaluated for its matrix hardness (MH) and matrix resilience (MR), was evaluated to be as strong as original bone, having a 99% MH and 98% MR property, to healthy human clavicle bones.
Full article
►▼
Show Figures
Open AccessReview
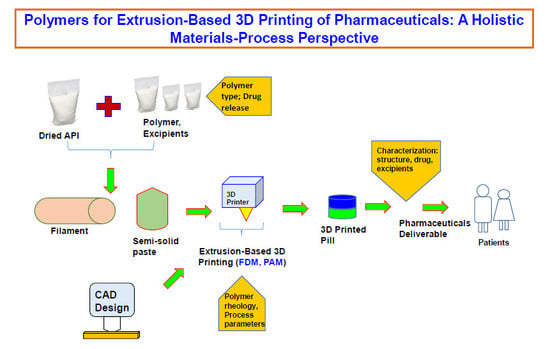
Polymers for Extrusion-Based 3D Printing of Pharmaceuticals: A Holistic Materials–Process Perspective
by
Mohammad A. Azad, Deborah Olawuni, Georgia Kimbell, Abu Zayed Md Badruddoza, Md. Shahadat Hossain and Tasnim Sultana
Cited by 288 | Viewed by 18761
Abstract
Three dimensional (3D) printing as an advanced manufacturing technology is progressing to be established in the pharmaceutical industry to overcome the traditional manufacturing regime of 'one size fits for all'. Using 3D printing, it is possible to design and develop complex dosage forms
[...] Read more.
Three dimensional (3D) printing as an advanced manufacturing technology is progressing to be established in the pharmaceutical industry to overcome the traditional manufacturing regime of 'one size fits for all'. Using 3D printing, it is possible to design and develop complex dosage forms that can be suitable for tuning drug release. Polymers are the key materials that are necessary for 3D printing. Among all 3D printing processes, extrusion-based (both fused deposition modeling (FDM) and pressure-assisted microsyringe (PAM)) 3D printing is well researched for pharmaceutical manufacturing. It is important to understand which polymers are suitable for extrusion-based 3D printing of pharmaceuticals and how their properties, as well as the behavior of polymer–active pharmaceutical ingredient (API) combinations, impact the printing process. Especially, understanding the rheology of the polymer and API–polymer mixtures is necessary for successful 3D printing of dosage forms or printed structures. This review has summarized a holistic materials–process perspective for polymers on extrusion-based 3D printing. The main focus herein will be both FDM and PAM 3D printing processes. It elaborates the discussion on the comparison of 3D printing with the traditional direct compression process, the necessity of rheology, and the characterization techniques required for the printed structure, drug, and excipients. The current technological challenges, regulatory aspects, and the direction toward which the technology is moving, especially for personalized pharmaceuticals and multi-drug printing, are also briefly discussed.
Full article
►▼
Show Figures
Open AccessArticle
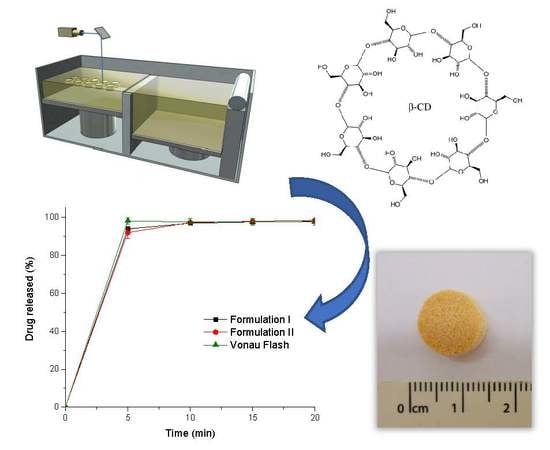
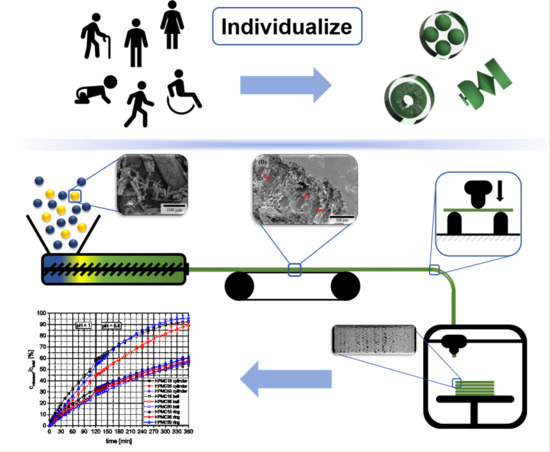
Selective Laser Sintering 3D Printing of Orally Disintegrating Printlets Containing Ondansetron
by
Nour Allahham, Fabrizio Fina, Carmen Marcuta, Lilia Kraschew, Wolfgang Mohr, Simon Gaisford, Abdul W. Basit and Alvaro Goyanes
Cited by 153 | Viewed by 9556
Abstract
The aim of this work was to explore the feasibility of using selective laser sintering (SLS) 3D printing (3DP) to fabricate orodispersable printlets (ODPs) containing ondansetron. Ondansetron was first incorporated into drug-cyclodextrin complexes and then combined with the filler mannitol. Two 3D printed
[...] Read more.
The aim of this work was to explore the feasibility of using selective laser sintering (SLS) 3D printing (3DP) to fabricate orodispersable printlets (ODPs) containing ondansetron. Ondansetron was first incorporated into drug-cyclodextrin complexes and then combined with the filler mannitol. Two 3D printed formulations with different levels of mannitol were prepared and tested, and a commercial ondansetron orally disintegrating tablet (ODT) product (Vonau
® Flash) was also investigated for comparison. Both 3D printed formulations disintegrated at ~15 s and released more than 90% of the drug within 5 min independent of the mannitol content; these results were comparable to those obtained with the commercial product. This work demonstrates the potential of SLS 3DP to fabricate orodispersible printlets with characteristics similar to a commercial ODT, but with the added benefit of using a manufacturing technology able to prepare medicines individualized to the patient.
Full article
►▼
Show Figures
Open AccessArticle
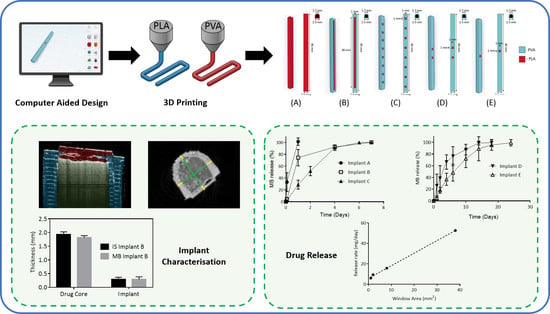
Development of a Biodegradable Subcutaneous Implant for Prolonged Drug Delivery Using 3D Printing
by
Sarah A. Stewart, Juan Domínguez-Robles, Victoria J. McIlorum, Elena Mancuso, Dimitrios A. Lamprou, Ryan F. Donnelly and Eneko Larrañeta
Cited by 148 | Viewed by 14406
Abstract
Implantable drug delivery devices offer many advantages over other routes of drug delivery. Most significantly, the delivery of lower doses of drug, thus, potentially reducing side-effects and improving patient compliance. Three dimensional (3D) printing is a flexible technique, which has been subject to
[...] Read more.
Implantable drug delivery devices offer many advantages over other routes of drug delivery. Most significantly, the delivery of lower doses of drug, thus, potentially reducing side-effects and improving patient compliance. Three dimensional (3D) printing is a flexible technique, which has been subject to increasing interest in the past few years, especially in the area of medical devices. The present work focussed on the use of 3D printing as a tool to manufacture implantable drug delivery devices to deliver a range of model compounds (methylene blue, ibuprofen sodium and ibuprofen acid) in two in vitro models. Five implant designs were produced, and the release rate varied, depending on the implant design and the drug properties. Additionally, a rate controlling membrane was produced, which further prolonged the release from the produced implants, signalling the potential use of these devices for chronic conditions.
Full article
►▼
Show Figures
Open AccessArticle
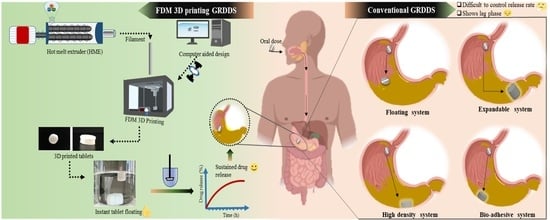
Fabrication of Intragastric Floating, Controlled Release 3D Printed Theophylline Tablets Using Hot-Melt Extrusion and Fused Deposition Modeling
by
Bhupendra Raj Giri, Eon Soo Song, Jaewook Kwon, Ju-Hyun Lee, Jun-Bom Park and Dong Wuk Kim
Cited by 92 | Viewed by 7728
Abstract
This work presents a novel approach for producing gastro-retentive floating tablets (GRFT) by coupling hot-melt extrusion (HME) and fused deposition three-dimensional printing (3DP). Filaments containing theophylline (THEO) within a hydroxypropyl cellulose (HPC) matrix were prepared using HME. 3DP tablets with different infill percentages
[...] Read more.
This work presents a novel approach for producing gastro-retentive floating tablets (GRFT) by coupling hot-melt extrusion (HME) and fused deposition three-dimensional printing (3DP). Filaments containing theophylline (THEO) within a hydroxypropyl cellulose (HPC) matrix were prepared using HME. 3DP tablets with different infill percentages and shell thickness were developed and evaluated to determine their drug content, floating behavior, dissolution, and physicochemical properties. The dissolution studies revealed a relationship between the infill percentage/shell thickness and the drug release behavior of the 3DP tablets. All the developed GRFTs possessed the ability to float for 10 h and exhibited zero-order release kinetics. The drug release could be described by the Peppas–Sahlin model, as a combination of Fickian diffusion and swelling mechanism. Drug crystallinity was found unaltered throughout the process. 3DP coupled with HME, could be an effective blueprint to produce controlled-release GRFTs, providing the advantage of simplicity and versatility compared to the conventional methods.
Full article
►▼
Show Figures
Open AccessArticle
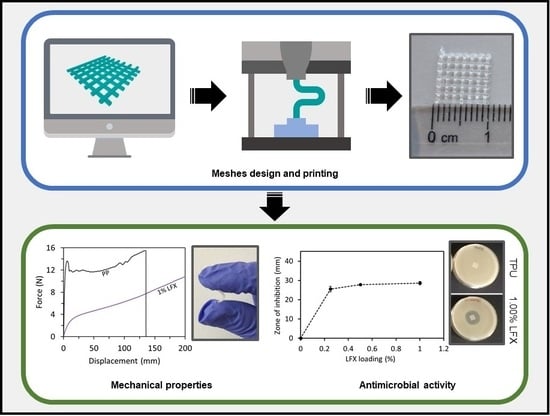
3D Printing of Drug-Loaded Thermoplastic Polyurethane Meshes: A Potential Material for Soft Tissue Reinforcement in Vaginal Surgery
by
Juan Domínguez-Robles, Caterina Mancinelli, Elena Mancuso, Inmaculada García-Romero, Brendan F. Gilmore, Luca Casettari, Eneko Larrañeta and Dimitrios A. Lamprou
Cited by 118 | Viewed by 11404
Abstract
Current strategies to treat pelvic organ prolapse (POP) or stress urinary incontinence (SUI), include the surgical implantation of vaginal meshes. Recently, there have been multiple reports of issues generated by these meshes conventionally made of poly(propylene). This material is not the ideal candidate,
[...] Read more.
Current strategies to treat pelvic organ prolapse (POP) or stress urinary incontinence (SUI), include the surgical implantation of vaginal meshes. Recently, there have been multiple reports of issues generated by these meshes conventionally made of poly(propylene). This material is not the ideal candidate, due to its mechanical properties leading to complications such as chronic pain and infection. In the present manuscript, we propose the use of an alternative material, thermoplastic polyurethane (TPU), loaded with an antibiotic in combination with fused deposition modelling (FDM) to prepare safer vaginal meshes. For this purpose, TPU filaments containing levofloxacin (LFX) in various concentrations (e.g., 0.25%, 0.5%, and 1%) were produced by extrusion. These filaments were used to 3D print vaginal meshes. The printed meshes were fully characterized through different tests/analyses such as fracture force studies, attenuated total reflection-Fourier transform infrared, thermal analysis, scanning electron microscopy, X-ray microcomputed tomography (μCT), release studies and microbiology testing. The results showed that LFX was uniformly distributed within the TPU matrix, regardless the concentration loaded. The mechanical properties showed that poly(propylene) (PP) is a tougher material with a lower elasticity than TPU, which seemed to be a more suitable material due to its elasticity. In addition, the printed meshes showed a significant bacteriostatic activity on both
Staphylococcus aureus and
Escherichia coli cultures, minimising the risk of infection after implanting them. Therefore, the incorporation of LFX to the TPU matrix can be used to prepare anti-infective vaginal meshes with enhanced mechanical properties compared with current PP vaginal meshes.
Full article
►▼
Show Figures
Open AccessArticle
Development of Bio-Active Patches Based on Pectin for the Treatment of Ulcers and Wounds Using 3D-Bioprinting Technology
by
Eleftherios G. Andriotis, Georgios K. Eleftheriadis, Christina Karavasili and Dimitrios G. Fatouros
Cited by 105 | Viewed by 11373
Abstract
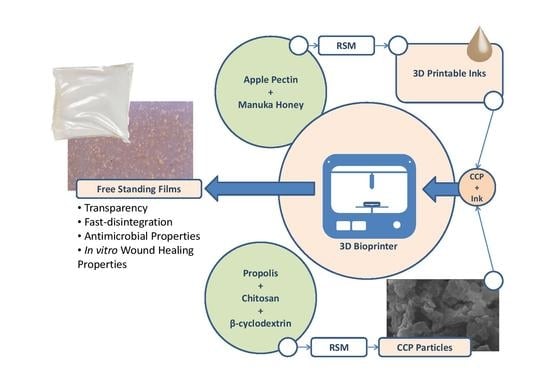
Biodegradable 3D-printable inks based on pectin have been developed as a system for direct and indirect wound-dressing applications, suitable for 3D printing technologies. The 3D-printable inks formed free-standing transparent films upon drying, with the latter exhibiting fast disintegration upon contact with aqueous media.
[...] Read more.
Biodegradable 3D-printable inks based on pectin have been developed as a system for direct and indirect wound-dressing applications, suitable for 3D printing technologies. The 3D-printable inks formed free-standing transparent films upon drying, with the latter exhibiting fast disintegration upon contact with aqueous media. The antimicrobial and wound-healing activities of the inks have been successfully enhanced by the addition of particles, comprised of chitosan and cyclodextrin inclusion complexes with propolis extract. Response Surface Methodology (RSM) was applied for the optimization of the inks (extrusion-printing pressure, shrinkage minimization over-drying, increased water uptake and minimization of the disintegration of the dry patches upon contact with aqueous media). Particles comprised of chitosan and cyclodextrin/propolis extract inclusion complexes (CCP), bearing antimicrobial properties, were optimized and integrated with the produced inks. The bioprinted patches were assessed for their cytocompatibility, antimicrobial activity and in vitro wound-healing properties. These studies were complemented with ex vivo skin adhesion measurements, a relative surface hydrophobicity and opacity measurement, mechanical properties, visualization, and spectroscopic techniques. The in vitro wound-healing studies revealed that the 3D-bioprinted patches enhanced the in vitro wound-healing process, while the incorporation of CCP further enhanced wound-healing, as well as the antimicrobial activity of the patches.
Full article
►▼
Show Figures
Open AccessArticle
Novel Gastroretentive Floating Pulsatile Drug Delivery System Produced via Hot-Melt Extrusion and Fused Deposition Modeling 3D Printing
by
Nagi Reddy Dumpa, Suresh Bandari and Michael A. Repka
Cited by 147 | Viewed by 11601
Abstract
This study was performed to develop novel core-shell gastroretentive floating pulsatile drug delivery systems using a hot-melt extrusion-paired fused deposition modeling (FDM) 3D printing and direct compression method. Hydroxypropyl cellulose (HPC) and ethyl cellulose (EC)-based filaments were fabricated using hot-melt extrusion technology and
[...] Read more.
This study was performed to develop novel core-shell gastroretentive floating pulsatile drug delivery systems using a hot-melt extrusion-paired fused deposition modeling (FDM) 3D printing and direct compression method. Hydroxypropyl cellulose (HPC) and ethyl cellulose (EC)-based filaments were fabricated using hot-melt extrusion technology and were utilized as feedstock material for printing shells in FDM 3D printing. The directly compressed theophylline tablet was used as the core. The tablet shell to form pulsatile floating dosage forms with different geometries (shell thickness: 0.8, 1.2, 1.6, and 2.0 mm; wall thickness: 0, 0.8, and 1.6 mm; and % infill density: 50, 75, and 100) were designed, printed, and evaluated. All core-shell tablets floated without any lag time and exhibited good floating behavior throughout the dissolution study. The lag time for the pulsatile release of the drug was 30 min to 6 h. The proportion of ethyl cellulose in the filament composition had a significant (
p < 0.05) effect on the lag time. The formulation (2 mm shell thickness, 1.6 mm wall thickness, 100% infill density, 0.5% EC) with the desired lag time of 6 h was selected as an optimized formulation. Thus, FDM 3D printing is a potential technique for the development of complex customized drug delivery systems for personalized pharmacotherapy.
Full article
►▼
Show Figures
Open AccessArticle
Stencil Printing—A Novel Manufacturing Platform for Orodispersible Discs
by
Henrika Wickström, Rajesh Koppolu, Ermei Mäkilä, Martti Toivakka and Niklas Sandler
Cited by 15 | Viewed by 6206
Abstract
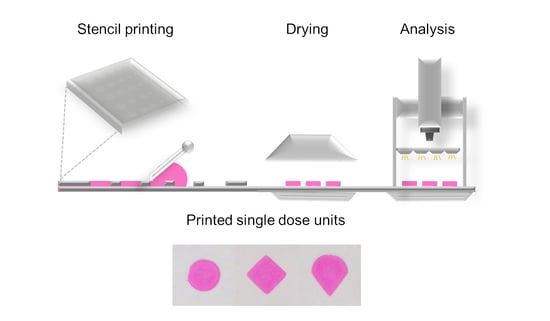
Stencil printing is a commonly used printing method, but it has not previously been used for production of pharmaceuticals. The aim of this study was to explore whether stencil printing of drug containing polymer inks could be used to manufacture flexible dosage forms
[...] Read more.
Stencil printing is a commonly used printing method, but it has not previously been used for production of pharmaceuticals. The aim of this study was to explore whether stencil printing of drug containing polymer inks could be used to manufacture flexible dosage forms with acceptable mass and content uniformity. Formulation development was supported by physicochemical characterization of the inks and final dosage forms. The printing of haloperidol (HAL) discs was performed using a prototype stencil printer. Ink development comprised of investigations of ink rheology in combination with printability assessment. The results show that stencil printing can be used to manufacture HAL doses in the therapeutic treatment range for 6–17 year-old children. The therapeutic HAL dose was achieved for the discs consisting of 16% of hydroxypropyl methylcellulose (HPMC) and 1% of lactic acid (LA). The formulation pH remained above pH 4 and the results imply that the drug was amorphous. Linear dose escalation was achieved by an increase in aperture area of the print pattern, while keeping the stencil thickness fixed. Disintegration times of the orodispersible discs printed with 250 and 500 µm thick stencils were below 30 s. In conclusion, stencil printing shows potential as a manufacturing method of pharmaceuticals.
Full article
►▼
Show Figures
Open AccessArticle
3D-Printed Solid Dispersion Drug Products
by
Suet Li Chew, Laura Modica de Mohac and Bahijja Tolulope Raimi-Abraham
Cited by 20 | Viewed by 7992
Abstract
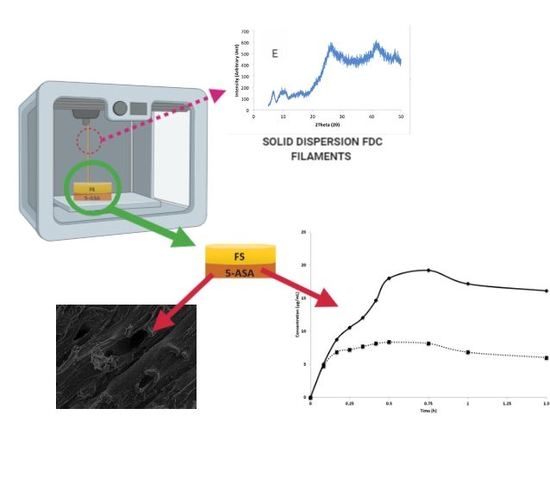
With the well-known advantages of additive manufacturing methods such as three-dimensional (3D) printing in drug delivery, it is disappointing that only one product has been successful in achieving regulatory approval in the past few years. Further research and development is required in this
[...] Read more.
With the well-known advantages of additive manufacturing methods such as three-dimensional (3D) printing in drug delivery, it is disappointing that only one product has been successful in achieving regulatory approval in the past few years. Further research and development is required in this area to introduce more 3D printed products into the market. Our study investigates the potential of fixed dose combination solid dispersion drug products generated via 3D printing. Two model drugs—fluorescein sodium (FS) and 5-aminosalicyclic acid (5-ASA)—were impregnated onto a polyvinyl alcohol (PVA) filament, and the influence of solvent choice in optimal drug loading as well as other influences such as the physicochemical and mechanical properties of the resultant filaments were investigated prior to development of the resultant drug products. Key outcomes of this work included the improvement of filament drug loading by one- to threefold due to solvent choice on the basis of its polarity and the generation of a 3D-printed product confirmed to be a solid dispersion fixed dose combination with the two model drugs exhibiting favourable in vitro dissolution characteristics.
Full article
►▼
Show Figures
Open AccessArticle
Additive Manufacturing of Personalized Pharmaceutical Dosage Forms via Stereolithography
by
Andrew V. Healy, Evert Fuenmayor, Patrick Doran, Luke M. Geever, Clement L. Higginbotham and John G. Lyons
Cited by 82 | Viewed by 8004
Abstract
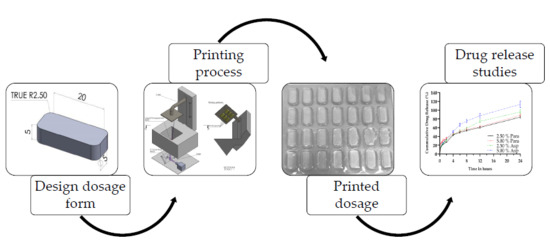
The introduction of three-dimensional printing (3DP) has created exciting possibilities for the fabrication of dosage forms, paving the way for personalized medicine. In this study, oral dosage forms of two drug concentrations, namely 2.50% and 5.00%, were fabricated via stereolithography (SLA) using a
[...] Read more.
The introduction of three-dimensional printing (3DP) has created exciting possibilities for the fabrication of dosage forms, paving the way for personalized medicine. In this study, oral dosage forms of two drug concentrations, namely 2.50% and 5.00%, were fabricated via stereolithography (SLA) using a novel photopolymerizable resin formulation based on a monomer mixture that, to date, has not been reported in the literature, with paracetamol and aspirin selected as model drugs. In order to produce the dosage forms, the ratio of poly(ethylene glycol) diacrylate (PEGDA) to poly(caprolactone) triol was varied with diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide (Irgacure TPO) utilized as the photoinitiator. The fabrication of 28 dosages in one print process was possible and the printed dosage forms were characterized for their drug release properties. It was established that both drugs displayed a sustained release over a 24-h period. The physical properties were also investigated, illustrating that SLA affords accurate printing of dosages with some statistically significant differences observed from the targeted dimensional range, indicating an area for future process improvement. The work presented in this paper demonstrates that SLA has the ability to produce small, individualized batches which may be tailored to meet patients’ specific needs or provide for the localized production of pharmaceutical dosage forms.
Full article
►▼
Show Figures
Open AccessArticle
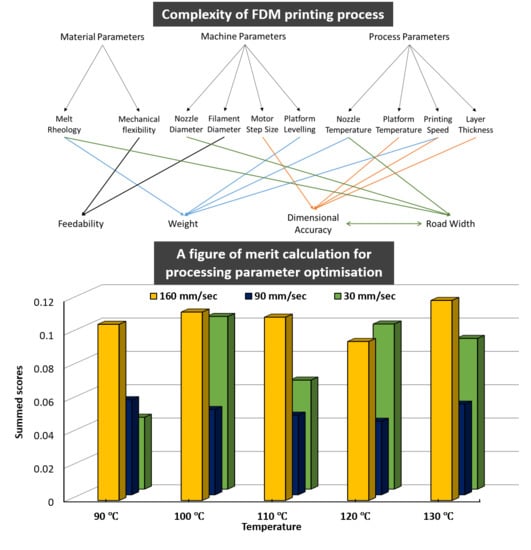
Impact of Processing Parameters on the Quality of Pharmaceutical Solid Dosage Forms Produced by Fused Deposition Modeling (FDM)
by
Muqdad Alhijjaj, Jehad Nasereddin, Peter Belton and Sheng Qi
Cited by 82 | Viewed by 6203
Abstract
Fused deposition modeling (FDM) three-dimensional (3D) printing is being increasingly explored as a direct manufacturing method to product pharmaceutical solid dosage forms. Despite its many advantages as a pharmaceutical formulation tool, it remains restricted to proof-of-concept formulations. The optimization of the printing process
[...] Read more.
Fused deposition modeling (FDM) three-dimensional (3D) printing is being increasingly explored as a direct manufacturing method to product pharmaceutical solid dosage forms. Despite its many advantages as a pharmaceutical formulation tool, it remains restricted to proof-of-concept formulations. The optimization of the printing process in order to achieve adequate precision and printing quality remains to be investigated. Demonstrating a thorough understanding of the process parameters of FDM and their impact on the quality of printed dosage forms is undoubtedly necessary should FDM advance from a proof-of-concept stage to an adapted pharmaceutical manufacturing tool. This article describes the findings of an investigation into a number of critical process parameters of FDM and their impact on quantifiable, pharmaceutically-relevant measures of quality. Polycaprolactone, one of the few polymers which is both suitable for FDM and is a GRAS (generally regarded as safe) material, was used to print internally-exposed grids, allowing examination of both their macroscopic and microstructural reproducibility of FDM. Of the measured quality parameters, dimensional authenticity of the grids was found to poorly match the target dimensions. Weights of the grids were found to significantly vary upon altering printing speed. Printing temperature showed little effect on weight. Weight uniformity per batch was found to lie within acceptable pharmaceutical quality limits. Furthermore, we report observing a microstructural distortion relating to printing temperature which we dub The First Layer Effect (FLE). Principal Component Analysis (PCA) was used to study factor interactions and revealed, among others, the existence of an interaction between weight/dosing accuracy and dimensional authenticity dictating a compromise between the two quality parameters. The Summed Standard Deviation (SSD) is proposed as a method to extract the optimum printing parameters given all the perceived quality parameters and the necessary compromises among them.
Full article
►▼
Show Figures
Open AccessArticle
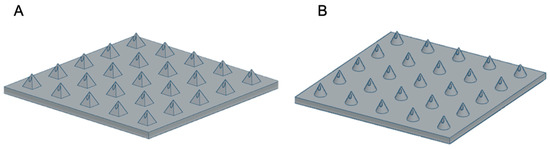
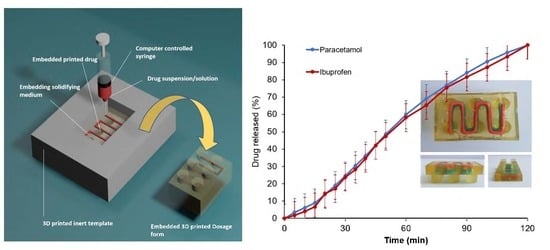
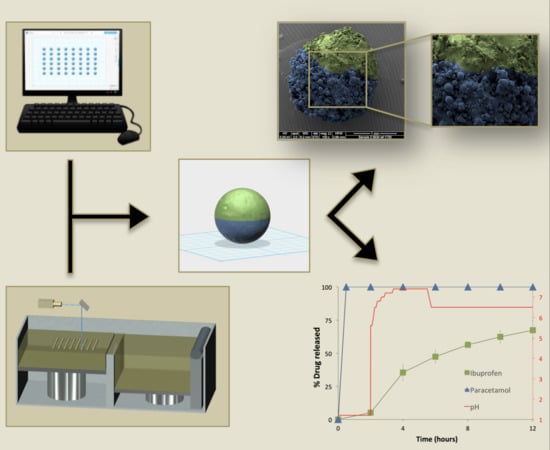
Embedded 3D Printing of Novel Bespoke Soft Dosage Form Concept for Pediatrics
by
Katarzyna Rycerz, Krzysztof Adam Stepien, Marta Czapiewska, Basel T. Arafat, Rober Habashy, Abdullah Isreb, Matthew Peak and Mohamed A. Alhnan
Cited by 81 | Viewed by 8417
Abstract
Embedded three-dimensional printing (e-3DP) is an emerging method for additive manufacturing where semi-solid materials are extruded within a solidifying liquid matrix. Here, we present the first example of employing e-3DP in the pharmaceutical field and demonstrate the fabrication of bespoke chewable dosage forms
[...] Read more.
Embedded three-dimensional printing (e-3DP) is an emerging method for additive manufacturing where semi-solid materials are extruded within a solidifying liquid matrix. Here, we present the first example of employing e-3DP in the pharmaceutical field and demonstrate the fabrication of bespoke chewable dosage forms with dual drug loading for potential use in pediatrics. Lego
TM-like chewable bricks made of edible soft material (gelatin-based matrix) were produced by directly extruding novel printing patterns of model drug ink (embedded phase) into a liquid gelatin-based matrix (embedding phase) at an elevated temperature (70 °C) to then solidify at room temperature. Dose titration of the two model drugs (paracetamol and ibuprofen) was possible by using specially designed printing patterns of the embedded phase to produce varying doses. A linearity [R
2 = 0.9804 (paracetamol) and 0.9976 (ibuprofen)] was achieved between percentage of completion of printing patterns and achieved doses using a multi-step method. The impact of embedded phase rheological behavior, the printing speed and the needle size of the embedded phase were examined. Owning to their appearance, modular nature, ease of personalizing dose and geometry, and tailoring and potential inclusion of various materials, this new dosage form concept holds a substantial promise for novel dosage forms in pediatrics.
Full article
►▼
Show Figures
Open AccessArticle
Customized Novel Design of 3D Printed Pregabalin Tablets for Intra-Gastric Floating and Controlled Release Using Fused Deposition Modeling
by
Shrawani Lamichhane, Jun-Bom Park, Dong Hwan Sohn and Sangkil Lee
Cited by 78 | Viewed by 7467
Abstract
Three-dimensional (3D) printing has been recently employed in the design and formulation of various dosage forms with the aim of on-demand manufacturing and personalized medicine. In this study, we formulated a floating sustained release system using fused deposition modeling (FDM). Filaments were prepared
[...] Read more.
Three-dimensional (3D) printing has been recently employed in the design and formulation of various dosage forms with the aim of on-demand manufacturing and personalized medicine. In this study, we formulated a floating sustained release system using fused deposition modeling (FDM). Filaments were prepared using hypromellose acetate succinate (HPMCAS), polyethylene glycol (PEG 400) and pregabalin as the active ingredient. Cylindrical tablets with infill percentages of 25%, 50% and 75% were designed and printed with the FDM printer. An optimized formulation (F6) was designed with a closed bottom layer and a partially opened top layer. Filaments and tablets were characterized by means of fourier-transform infrared spectroscopy (FTIR), differential scanning calorimetry (DSC), X-ray powder diffraction (XRPD), and thermogravimetric analysis (TGA). The results show that the processing condition did not have a significant effect on the stability of the drug and the crystallinity of the drug remained even after printing. A dissolution study revealed that drug release is faster in an open system with low infill percentage compared to closed systems and open systems with a high infill ratio. The optimized formulation (F6) with partially opened top layer showed zero-order drug release. The results show that FDM printing is suitable for the formulation of floating dosage form with the desired drug release profile.
Full article
►▼
Show Figures
Open AccessArticle
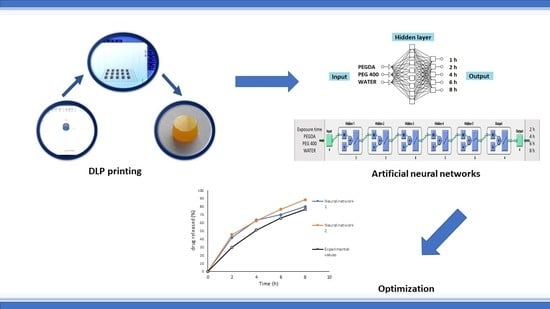
Optimization and Prediction of Ibuprofen Release from 3D DLP Printlets Using Artificial Neural Networks
by
Marijana Madzarevic, Djordje Medarevic, Aleksandra Vulovic, Tijana Sustersic, Jelena Djuris, Nenad Filipovic and Svetlana Ibric
Cited by 68 | Viewed by 6437
Abstract
The aim of this work was to investigate effects of the formulation factors on tablet printability as well as to optimize and predict extended drug release from cross-linked polymeric ibuprofen printlets using an artificial neural network (ANN). Printlets were printed using digital light
[...] Read more.
The aim of this work was to investigate effects of the formulation factors on tablet printability as well as to optimize and predict extended drug release from cross-linked polymeric ibuprofen printlets using an artificial neural network (ANN). Printlets were printed using digital light processing (DLP) technology from formulations containing polyethylene glycol diacrylate, polyethylene glycol, and water in concentrations according to D-optimal mixture design and 0.1%
w/
w riboflavin and 5%
w/
w ibuprofen. It was observed that with higher water content longer exposure time was required for successful printing. For understanding the effects of excipients and printing parameters on drug dissolution rate in DLP printlets two different neural networks were developed with using two commercially available softwares. After comparison of experimental and predicted values of in vitro dissolution at the corresponding time points for optimized formulation, the
R2 experimental vs. predicted value was 0.9811 (neural network 1) and 0.9960 (neural network 2). According to difference f
1 and similarity factor f
2 (f
1 = 14.30 and f
2 = 52.15) neural network 1 with supervised multilayer perceptron, backpropagation algorithm, and linear activation function gave a similar dissolution profile to obtained experimental results, indicating that adequate ANN is able to set out an input–output relationship in DLP printing of pharmaceutics.
Full article
►▼
Show Figures
Open AccessReview
Application of Micro-Scale 3D Printing in Pharmaceutics
by
Andrew Kjar and Yu Huang
Cited by 65 | Viewed by 11899
Abstract
3D printing, as one of the most rapidly-evolving fabrication technologies, has released a cascade of innovation in the last two decades. In the pharmaceutical field, the integration of 3D printing technology has offered unique advantages, especially at the micro-scale. When printed at a
[...] Read more.
3D printing, as one of the most rapidly-evolving fabrication technologies, has released a cascade of innovation in the last two decades. In the pharmaceutical field, the integration of 3D printing technology has offered unique advantages, especially at the micro-scale. When printed at a micro-scale, materials and devices can provide nuanced solutions to controlled release, minimally invasive delivery, high-precision targeting, biomimetic models for drug discovery and development, and future opportunities for personalized medicine. This review aims to cover the recent advances in this area. First, the 3D printing techniques are introduced with respect to the technical parameters and features that are uniquely related to each stage of pharmaceutical development. Then specific micro-sized pharmaceutical applications of 3D printing are summarized and grouped according to the provided benefits. Both advantages and challenges are discussed for each application. We believe that these technologies provide compelling future solutions for modern medicine, while challenges remain for scale-up and regulatory approval.
Full article
►▼
Show Figures
Open AccessArticle
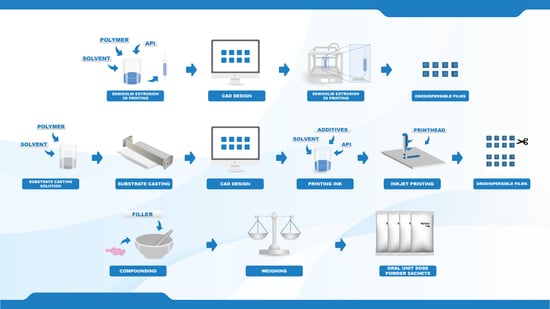
Towards Printed Pediatric Medicines in Hospital Pharmacies: Comparison of 2D and 3D-Printed Orodispersible Warfarin Films with Conventional Oral Powders in Unit Dose Sachets
by
Heidi Öblom, Erica Sjöholm, Maria Rautamo and Niklas Sandler
Cited by 115 | Viewed by 11068
Abstract
To date, the lack of age-appropriate medicines for many indications results in dose manipulation of commercially available dosage forms, commonly resulting in inaccurate doses. Various printing technologies have recently been explored in the pharmaceutical field due to the flexible and precise nature of
[...] Read more.
To date, the lack of age-appropriate medicines for many indications results in dose manipulation of commercially available dosage forms, commonly resulting in inaccurate doses. Various printing technologies have recently been explored in the pharmaceutical field due to the flexible and precise nature of the techniques. The aim of this study was, therefore, to compare the currently used method to produce patient-tailored warfarin doses at HUS Pharmacy in Finland with two innovative printing techniques. Dosage forms of various strengths (0.1, 0.5, 1, and 2 mg) were prepared utilizing semisolid extrusion 3D printing, inkjet printing and the established compounding procedure for oral powders in unit dose sachets (OPSs). Orodispersible films (ODFs) drug-loaded with warfarin were prepared by means of printing using hydroxypropylcellulose as a film-forming agent. The OPSs consisted of commercially available warfarin tablets and lactose monohydrate as a filler. The ODFs resulted in thin and flexible films showing acceptable ODF properties. Moreover, the printed ODFs displayed improved drug content compared to the established OPSs. All dosage forms were found to be stable over the one-month stability study and suitable for administration through a naso-gastric tube, thus, enabling administration to all possible patient groups in a hospital ward. This work demonstrates the potential of utilizing printing technologies for the production of on-demand patient-specific doses and further discusses the advantages and limitations of each method.
Full article
►▼
Show Figures
Open AccessArticle
Fused Deposition Modeling 3D Printing: Test Platforms for Evaluating Post-Fabrication Chemical Modifications and In-Vitro Biological Properties
by
Petra Arany, Eszter Róka, Laurent Mollet, Anthony W. Coleman, Florent Perret, Beomjoon Kim, Renátó Kovács, Adrienn Kazsoki, Romána Zelkó, Rudolf Gesztelyi, Zoltán Ujhelyi, Pálma Fehér, Judit Váradi, Ferenc Fenyvesi, Miklós Vecsernyés and Ildikó Bácskay
Cited by 16 | Viewed by 5433
Abstract
3D printing is attracting considerable interest for its capacity to produce prototypes and small production runs rapidly. Fused deposit modeling (FDM) was used to produce polyvalent test plates for investigation of the physical, chemical, and in-vitro biological properties of printed materials. The polyvalent
[...] Read more.
3D printing is attracting considerable interest for its capacity to produce prototypes and small production runs rapidly. Fused deposit modeling (FDM) was used to produce polyvalent test plates for investigation of the physical, chemical, and in-vitro biological properties of printed materials. The polyvalent test plates (PVTPs) are poly-lactic acid cylinders, 14 mm in diameter and 3 mm in height. The polymer ester backbone was surface modified by a series of ramified and linear oligoamines to increase its hydrophilicity and introduce a positive charge. The chemical modification was verified by FT-IR spectroscopy, showing the introduction of amide and amine functions, and contact angle measurements confirmed increased hydrophilicity. Morphology studies (SEM, optical microscopy) indicated that the modification of PVTP possessed a planar morphology with small pits. Positron annihilation lifetime spectroscopy demonstrated that the polymeric free volume decreased on modification. An MTT-based prolonged cytotoxicity test using Caco-2 cells showed that the PVTPs are non-toxic at the cellular level. The presence of surface oligoamines on the PVTPs reduced biofilm formation by
Candida albicans SC5314 significantly. The results demonstrate that 3D printed objects may be modified at their surface by a simple amidation reaction, resulting in a reduced propensity for biofilm colonization and cellular toxicity.
Full article
►▼
Show Figures
Open AccessArticle
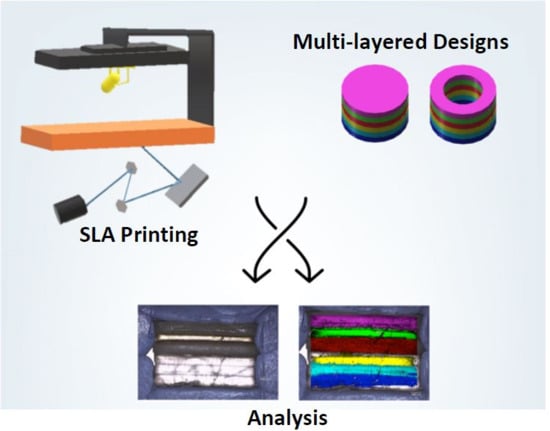
3D Printing of a Multi-Layered Polypill Containing Six Drugs Using a Novel Stereolithographic Method
by
Pamela Robles-Martinez, Xiaoyan Xu, Sarah J. Trenfield, Atheer Awad, Alvaro Goyanes, Richard Telford, Abdul W. Basit and Simon Gaisford
Cited by 303 | Viewed by 18426
Abstract
Three-dimensional printing (3DP) has demonstrated great potential for multi-material fabrication because of its capability for printing bespoke and spatially separated material conformations. Such a concept could revolutionise the pharmaceutical industry, enabling the production of personalised, multi-layered drug products on demand. Here, we developed
[...] Read more.
Three-dimensional printing (3DP) has demonstrated great potential for multi-material fabrication because of its capability for printing bespoke and spatially separated material conformations. Such a concept could revolutionise the pharmaceutical industry, enabling the production of personalised, multi-layered drug products on demand. Here, we developed a novel stereolithographic (SLA) 3D printing method that, for the first time, can be used to fabricate multi-layer constructs (polypills) with variable drug content and/or shape. Using this technique, six drugs, including paracetamol, caffeine, naproxen, chloramphenicol, prednisolone and aspirin, were printed with different geometries and material compositions. Drug distribution was visualised using Raman microscopy, which showed that whilst separate layers were successfully printed, several of the drugs diffused across the layers depending on their amorphous or crystalline phase. The printed constructs demonstrated excellent physical properties and the different material inclusions enabled distinct drug release profiles of the six actives within dissolution tests. For the first time, this paper demonstrates the feasibility of SLA printing as an innovative platform for multi-drug therapy production, facilitating a new era of personalised polypills.
Full article
►▼
Show Figures
Open AccessArticle
Influence of High, Disperse API Load on Properties along the Fused-Layer Modeling Process Chain of Solid Dosage Forms
by
Marius Tidau, Arno Kwade and Jan Henrik Finke
Cited by 22 | Viewed by 5666
Abstract
In order to cope with the increasing number of multimorbid patients due to demographic changes, individualized polypill solutions must be developed. One promising tool is fused layer modeling (FLM) of dosage forms with patient-specific dose combinations and release individualization. As there are few
[...] Read more.
In order to cope with the increasing number of multimorbid patients due to demographic changes, individualized polypill solutions must be developed. One promising tool is fused layer modeling (FLM) of dosage forms with patient-specific dose combinations and release individualization. As there are few approaches reported that systematically investigate the influence of high disperse active pharmaceutical ingredient (API) loads in filaments needed for FLM, this was the focus for the present study. Different filaments based on polyethylene oxide and hypromellose (HPMC) with different loads of theophylline as model API (up to 50 wt.%) were extruded with a twin-screw extruder and printed to dosage forms. Along the process chain, the following parameters were investigated: particle size and shape of theophylline; mechanical properties, microstructure, mass and content uniformity of filaments as well as dosage forms and the theophylline release from selected dosage forms. Especially for HPMC, increasing theophylline load enhanced the flexural strength of filaments whilst the FLM accuracy decreased inducing defects in microstructure. Theophylline load had no significant effect on the dissolution profile of HPMC-based dosage forms. Therefore, a thorough analysis of particle-induced effects is necessary to correlate mechanical properties of filaments, printability, and the dosage-and-release profile adjustment.
Full article
►▼
Show Figures
Open AccessArticle
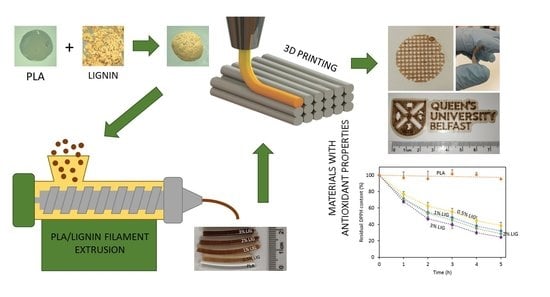
Antioxidant PLA Composites Containing Lignin for 3D Printing Applications: A Potential Material for Healthcare Applications
by
Juan Domínguez-Robles, Niamh K. Martin, Mun Leon Fong, Sarah A. Stewart, Nicola J. Irwin, María Isabel Rial-Hermida, Ryan F. Donnelly and Eneko Larrañeta
Cited by 244 | Viewed by 16593
Abstract
Lignin (LIG) is a natural biopolymer with well-known antioxidant capabilities. Accordingly, in the present work, a method to combine LIG with poly(lactic acid) (PLA) for fused filament fabrication applications (FFF) is proposed. For this purpose, PLA pellets were successfully coated with LIG powder
[...] Read more.
Lignin (LIG) is a natural biopolymer with well-known antioxidant capabilities. Accordingly, in the present work, a method to combine LIG with poly(lactic acid) (PLA) for fused filament fabrication applications (FFF) is proposed. For this purpose, PLA pellets were successfully coated with LIG powder and a biocompatible oil (castor oil). The resulting pellets were placed into an extruder at 200 °C. The resulting PLA filaments contained LIG loadings ranging from 0% to 3% (
w/
w). The obtained filaments were successfully used for FFF applications. The LIG content affected the mechanical and surface properties of the overall material. The inclusion of LIG yielded materials with lower resistance to fracture and higher wettabilities. Moreover, the resulting 3D printed materials showed antioxidant capabilities. By using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method, the materials were capable of reducing the concentration of this compound up to ca. 80% in 5 h. This radical scavenging activity could be potentially beneficial for healthcare applications, especially for wound care. Accordingly, PLA/LIG were used to design meshes with different designs for wound dressing purposes. A wound healing model compound, curcumin (CUR), was applied in the surface of the mesh and its diffusion was studied. It was observed that the dimensions of the meshes affected the permeation rate of CUR. Accordingly, the design of the mesh could be modified according to the patient’s needs.
Full article
►▼
Show Figures
Open AccessArticle
3D Printed Pellets (Miniprintlets): A Novel, Multi-Drug, Controlled Release Platform Technology
by
Atheer Awad, Fabrizio Fina, Sarah J. Trenfield, Pavanesh Patel, Alvaro Goyanes, Simon Gaisford and Abdul W. Basit
Cited by 201 | Viewed by 15232
Abstract
Selective laser sintering (SLS) is a single-step three-dimensional printing (3DP) process that can be leveraged to engineer a wide array of drug delivery systems. The aim of this work was to utilise SLS 3DP, for the first time, to produce small oral dosage
[...] Read more.
Selective laser sintering (SLS) is a single-step three-dimensional printing (3DP) process that can be leveraged to engineer a wide array of drug delivery systems. The aim of this work was to utilise SLS 3DP, for the first time, to produce small oral dosage forms with modified release properties. As such, paracetamol-loaded 3D printed multiparticulates, termed miniprintlets, were fabricated in 1 mm and 2 mm diameters. Despite their large surface area compared with a conventional monolithic tablet, the ethyl cellulose-based miniprintlets exhibited prolonged drug release patterns. The possibility of producing miniprintlets combining two drugs, namely paracetamol and ibuprofen, was also investigated. By varying the polymer, the dual miniprintlets were programmed to achieve customised drug release patterns, whereby one drug was released immediately from a Kollicoat Instant Release matrix, whilst the effect of the second drug was sustained over an extended time span using ethyl cellulose. Herein, this work has highlighted the versatility of SLS 3DP to fabricate small and intricate formulations containing multiple active pharmaceutical ingredients with distinct release properties.
Full article
►▼
Show Figures
Open AccessReview
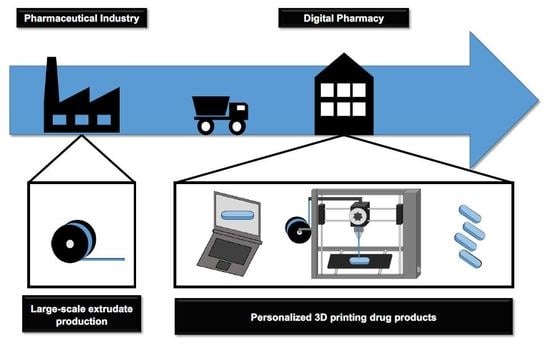
The Digital Pharmacies Era: How 3D Printing Technology Using Fused Deposition Modeling Can Become a Reality
by
Maisa R. P. Araújo, Livia L. Sa-Barreto, Tais Gratieri, Guilherme M. Gelfuso and Marcilio Cunha-Filho
Cited by 151 | Viewed by 13422
Abstract
The pharmaceutical industry is set to join the fourth industrial revolution with the 3D printing of medicines. The application of 3D printers in compounding pharmacies will turn them into digital pharmacies, wrapping up the telemedicine care cycle and definitively modifying the pharmacotherapeutic treatment
[...] Read more.
The pharmaceutical industry is set to join the fourth industrial revolution with the 3D printing of medicines. The application of 3D printers in compounding pharmacies will turn them into digital pharmacies, wrapping up the telemedicine care cycle and definitively modifying the pharmacotherapeutic treatment of patients. Fused deposition modeling 3D printing technology melts extruded drug-loaded filaments into any dosage form; and allows the obtainment of flexible dosages with different shapes, multiple active pharmaceutical ingredients and modulated drug release kinetics—in other words, offering customized medicine. This work aimed to present an update on this technology, discussing its challenges. The co-participation of the pharmaceutical industry and compounding pharmacies seems to be the best way to turn this technology into reality. The pharmaceutical industry can produce drug-loaded filaments on a large scale with the necessary quality and safety guarantees; while digital pharmacies can transform the filaments into personalized medicine according to specific prescriptions. For this to occur, adaptations in commercial 3D printers will need to meet health requirements for drug products preparation, and it will be necessary to make advances in regulatory gaps and discussions on patent protection. Thus, despite the conservatism of the sector, 3D drug printing has the potential to become the biggest technological leap ever seen in the pharmaceutical segment, and according to the most optimistic prognostics, it will soon be within reach.
Full article
►▼
Show Figures