Modulating the Blood–Brain Barrier: A Comprehensive Review
Abstract
:1. Introduction
| Physicochemical Properties | Value | Reference |
|---|---|---|
| Molecular weight | <500 Da | Lipinski et al. [2] |
| Molecular weight | <400 Da | Levin [3] |
| Molecular weight | <450 Da | Atkinson et al. [4] |
| LogP | <5 | Lipinski et al. [2] |
| LogP | 1.5–2.7 | Hansch et leo [5] |
| LogD | 1–3 | Van de Waterbeemd et al. [6] |
| Hydrogen bond donors | <5 | Lipinski et al. [2] |
| Hydrogen bond acceptors | <10 | Lipinski et al. [2] |
| Hydrogen bonds | <8 | Pajouhesh et Lenz [7] |
| Polar Hydrogen atoms | 0–1 | Ghose et al. [8] |
| No. Nitrogens | 1–2 | Ghose et al. [8] |
| No. Nitrogens + Oxygens | 2–4 | Ghose et al. [8] |
| Polar surface area (PSA) | <90 A2 | Hitchcock et Pennington [9] |
| Polar surface area (PSA) | <60–70 A2 | Kelder et al. [10] |
| Polar surface area (PSA) | 25−60 Å2 | Ghose et al. [8] |
| Solvent accessible surface area | 455−575 Å2 | Ghose et al. [8] |
| pKa | 4–10 | Fischer et al. [11] |
| Carboxylic acid functional groups | None, unless AA residue | Ghose et al. [8] |
| Rotatable bonds | <5 | Pajouhesh et Lenz [7] |
| Rotatable bonds | 1–4 | Ghose et al. [8] |
| Molecular volume | 740−970 Å3 | Ghose et al. [8] |
| Cytochrome P450 Inhibition | <50% at 30 μM | Pajouhesh et Lenz [7] |
| CYP2D6 metabolism | Low | Pajouhesh et Lenz [7] |
| CYP3A4 inducer | Not potent | Pajouhesh et Lenz [7] |
| Serum albumin affinity | Kd < 10 μM | Raub et al. [12] |
| P-Glycoprotein affinity | None to low | Raub et al. [12] |
| Aqueous solubility | >60 μg/mL | Pajouhesh et Lenz [7] |
| Effective permeability | 1 × 10−6 cm/s | Pajouhesh et Lenz [7] |
| CNS MPO | ≥4 | Wager et al. [13] |
2. Current Agents for Modulating the BBB
2.1. Focused Ultrasound
2.2. Development of Focused Ultrasound BBB modulation
2.2.1. MRgFUS BBB Modulation—Targeted Drug Delivery and Gene Therapy
2.2.2. MRgFUS BBB-Modulating Clinical Studies
| BBB Modulator | BBB Permeability Result | Onset of Action | Time to Recover | Tracer(s) Used | NP Size (nm) | Cell Line/Animal Tested | Administration |
|---|---|---|---|---|---|---|---|
| MRgFUS [32] | 3.8-fold increase of Evans blue dye accumulation in healthy brain tissue 2.1-fold increase of Evans blue dye accumulation in brain tumour tissue 1.7-fold increase in TMZ CSF/plasma ratio | <60 s | N.R | TMZ 194 Da Evans blue dye 961 Da | N/A | 7–8 week old Male Fischer 344 rats (180 g) | Intravenous injection |
| MRgFUS [33] | 1.4-fold slowing of 9 L gliosarcoma tumour growth | <120 s | N.R | Liposomal Doxorubicin (LDox) 544 Da | 100 | Male Sprague Dawley rats (~200 g) | Tail vein injection |
| MRgFUS [35] | 22% increase in striatum permeability 26% increase in hippocampus permeability | <15 min | N.R | GFP-ECNPCs | N/A | Sprague Dawley rats (200–250 g) | Carotid artery injection (stem cells) Tail vein injection (microbubbles) |
| MRgFUS [47] | 1.2-fold increase in free CDDP permeability 2.1-fold increase in AuNP | <24 h | <24 h | Cisplatin (CDDP) 300 Da AuNP-UP-CDDP (9 nm) | 9 | NSG mice | Tail vein injection (All) |
| MRgFUS [49] | 6-fold increase in Cisplatin across the BTB in 9 L glioma rat model 28-fold increase in Cisplatin across the BTB in F98 glioma rat model | <1 h | <1 h | Cisplatin (CDDP) 300 Da PAA-PEG-CDDP | 60 | Female Fisher 344 rats (200–220 g) | Tail vein perfusion |
| MRgFUS [56] | 50–75% of neurons transduced with GFP in the right striatum | <2 weeks | <2 weeks | ~20 nm Virus GFP plasmid (1–5 MDa) | 20 | Ten-week-old male Sprague Dawley rats (250–300 g) | Tail vein perfusion |
| MRgFUS [58] | 7.7-fold increase in TMZ in tumour tissue 1.5-fold increase in LDox in tumour tissue | <24 h | <24 h | Liposomal Doxorubicin (LDox) 544 Da TMZ 194 Da | 250–2500 | Human phase I clinical trial (5 patient population) | Intravenous injection (LDox) Oral administration (TMZ) |
| MRgFUS [59] | ~15% increase in Gadolinium leakage at target site | 0 min | <24 h | Gadolinium contrast agent (unspecified) (545–975 Da) | N/A | Human phase I clinical trial (4 patient population) | Intravenous injection |
3. Small Molecule BBB Modulators
3.1. Hyperosmolar Agents
3.2. Inflammatory Mediators
3.3. Alkylglycerols
3.4. Sodium Caprate (C10)
3.5. Regadenoson
3.6. Fingolimod
3.7. NS1619
3.8. NEO100
3.9. M01
4. Peptide and Peptidomimetic BBB Modulators
4.1. RMP-7
4.2. Zonula Toxin and Analogues
4.3. PN-159
4.4. HAV-6, C-CPE and Their Derivatives
4.5. Claudin Extracellular Loop Mimics
4.6. Occludin ECLs—Epithelial Disruptors
5. Protein BBB Modulators
5.1. Angubindin-1
5.2. Gintonin
5.3. Antibodies
6. Oligonucleotide-Based Gene Silencing
6.1. RNAi
6.2. Antisense Therapy
| BBB Modulator | BBB Permeability Result | Onset of Action | Time to Recover | Tracer(s) Used | Cell Line/Animal Tested | Administration |
|---|---|---|---|---|---|---|
| Small Molecules | ||||||
| Hyperosmolar Agents | ||||||
| Mannitol a (45 mL, 25% w/v) [61] | 100-fold increase of MTX in Brain tissue | <30 min | N.R | Methotrexate (MTX) 454 Da | Adult mongrel dogs (20–25 kg) | Internal carotid artery injection |
| Mannitol a (45 mL, 25% w/v) [61] | 10-fold increase of MTX in Brain tissue | <30 min | N.R | Methotrexate (MTX) 454 Da | Adult mongrel dogs (20–25 kg) | Femoral vein injection |
| Mannitol (180–360 mL, 25% w/v) [63] | 1000% increase in BBB permeability 60% increase in BTB permeability | 4 min (brain) 4 min (tumour) | 43 min (brain) 35 min (tumour) | Methotrexate (MTX) 454 Da | Thirteen glioblastoma multiforme patients | Intracarotid injection (All) |
| Mannitol a (30 mL, 25% w/v) [65] | 10-fold increase in [68Ga]EDTA | 30 s | 10 min | [68Ga]EDTA 356 Da | Five adult rhesus monkeys (5–10 kg) | Intracarotid injection (mannitol) Intravenous (68Ga EDTA) |
| Mannitol a (30 mL, 25% w/v) [66] | 2.5 increase in influx constant | 30 s | 2 h (extrapolated) | Rubidium 82 82 Da | Six adult male baboons (25–30 kg) | Intracarotid injection (mannitol) Peripheral intravenous injection (Rubidium 82) |
| Mannitol a (8 mL/40 s, 25% w/v) [73] | 5-fold increase in EBD brain accumulation | <40 s | >1 h | Evans Blue Dye (EBD) 961 Da | New Zealand white rabbits | Intracarotid injection (mannitol) Intravenous injection (EBD) |
| Mannitol a,b (2.25 mL/25 s, 25% w/v) [75] | ~4 to 55-fold increase in siRNA in brain tissue relative to saline control | <48 h | <48 h | Cy3-PD-hsiRNA ~13–15 kDa | 8–12 week male Sprague-Dawley rats (~325 g) | Intracarotid injection (All) |
| Arabinose a,b (2 g/Rat) [88] | 19-fold increase in brain permeability | <15 min | 2 h | [14C]Sucrose 342 Da | Male adult Osborn-Mendel strain rats (250–350 g) | Right carotid artery perfusion |
| Inflammatory Mediators | ||||||
| Histamine (100 μM) [100] | 20% drop in TEER | 5 min | >30 min | NTU | Co-culture model: HUVEC-304 C6 glioma cells (12-well) | In vitro |
| Histamine c (10 μM) [101] | 4-fold increase in Evans blue albumin (EBA) flux | <15 min | >2 h | EBA 67 kDa | Co-culture model: Bovine BCECs Primary rat astrocytes (6 well) | In vitro |
| Leukotriene D4 b (6 pmol/Mouse) [122] | 1.3-fold increase in brain:serum % of fluorescence marker | <35 min | >35 min | Sodium Fluorescein 355 Da | Adult male Swiss mice (25 ± 3.5 g) | ICV injection |
| Alkylglycerols | ||||||
| 1-O-pentylglycerol a (39 mg/Rat) [125] | Increase in tracer permeabilities: Methotrexate (230-fold) Cisplatin (125-fold) Vancomycin (15-fold) Gentamicin (12-fold) | <3 min | 15 min | Methotrexate (MTX) 454 Da Cisplatin (CDDP) 300 Da Vancomycin (VCM) 1449 Da Gentamicin (GTM) 478 Da | Male Wistar rats (250–320 g) | Right internal carotid artery injection |
| 1-O-pentylglycerol d (39–57 mg/Rat) [126] | 17-fold increase in Erucylphosphocholine (EPC) | <5 min | N.R | EPC 490 Da | Male Wistar rats (230–305 g) | Intracarotid bolus injection |
| 1-O-pentylglycerol a,b (90 ± 10 mg/kg) [127] | 6.5-fold increase in sodium fluorescein 2.7-fold increase in lissamine-rhodamine B200 (RB 200) albumin | <8 min | N.R | Sodium Fluorescein 367 Da RB 200 albumin 70 kDa | Wistar rats (180–220 g) | Intracarotid injection |
| 2-O-hexyldiglycerol a,b (1.2 mL/18 s, 100 mM) [129] | ~1.9-fold increase in RB 200 γ-globulin brain permeability | ≤10 min | ~24 h | RB 200 γ-globulin ~150 kDa | Wistar rats (180–220 g) | Intracarotid injection |
| Other | ||||||
| Sodium Caprate b (7.5 mM) [134] | ~2.6-fold increase in Lucifer Yellow permeability | <10 min | >40 min | Lucifer Yellow 457 Da | Monoculture model: MDCK-II cells (24 well) | In vitro |
| Sodium Caprate a,b (20 mM) [138] | Increase in tracer BBB permeabilities: Mannitol 7-fold Sucrose 6.4-fold PEG 900 5.6-fold PEG 4000 3.6-fold FITC dextran 4000 3.3-fold FITC dextran 20,000 3.2-fold FITC dextran 70,000 2.2-fold | 2–5 mins | >15 min | Mannitol 182 Da Sucrose 342 Da PEG 900 900 Da PEG 4000 4000 Da FITC dextran 4K 4400 Da FITC dextran 20K 19,600 Da FITC dextran 70K 71,200 Da | Male Wistar rats (200–250 g) | Internal carotid artery perfusion |
| Sodium Caprate a (8.7 mg/Rat) [139] | 10-fold increase in Mannitol brain permeability | 30–90 s | 1 h | Mannitol 182 Da | Adult sprague dawley rats (360–380 g) | Left internal carotid artery infusion |
| Regadenoson b (0.5 μg/kg) (3 doses, 5 min apart) [143] | Approx. 3-fold increase in dextran BBB permeability | <35 min | 35 min | Dextran 10 kDa | Sprague Dawley rats female, 8 weeks (200–220 g) | Retro-orbital intravenous injection |
| Regadenoson b (50 μg/kg) [143] | Approx. 4-fold increase in dextran BBB permeability | <35 min | 35 min | Dextran 10 kDa | Sprague Dawley rats female, 8 weeks (200–220 g) | Retro-orbital intravenous injection |
| Regadenoson c (50 μg/kg) [144] | Approx. 5, 10 and 11-fold increase in epirubicin within the cerebellum, hippocampus and cortex respectively | 5–15 min | 30 min | Epirubicin 544 Da | Wild type mice (unspecified) | Intravenous injection |
| Regadenoson (0.5 μg/kg) [148] | 60% increase in temozolomide BBB permeability | <1 h | N.R | Temozolomide 194 Da | Female F344 rats (150–170 g) | Intravenous tail injection |
| Fingolimod b (5 mg/kg) [151] | 2.7-fold increase in Alexa Fluor 555–cadaverine (AFC) leakage | <6 h | <7 days | AFC 1 kDa | Wild type mice | Oral gavage |
| NIBR-0213 b (60 mg/kg) [151] | 5-fold increase in Alexa Fluor 555–cadaverine (AFC) leakage | <6 h | <48 h | AFC 1 kDa | Wild type mice | Oral gavage |
| NS1619 b (10 μM) [157] | 40% drop in TEER 4-fold increase in horseradish peroxidase (HRP) flux | 1–2 h | 4–6 h | HRP (44 kDa) | Co-culture model: Rat BMECs C6 glioma cells (24 well) | In vitro |
| M01 b (2.9 µmol/kg) [161] | 3.9-fold increase in Fluorescein levels within cerebrum | <3 h | 24–48 h | Sodium Fluorescein 367 Da | Adult C57BL/6N mice | Intravenous tail injection |
| Peptides, Peptidomimetics & Proteins | ||||||
| RMP-7 d (1.5 µg/kg) [171] | 2.7-fold increase in tumour permeability of carboplatin | <20 min | 35–65 min | Carboplatin 373 Da | Female Wistar rats (180–230 g) | Intracarotid infusion (RMP-7) |
| RMP-7 d (1.5 µg/kg) [173] | 4-fold increase in 70 kDa dextran | <5 min | 25 min | Dextran 70k 70 kDa | Wistar rats RG2 glioma model | Intra-arterial infusion |
| Zonula occluden toxin b (2 μg/mL) [186] | 2-fold increase in sucrose, doxorubicin and paclitaxel across BBB monolayer 1.3-fold increase in insulin across BBB monolayer 32% drop in TEER | 30 min | 80 min | Sucrose 342 Da Doxorubicin 544 Da Paclitaxel 854 Da Insulin 5734 Da | Bovine BMEC monolayer | In vitro |
| ∆G b (600 μg ∆G/kg) (MTX) (800 μg ∆G/kg) (PTX) [188] | 7-fold increase in brain:plasma ratio (MTX) 2.5 increase in brain:plasma ratio (PTX) | <5 min | N.R | Sucrose 342 Da Methotrexate (MTX) 454 Da Paclitaxel (PTX) 854 Da | Male Sprague–Dawley rats (225–275 g) | Intracarotid cannula |
| ADT-6 b (2 mM) [194] | 60% reduction in TEER 1.5-fold increase in fluorescein flux No significant increase in albumin flux | <1 h | 1–24 h | Fluorescein 376 Da Albumin 65 kDa | Triple Culture BBB: Primary BMECs Glial cells Pericytes (12 well) | In vitro |
| HAV-6 b (2 mM) [194] | 60% reduction in TEER No significant increase in fluorescein flux No significant increase in albumin flux | <1 h | 1–24 h | Fluorescein 376 Da Albumin 65 kDa | Triple Culture BBB: Primary BMECs Glial cells Pericytes (12 well) | In vitro |
| C-CPE b (1 mM) [194] | 28% reduction in TEER No significant increase in fluorescein flux No significant increase in albumin flux | <1 h | 1–24 h | Fluorescein 376 Da Albumin 65 kDa | Triple Culture BBB: Primary BMECs Glial cells Pericytes (12 well) | In vitro |
| 7-mer b,c (100 μM) [194] | 49% reduction in TEER 5.5-fold increase in fluorescein flux 3.5-fold increase in albumin flux | <1 h | 1–24 h | Fluorescein 376 Da Albumin 65 kDa | Triple Culture BBB: Primary BMECs Glial cells Pericytes (12 well) | In vitro |
| AT-1002 b (2 mM) [194] | 48% reduction in TEER 6.5-fold increase in fluorescein flux 6-fold increase in albumin flux | <1 h | 1–24 h | Fluorescein 376 Da Albumin 65 kDa | Triple Culture BBB: Primary BMECs Glial cells Pericytes (12 well) | In vitro |
| PN-159 b (10 μM) [194] | 68% reduction in TEER 11-fold increase in fluorescein flux 9.5-fold increase in albumin flux | <1 h | 1–24 h | Fluorescein 376 Da Albumin 65 kDa | Triple Culture BBB: Primary BMECs Glial cells Pericytes (12 well) | In vitro |
| HAV-6 b,e (10 μmol/kg) [195] | 2.7-fold increase in Galbumin flux (posterior brain) 3.5-fold increase in Galbumin flux (midbrain) 3.2-fold increase in Galbumin flux (anterior brain) | <3 min | <10 min | Galbumin 65 kDa | Balb/c mice | Tail vein injection |
| ADTC5 b,e 7.7 mg/kg (Galbumin and IRdye) 30 mg/kg (cIBR7 assay only) [195] | 3-fold increase in Galbumin flux (posterior brain) 4.8-fold increase in Galbumin flux (midbrain) 3.5-fold increase in Galbumin flux (anterior brain) 2.8-fold increase in IRdye800cw-cLABL brain to plasma fluorescence 4-fold increase in cIBR7 brain levels | <3 min | <40 min | Galbumin 65 kDa IRdye800cw-cLABL 2.2 kDa cIBR7 775 Da | Balb/c mice (cIBR7 and Galbumin) male Sprague–Dawley rats (300–400 g) (IRdye assay) | jugular vein cannulation (IRdye assay) Tail vein injection (cIBR7 and Galbumin) |
| cHAVc3 b,e (6.6 mg/kg) [195] | 2-fold increase in Galbumin flux (posterior brain) 4.2-fold increase in Galbumin flux (midbrain) 3.2-fold increase in Galbumin flux (anterior brain) | <3 min | >51 min | Galbumin 65 kDa | Balb/c mice | Tail vein injection |
| cCPE-Y306W/S313H b (10 μg/mL) [199] | 1.9-fold increase in Carboxyfluorecein flux 68% drop in TEER | <5 h | 35 h | CF 376 Da | Monoculture BBB: pPBMEC (12 well) | In vitro |
| cCPE-Y306W/S313H b,f (10 μg/mL, in vitro) (5 ng/larva, in vivo) [200] | 60% drop in TEER (in vitro) 4.3 increase in Rhod B-Dex flux (cerebellar central artery) (in vivo) 4.6 increase in Rhod B-Dex flux (middle mesencephalic central artery) (in vivo) 5.3 increase in Rhod B-Dex flux (metencephalic artery) (in vivo) | <3 h (in vitro) <1 h (in vivo) | 48 h (in vitro) 3–4 h (in vivo) | Rhod B-Dex 10 kDa | Monoculture BBB: bEnd.3 Zebrafish Larvae (in vivo) | In vitroposterior cardinal vein injection (In vivo) |
| C1C2 b (200 μM) [202] | 50% drop in TEER 8.25-fold increase in lucifer yellow 7-fold increase in AlexaFluor 680-dextran | <2 h | >24 h | Lucifer Yellow 444 Da AlexaFluor-680 3 kDa | Monoculture BBB: Primary mouse BMECs (24 well) | In vitro |
| D-aa-C5C2 b,g In vivo(3.5 μmol/kg) In vitro(300 μM) [208] | 55% drop in TEER (in vitro-endo) 1.4-fold increase in Gd-DTPA (in vivo) 4-fold increase in Doxorubicin (in vitro—epithelial) 5.5-fold Lucifer Yellow flux (in vitro—epithelial) 3.75-fold increase in Fluorescein-Dex flux (in vitro—epithelial) | <4 h (in vivo) <12 h (in vitro) | 4–12 h (in vivo) >48 h (in vitro) | Doxorubicin 544 Da Gd-DTPA 547 Da Lucifer Yellow 457 Da Fluorescein-Dex 10 kDa | Monoculture BBB: bEND.3 cells (12 well) Monoculture Epithelial: MDCKII cells (Cld-5 transfected) (12 well) Animal model: C57BL/6N mice, 10–19 weeks old (18–23 g) | In vitroTail vein injection (in vivo) |
| cCPE-Y306W/S313H b In vivo(360 nmol/kg) In vitro(120 μg/mL) [214] | 97% drop in TEER 5.6-fold increase in ASO brain levels | <2 h (in vitro) <1 h (in vivo) | >120 h (in vitro) N.R (in vivo) | ASO (16 NCT’s) 5.3 kDa | Triple Culture BBB: Primary rat BCEC’s Primary rat Pericytes Primary rat astrocytes (24 well) Animal model: Wild-type female C57BL/6 mice (8–11 weeks) | Intravenous injection |
| Angubindin-1 b (10 mg/kg) [214] | 90% drop in TEER (in vitro) 20-fold increase in ASO brain levels (in vivo) | <2 h (in vitro) <1 h (in vivo) | 120 h (in vitro) <24 h (in vivo) | ASO (16 NCT’s) 5.3 kDa | Triple Culture BBB: Primary rat BCEC’s Primary rat Pericytes Primary rat astrocytes (24 well) Animal model: Wild-type female C57BL/6 mice (8–11 weeks) | Intravenous injection |
| Gintonin b (100 μg/mL, in vitro) (10 mg/kg, in vivo) [215] | ~110-fold increase in Texas red-Dextran (in vitro) Approx. 4-fold increase in FITC Dextran Brain levels (in vivo) 41% increase in EPO levels in the CSF (in vivo) | <1 min (in vitro) <5 min (in vivo) | 15–30 min (in vitro) >30 min (in vivo) | Texas red-Dextran (70 kDa) FITC-Dextran (10 kDa) EPO (34 kDa) | Monoculture BBB: HBMECs (24 well) Animal model: 8 Week Male Sprague Dawley rats (220–250 g) | Retro-orbital vein injection |
| M48 b (150 μg/mL) [217] | 98% drop in TEER 3-fold increase in P(app) (Fluorescein) 3.5-fold increase in P(app) (Fluorescein-Dex) | <3 h | 12–24 h | Fluorescein 376 Da Fluorescein-Dex 4 kDa | Triple Culture BBB: CMBMECs Rat cerebral astrocytes Rat cerebral pericytes (24 well) | In vitro |
| R9 b (150 μg/mL) [217] | 95% drop in TEER 3.75-fold increase in P(app) (Fluorescein) 5.75-fold increase in P(app) (Fluorescein-Dex) | <3 h | 12–24 h | Fluorescein 376 Da Fluorescein-Dex 4 kDa | Triple Culture BBB: CMBMECs Rat cerebral astrocytes Rat cerebral pericytes (24 well) | In vitro |
| Oligonucleotides | ||||||
| Claudin-5 + Occludin siRNA b,h (10 pmol(each)/well) (20 μg(each)/mouse, 1 mg(each)/kg) [153] | 2.8-fold increase in apical to basolateral permeability of FITC amyloid-β peptide (in vitro) 2.5-fold increase in apical to basolateral Papp of FITC amyloid-β peptide (in vitro) 17-fold increase in basolateral to apical permeability of FITC amyloid-β peptide (in vitro) 20-fold increase in basolateral to apical Papp of FITC amyloid-β peptide (in vitro) 2.4-fold increase in 3k biotin-dextran (in vivo) | <72 h (in vitro) (in vivo) | >74 h (in vitro) >72 h (in vivo) | Biotin-dextran 3 kDa | Monoculture BBB model: Bend.3 (24 well) Animal model: Tg2576 mice (~20 g) | Transwell luminal surface (in vitro) Transwell abluminal surface (in vitro) Tail vein injection (in vivo) |
| Claudin-5 siRNA b,h (10 pmol/well) (20 μg/mouse, 1 mg/kg) [153] | 2.5-fold increase in apical to basolateral permeability of FITC amyloid-β peptide (in vitro) 2-fold increase in apical to basolateral Papp of FITC amyloid-β peptide (in vitro) 8.5-fold increase in basolateral to apical permeability of FITC amyloid-β peptide (in vitro) 8-fold increase in basolateral to apical Papp of FITC amyloid-β peptide (in vitro) 1.3-fold increase in 3k biotin-dextran (in vivo) | <72 h (in vitro) (in vivo) | >74 h (in vitro) >72 h (in vivo) | Biotin-dextran 3 kDa | Monoculture BBB model: Bend.3 (24 well) Animal model: Tg2576 mice (~20 g) | Transwell luminal surface (in vitro) Transwell abluminal surface (in vitro) Tail vein injection (in vivo) |
| Occludin siRNA b,h (10 pmol/well) (20 μg/mouse, 1 mg/kg) [153] | 2.6-fold increase in apical to basolateral permeability of FITC amyloid-β peptide (in vitro) 2.3-fold increase in apical to basolateral Papp of FITC amyloid-β peptide (in vitro) 11-fold increase in basolateral to apical permeability of FITC amyloid-β peptide (in vitro) 10-fold increase in basolateral to apical Papp of FITC amyloid-β peptide (in vitro) No significant increase in 3k biotin-dextran (in vivo) | <72 h (in vitro) (in vivo) | >74 h (in vitro) >72 h (in vivo) | Biotin-dextran 3 kDa | Monoculture BBB model: Bend.3 (24 well) Animal model: Tg2576 mice (~20 g) | Transwell luminal surface (in vitro) Transwell abluminal surface (in vitro) Tail vein injection (in vivo) |
| Claudin-5 shRNA c,i (2 µL AAV sln) [223] | 6.5-fold increase in Biotin (hippocampus) 3.6-fold increase in Biotin (mPFC) | <24 h | N.R | Biotin 600 Da | C57/BL6J mice (8–12 weeks) | Stereotaxic injection into hippocampus or mPFC |
| Claudin-5 siRNA b (20 μg/mouse) [224] | 1.25-fold increase in Gd-DTPA | <24 h | 3–7 days | Gd-DTPA 742 Da | C57/BL6 mice (20–30 g) | Tail vein injection |
| 13-mer Toc-HDO c,i (50 mg/kg) [228] | 55% reduction in efflux rate | <72 h | >72 h | 99mTc-ECD 436 Da | Wild-type C57BL/6 mice (7–10 weeks) | Intravenous injection |
6.2.1. Future Prospects of BBB Modulation
Focused Ultrasound
Small Molecules and Peptides
Oligonucleotides
A Combined Approach
BBB Modulation and Sterile Inflammation
| Small Molecule | Chemical Formula | Molecular Mass (Da) | Chemical Structure |
| Hyperosmolar Agents | |||
| Mannitol | C6H14O6 | 182.17 |  |
| Arabinose | C5H10O5 | 150.13 | 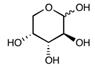 |
| Inflammatory Mediators | |||
| Histamine | C5H9N3 | 111.15 |  |
| Leukotriene C4 | C30H47N3O9S | 625.77 |  |
| Leukotriene D4 | C25H40N2O6S | 496.66 |  |
| Leukotriene E4 | C23H37NO5S | 439.61 | 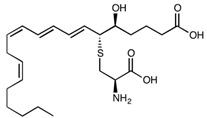 |
| Alkylglycerols | |||
| 1-O-Pentylglycerol | C8H18O3 | 162.23 |  |
| 2-O-hexyldiglycerol | C12H26O5 | 250.33 | 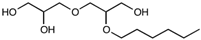 |
| Other | |||
| Sodium Caprate | C10H19NaO2 | 194.25 |  |
| Regadenoson | C15H18N8O5 | 390.35 |  |
| Fingolimod | C19H33NO2 | 307.47 |  |
| NIBR-0213 | C27H29ClN2O3 | 464.98 | 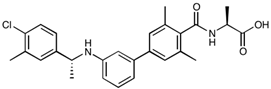 |
| NS1619 | C15H8F6N2O2 | 362.23 | 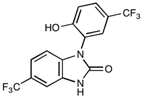 |
| NEO100 | C10H16O | 152.23 | 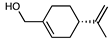 |
| M01 | C30H28N4O5 | 524.57 |  |
| Peptide/Peptidomimetic | Peptide Sequence | Molecular Mass (Da) | Chemical Structure |
| ADT-6 | Ac-ADTPPV-NH2 | 639.70 | 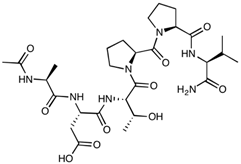 |
| ADTC5 | Cyclo(1,7)Ac-CDTPPVC-NH2 | 772.89 |  |
| AT-1002 | FCIGRL | 707.88 | 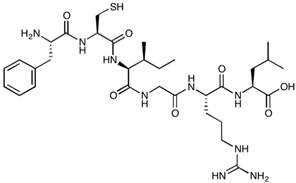 |
| AT-1002 (Allyl-Gly) | H-Phe-(Allyl)Gly-Ile-Gly-Arg-Leu-OH | 701.86 |  |
| Bradykinin | RPPGFSPFR | 1060.21 |  |
| cCPE | SSYSGNYPYSILFQKF | 1901.08 |  |
| cCPE-Y306W/S313H | SHYSGNYPWSILFQKF | 1974.18 |  |
| C1C2 | SSVSQSTGQIQSKVDSLLNLNSTQATR-NH2 | 2835.05 |  |
| cHAVc3 | Cyclo(1,6)Ac-CSHAVC-NH2 | 657.76 | 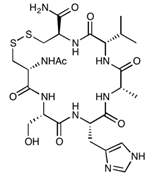 |
| HAV-6 | Ac-SHAVSS-NH2 | 627.65 | 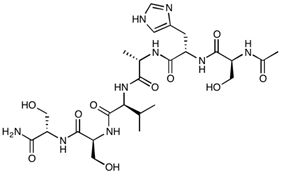 |
| PN-159 | KLALKLALKALKAALKLA-NH2 | 1876.46 |  |
| RMP-7 | H-Arg-Pro-Hyp-Gly-2Thi-Ser-Pro-βTyr(Me)-Arg-OH | 1098.28 | 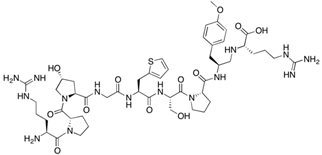 |
| 7-mer (PN78) | FDFWITP | 925.04 | 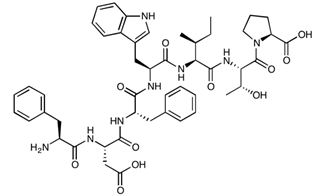 |
| Protein | Molecular Mass (Da) | Amino Acid Sequence | |
| Zonula occluden toxin | 44,903.41 |  | |
| ∆G | 12,852.67 |  | |
| Angubindin-1 | 27,020.19 |  | |
| Oligonucleotide | Molecular Mass (Da) | Oligonucleotide Sequence | |
| Claudin-5 siRNA | 13,352.40 |  | |
| Occludin siRNA | 13,307.37 | 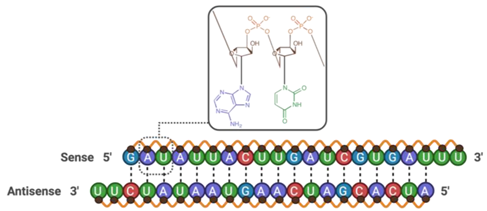 | |
| 13-mer Toc-HDO | 8984.37 |  | |
 Guanine,
Guanine,  Adenine,
Adenine,  Thymine,
Thymine,  Uracil,
Uracil,  Cytosine,
Cytosine,  Ribose sugar unit,
Ribose sugar unit,  Deoxyribose Sugar unit,
Deoxyribose Sugar unit,  2′-O-methyl ribose,
2′-O-methyl ribose,  Locked deoxyribose,
Locked deoxyribose,  Phosphothioate bonds,
Phosphothioate bonds,  Phosphonate bonds,
Phosphonate bonds,  α-Tocopherol.
α-Tocopherol.| Title | Condition(s) | BBBD Method | Phase | Enrolment | Start Date | Status |
|---|---|---|---|---|---|---|
| Focused Ultrasound (Non-combination studies) | ||||||
| Assessment of Initial Efficacy and Safety of High Intensity Focused Ultrasound ‘ExAblate 4000 Type 2’ for Blood-Brain Barrier Disruption in Patients With Alzheimer’s Disease | Alzheimer’s Disease | Exablate | N.A. | 6 | 10 April 2020 | Active, not recruiting |
| Blood-Brain Barrier Opening in Alzheimer’ Disease (BOREAL1) | Alzheimer’s Disease | SonoCloud | I/II | 10 | 26 June 2017 | Active, not recruiting |
| Blood-Brain Barrier Opening Using MR-Guided Focused Ultrasound in Patients With Amyotrophic Lateral Sclerosis | Amyotrophic Lateral Sclerosis | MRgFUS | N.A. | 8 | 13 April 2018 | Active, not recruiting |
| Non-invasive Blood-brain Barrier Opening in Alzheimer’s Disease Patients Using Focused Ultrasound | Alzheimer’s Disease | MRgFUS | N.A. | 6 | 1 October 2020 | Recruiting |
| A Study to Evaluate Temporary Blood-Brain Barrier Disruption in Patients With Parkinson’s Disease Dementia | Parkinson’s Disease Dementia | MRgFUS | N.A. | 10 | 26 November 2018 | Active, not recruiting |
| The Use of Focused Ultrasound and Microbubble Infusion for Altering Brain Perfusion and the Blood-Brain Barrier | Low Grade Glioma | MRgFUS | N.A. | 15 | 1 February 2020 | Not yet recruiting |
| Assessment of Safety and Feasibility of ExAblate Blood-Brain Barrier (BBB) Disruption for Treatment of Glioma | Glioblastoma | ExAblate | N.A. | 20 | 16 October 2018 | Recruiting |
| Assessment of Safety and Feasibility of ExAblate Blood-Brain Barrier (BBB) Disruption | Glioma | ExAblate | N.A. | 20 | 26 March 2019 | Recruiting |
| ExAblate Blood-Brain Barrier Opening for Treatment of Alzheimer’s Disease | Alzheimer’s Disease | ExAblate | N.A. | 30 | 6 December 2018 | Recruiting |
| ExAblate Blood-Brain Barrier (BBB) Disruption for the Treatment of Alzheimer’s Disease | Alzheimer’s Disease | ExAblate | N.A. | 20 | 28 September 2018 | Recruiting |
| ExAblate Blood-Brain Barrier Disruption (BBBD) for Planned Surgery in Suspected Infiltrating Glioma | Glioma | ExAblate | N.A. | 15 | 18 October 2018 | Active, not recruiting |
| ExAblate Blood-Brain Barrier Disruption for Glioblastoma in Patients Undergoing Standard Chemotherapy | Glioblastoma multiforme | ExAblate | N.A. | 10 | 28 August 2018 | Recruiting |
| Blood-Brain Barrier Disruption (BBBD) Using MRgFUS in the Treatment of Her2-positive Breast Cancer Brain Metastases | Breast cancer Brain metastases | ExAblate | N.A. | 10 | 18 September 2019 | Recruiting |
| Safety and Effectiveness of Blood-Brain Barrier Disruption (BBBD) in Subjects With Suspected Infiltrating Glioma (BBBD) | Glioma | ExAblate | N.A. | 120 | 1 December 2021 | Not yet recruiting |
| Assessment of Safety and Feasibility of ExAblate Blood-Brain Barrier (BBB) Disruption in GBM Patients | Glioma | ExAblate | N.A. | 5 | 15 September 2021 | Not yet recruiting |
| Focused Ultrasound (Drug-device Combination studies) | ||||||
| Exablate Blood-Brain Barrier Disruption With Carboplatin for the Treatment of rGBM | Glioblastoma | Exablate | I/II | 40 | 13 October 2020 | Recruiting |
| Ultrasound-based Blood-brain Barrier Opening and Albumin-bound Paclitaxel for Recurrent Glioblastoma (SC9/ABX) | Glioblastoma | SonoCloud-9 | I/II | 37 | August 2020 | Recruiting |
| Blood-Brain-Barrier Disruption With Cerezyme in Patient’s With Parkinson’s Disease Dementia | Parkinson disease dementia | ExAblate | N.A. | 6 | 16 July 2020 | Active, not recruiting |
| Blood-Brain Barrier Disruption Using Transcranial MRI-Guided Focused Ultrasound | Brain Tumor | ExAblate | N.A. | 10 | October 2014 | Active, not recruiting |
| Exablate Blood-Brain Barrier Disruption for the Treatment of rGBM in Subjects Undergoing Carboplatin Monotherapy | Glioblastoma | ExAblate | I/II | 30 | 6 November 2020 | Recruiting |
| Safety and Efficacy of Transient Opening of the Blood-brain Barrier (BBB) With the SonoCloud-9 | Adult glioblastoma | SonoCloud-9 | I/IIa | 30 | 18 February 2019 | Active, not recruiting |
| Safety and Efficacy of Sonocloud Device Combined With Nivolumab in Brain Metastases From Patients With Melanoma | Metastatic melanoma | SonoCloud | I/II | 21 | 24 October 2019 | Recruiting |
| Efficacy and Safety of NaviFUS System add-on Bevacizumab (BEV) in Recurrent GBM Patients | Glioblastoma | NaviFUS system | N.A. | 10 | 21 July 2020 | Recruiting |
| Non-Invasive Focused Ultrasound (FUS) With Oral Panobinostat in Children With Progressive Diffuse Midline Glioma (DMG) | Diffuse midline glioma | FUS | I | 15 | July 2021 | Recruiting |
| Innovative SonoCloud-9 Device for Blood Brain Barrier Opening in First Line Temozolomide Glioblastoma Patients. (SonoFIRST) | Glioblastoma | SonoCloud-9 | II | 66 | 11 September 2021 | Recruiting |
| Laser Heat Ablation | ||||||
| Using MRI-Guided Laser Heat Ablation to Induce Disruption of the Peritumoral Blood-Brain Barrier to Enhance Delivery and Efficacy of Treatment of Pediatric Brain Tumors | Glioma | MRI-guided laser heat ablation | II | 12 | 14 August 2015 | Recruiting |
| MK-3475 in Combination With MRI-guided Laser Ablation in Recurrent Malignant Gliomas | Malignant glioma | MRI-guided laser heat ablation | I/II | 58 | 5 August 2015 | Active, not recruiting |
| Surgery | ||||||
| Surgical Tissue Flap to Bypass the Blood-Brain Barrier in GBM | Glioblastoma multiforme | Temporoparietal fascial or Pericranial surgical flap | N.A. | 10 | 27 July 2018 | Recruiting |
| Laparoscopically Harvested Omental Free Tissue Autograft to Bypass the Blood-Brain Barrier (BBB) in Human Recurrent Glioblastoma Multiforme (rGBM) | Glioma | Laparoscopically harvested omental free flap | I | 10 | 6 January 2020 | Recruiting |
| Small Molecule | ||||||
| Determining Dose of Regadenoson Most Likely to Transiently Alter the Integrity of the Blood-Brain Barrier in Patients With High Grade Gliomas | High grade glioma | Regadenoson | I | 45 | 6 December 2019 | Recruiting |
| Melphalan, Carboplatin, Mannitol, and Sodium Thiosulfate in Treating Patients With Recurrent or Progressive CNS Embryonal or Germ Cell Tumors | CNS tumours | Mannitol | I/II | 55 | 9 July 2009 | Active, not recruiting |
| Carboplatin, Melphalan, Etoposide Phosphate, Mannitol, and Sodium Thiosulfate in Treating Patients With Previously Treated Brain Tumors | Glioma | Mannitol | I/II | 43 | 15 September 2005 | Recruiting |
| Methotrexate, Mannitol, Rituximab, and Carboplatin in Treating Patients With Newly Diagnosed Primary Central Nervous System Lymphoma | CNS lymphoma | Mannitol | I/II | 81 | 14 October 2005 | Recruiting |
| Super-selective Intra-arterial Repeated Infusion of Cetuximab for the Treatment of Newly Diagnosed Glioblastoma | Glioblastoma | Mannitol (SIACI) | I/II | 33 | June 16 | Recruiting |
| Super-selective Intra-arterial Cerebral Infusion of Trastuzumab for the Treatment of Cerebral Metastases of HER2/Neu Positive Breast Cancer | Neoplasm metastasis | Mannitol (SIACI) | I | 48 | August-2021 | Recruiting |
| Super-Selective Intraarterial Cerebral Infusion Of Temozolomide (Temodar) For Treatment Of Newly Diagnosed GBM And AA | Glioma | Mannitol (SIACI) | I | 30 | August 2010 | Active, not recruiting |
| Repeated Super-selective Intraarterial Cerebral Infusion Of Bevacizumab Plus Carboplatin For Treatment Of Relapsed/Refractory GBM And Anaplastic Astrocytoma | Glioma | Mannitol (SIACI) | I/II | 54 | September 2011 | Suspended |
| Repeated Super-selective Intraarterial Cerebral Infusion of Bevacizumab (Avastin) for Treatment of Relapsed GBM and AA | Glioma | Mannitol (SIACI) | I/II | 54 | October 2010 | Recruiting |
| Repeated Super-Selective Intraarterial Cerebral Infusion of Bevacizumab (Avastin) for Treatment of Newly Diagnosed GBM | Glioblastoma multiforme | Mannitol (SIACI) | I/II | 25 | February 2013 | Recruiting |
| Intraarterial Infusion Of Erbitux and Bevacizumab For Relapsed/Refractory Intracranial Glioma In Patients Under 22 | Glioma | Mannitol (SIACI) | I/II | 30 | June 2013 | Recruiting |
| Super Selective Intra-arterial Repeated Infusion of Cetuximab (Erbitux) With Reirradiation for Treatment of Relapsed/Refractory GBM, AA, and AOA | Glioma | Mannitol (SIACI) | II | 37 | May 2016 | Recruiting |
| IA Carboplatin + Radiotherapy in Relapsing GBM | Glioblastoma multiforme | Intra-arterial chemotherapy | II | 35 | 10 July 2018 | Recruiting |
| Miscellaneous | ||||||
| TMS Electrochemotherapy for Glioblastoma Multiforme | Glioblastoma | TMS | II | 20 | January 2015 | Suspended |
| The Danish Neuropsychological Study on the Adverse Effects of ECT | Depressive disorder | Electroconvulsive therapy | N.A. | 290 | 12 November 2020 | Recruiting |
| CED of MTX110 Newly Diagnosed Diffuse Midline Gliomas | Gliomas | Convection enhanced delivery | I | 9 | 10 March2020 | Recruiting |
7. Conclusions
| Trial | Condition(s) | BBBD Method | Phase | Enrolment | Start Date | End Date |
|---|---|---|---|---|---|---|
| Ultrasound | ||||||
| Evaluation of Blood-Brain Barrier Integrity and Structural Abnormalities in MPS IIIB Patients Using Multimodal Magnetic Resonance Imaging | MPS IIIB (Sanfilippo B Syndrome) | MRgFUS | N.A | 5 | December 2013 | May 2014 |
| Blood-Brain-Barrier Opening Using Focused Ultrasound With IV Contrast Agents in Patients With Early Alzheimer’s Disease | Alzheimer’s Disease | ExAblate | I | 6 | December 2016 | December 2017 |
| Safety of BBB Opening With the SonoCloud | Glioma | SonoCloud | I/II | 27 | July 2014 | July 2018 |
| Safety of BBB Disruption Using NaviFUS System in Recurrent Glioblastoma Multiforme (GBM) Patients | Glioblastoma multiforme | NaviFUS system | N.A | 6 | August 2018 | June 2019 |
| Laser Heat Ablation | ||||||
| MRI-Guided Laser Surgery and Doxorubicin Hydrochloride in Treating Patients With Recurrent Glioblastoma Multiforme | Glioblastoma | MRI-guided laser heat ablation | I/IIa | 37 | August 2013 | May 2018 |
| Small Molecule | ||||||
| Methotrexate, Cyclophosphamide, and Etoposide Phosphate Given With Osmotic Blood-Brain Barrier Disruption Plus Dexamethasone and Cytarabine in Treating Patients With Primary CNS Lymphoma | Lymphoma | Osmotic BBBD (unspecified agent) | II | 22 | January 2000 | July 2006 |
| Brain Interstitium Temozolomide Concentration Pre and Post Regadenoson Administration | Blood–brain barrier defect | Regadenoson | I | 6 | February 2015 | February 2018 |
| Super-Selective Intraarterial Cerebral Infusion of Cetuximab (Erbitux) for Treatment of Relapsed/Refractory GBM and AA | Glioma | Mannitol (SIACI) | I | 15 | December 2009 | January 2016 |
| Super-Selective Intraarterial Intracranial Infusion of Avastin (Bevacizumab) | Glioma | Mannitol (SIACI) | I | 30 | July 2009 | January 2014 |
| Low-dose Intra-arterial Bevacizumab for Edema and Radiation Necrosis Therapeutic Intervention (LIBERTI) | Radiation Necrosis | Mannitol (IA) | II | 10 | November 2016 | June 2019 |
| Peptides | ||||||
| A Pediatric Phase I Trial of RMP-7 and Carboplatin in Brain Tumors | Gliomas | RMP-7 | I | 30 | April 1996 | March 2000 |
| The Safety and Effectiveness of RMP-7 Plus Amphotericin B in Patients With HIV and Cryptococcal Meningitis | Viral/Fungal Infections | RMP-7 | I | N.R | August 2001 * | June2005 * |
| Lobradimil and Carboplatin in Treating Children With Brain Tumors | Brain and CNS Tumors | RMP-7 | II | 146 (max) * | March 1998 | April 2003 |
| Radiation Therapy Plus Carboplatin and Lobradimil in Treating Children With Newly Diagnosed Brain Stem Gliomas | Brain and CNS Tumors | RMP-7 | I | 13 | February 2001 | September 2005 |
| Transcranial Magnetic Stimulation | ||||||
| Effect of Deep TMS on the Permeability of the BBB in Patients With Glioblastoma Multiforme: a Pilot Study | Glioblastoma multiforme of the brain | dTMS | II | 15 | November 2014 | May 2015 |
| Electroconvulsive Therapy | ||||||
| Exploring Effects of Electroconvulsive Therapy on the Human Brain in Depression—The Danish ECT/MRI Study | Major depressive disorder | Electroconvulsive therapy | N.A | 60 | August 2017 | June 2020 |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, D.; Wang, C.; Wang, L.; Chen, Y. A comprehensive review in improving delivery of small-molecule chemotherapeutic agents overcoming the blood-brain/brain tumor barriers for glioblastoma treatment. Drug Deliv. 2019, 26, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Levin, V.A. Relationship of octanol/water partition coefficient and molecular weight to rat brain capillary permeability. J. Med. Chem. 1980, 23, 682–684. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, F.; Cole, S.; Green, C.; van de Waterbeemd, H. Lipophilicity and Other Parameters Affecting Brain Penetration. Curr. Med. Chem. Nerv. Syst. Agents 2002, 2, 229–240. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A. Substituent Constants for Correlation Analysis in Chemistry and Biology; Wiley: Hoboken, NJ, USA, 1979. [Google Scholar]
- Van De Waterbeemd, H.; Camenisch, G.; Folkers, G.; Chretien, J.R.; Raevsky, O.A. Estimation of Blood-Brain Barrier Crossing of Drugs Using Molecular Size and Shape, and H-Bonding Descriptors. J. Drug Target. 1998, 6, 151–165. [Google Scholar] [CrossRef]
- Pajouhesh, H.; Lenz, G.R. Medicinal chemical properties of successful central nervous system drugs. NeuroRX 2005, 2, 541–553. [Google Scholar] [CrossRef] [Green Version]
- Ghose, A.K.; Herbertz, T.; Hudkins, R.L.; Dorsey, B.D.; Mallamo, J.P. Knowledge-Based, Central Nervous System (CNS) Lead Selection and Lead Optimization for CNS Drug Discovery. ACS Chem. Neurosci. 2012, 3, 50–68. [Google Scholar] [CrossRef] [Green Version]
- Hitchcock, S.A.; Pennington, L.D. Structure–Brain Exposure Relationships. J. Med. Chem. 2006, 49, 7559–7583. [Google Scholar] [CrossRef]
- Kelder, J.; Grootenhuis, P.D.J.; Bayada, D.M.; Delbressine, L.P.C.; Ploemen, J. Polar Molecular Surface as a Dominating Determinant for Oral Absorption and Brain Penetration of Drugs. Pharm. Res. 1999, 16, 1514–1519. [Google Scholar] [CrossRef]
- Fischer, H.; Gottschlich, R.; Seelig, A. Blood-Brain Barrier Permeation: Molecular Parameters Governing Passive Diffusion. J. Membr. Biol. 1998, 165, 201–211. [Google Scholar] [CrossRef] [Green Version]
- Raub, T.J.; Lutzke, B.S.; Andrus, P.K.; Sawada, G.A.; Staton, B.A. Early Preclinical Evaluation of Brain Exposure in Support of Hit Identification and Lead Optimization. In Optimizing the “Drug-Like” Properties of Leads in Drug Discovery; Springer: New York, NY, USA, 2006; pp. 355–410. [Google Scholar]
- Wager, T.T.; Hou, X.; Verhoest, P.R.; Villalobos, A. Moving beyond Rules: The Development of a Central Nervous System Multiparameter Optimization (CNS MPO) Approach to Enable Alignment of Druglike Properties. ACS Chem. Neurosci. 2010, 1, 435–449. [Google Scholar] [CrossRef] [Green Version]
- Löscher, W.; Potschka, H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRX 2005, 2, 86–98. [Google Scholar] [CrossRef]
- Armulik, A.; Genové, G.; Betsholtz, C. Pericytes: Developmental, Physiological, and Pathological Perspectives, Problems, and Promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attwell, D.; Buchan, A.; Charpak, S.; Lauritzen, M.; MacVicar, B.; Newman, E.A. Glial and neuronal control of brain blood flow. Nat. Cell Biol. 2010, 468, 232–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, G.R.J.; Howarth, C.; MacVicar, B.A. Bidirectional control of arteriole diameter by astrocytes. Exp. Physiol. 2011, 96, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRX 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Dowden, H.; Munro, J. Trends in clinical success rates and therapeutic focus. Nat. Rev. Drug Discov. 2019, 18, 495–496. [Google Scholar] [CrossRef]
- Gribkoff, V.K.; Kaczmarek, L.K. The need for new approaches in CNS drug discovery: Why drugs have failed, and what can be done to improve outcomes. Neuropharmacology 2017, 120, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Sardi, I.; Fantappiè, O.; la Marca, G.; Giovannini, M.G.; Iorio, A.L.; Da Ros, M.; Malvagia, S.; Cardellicchio, S.; Giunti, L.; de Martino, M.; et al. Delivery of doxorubicin across the blood–brain barrier by ondansetron pretreatment: A study in vitro and in vivo. Cancer Lett. 2014, 353, 242–247. [Google Scholar] [CrossRef]
- Voulgaris, S.; Partheni, M.; Karamouzis, M.; Dimopoulos, P.; Papadakis, N.; Kalofonos, H. Intratumoral Doxorubicin in Patients With Malignant Brain Gliomas. Am. J. Clin. Oncol. 2002, 25, 60–64. [Google Scholar] [CrossRef]
- Cheng, Y.; Dai, Q.; Morshed, R.A.; Fan, X.; Wegscheid, M.L.; Wainwright, D.; Han, Y.; Zhang, L.; Auffinger, B.; Tobias, A.L.; et al. Blood-Brain Barrier Permeable Gold Nanoparticles: An Efficient Delivery Platform for Enhanced Malignant Glioma Therapy and Imaging. Small 2014, 10, 5137–5150. [Google Scholar] [CrossRef] [PubMed]
- Zafir-Lavie, I.; Sherbo, S.; Goltsman, H.; Badinter, F.; Yeini, E.; Ofek, P.; Miari, R.; Tal, O.; Liran, A.; Shatil, T.; et al. Successful intracranial delivery of trastuzumab by gene-therapy for treatment of HER2-positive breast cancer brain metastases. J. Control. Release 2018, 291, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, S.I.; Hori, M.; Klatzo, I. Testing of a hypothesis for osmotic opening of the blood-brain barrier. Am. J. Physiol. Content 1972, 223, 323–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tempany, C.M.C.; McDannold, N.J.; Hynynen, K.; Jolesz, F.A. Focused Ultrasound Surgery in Oncology: Overview and Principles. Radiology 2011, 259, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, S.D.; Chiu, A.; Yoo, S.-S.; Park, S. Estimation of the spatial profile of neuromodulation and the temporal latency in motor responses induced by focused ultrasound brain stimulation. NeuroReport 2014, 25, 475–479. [Google Scholar] [CrossRef] [PubMed]
- King, R.L.; Brown, J.R.; Newsome, W.T.; Pauly, K.B. Effective Parameters for Ultrasound-Induced In Vivo Neurostimulation. Ultrasound Med. Biol. 2013, 39, 312–331. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.-S.; Bystritsky, A.; Lee, J.-H.; Zhang, Y.; Fischer, K.; Min, B.-K.; McDannold, N.J.; Pascual-Leone, A.; Jolesz, F.A. Focused ultrasound modulates region-specific brain activity. NeuroImage 2011, 56, 1267–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, B.-K.; Bystritsky, A.; Jung, K.-I.; Fischer, K.; Zhang, Y.; Maeng, L.-S.; Park, S.I.; Chung, Y.-A.; A Jolesz, F.; Yoo, S.-S. Focused ultrasound-mediated suppression of chemically-induced acute epileptic EEG activity. BMC Neurosci. 2011, 12, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fishman, P.S.; Frenkel, V. Focused Ultrasound: An Emerging Therapeutic Modality for Neurologic Disease. Neurotherapy 2017, 14, 393–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, K.-C.; Chu, P.-C.; Wang, H.-Y.J.; Huang, C.-Y.; Chen, P.-Y.; Tsai, H.-C.; Lu, Y.-J.; Lee, P.-Y.; Tseng, I.-C.; Feng, L.-Y.; et al. Focused Ultrasound-Induced Blood–Brain Barrier Opening to Enhance Temozolomide Delivery for Glioblastoma Treatment: A Preclinical Study. PLoS ONE 2013, 8, e58995. [Google Scholar] [CrossRef] [Green Version]
- Treat, L.H.; McDannold, N.; Zhang, Y.; Vykhodtseva, N.; Hynynen, K. Improved Anti-Tumor Effect of Liposomal Doxorubicin After Targeted Blood-Brain Barrier Disruption by MRI-Guided Focused Ultrasound in Rat Glioma. Ultrasound Med. Biol. 2012, 38, 1716–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkins, R.; Burgess, A.; Ganguly, M.; Francia, G.; Kerbel, R.; Wels, W.S.; Hynynen, K. Focused Ultrasound Delivers Targeted Immune Cells to Metastatic Brain Tumors. Cancer Res. 2013, 73, 1892–1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgess, A.; Ayala-Grosso, C.A.; Ganguly, M.; Jordão, J.F.; Aubert, I.; Hynynen, K. Targeted Delivery of Neural Stem Cells to the Brain Using MRI-Guided Focused Ultrasound to Disrupt the Blood-Brain Barrier. PLoS ONE 2011, 6, e27877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkins, R.; Burgess, A.; Kerbel, R.; Wels, W.S.; Hynynen, K. Early treatment of HER2-amplified brain tumors with targeted NK-92 cells and focused ultrasound improves survival. Neuro-Oncology 2016, 18, 974–981. [Google Scholar] [CrossRef] [Green Version]
- Elias, W.J.; Huss, D.; Voss, T.; Loomba, J.; Khaled, M.; Zadicario, E.; Frysinger, R.C.; Sperling, S.; Wylie, S.; Monteith, S.J.; et al. A Pilot Study of Focused Ultrasound Thalamotomy for Essential Tremor. N. Engl. J. Med. 2013, 369, 640–648. [Google Scholar] [CrossRef]
- Magara, A.; Bühler, R.; Moser, D.; Kowalski, M.; Pourtehrani, P.; Jeanmonod, D. First experience with MR-guided focused ultrasound in the treatment of Parkinson’s disease. J. Ther. Ultrasound 2014, 2, 11. [Google Scholar] [CrossRef] [Green Version]
- Jeanmonod, D.; Werner, B.; Morel, A.; Michels, L.; Zadicario, E.; Schiff, G.; Martin, E. Transcranial magnetic resonance imaging–guided focused ultrasound: Noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurg. Focus 2012, 32, E1. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, J.F.; Herrick, J.F. Biologic reactions to cavitation, a consideration for ultrasonic therapy. Arch. Phys. Med. Rehabil. 1953, 34. [Google Scholar]
- Vykhodtseva, N.; Hynynen, K.; Damianou, C. Histologic effects of high intensity pulsed ultrasound exposure with subharmonic emission in rabbit brain in vivo. Ultrasound Med. Biol. 1995, 21, 969–979. [Google Scholar] [CrossRef]
- Hynynen, K.; A Jolesz, F. Demonstration of Potential Noninvasive Ultrasound Brain Therapy Through an Intact Skull. Ultrasound Med. Biol. 1998, 24, 275–283. [Google Scholar] [CrossRef]
- Hynynen, K.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.A. Noninvasive MR Imaging–guided Focal Opening of the Blood-Brain Barrier in Rabbits. Radiology 2001, 220, 640–646. [Google Scholar] [CrossRef]
- Hills, B.A.; James, P.B. Microbubble damage to the blood-brain barrier: Relevance to decompression sickness. Undersea Biomed. Res. 1991, 18, 111–116. [Google Scholar]
- Ivanov, A.I.; Christodoulou, J.; Parkinson, J.; Barnham, K.J.; Tucker, A.; Woodrow, J.; Sadler, P.J. Cisplatin Binding Sites on Human Albumin. J. Biol. Chem. 1998, 273, 14721–14730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urien, S.; Lokiec, F. Population pharmacokinetics of total and unbound plasma cisplatin in adult patients. Br. J. Clin. Pharmacol. 2004, 57, 756–763. [Google Scholar] [CrossRef] [Green Version]
- Coluccia, D.; Figueiredo, C.A.; Wu, M.Y.; Riemenschneider, A.N.; Diaz, R.; Luck, A.; Smith, C.; Das, S.; Ackerley, C.; O’Reilly, M.; et al. Enhancing glioblastoma treatment using cisplatin-gold-nanoparticle conjugates and targeted delivery with magnetic resonance-guided focused ultrasound. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Earhart, R.H.; A Martin, P.; Tutsch, K.D.; Ertürk, E.; Wheeler, R.H.; E Bull, F. Improvement in the therapeutic index of cisplatin (NSC 119875) by pharmacologically induced chloruresis in the rat. Cancer Res. 1983, 43, 1187–1194. [Google Scholar]
- Timbie, K.F.; Afzal, U.; Date, A.; Zhang, C.; Song, J.; Miller, G.W.; Suk, J.S.; Hanes, J.; Price, R.J. MR image-guided delivery of cisplatin-loaded brain-penetrating nanoparticles to invasive glioma with focused ultrasound. J. Control. Release 2017, 263, 120–131. [Google Scholar] [CrossRef]
- Noroozian, Z.; Xhima, K.; Huang, Y.; Kaspar, B.K.; Kügler, S.; Hynynen, K.; Aubert, I. MRI-Guided Focused Ultrasound for Targeted Delivery of rAAV to the Brain. In Methods in Molecular Biology; Springer: Amsterdam, The Netherlands, 2019; Volume 1950, pp. 177–197. [Google Scholar]
- Alonso, A.; Reinz, E.; Leuchs, B.; Kleinschmidt, J.; Fatar, M.; Geers, B.; Lentacker, I.; Hennerici, M.G.; de Smedt, S.C.; Meairs, S. Focal Delivery of AAV2/1-transgenes Into the Rat Brain by Localized Ultrasound-induced BBB Opening. Mol. Ther. Nucleic Acids 2013, 2, e73. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.; Wei, K.-C.; Huang, C.-Y.; Wen, C.-J.; Yen, T.-C.; Liu, C.-L.; Lin, Y.-T.; Chen, J.-C.; Shen, C.-R. Noninvasive and Targeted Gene Delivery into the Brain Using Microbubble-Facilitated Focused Ultrasound. PLoS ONE 2013, 8, e57682. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Kugelman, T.; Buch, A.; Herman, M.; Han, Y.; Karakatsani, M.E.; Hussaini, S.A.; Duff, K.; Konofagou, E.E. Non-invasive, Focused Ultrasound-Facilitated Gene Delivery for Optogenetics. Sci. Rep. 2017, 7, 39955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanimirovic, D.B.; Sandhu, J.K.; Costain, W.J. Emerging Technologies for Delivery of Biotherapeutics and Gene Therapy across the Blood–Brain Barrier. BioDrugs 2018, 32, 547–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stavarache, M.A.; Petersen, N.; Jurgens, E.M.; Milstein, E.R.; Rosenfeld, Z.B.; Ballon, D.J.; Kaplitt, M.G. Safe and stable noninvasive focal gene delivery to the mammalian brain following focused ultrasound. J. Neurosurg. 2019, 130, 989–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, E.-J.; Zhang, Y.-Z.; Vykhodtseva, N.; McDannold, N. Ultrasound-mediated blood-brain/blood-tumor barrier disruption improves outcomes with trastuzumab in a breast cancer brain metastasis model. J. Control. Release 2012, 163, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Lipsman, N.; Meng, Y.; Bethune, A.J.; Huang, Y.; Lam, B.; Masellis, M.; Herrmann, N.; Heyn, C.; Aubert, I.; Boutet, A.; et al. Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mainprize, T.; Lipsman, N.; Huang, Y.; Meng, Y.; Bethune, A.; Ironside, S.; Heyn, C.; Alkins, R.; Trudeau, M.; Sahgal, A.; et al. Blood-Brain Barrier Opening in Primary Brain Tumors with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study. Sci. Rep. 2019, 9, 321. [Google Scholar] [CrossRef] [Green Version]
- Abrahao, A.; Meng, Y.; Llinas, M.; Huang, Y.; Hamani, C.; Mainprize, T.; Aubert, I.; Heyn, C.; Black, S.E.; Hynynen, K.; et al. First-in-human trial of blood–brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, A.; Tatter, S.B.; Debinski, W. Neurosurgical Techniques for Disruption of the Blood–Brain Barrier for Glioblastoma Treatment. Pharmaceutics 2015, 7, 175–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuwelt, E.A.; Frenkel, E.P.; Rapoport, S.; Barnett, P. Effect of Osmotic Blood-Brain Barrier Disruption on Methotrexate Pharmacokinetics in the Dog. Neurosurgery 1980, 7, 36–43. [Google Scholar] [CrossRef]
- Wang, M.; Etu, J.; Joshi, S. Enhanced Disruption of the Blood Brain Barrier by Intracarotid Mannitol Injection during Transient Cerebral Hypoperfusion in Rabbits. J. Neurosurg. Anesthesiol. 2007, 19, 249–256. [Google Scholar] [CrossRef]
- Zünkeler, B.; Carson, R.E.; Olson, J.; Blasberg, R.G.; DeVroom, H.; Lutz, R.J.; Saris, S.C.; Wright, D.C.; Kammerer, W.; Patronas, N.J.; et al. Quantification and pharmacokinetics of blood-brain barrier disruption in humans. J. Neurosurg. 1996, 85, 1056–1065. [Google Scholar] [CrossRef]
- Rapoport, S.I. Modulation of Blood-Brain Barrier Permeability. J. Drug Target. 1996, 3, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.M.; Goble, J.C.; Bird, J.H.; Girton, M.E.; Doppman, J.L.; Rapoport, S.I.; Barranger, J.A. Measurement of blood-brain barrier permeability with positron emission tomography and [68Ga]EDTA. J. Cereb. Blood Flow Metab. 1984, 4, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Zünkeler, B.; Carson, R.E.; Olson, J.; Blasberg, R.G.; Girton, M.; Bacher, J.; Herscovitch, P.; Oldfield, E.H. Hyperosmolar blood-brain barrier disruption in baboons: An in vivo study using positron emission tomography and rubidium-82. J. Neurosurg. 1996, 84, 494–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzales-Portillo, G.S.; Sanberg, P.R.; Franzblau, M.; Gonzales-Portillo, C.; Diamandis, T.; Staples, M.; Sanberg, C.D.; Borlongan, C.V. Mannitol-Enhanced Delivery of Stem Cells and Their Growth Factors across the Blood–Brain Barrier. Cell Transplant. 2014, 23, 531–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doolittle, N.D.; Miner, M.E.; Hall, W.A.; Siegal, T.; Jerome, E.; Osztie, E.; McAllister, L.D.; Bubalo, J.S.; Kraemer, D.F.; Fortin, D.; et al. Safety and efficacy of a multicenter study using intraarterial chemotherapy in conjunction with osmotic opening of the blood-brain barrier for the treatment of patients with malignant brain tumors. Cancer 2000, 88, 637–647. [Google Scholar] [CrossRef]
- Bellavance, M.-A.; Blanchette, M.; Fortin, D. Recent Advances in Blood–Brain Barrier Disruption as a CNS Delivery Strategy. AAPS J. 2008, 10, 166–177. [Google Scholar] [CrossRef] [Green Version]
- Guillaume, D.J.; Doolittle, N.D.; Gahramanov, S.; Hedrick, N.A.; Delashaw, J.B.; Neuwelt, E.A. Intra-arterial chemotherapy with osmotic blood-brain barrier disruption for aggressive oligoden-droglial tumors: Results of a phase I study. Neurosurgery 2010, 66, 48–58. [Google Scholar] [CrossRef]
- Neuwelt, E.A.; Glasberg, M.; Frenkel, E.; Barnett, P. Neurotoxicity of chemotherapeutic agents after blood-brain barrier modification: Neuropathological studies. Ann. Neurol. 1983, 14, 316–324. [Google Scholar] [CrossRef]
- Tomiwa, K.; Hazama, F.; Mikawa, H. Neurotoxicity of vincristine after the osmotic opening of the blood-brain barrier. Neuropathol. Appl. Neurobiol. 1983, 9, 345–354. [Google Scholar] [CrossRef]
- Joshi, S.; Ergin, A.; Wang, M.; Reif, R.; Zhang, J.; Bruce, J.N.; Bigio, I.J. Inconsistent blood brain barrier disruption by intraarterial mannitol in rabbits: Implications for chemotherapy. J. Neuro-Oncol. 2011, 104, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Kiviniemi, V.; Korhonen, V.; Kortelainen, J.; Rytky, S.; Keinänen, T.; Tuovinen, T.; Isokangas, M.; Sonkajärvi, E.; Siniluoto, T.; Nikkinen, J.; et al. Real-time monitoring of human blood-brain barrier disruption. PLoS ONE 2017, 12, e0174072. [Google Scholar] [CrossRef] [PubMed]
- Godinho, B.M.; Henninger, N.; Bouley, J.; Alterman, J.F.; Haraszti, R.A.; Gilbert, J.W.; Sapp, E.; Coles, A.H.; Biscans, A.; Nikan, M.; et al. Transvascular Delivery of Hydrophobically Modified siRNAs: Gene Silencing in the Rat Brain upon Disruption of the Blood-Brain Barrier. Mol. Ther. 2018, 26, 2580–2591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norouzi, M.; Yathindranath, V.; Thliveris, J.A.; Miller, D.W. Salinomycin-Loaded Iron Oxide Nanoparticles for Glioblastoma Therapy. Nanomaterials 2020, 10, 477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikan, M.; Osborn, M.F.; Coles, A.H.; Biscans, A.; Godinho, B.M.; Haraszti, R.A.; Sapp, E.; Echeverria, D.; DiFiglia, M.; Aronin, N.; et al. Synthesis and Evaluation of Parenchymal Retention and Efficacy of a Metabolically Stable O-Phosphocholine-N-docosahexaenoyl-l-serine siRNA Conjugate in Mouse Brain. Bioconjugate Chem. 2017, 28, 1758–1766. [Google Scholar] [CrossRef]
- Godinho, B.M.; Gilbert, J.W.; Haraszti, R.A.; Coles, A.H.; Biscans, A.; Roux, L.; Nikan, M.; Echeverria, D.; Hassler, M.; Khvorova, A. Pharmacokinetic Profiling of Conjugated Therapeutic Oligonucleotides: A High-Throughput Method Based Upon Serial Blood Microsampling Coupled to Peptide Nucleic Acid Hybridization Assay. Nucleic Acid Ther. 2017, 27, 323–334. [Google Scholar] [CrossRef]
- D’Amico, R.S.; Khatri, D.; Reichman, N.; Patel, N.V.; Wong, T.; Fralin, S.R.; Li, M.; Ellis, J.A.; Ortiz, R.; Langer, D.J.; et al. Super selective intra-arterial cerebral infusion of modern chemotherapeutics after blood–brain barrier disruption: Where are we now, and where we are going. J. Neuro-Oncol. 2020, 147, 261–278. [Google Scholar] [CrossRef]
- Boockvar, J.A.; Tsiouris, A.J.; Hofstetter, C.P.; I Kovanlikaya, I.; Fralin, S.; Kesavabhotla, K.; Seedial, S.M.; Pannullo, S.C.; Schwartz, T.H.; E Stieg, P.; et al. Safety and maximum tolerated dose of superselective intraarterial cerebral infusion of bevacizumab after osmotic blood-brain barrier disruption for recurrent malignant glioma. J. Neurosurg. 2011, 114, 624–632. [Google Scholar] [CrossRef]
- Burkhardt, J.-K.; Riina, H.; Shin, B.J.; Christos, P.; Kesavabhotla, K.; Hofstetter, C.P.; Tsiouris, A.J.; Boockvar, J.A. Intra-Arterial Delivery of Bevacizumab after Blood-Brain Barrier Disruption for the Treatment of Recurrent Glioblastoma: Progression-Free Survival and Overall Survival. World Neurosurg. 2012, 77, 130–134. [Google Scholar] [CrossRef] [Green Version]
- Jeon, J.; Kovanlikaya, I.; Boockvar, J.; Mao, X.; Shin, B.; Burkhardt, J.K.; Kesavabhotla, K.; Christos, P.; Riina, H.; Shungu, D.; et al. Metabolic Response of Glioblastoma to Superselective Intra-Arterial Cerebral Infusion of Bevacizumab: A Proton MR Spectroscopic Imaging Study. Am. J. Neuroradiol. 2012, 33, 2095–2102. [Google Scholar] [CrossRef] [Green Version]
- Shin, B.J.; Burkhardt, J.-K.; Riina, H.A.; Boockvar, J.A. Superselective Intra-Arterial Cerebral Infusion of Novel Agents After Blood–Brain Disruption for the Treatment of Recurrent Glioblastoma Multiforme: A Technical Case Series. Neurosurg. Clin. N. Am. 2012, 23, 323–329. [Google Scholar] [CrossRef]
- Galla, N.; Chiang, G.; Chakraborty, S.; Singh, R.; Tsiouris, A.J.; Boockvar, J.; Kovanlikaya, I. Apparent diffusion coefficient changes predict survival after intra-arterial bevacizumab treatment in recurrent glioblastoma. Neuroradiology 2017, 59, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Filippi, C.G.; Burkhardt, J.-K.; Fralin, S.; Ray, A.; Wong, T.; Ortiz, R.; Langer, D.J.; A Boockvar, J. Durability of single dose intra-arterial bevacizumab after blood/brain barrier disruption for recurrent glioblastoma. J. Exp. Ther. Oncol. 2016, 11, 261–267. [Google Scholar] [PubMed]
- Kulason, K.O.; Schneider, J.R.; Chakraborty, S.; Filippi, C.G.; Pramanik, B.; Wong, T.; Fralin, S.; Tan, K.; Ray, A.; Alter, R.A.; et al. Superselective intraarterial cerebral infusion of cetuximab with blood brain barrier disruption combined with Stupp Protocol for newly diagnosed glioblastoma. J. Exp. Ther. Oncol. 2018, 12, 223–229. [Google Scholar]
- Dobrogowska, D.; Vorbrodt, A. Immunogold localization of tight junctional proteins in normal and osmotically-affected rat blood-brain barrier. J. Mol. Histol. 2003, 35, 529–539. [Google Scholar] [CrossRef]
- Rapoport, S.I.; Fredericks, W.R.; Ohno, K.; Pettigrew, K.D. Quantitative aspects of reversible osmotic opening of the blood-brain barrier. Am. J. Physiol. Integr. Comp. Physiol. 1980, 238, R421–R431. [Google Scholar] [CrossRef]
- Cosolo, W.C.; Martinello, P.; Louis, W.J.; Christophidis, N. Blood-brain barrier disruption using mannitol: Time course and electron microscopy studies. Am. J. Physiol. Integr. Comp. Physiol. 1989, 256, R443–R447. [Google Scholar] [CrossRef]
- Rapoport, S.I.; Suzuki, M.; Iwasaki, Y. Microinfarction: Osmotic BBB opening or microcrystals in infusate? J. Neurosurg. 1991, 74, 685. [Google Scholar]
- Tomiwa, K.; Hazama, F.; Mikawa, H. Reversible osmotic opening of the blood-brain barrier Prevention of Tissue Damage with Filtration of the Perfusate. Pathol. Int. 1982, 32, 427–435. [Google Scholar] [CrossRef]
- Sedeyn, J.C.; Wu, H.; Hobbs, R.D.; Levin, E.C.; Nagele, R.; Venkataraman, V. Histamine Induces Alzheimer’s Disease-Like Blood Brain Barrier Breach and Local Cellular Responses in Mouse Brain Organotypic Cultures. BioMed Res. Int. 2015, 2015, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahlen, S.E.; Bjork, J.; Hedqvist, P.; Arfors, K.E.; Hammarstrom, S.; Lindgren, J.A.; Samuelsson, B. Leukotrienes promote plasma leakage and leukocyte adhesion in postcapillary venules: In vivo effects with relevance to the acute inflammatory response. Proc. Natl. Acad. Sci. USA 1981, 78, 3887–3891. [Google Scholar] [CrossRef] [Green Version]
- Stromberga, Z.; Chess-Williams, R.; Moro, C. Histamine modulation of urinary bladder urothelium, lamina propria and detrusor contractile activity via H1 and H2 receptors. Sci. Rep. 2019, 9, 3899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panula, P.; Chazot, P.L.; Cowart, M.; Gutzmer, R.; Leurs, R.; Liu, W.L.S.; Stark, H.; Thurmond, R.L.; Haas, H.L. International Union of Basic and Clinical Pharmacology. XCVIII. Histamine Receptors. Pharmacol. Rev. 2015, 67, 601–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.; Diehl, S.; Noubade, R.; Ledoux, J.; Nelson, M.; Spach, K.; Zachary, J.F.; Blankenhorn, E.P.; Teuscher, C. Endothelial histamine H1 receptor signaling reduces blood-brain barrier permeability and susceptibility to autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2010, 107, 18967–18972. [Google Scholar] [CrossRef] [Green Version]
- Teuscher, C.; Subramanian, M.; Noubade, R.; Gao, J.F.; Offner, H.; Zachary, J.F.; Blankenhorn, E.P. Central histamine H3 receptor signaling negatively regulates susceptibility to autoimmune inflammatory disease of the CNS. Proc. Natl. Acad. Sci. USA 2007, 104, 10146–10151. [Google Scholar] [CrossRef] [Green Version]
- Del Rio, R.; Noubade, R.; Saligrama, N.; Wall, E.H.; Krementsov, D.; Poynter, M.; Zachary, J.F.; Thurmond, R.L.; Teuscher, C. Histamine H4 Receptor Optimizes T Regulatory Cell Frequency and Facilitates Anti-Inflammatory Responses within the Central Nervous System. J. Immunol. 2011, 188, 541–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbott, N.J. Inflammatory Mediators and Modulation of Blood–Brain Barrier Permeability. Cell. Mol. Neurobiol. 2000, 20, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Hurst, R.D.; Clark, J.B. Alterations in transendothelial electrical resistance by vasoactive agonists and cyclic AMP in a blood-brain barrier model system. Neurochem. Res. 1998, 23, 149–154. [Google Scholar] [CrossRef]
- Deli, M.A.; Dehouck, M.-P.; Cecchelli, R.; Ábrahám, C.S.; Joó, F. Histamine induces a selective albumin permeation through the blood-brain barrierin vitro. Inflamm. Res. 1995, 44, S56–S57. [Google Scholar] [CrossRef]
- Gaillard, P.J.; de Boer, A.G. Relationship between permeability status of the blood–brain barrier and in vitro permeability coefficient of a drug. Eur. J. Pharm. Sci. 2000, 12, 95–102. [Google Scholar] [CrossRef]
- Patnaik, R.; Sharma, A.; Skaper, S.D.; Muresanu, D.F.; Lafuente, J.V.; Castellani, R.J.; Nozari, A.; Sharma, H.S. Histamine H3 Inverse Agonist BF 2649 or Antagonist with Partial H4 Agonist Activity Clobenpropit Reduces Amyloid Beta Peptide-Induced Brain Pathology in Alzheimer’s Disease. Mol. Neurobiol. 2017, 55, 312–321. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Z.; Chen, R.; Zhang, J.; Hu, X.; He, C.; Su, Q.; Ma, H.; Ren, H.; Qian, M.; et al. Immethridine, histamine H3-receptor (H3R) agonist, alleviated experimental autoimmune encephalomyelitis via inhibiting the function of dendritic cells. Oncotarget 2017, 8, 75038–75049. [Google Scholar] [CrossRef] [Green Version]
- Kitbunnadaj, R.; Zuiderveld, O.P.; Christophe, B.; Hulscher, S.; Menge, W.M.P.B.; Gelens, E.; Snip, E.; Bakker, R.A.; Célanire, S.; Gillard, M.; et al. Identification of 4-(1H-Imidazol-4(5)-ylmethyl)pyridine (Immethridine) as a Novel, Potent, and Highly Selective Histamine H3Receptor Agonist. J. Med. Chem. 2004, 47, 2414–2417. [Google Scholar] [CrossRef] [PubMed]
- Varaschin, R.K.; Rosenberg, M.J.; Hamilton, D.A.; Savage, D.D. Differential Effects of the Histamine H3Receptor Agonist Methimepip on Dentate Granule Cell Excitability, Paired-Pulse Plasticity and Long-Term Potentiation in Prenatal Alcohol-Exposed Rats. Alcohol. Clin. Exp. Res. 2014, 38, 1902–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitbunnadaj, R.; Hashimoto, T.; Poli, E.; Zuiderveld, O.P.; Menozzi, A.; Hidaka, R.; de Esch, I.J.P.; Bakker, R.A.; Menge, W.M.P.B.; Yamatodani, A.; et al. N-Substituted Piperidinyl Alkyl Imidazoles: Discovery of Methimepip as a Potent and Selective Histamine H3 Receptor Agonist. J. Med. Chem. 2005, 48, 2100–2107. [Google Scholar] [CrossRef]
- Fletcher, L. Guilford acquires Gliatech in strategic deal. Nat. Biotechnol. 2000, 18, 710. [Google Scholar] [CrossRef]
- Krueger, K.M.; Witte, D.G.; Ireland-Denny, L.; Miller, T.R.; Baranowski, J.L.; Buckner, S.; Milicic, I.; Esbenshade, T.A.; Hancock, A.A. G Protein-Dependent Pharmacology of Histamine H3 Receptor Ligands: Evidence for Heterogeneous Active State Receptor Conformations. J. Pharmacol. Exp. Ther. 2005, 314, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Kovacova, E.; Gavliakova, S.; Buday, T.; Plevkova, J. Effect of histamine H3 receptor selective agonist imetit on cough and symptoms of allergic rhinitis in animal model of upper airway cough syndrome. Clin. Transl. Allergy 2015, 5, P13. [Google Scholar] [CrossRef] [Green Version]
- McLeod, R.L.; Gertner, S.B.; Hey, J.A. Production by R-α-methylhistamine of a histamine H3 receptor-mediated decrease in basal vascular resistance in guinea-pigs. Br. J. Pharmacol. 1993, 110, 553–558. [Google Scholar] [CrossRef]
- Chiba, S.; Itateyama, E.; Sakata, T.; Yoshimatsu, H. Acute central administration of immepip, a histamine H3 receptor agonist, suppresses hypothalamic histamine release and elicits feeding behavior in rats. Brain Res. Bull. 2009, 79, 37–40. [Google Scholar] [CrossRef]
- Wulff, B.S.; Hastrup, S.; Rimvall, K. Characteristics of recombinantly expressed rat and human histamine H3 receptors. Eur. J. Pharmacol. 2002, 453, 33–41. [Google Scholar] [CrossRef]
- Baldi, E.; Bucherelli, C.; Schunack, W.; Cenni, G.; Blandina, P.; Passani, M.B. The H3 receptor protean agonist proxyfan enhances the expression of fear memory in the rat. Neuropharmacology 2005, 48, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Gbahou, F.; Rouleau, A.; Morisset, S.; Parmentier, R.; Crochet, S.; Lin, J.-S.; Ligneau, X.; Tardivel-Lacombe, J.; Stark, H.; Schunack, W.; et al. Protean agonism at histamine H3 receptors in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 11086–11091. [Google Scholar] [CrossRef] [Green Version]
- Kanaoka, Y.; Boyce, J.A. Cysteinyl Leukotrienes and Their Receptors: Cellular Distribution and Function in Immune and Inflammatory Responses. J. Immunol. 2004, 173, 1503–1510. [Google Scholar] [CrossRef] [Green Version]
- White, M. Mediators of inflammation and the inflammatory process. J. Allergy Clin. Immunol. 1999, 103, S378–S381. [Google Scholar] [CrossRef]
- Mawhin, M.-A.; Tilly, P.; Zirka, G.; Charles, A.-L.; Slimani, F.; Vonesch, J.-L.; Michel, J.-B.; Bäck, M.; Norel, X.; Fabre, J.-E. Neutrophils recruited by leukotriene B4 induce features of plaque destabilization during endotoxaemia. Cardiovasc. Res. 2018, 114, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Barrow, A.; Fleming, V.; Hunter, M.G.; Ogg, G.; Klenerman, P.; Pettipher, R. Leukotriene E4Activates Human Th2 Cells for Exaggerated Proinflammatory Cytokine Production in Response to Prostaglandin D2. J. Immunol. 2011, 188, 694–702. [Google Scholar] [CrossRef] [Green Version]
- Haeggström, J.Z.; Funk, C.D. Lipoxygenase and Leukotriene Pathways: Biochemistry, Biology, and Roles in Disease. Chem. Rev. 2011, 111, 5866–5898. [Google Scholar] [CrossRef]
- Wang, M.-L.; Huang, X.-J.; Fang, S.-H.; Yuan, Y.-M.; Zhang, W.-P.; Lu, Y.-B.; Ding, Q.; Wei, E.-Q. Leukotriene D4 induces brain edema and enhances CysLT2 receptor-mediated aquaporin 4 expression. Biochem. Biophys. Res. Commun. 2006, 350, 399–404. [Google Scholar] [CrossRef]
- Lenz, Q.; Arroyo, D.; Temp, F.; Poersch, A.; Masson, C.; Jesse, A.; Marafiga, J.; Reschke, C.; Iribarren, P.; Mello, C. Cysteinyl leukotriene receptor (CysLT) antagonists decrease pentylenetetrazol-induced seizures and blood–brain barrier dysfunction. Neuroscience 2014, 277, 859–871. [Google Scholar] [CrossRef]
- Black, K.L.; Baba, T.; Pardridge, W.M. Enzymatic barrier protects brain capillaries from leukotriene C4. J. Neurosurg. 1994, 81, 745–751. [Google Scholar] [CrossRef]
- Chio, C.-C.; Lin, S.-J.; Lin, M.-T. Leukotriene E4 selectively increase the delivery of methotrexate to the C6 gliomas in rats. J. Neuro-Oncol. 1995, 25, 89–95. [Google Scholar] [CrossRef]
- Jendrossek, V.; Erdlenbruch, B.; Lakomek, M. Transient and controllable opening of the blood-brain barrier to cytostatic and antibiotic agents by alkylglycerols in rats. Exp. Brain Res. 2000, 135, 417–422. [Google Scholar] [CrossRef]
- Erdlenbruch, B.; Jendrossek, V.; Kugler, W.; Eibl, H.; Lakomek, M. Increased delivery of erucylphosphocholine to C6 gliomas by chemical opening of the blood-brain barrier using intracarotid pentylglycerol in rats. Cancer Chemother. Pharmacol. 2002, 50, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Erdlenbruch, B.; Alipour, M.; Fricker, G.; Miller, D.S.; Kugler, W.; Eibl, H.; Lakomek, M. Alkylglycerol opening of the blood-brain barrier to small and large fluorescence markers in normal and C6 glioma-bearing rats and isolated rat brain capillaries. Br. J. Pharmacol. 2003, 140, 1201–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdlenbruch, B.; Kugler, W.; Schinkhof, C.; Neurath, H.; Eibl, H.; Lakomek, M. Blood–brain barrier opening with alkylglycerols: Biodistribution of 1-O-pentylglycerol after intravenous and intracarotid administration in rats. J. Drug Target. 2005, 13, 143–150. [Google Scholar] [CrossRef]
- Hülper, P.; Dullin, C.; Kugler, W.; Lakomek, M.; Erdlenbruch, B. Monitoring Proteins Using In Vivo Near-Infrared Time-Domain Optical Imaging after 2-O-Hexyldiglycerol-Mediated Transfer to the Brain. Mol. Imaging Biol. 2011, 13, 275–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greene, C.; Hanley, N.; Campbell, M. Claudin-5: Gatekeeper of neurological function. Fluids Barriers CNS 2019, 16, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hülper, P.; Veszelka, S.; Walter, F.R.; Wolburg, H.; Fallier-Becker, P.; Piontek, J.; E Blasig, I.; Lakomek, M.; Kugler, W.; A Deli, M. Acute effects of short-chain alkylglycerols on blood-brain barrier properties of cultured brain endothelial cells. Br. J. Pharmacol. 2013, 169, 1561–1573. [Google Scholar] [CrossRef]
- Ibegbu, M.; Boussahel, A.; Cragg, S.; Tsibouklis, J.; Barbu, E. Nanoparticles of alkylglyceryl dextran and poly(ethyl cyanoacrylate) for applications in drug delivery: Preparation and characterization. Int. J. Polym. Mater. 2017, 66, 265–279. [Google Scholar] [CrossRef] [Green Version]
- Toman, P.; Lien, C.-F.; Ahmad, Z.; Dietrich, S.; Smith, J.; An, Q.; Molnár, É.; Pilkington, G.J.; Gorecki, D.; Tsibouklis, J.; et al. Nanoparticles of alkylglyceryl-dextran- graft -poly(lactic acid) for drug delivery to the brain: Preparation and in vitro investigation. Acta Biomater. 2015, 23, 250–262. [Google Scholar] [CrossRef] [Green Version]
- Del Vecchio, G.; Tscheik, C.; Tenz, K.; Helms, H.C.; Winkler, L.; Blasig, R.; Blasig, I.E. Sodium Caprate Transiently Opens Claudin-5-Containing Barriers at Tight Junctions of Epithelial and Endothelial Cells. Mol. Pharm. 2012, 9, 2523–2533. [Google Scholar] [CrossRef] [PubMed]
- Lindmark, T.; Nikkilä, T.; Artursson, P. Mechanisms of absorption enhancement by medium chain fatty acids in intestinal epithelial Caco-2 cell monolayers. J. Pharmacol. Exp. Ther. 1995, 275, 958–964. [Google Scholar] [PubMed]
- Motohiro, T.; Aramaki, M.; Oda, K.; Kawakami, A.; Tanaka, K.; Koga, T.; Shimada, Y.; Tomita, S.; Sakata, Y.; Fujimoto, T.; et al. Antibiotic Suppositories. Pediatr. Int. 1986, 28, 535–543. [Google Scholar] [CrossRef]
- Maher, S.; Leonard, T.W.; Jacobsen, J.; Brayden, D. Safety and efficacy of sodium caprate in promoting oral drug absorption: From in vitro to the clinic. Adv. Drug Deliv. Rev. 2009, 61, 1427–1449. [Google Scholar] [CrossRef]
- Ohnishi, T.; Aida, K.; Awazu, S. Enhancement of Blood-brain Barrier Permeability by Sodium Caprate. J. Pharm. Pharmacol. 2010, 51, 1015–1018. [Google Scholar] [CrossRef]
- Preston, E.; Slinn, J.; Vinokourov, I.; Stanimirovic, D. Graded reversible opening of the rat blood–brain barrier by intracarotid infusion of sodium caprate. J. Neurosci. Methods 2008, 168, 443–449. [Google Scholar] [CrossRef]
- Brandhonneur, N.; Dollo, G.; Ratajczak-Enselme, M.; Deniau, A.L.; Chevanne, F.; Estèbe, J.P.; Legrand, A.; Le Corre, P. Ex vivo and in vivo diffusion of ropivacaine through spinal meninges: Influence of absorption enhancers. Int. J. Pharm. 2011, 404, 36–41. [Google Scholar] [CrossRef]
- Nitta, T.; Hata, M.; Gotoh, S.; Seo, Y.; Sasaki, H.; Hashimoto, N.; Furuse, M.; Tsukita, S. Size-selective loosening of the blood-brain barrier in claudin-5—Deficient mice. J. Cell Biol. 2003, 161, 653–660. [Google Scholar] [CrossRef]
- Uchida, Y.; Ohtsuki, S.; Katsukura, Y.; Ikeda, C.; Suzuki, T.; Kamiie, J.; Terasaki, T. Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J. Neurochem. 2011, 117, 333–345. [Google Scholar] [CrossRef]
- Carman, A.; Mills, J.H.; Krenz, A.; Kim, D.-G.; Bynoe, M.S. Adenosine Receptor Signaling Modulates Permeability of the Blood-Brain Barrier. J. Neurosci. 2011, 31, 13272–13280. [Google Scholar] [CrossRef]
- Kim, D.-G.; Bynoe, M.S. A2A adenosine receptor modulates drug efflux transporter P-glycoprotein at the blood-brain barrier. J. Clin. Investig. 2016, 126, 1717–1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bynoe, M.S.; Viret, C.; Yan, A.; Kim, D.-G. Adenosine receptor signaling: A key to opening the blood–brain door. Fluids Barriers CNS 2015, 12, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, S.; Weingart, J.; Nduom, E.K.; Harfi, T.T.; George, R.T.; McAreavey, D.; Ye, X.; Anders, N.M.; Peer, C.; Figg, W.D.; et al. The effect of an adenosine A2A agonist on intra-tumoral concentrations of temozolomide in patients with recurrent glioblastoma. Fluids Barriers CNS 2018, 15, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, S.; George, R.T.; Lodge, M.A.; Piotrowski, A.; Wahl, R.L.; Gujar, S.K.; Grossman, S.A. The effect of regadenoson on the integrity of the human blood-brain barrier, a pilot study. J. Neuro-Oncol. 2017, 132, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.; Anders, N.M.; Mangraviti, A.; Wanjiku, T.M.; Sankey, E.W.; Liu, A.; Brem, H.; Tyler, B.; Rudek, M.A.; Grossman, S.A. The effect of regadenoson-induced transient disruption of the blood–brain barrier on temozolomide delivery to normal rat brain. J. Neuro-Oncol. 2015, 126, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Thompson, C.A. FDA approves pharmacologic stress agent. Am. J. Health Pharm. 2008, 65, 890. [Google Scholar] [CrossRef]
- Proia, R.; Hla, T. Emerging biology of sphingosine-1-phosphate: Its role in pathogenesis and therapy. J. Clin. Investig. 2015, 125, 1379–1387. [Google Scholar] [CrossRef] [Green Version]
- Yanagida, K.; Liu, C.H.; Faraco, G.; Galvani, S.; Smith, H.K.; Burg, N.; Anrather, J.; Sanchez, T.; Iadecola, C.; Hla, T. Size-selective opening of the blood–brain barrier by targeting endothelial sphingosine 1–phosphate receptor 1. Proc. Natl. Acad. Sci. USA 2017, 114, 4531–4536. [Google Scholar] [CrossRef] [Green Version]
- Annunziata, P.; Cioni, C.; Masi, G.; Tassi, M.; Marotta, G.; Severi, S. Fingolimod reduces circulating tight-junction protein levels and in vitro peripheral blood mononuclear cells migration in multiple sclerosis patients. Sci. Rep. 2018, 8, 15371. [Google Scholar] [CrossRef]
- Keaney, J.; Walsh, D.M.; O’Malley, T.; Hudson, N.; Crosbie, D.E.; Loftus, T.; Sheehan, F.; McDaid, J.; Humphries, M.M.; Callanan, J.J.; et al. Autoregulated paracellular clearance of amyloid-β across the blood-brain barrier. Sci. Adv. 2015, 1, e1500472. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Hla, T. S1P Control of Endothelial Integrity. Curr. Top. Microbiol. Immunol. 2014, 378, 85–105. [Google Scholar] [CrossRef] [Green Version]
- Sohet, F.; Lin, C.; Munji, R.N.; Lee, S.Y.; Ruderisch, N.; Soung, A.; Arnold, T.D.; Derugin, N.; Vexler, Z.S.; Yen, F.T.; et al. LSR/angulin-1 is a tricellular tight junction protein involved in blood–brain barrier formation. J. Cell Biol. 2015, 208, 703–711. [Google Scholar] [CrossRef] [Green Version]
- Niaudet, C.; Hofmann, J.J.; Mäe, M.A.; Jung, B.; Gaengel, K.; Vanlandewijck, M.; Ekvärn, E.; Salvado, M.D.; Mehlem, A.; Al Sayegh, S.; et al. Gpr116 Receptor Regulates Distinctive Functions in Pneumocytes and Vascular Endothelium. PLoS ONE 2015, 10, e0137949. [Google Scholar] [CrossRef]
- Gu, Y.-T.; Xue, Y.-X.; Wang, Y.-F.; Wang, J.-H.; Shangguan, Q.-R.; Zhang, J.-X.; Qin, L.-J. Role of ROS/RhoA/PI3K/PKB Signaling in NS1619-Mediated Blood–Tumor Barrier Permeability Increase. J. Mol. Neurosci. 2012, 48, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; I Marín-Ramos, N.; He, H.; Zeng, S.; Cho, H.-Y.; Swenson, S.D.; Zheng, L.; Epstein, A.L.; Schönthal, A.H.; Hofman, F.M.; et al. NEO100 enables brain delivery of blood–brain barrier impermeable therapeutics. Neuro-Oncology 2021, 23, 63–75. [Google Scholar] [CrossRef]
- Neonc Technologies, Inc. An Open-Label, Phase 1/2A Dose Escalation Study of Safety and Efficacy of NEO100 in Recurrent Grade IV Glioma. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT02704858 (accessed on 10 May 2021).
- Da Fonseca, C.O.; Simão, M.; Lins, I.R.; Caetano, R.O.; Futuro, D.; Quirico-Santos, T. Efficacy of monoterpene perillyl alcohol upon survival rate of patients with recurrent glioblastoma. J. Cancer Res. Clin. Oncol. 2010, 137, 287–293. [Google Scholar] [CrossRef]
- Breitkreuz-Korff, O.; Tscheik, C.; Del Vecchio, G.; Dithmer, S.; Walther, W.; Orthmann, A.; Wolburg, H.; Haseloff, R.F.; Schröder, L.; Blasig, I.E.; et al. M01 as a novel drug enhancer for specifically targeting the blood-brain barrier. J. Control. Release 2021, 338, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, S.; Sato, S.; Yamaguchi, H.; Kamoi, M.; Asashima, T.; Terasaki, T. Exogenous expression of claudin-5 induces barrier properties in cultured rat brain capillary endothelial cells. J. Cell. Physiol. 2006, 210, 81–86. [Google Scholar] [CrossRef]
- Brayden, D.; Hill, T.; Fairlie, D.; Maher, S.; Mrsny, R. Systemic delivery of peptides by the oral route: Formulation and medicinal chemistry approaches. Adv. Drug Deliv. Rev. 2020, 157, 2–36. [Google Scholar] [CrossRef]
- Guo, Y.; Ramachandran, C.; Satpathy, M.; Srinivas, S.P. Histamine-induced Myosin Light Chain Phosphorylation Breaks down the Barrier Integrity of Cultured Corneal Epithelial Cells. Pharm. Res. 2007, 24, 1824–1833. [Google Scholar] [CrossRef]
- Miranda, A.; Blanco-Prieto, M.J.; Sousa, J.; Pais, A.; Vitorino, C. Breaching barriers in glioblastoma. Part II: Targeted drug delivery and lipid nanoparticles. Int. J. Pharm. 2017, 531, 389–410. [Google Scholar] [CrossRef]
- Heidenreich, K.; Corser-Jensen, C. 5-Lipoxygenase-Activating Protein Inhibitors: Promising Drugs for Treating Acute and Chronic Neuroinflammation Following Brain Injury. In New Therapeutics for Traumatic Brain Injury: Prevention of Secondary Brain Damage and Enhancement of Repair and Regeneration; Elsevier: Amsterdam, The Netherlands, 2017; pp. 199–210. [Google Scholar]
- Saaber, D.; Wollenhaupt, S.; Baumann, K.; Reichl, S. Recent progress in tight junction modulation for improving bioavailability. Expert Opin. Drug Discov. 2014, 9, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Wysolmerski, R.B.; Lagunoff, D. Regulation of permeabilized endothelial cell retraction by myosin phosphorylation. Am. J. Physiol. Physiol. 1991, 261, C32–C40. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, J.H.; De Lanerolle, P.; Wilson, E.; Ma, W.; Yuan, S.Y. Myosin light chain kinase transference induces myosin light chain activation and endothelial hyperpermeability. Am. J. Physiol. Physiol. 2000, 279, C1285–C1289. [Google Scholar] [CrossRef]
- Straub, J.A.; Akiyama, A.; Parmar, P. In Vitro Plasma Metabolism of RMP-7. Pharm. Res. 1994, 11, 1673–1676. [Google Scholar] [CrossRef]
- Matsukado, K.; Inamura, T.; Nakano, S.; Fukui, M.; Bartus, R.T.; Black, K.L. Enhanced Tumor Uptake of Carboplatin and Survival in Glioma-bearing Rats by Intracarotid Infusion of Bradykinin Analog, RMP-7. Neurosurgery 1996, 39, 125–134. [Google Scholar] [CrossRef]
- Elliott, P.J.; Hayward, N.J.; Dean, R.L.; Blunt, D.G.; Bartus, R.T. Intravenous RMP-7 selectively increases uptake of car-boplatin into rat brain tumors. Cancer Res. 1996, 56, 3998–4005. [Google Scholar]
- Bartus, R.; Elliott, P.; Hayward, N.; Dean, R.; McEwen, E.; Fisher, S. Permeability of the blood brain barrier by the bradykinin agonist, RMP-7: Evidence for a sensitive, auto-regulated, receptor-mediated system. Immunopharmacology 1996, 33, 270–278. [Google Scholar] [CrossRef]
- Ford, J.; Osborn, C.; Barton, T.; Bleehen, N. A phase I study of intravenous RMP-7 with carboplatin in patients with progression of malignant glioma. Eur. J. Cancer 1998, 34, 1807–1811. [Google Scholar] [CrossRef]
- Warren, K.E.; Patel, M.C.; Aikin, A.A.; Widemann, B.; Libucha, M.; Adamson, P.C.; Neuwirth, R.; Benziger, D.; O’Toole, T.; Ford, K.; et al. Phase I trial of lobradimil (RMP-7) and carboplatin in children with brain tumors. Cancer Chemother. Pharmacol. 2001, 48, 275–282. [Google Scholar] [CrossRef]
- Packer, R.J.; Krailo, M.; Mehta, M.; Warren, K.; Allen, J.; Jakacki, R.; Villablanca, J.G.; Chiba, A.; Reaman, G. Phase 1 study of concurrent RMP-7 and carboplatin with radiotherapy for children with newly diagnosed brainstem gliomas. Cancer 2005, 104, 1281–1287. [Google Scholar] [CrossRef]
- Gregor, A.; Lind, M.; Newman, H.; Grant, R.; Hadley, D.; Barton, T.; Osborn, C. Phase II Studies of RMP-7 and Carboplatin in the Treatment of Recurrent High Grade Glioma. J. Neuro-Oncol. 1999, 44, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Prados, M.D.; Schold, S.C.; Fine, H.A.; Jaeckle, K.; Hochberg, F.; Mechtler, L.; Fetell, M.R.; Phuphanich, S.; Feun, L.; Janus, T.J.; et al. A randomized, double-blind, placebo-controlled, phase 2 study of RMP-7 in combination with carboplatin administered intravenously for the treatment of recurrent malignant glioma. Neuro-Oncol. 2003, 5, 96–103. [Google Scholar] [CrossRef]
- Emerich, D.F.; Snodgrass, P.; Dean, R.; Agostino, M.; Hasler, B.; Pink, M.; Xiong, H.; Kim, B.S.; Bartus, R.T. Enhanced delivery of carboplatin into brain tumours with intravenous CereportTM (RMP-7): Dramatic differences and insight gained from dosing parameters. Br. J. Cancer 1999, 80, 964–970. [Google Scholar] [CrossRef]
- Warren, K.; Jakacki, R.; Widemann, B.; Aikin, A.; Libucha, M.; Packer, R.; Vezina, G.; Reaman, G.; Shaw, D.; Krailo, M.; et al. Phase II trial of intravenous lobradimil and carboplatin in childhood brain tumors: A report from the Children’s Oncology Group. Cancer Chemother. Pharmacol. 2006, 58, 343–347. [Google Scholar] [CrossRef]
- Fasano, A.; Baudry, B.; Pumplin, D.W.; Wasserman, S.S.; Tall, B.; Ketley, J.M.; Kaper, J. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc. Natl. Acad. Sci. USA 1991, 88, 5242–5246. [Google Scholar] [CrossRef] [Green Version]
- Fasano, A. Zonulin and Its Regulation of Intestinal Barrier Function: The Biological Door to Inflammation, Autoimmunity, and Cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef] [Green Version]
- Uzzau, S.; Cappuccinelli, P.; Fasano, A. Expression of Vibrio cholerae zonula occludens toxin and analysis of its subcellular localization. Microb. Pathog. 1999, 27, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Fiorentini, C.; Donelli, G.; Uzzau, S.; Kaper, J.; Margaretten, K.; Ding, X.; Guandalini, S.; Comstock, L.; E Goldblum, S. Zonula occludens toxin modulates tight junctions through protein kinase C-dependent actin reorganization, in vitro. J. Clin. Investig. 1995, 96, 710–720. [Google Scholar] [CrossRef]
- Cox, D. Enhancing the permeation of marker compounds and enaminone anticonvulsants across Caco-2 monolayers by modulating tight junctions using zonula occludens toxin. Eur. J. Pharm. Biopharm. 2001, 52, 145–150. [Google Scholar] [CrossRef]
- Karyekar, C.S.; Fasano, A.; Raje, S.; Lu, R.; Dowling, T.C.; Eddington, N.D. Zonula Occludens Toxin Increases the Permeability of Molecular Weight Markers and Chemotherapeutic Agents Across the Bovine Brain Microvessel Endothelial Cells. J. Pharm. Sci. 2003, 92, 414–423. [Google Scholar] [CrossRef]
- Di Pierro, M.; Lu, R.; Uzzau, S.; Wang, W.; Margaretten, K.; Pazzani, C.; Maimone, F.; Fasano, A. Zonula Occludens Toxin Structure-Function Analysis. J. Biol. Chem. 2001, 276, 19160–19165. [Google Scholar] [CrossRef] [Green Version]
- Menon, D.; Karyekar, C.S.; Fasano, A.; Lu, R.; Eddington, N.D. Enhancement of brain distribution of anticancer agents using ΔG, the 12kDa active fragment of ZOT. Int. J. Pharm. 2005, 306, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Fasano, A.; Eddington, N. Effect of the six-mer synthetic peptide (AT1002) fragment of zonula occludens toxin on the intestinal absorption of cyclosporin A. Int. J. Pharm. 2008, 351, 8–14. [Google Scholar] [CrossRef]
- Goldblum, S.E.; Rai, U.; Tripathi, A.; Thakar, M.; De Leo, L.; Di Toro, N.; Not, T.; Ramachandran, R.; Puche, A.C.; Hollenberg, M.D.; et al. The active Zot domain (aa 288–293) increases ZO-1 and myosin 1C serine/threonine phosphorylation, alters interaction between ZO-1 and its binding partners, and induces tight junction disassembly through proteinase activated receptor 2 activation. FASEB J. 2011, 25, 144–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Oliver, E.; Kitchens, K.M.; Vere, J.; Alkan, S.S.; Tamiz, A.P. Structure–activity relationship studies of permeability modulating peptide AT-1002. Bioorganic Med. Chem. Lett. 2008, 18, 4584–4586. [Google Scholar] [CrossRef] [PubMed]
- Leffler, D.A.; Kelly, C.P.; Abdallah, H.Z.; Colatrella, A.M.; A Harris, L.; Leon, F.; Arterburn, L.A.; Paterson, B.M.; Lan, Z.H.; Murray, J. A Randomized, Double-Blind Study of Larazotide Acetate to Prevent the Activation of Celiac Disease during Gluten Challenge. Am. J. Gastroenterol. 2012, 107, 1554–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, P.H.; Quay, S.C. Advances in nasal drug delivery through tight junction technology. Expert Opin. Drug Deliv. 2005, 2, 281–298. [Google Scholar] [CrossRef]
- Bocsik, A.; Walter, F.R.; Gyebrovszki, A.; Fülöp, L.; Blasig, I.; Dabrowski, S.; Ötvös, F.; Tóth, A.; Rákhely, G.; Veszelka, S.; et al. Reversible Opening of Intercellular Junctions of Intestinal Epithelial and Brain Endothelial Cells with Tight Junction Modulator Peptides. J. Pharm. Sci. 2016, 105, 754–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulapane, K.R.; On, N.; Kiptoo, P.; Williams, T.D.; Miller, N.W.; Siahaan, T.J. Improving Brain Delivery of Biomolecules via BBB Modulation in Mouse and Rat: Detection using MRI, NIRF, and Mass Spectrometry. Nanotheranostics 2017, 1, 217–231. [Google Scholar] [CrossRef] [Green Version]
- Perez, T.D.; Nelson, W.J. Cadherin Adhesion: Mechanisms and Molecular Interactions. Handb. Exp. Pharmacol. 2004, 165, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Morita, K.; Furuse, M.; Fujimoto, K.; Tsukita, S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc. Natl. Acad. Sci. USA 1999, 96, 511–516. [Google Scholar] [CrossRef] [Green Version]
- Protze, J.; Eichner, M.; Piontek, A.; Dinter, S.; Rossa, J.; Blecharz, K.G.; Vajkoczy, P.; Piontek, J.; Krause, G. Directed structural modification of Clostridium perfringens enterotoxin to enhance binding to claudin-5. Cell. Mol. Life Sci. 2014, 72, 1417–1432. [Google Scholar] [CrossRef]
- Neuhaus, W.; Piontek, A.; Protze, J.; Eichner, M.; Mahringer, A.; Subileau, E.-A.; Lee, I.-F.M.; Schulzke, J.D.; Krause, G.; Piontek, J. Reversible opening of the blood-brain barrier by claudin-5-binding variants of Clostridium perfringens enterotoxin’s claudin-binding domain. Biomaterials 2018, 161, 129–143. [Google Scholar] [CrossRef]
- Liao, Z.; Yang, Z.; Piontek, A.; Eichner, M.; Krause, G.; Li, L.; Piontek, J.; Zhang, J. Specific binding of a mutated fragment of Clostridium perfringens enterotoxin to endothelial claudin-5 and its modulation of cerebral vascular permeability. Neuroscience 2016, 327, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Mrsny, R.J.; Brown, G.T.; Gerner-Smidt, K.; Buret, A.G.; Meddings, J.B.; Quan, C.; Koval, M.; Nusrat, A. A Key Claudin Extracellular Loop Domain is Critical for Epithelial Barrier Integrity. Am. J. Pathol. 2008, 172, 905–915. [Google Scholar] [CrossRef] [Green Version]
- Staat, C.; Coisne, C.; Dabrowski, S.; Stamatovic, S.M.; Andjelkovic, A.V.; Wolburg, H.; Engelhardt, B.; Blasig, I.E. Mode of action of claudin peptidomimetics in the transient opening of cellular tight junction barriers. Biomaterials 2015, 54, 9–20. [Google Scholar] [CrossRef]
- Dabrowski, S.; Staat, C.; Zwanziger, D.; Sauer, R.-S.; Bellmann, C.; Günther, R.; Krause, E.; Haseloff, R.F.; Rittner, H.; Blasig, I.E. Redox-sensitive structure and function of the first extracellular loop of the cell-cell contact protein claudin-1: Lessons from molecular structure to animals. Antioxid. Redox Signal. 2015, 22, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piorntek, J.; Winkler, L.; Wolburg, H.; Müller, S.L.; Zuleger, N.; Piehl, C.; Wiesner, B.; Krause, G.; Blasig, I.E. Formation of tight junction: Determinants of homophilic interaction between classic claudins. FASEB J. 2008, 22, 146–158. [Google Scholar] [CrossRef] [Green Version]
- Zwanziger, D.; Staat, C.; Andjelkovic, A.V.; Blasig, I.E. Claudin-derived peptides are internalized via specific endocytosis pathways. Ann. N. Y. Acad. Sci. 2012, 1257, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwanziger, D.; Hackel, D.; Staat, C.; Böcker, A.; Brack, A.; Beyermann, M.; Rittner, H.; Blasig, I.E. A Peptidomimetic Tight Junction Modulator to Improve Regional Analgesia. Mol. Pharm. 2012, 9, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Sladojevic, N.; Stamatovic, S.M.; Johnson, A.M.; Choi, J.; Hu, A.; Dithmer, S.; Blasig, I.E.; Keep, R.F.; Andjelkovic, A.V. Claudin-1-Dependent Destabilization of the Blood–Brain Barrier in Chronic Stroke. J. Neurosci. 2018, 39, 743–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dithmer, S.; Staat, C.; Müller, C.; Ku, M.-C.; Pohlmann, A.; Niendorf, T.; Gehne, N.; Fallier-Becker, P.; Kittel, Á.; Walter, F.R.; et al. Claudin peptidomimetics modulate tissue barriers for enhanced drug delivery. Ann. N. Y. Acad. Sci. 2017, 1397, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.; Gumbiner, B.M. A Synthetic Peptide Corresponding to the Extracellular Domain of Occludin Perturbs the Tight Junction Permeability Barrier. J. Cell Biol. 1997, 136, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Tavelin, S.; Hashimoto, K.; Malkinson, J.; Lazorova, L.; Toth, I.; Artursson, P. A New Principle for Tight Junction Modulation Based on Occludin Peptides. Mol. Pharmacol. 2003, 64, 1530–1540. [Google Scholar] [CrossRef]
- Greene, C.; Campbell, M. Tight junction modulation of the blood brain barrier: CNS delivery of small molecules. Tissue Barriers 2016, 4, e1138017. [Google Scholar] [CrossRef] [Green Version]
- Berndt, P.; Winkler, L.; Cording, J.; Breitkreuz-Korff, O.; Rex, A.; Dithmer, S.; Rausch, V.; Blasig, R.; Richter, M.; Sporbert, A.; et al. Tight junction proteins at the blood–brain barrier: Far more than claudin-5. Cell. Mol. Life Sci. 2019, 76, 1987–2002. [Google Scholar] [CrossRef]
- Nagahama, M.; Yamaguchi, A.; Hagiyama, T.; Ohkubo, N.; Kobayashi, K.; Sakurai, J. Binding and Internalization of Clostridium perfringens Iota-Toxin in Lipid Rafts. Infect. Immun. 2004, 72, 3267–3275. [Google Scholar] [CrossRef] [Green Version]
- Zeniya, S.; Kuwahara, H.; Daizo, K.; Watari, A.; Kondoh, M.; Yoshida-Tanaka, K.; Kaburagi, H.; Asada, K.; Nagata, T.; Nagahama, M.; et al. Angubindin-1 opens the blood–brain barrier in vivo for delivery of antisense oligonucleotide to the central nervous system. J. Control. Release 2018, 283, 126–134. [Google Scholar] [CrossRef]
- Kim, D.-G.; Jang, M.; Choi, S.-H.; Kim, H.-J.; Jhun, H.; Kim, H.-C.; Rhim, H.; Cho, I.-H.; Nah, S.-Y. Gintonin, a ginseng-derived exogenous lysophosphatidic acid receptor ligand, enhances blood-brain barrier permeability and brain delivery. Int. J. Biol. Macromol. 2018, 114, 1325–1337. [Google Scholar] [CrossRef]
- Choi, S.-H.; Lee, B.-H.; Kim, H.-J.; Jung, S.-W.; Kim, H.; Shin, H.-C.; Lee, J.; Kim, H.-C.; Rhim, H.; Hwang, S.-H.; et al. Ginseng Gintonin Activates the Human Cardiac Delayed Rectifier K+ Channel: Involvement of Ca2+/Calmodulin Binding Sites. Mol. Cells 2014, 37, 656–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, Y.; Shirakura, K.; Okada, Y.; Takeda, H.; Endo, K.; Tamura, M.; Watari, A.; Sadamura, Y.; Sawasaki, T.; Doi, T.; et al. Claudin-5-Binders Enhance Permeation of Solutes across the Blood-Brain Barrier in a Mammalian Model. J. Pharmacol. Exp. Ther. 2017, 363, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Sasaki, H.; Furuse, M.; Tsukita, S. Endothelial Claudin. J. Cell Biol. 1999, 147, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Rahner, C.; Mitic, L.L.; Anderson, J. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology 2001, 120, 411–422. [Google Scholar] [CrossRef]
- Ullman, J.C.; Arguello, A.; Getz, J.A.; Bhalla, A.; Mahon, C.S.; Wang, J.; Giese, T.; Bedard, C.; Kim, D.J.; Blumenfeld, J.R.; et al. Brain delivery and activity of a lysosomal enzyme using a blood-brain barrier transport vehicle in mice. Sci. Transl. Med. 2020, 12, 1–13. [Google Scholar] [CrossRef]
- Kariolis, M.S.; Wells, R.C.; Getz, J.A.; Kwan, W.; Mahon, C.S.; Tong, R.; Kim, D.J.; Srivastava, A.; Bedard, C.; Henne, K.R.; et al. Brain delivery of therapeutic proteins using an Fc fragment blood-brain barrier transport vehicle in mice and monkeys. Sci. Transl. Med. 2020, 12, eaay1359. [Google Scholar] [CrossRef] [PubMed]
- Willson, J. Transferrin’ across the blood-brain barrier. Nat. Rev. Drug Discov. 2020, 19, 444. [Google Scholar] [CrossRef]
- Greene, C.; Kealy, J.; Humphries, M.M.; Gong, Y.; Hou, J.; Hudson, N.; Cassidy, L.M.; Martiniano, R.; Shashi, V.; Hooper, S.R.; et al. Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol. Psychiatry 2018, 23, 2156–2166. [Google Scholar] [CrossRef]
- Campbell, M.; Kiang, A.-S.; Kenna, P.F.; Kerskens, C.; Blau, C.; O’Dwyer, L.; Tivnan, A.; Kelly, J.A.; Brankin, B.; Farrar, G.-J.; et al. RNAi-mediated reversible opening of the blood-brain barrier. J. Gene Med. 2008, 10, 930–947. [Google Scholar] [CrossRef]
- Van Itallie, C.M.; Fanning, A.S.; Bridges, A.; Anderson, J.M. ZO-1 Stabilizes the Tight Junction Solute Barrier through Coupling to the Perijunctional Cytoskeleton. Mol. Biol. Cell 2009, 20, 3930–3940. [Google Scholar] [CrossRef] [Green Version]
- Gomes, M.J.; Kennedy, P.J.; Martins, S.; Sarmento, B. Delivery of siRNA silencing P-gp in peptide-functionalized nanoparticles causes efflux modulation at the blood–brain barrier. Nanomedicine 2017, 12, 1385–1399. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, C.; Wood, M.J.A. Antisense oligonucleotides: The next frontier for treatment of neurological disorders. Nat. Rev. Neurol. 2018, 14, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, H.; Song, J.; Shimoura, T.; Yoshida-Tanaka, K.; Mizuno, T.; Mochizuki, T.; Zeniya, S.; Li, F.; Nishina, K.; Nagata, T.; et al. Modulation of blood-brain barrier function by a heteroduplex oligonucleotide in vivo. Sci. Rep. 2018, 8, 4377. [Google Scholar] [CrossRef] [PubMed]
- Asami, Y.; Yoshioka, K.; Nishina, K.; Nagata, T.; Yokota, T. Drug delivery system of therapeutic oligonucleotides. Drug Discov. Ther. 2016, 10, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Focused Ultrasound Foundation. Focused Ultrasound & Blood-Brain Barrier Workshop; Focused Ultrasound Foundation: Washington, DC, USA, 2017. [Google Scholar]
- Carpentier, A.; Canney, M.; Vignot, A.; Reina, V.; Beccaria, K.; Horodyckid, C.; Karachi, C.; Leclercq, D.; Lafon, C.; Chapelon, J.-Y.; et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl. Med. 2016, 8, 343re2. [Google Scholar] [CrossRef]
- Idbaih, A.; Canney, M.; Belin, L.; Desseaux, C.; Vignot, A.; Bouchoux, G.; Asquier, N.; Law-Ye, B.; Leclercq, D.; Bissery, A.; et al. Safety and Feasibility of Repeated and Transient Blood–Brain Barrier Disruption by Pulsed Ultrasound in Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2019, 25, 3793–3801. [Google Scholar] [CrossRef] [Green Version]
- Foley, C.P.; Rubin, D.G.; Santillan, A.; Sondhi, D.; Dyke, J.; Gobin, Y.P.; Crystal, R.G.; Ballon, D. Intra-arterial delivery of AAV vectors to the mouse brain after mannitol mediated blood brain barrier disruption. J. Control. Release 2014, 196, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Mccarty, D.M.; DiRosario, J.; Gulaid, K.; Muenzer, J.; Fu, H. Mannitol-facilitated CNS entry of rAAV2 vector significantly delayed the neurological disease progression in MPS IIIB mice. Gene Ther. 2009, 16, 1340–1352. [Google Scholar] [CrossRef]
- A Neuwelt, E.; A Dahlborg, S. Chemotherapy administered in conjunction with osmotic blood-brain barrier modification in patients with brain metastases. J. Neuro-Oncol. 1987, 4, 195–207. [Google Scholar] [CrossRef]
- Joshi, S.; Ellis, J.A.; Ornstein, E.; Bruce, J.N. Intraarterial drug delivery for glioblastoma mutiforme. J. Neuro-Oncol. 2015, 124, 333–343. [Google Scholar] [CrossRef]
- Chakraborty, S.; Filippi, C.G.; Wong, T.; Ray, A.; Fralin, S.; Tsiouris, A.J.; Praminick, B.; Demopoulos, A.; McCrea, H.J.; Bodhinayake, I.; et al. Superselective intraarterial cerebral infusion of cetuximab after osmotic blood/brain barrier disruption for recurrent malignant glioma: Phase I study. J. Neuro-Oncol. 2016, 128, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, J.-K.; Shin, B.J.; Schlaff, C.D.; Riina, H.; A Boockvar, J. Cost analysis of intra-arterial versus intra-venous delivery of bevacizumab for the treatment of recurrent glioblastoma multiforme. J. Exp. Ther. Oncol. 2011, 9, 183–186. [Google Scholar]
- Linville, R.M.; Komin, A.; Lan, X.; DeStefano, J.G.; Chu, C.; Liu, G.; Walczak, P.; Hristova, K.; Searson, P.C. Reversible blood-brain barrier opening utilizing the membrane active peptide melittin in vitro and in vivo. Biomaterials 2021, 275, 120942. [Google Scholar] [CrossRef] [PubMed]
- Habermann, E. Bee and Wasp Venoms. Science 1972, 177, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guan, S.-M.; Sun, W.; Fu, H. Melittin, the Major Pain-Producing Substance of Bee Venom. Neurosci. Bull. 2016, 32, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Ceremuga, M.; Stela, M.; Janik, E.; Gorniak, L.; Synowiec, E.; Sliwinski, T.; Sitarek, P.; Saluk-Bijak, J.; Bijak, M. Melittin—A Natural Peptide from Bee Venom Which Induces Apoptosis in Human Leukaemia Cells. Biomolecules 2020, 10, 247. [Google Scholar] [CrossRef] [Green Version]
- Gajski, G.; Garaj-Vrhovac, V. Melittin: A lytic peptide with anticancer properties. Environ. Toxicol. Pharmacol. 2013, 36, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Kreinest, T.; Volkmer, I.; Staege, M.S. Melittin Increases Cisplatin Sensitivity and Kills KM-H2 and L-428 Hodgkin Lymphoma Cells. Int. J. Mol. Sci. 2020, 22, 343. [Google Scholar] [CrossRef] [PubMed]
- Lima, W.G.; de Brito, J.C.M.; Cardoso, V.N.; Fernandes, S.O.A. In-depth characterization of antibacterial activity of melittin against Staphylococcus aureus and use in a model of non-surgical MRSA-infected skin wounds. Eur. J. Pharm. Sci. 2021, 156, 105592. [Google Scholar] [CrossRef]
- Ombredane, A.S.; de Andrade, L.R.; Bonadio, R.S.; Pinheiro, W.O.; de Azevedo, R.B.; Joanitti, G.A. Melittin sensitizes skin squamous carcinoma cells to 5-fluorouracil by affecting cell proliferation and survival. Exp. Dermatol. 2021, 30, 710–716. [Google Scholar] [CrossRef]
- Pulgar, V.M. Transcytosis to Cross the Blood Brain Barrier, New Advancements and Challenges. Front. Neurosci. 2019, 12, 1019. [Google Scholar] [CrossRef]
- PepGen. Available online: https://pepgen.com/ (accessed on 13 May 2021).
- Oliveira, S.; Storm, G.; Schiffelers, R.M. Targeted Delivery of siRNA. J. Biomed. Biotechnol. 2006, 2006, 1–9. [Google Scholar] [CrossRef]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liang, X.-J. Therapeutic siRNA: State of the art. Signal Transduct. Target. Ther. 2020, 5, 1–25. [Google Scholar] [CrossRef]
- Kopatz, I.; Remy, J.-S.; Behr, J.-P. A model for non-viral gene delivery: Through syndecan adhesion molecules and powered by actin. J. Gene Med. 2004, 6, 769–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominska, M.; Dykxhoorn, D.M. Breaking down the barriers: siRNA delivery and endosome escape. J. Cell Sci. 2010, 123, 1183–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudolph, C.; Lausier, J.; Naundorf, S.; Rosenecker, J. In vivo gene delivery to the lung using polyethylenimine and fractured polyamidoamine dendrimers. J. Gene Med. 2000, 2, 269–278. [Google Scholar] [CrossRef]
- Yuan, W.; Li, G.; Fu, B.M. Effect of Surface Charge of Immortalized Mouse Cerebral Endothelial Cell Monolayer on Transport of Charged Solutes. Ann. Biomed. Eng. 2010, 38, 1463–1472. [Google Scholar] [CrossRef]
- Campbell, M.; Humphries, M.M.; Kiang, A.-S.; Nguyen, A.T.H.; Gobbo, O.L.; Tam, L.C.S.; Suzuki, M.; Hanrahan, F.; Ozaki, E.; Farrar, G.-J.; et al. Systemic low-molecular weight drug delivery to pre-selected neuronal regions. EMBO Mol. Med. 2011, 3, 235–245. [Google Scholar] [CrossRef]
- de Gooijer, M.C.; Kemper, E.M.; Buil, L.C.; Çitirikkaya, C.H.; Buckle, T.; Beijnen, J.H.; van Tellingen, O. ATP-binding cassette transporters restrict drug delivery and efficacy against brain tumors even when blood-brain barrier integrity is lost. Cell Rep. Med. 2021, 2, 100184. [Google Scholar] [CrossRef]
- Sinharay, S.; Tu, T.-W.; Kovacs, Z.I.; Schreiber-Stainthorp, W.; Sundby, M.; Zhang, X.; Papadakis, G.Z.; Reid, W.C.; Frank, J.A.; Hammoud, D.A. In vivo imaging of sterile microglial activation in rat brain after disrupting the blood-brain barrier with pulsed focused ultrasound: [18F]DPA-714 PET study. J. Neuroinflamm. 2019, 16, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pascal, A.; Li, N.; Lechtenberg, K.J.; Rosenberg, J.; Airan, R.D.; James, M.L.; Bouley, D.M.; Pauly, K.B. Histologic evaluation of activation of acute inflammatory response in a mouse model following ultrasound-mediated blood-brain barrier using different acoustic pressures and microbubble doses. Nanotheranostics 2020, 4, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Jordão, J.F.; Thévenot, E.; Markham-Coultes, K.; Scarcelli, T.; Weng, Y.-Q.; Xhima, K.; O’Reilly, M.; Huang, Y.; McLaurin, J.; Hynynen, K.; et al. Amyloid-β plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp. Neurol. 2013, 248, 16–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacs, Z.I.; Kim, S.; Jikaria, N.; Qureshi, F.; Milo, B.; Lewis, B.K.; Bresler, M.; Burks, S.R.; Frank, J.A. Disrupting the blood–brain barrier by focused ultrasound induces sterile inflammation. Proc. Natl. Acad. Sci. USA 2017, 114, E75–E84. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, Z.I.; Tu, T.-W.; Sundby, M.; Qureshi, F.; Lewis, B.K.; Jikaria, N.; Burks, S.R.; Frank, J.A. MRI and histological evaluation of pulsed focused ultrasound and microbubbles treatment effects in the brain. Theranostics 2018, 8, 4837–4855. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.; Bendayan, R.; Hynynen, K. Acute effects of focused ultrasound-induced increases in blood-brain barrier permeability on rat microvascular transcriptome. Sci. Rep. 2017, 7, srep45657. [Google Scholar] [CrossRef]
- McMahon, D.; Hynynen, K. Acute Inflammatory Response Following Increased Blood-Brain Barrier Permeability Induced by Focused Ultrasound is Dependent on Microbubble Dose. Theranostics 2017, 7, 3989–4000. [Google Scholar] [CrossRef]
- Ji, R.; Karakatsani, M.E.; Burgess, M.; Smith, M.; Murillo, M.F.; Konofagou, E.E. Cavitation-modulated inflammatory response following focused ultrasound blood-brain barrier opening. J. Control. Release 2021, 337, 458–471. [Google Scholar] [CrossRef]
- Burks, S.R.; Kersch, C.N.; Witko, J.A.; Pagel, M.A.; Sundby, M.; Muldoon, L.L.; Neuwelt, E.A.; Frank, J.A. Blood–brain barrier opening by intracarotid artery hyperosmolar mannitol induces sterile inflammatory and innate immune responses. Proc. Natl. Acad. Sci. USA 2021, 118, 2021915118. [Google Scholar] [CrossRef]
- Maggs, L.; Cattaneo, G.; Dal, A.E.; Moghaddam, A.S.; Ferrone, S. CAR T Cell-Based Immunotherapy for the Treatment of Glioblastoma. Front. Neurosci. 2021, 15, 662064. [Google Scholar] [CrossRef]
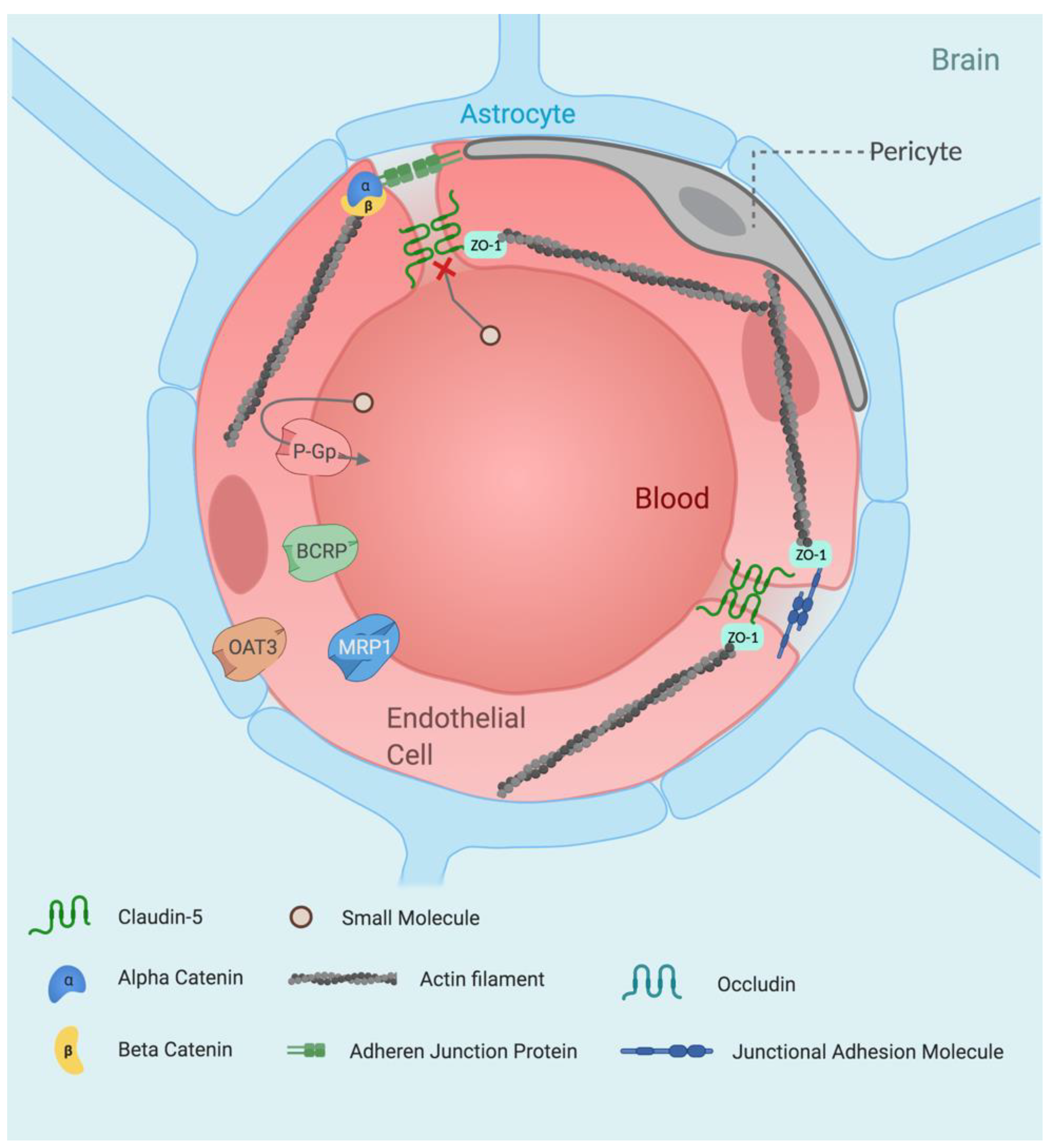



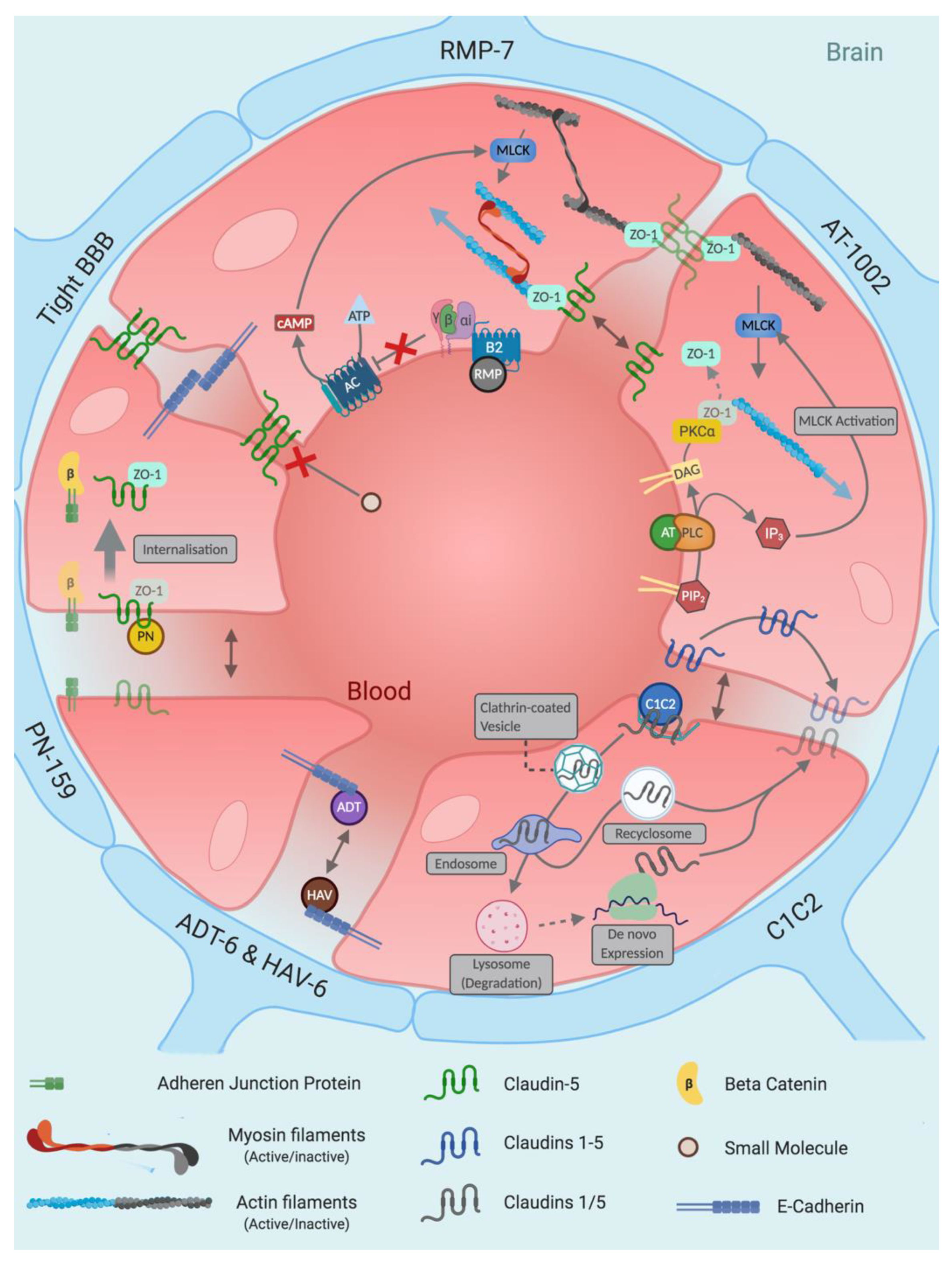


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whelan, R.; Hargaden, G.C.; Knox, A.J.S. Modulating the Blood–Brain Barrier: A Comprehensive Review. Pharmaceutics 2021, 13, 1980. https://doi.org/10.3390/pharmaceutics13111980
Whelan R, Hargaden GC, Knox AJS. Modulating the Blood–Brain Barrier: A Comprehensive Review. Pharmaceutics. 2021; 13(11):1980. https://doi.org/10.3390/pharmaceutics13111980
Chicago/Turabian StyleWhelan, Rory, Grainne C. Hargaden, and Andrew J. S. Knox. 2021. "Modulating the Blood–Brain Barrier: A Comprehensive Review" Pharmaceutics 13, no. 11: 1980. https://doi.org/10.3390/pharmaceutics13111980
APA StyleWhelan, R., Hargaden, G. C., & Knox, A. J. S. (2021). Modulating the Blood–Brain Barrier: A Comprehensive Review. Pharmaceutics, 13(11), 1980. https://doi.org/10.3390/pharmaceutics13111980






