1. Introduction
Fucoidan is a fucose-containing sulphated polysaccharide extracted from brown seaweeds. The biological functions of this marine polysaccharide include anticancer, antivirus, anti-coagulant, and modulation on diabetic and metabolic syndrome, have been extensively studied [
1]. With advantages such as being high solubility in aqueous solution and a favorable safety profile, fucoidan has been applied to cancer treatments in preclinical and clinical studies [
2,
3,
4].
The therapeutic effects of fucoidan have been demonstrated in abundant cancer types including acute leukemia [
5], lymphoma [
6], head and neck [
7], lung [
8], breast [
9], hepatoblastoma [
10], prostate [
11], and ovarian cancer [
10] in animal studies. The mechanisms or reactions discovered in these cancers were mostly associated with the induction of apoptosis and the inhibition of cell cycle. Moreover, fucoidan possesses antimetastatic ability [
12], inhibiting the formation of tumor nodules in the lungs of metastatic 4T1 tumor-bearing animal model [
9]. Fucoidan has also been used as a complementary therapy for patients in clinical studies, and the results demonstrated that fucoidan may augment the therapeutic index [
2].
In current applications, fucoidan is mostly administered via oral delivery. However, obstacles such as the relatively low bioavailability [
13] and the fast clearance rate [
14] have restricted the effective accumulation of fucoidan within the tumor, and thus limited its therapeutic efficacy. Engineered nanoparticles with the tailorable properties such as size, zeta potential, surface coating, and shape can optimize the pharmacokinetic behavior and the ability of one material to accumulate in a tumor [
15,
16]. As reported by Abdollah et al., the coating of fucoidan on the surface of nanoparticles can significantly extend its circulation time in the bloodstream [
17], thus offering more opportunities for the nanomedicines to accumulate within the tumor.
In this study, we developed a fucoidan-based nanoparticle (FuNP), explored its safety profile and its potential to inhibit tumor progression via parenteral administration route. Herein, we synthesized the colloidally stable FuNPs using the emulsion method. The compositions we used to stabilize the structure are listed as approved excipients by USFDA. The safety profiles of fucoidan and FuNPs injected via parenteral route were evaluated in mice, and the therapeutic efficacy of FuNPs was assessed in MDA-MB-231-tumor bearing mice. We demonstrated that FuNPs possessed a favorable safety profile and the potency to inhibit tumor progression as well as metastasis. This evidence paves the way for further translational applications of FuNPs.
2. Materials and Methods
2.1. Materials and Reagents
Poly(d,l-lactide-co-glycolide) (PLGA), soybean oil, and Poly(lactide-co-glycolide)-Rhodamine B were purchased from Merck (Germany). Anti-F-actin antibody and 2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride, 4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI) were purchased from BD Biosciences (USA). Fucoidan from fucus vesiculosus was purchased from Marinova (Tasmania, Australia).
2.2. Preparation of Fucoidan Nanoparticles (FuNPs)
FuNPs were synthesized by emulsification process. The aqueous phase with fucoidan and organic phase (i.e., dichloromethane, DCM) with PLGA plus soybean oil were mixed and sonicated using the ultrasonic homogenizer (UP200S with S2 probe, Hielscher, Germany) under ice bath. After forming a one-phase emulsion, the sample was subjected to the rotary evaporator to remove the DCM. The aqueous solution after evaporation was purified using a centrifuge.
2.3. Size Selection of FuNPs Using Centrifuge
The as-synthesized FuNPs were subjected to size selection to separate the large and small FuNPs. The group without the exclusion of large FuNPs was centrifuged at 14,000× g for 15 min. We then removed the suspension and redispersed them in water. To collect the large FuNPs, the as-synthesized FuNPs were centrifuged at 800× g for 5 min. The pellets represented the larger FuNPs were collected and redispersed in water, while the suspension was further centrifuged at 14,000× g for 15 min. The pellet as the smaller FuNPs was redispersed in water and stored in 4 °C for further use.
2.4. Characterization of FuNPs
The morphology and structure of FuNPs were characterized using a scanning electron microscopy (SEM, JSM-6700F, JOEL, Akishima, Japan) and a transmission electron microscopy (TEM, JEOL JEM-2100F, JEOL, Akishima, Japan). Dynamic light scattering was used (DLS, Litesizer 500, Anton Paar, Graz, Austria) to measure the size and zeta potential of FuNPs.
2.5. Stability Test
FuNPs were transferred from water to PBS for the stability test. In brief, the FuNPs were stored in 4 °C and 37 °C, respectively, and the size and zeta potential of the FuNPs were analyzed at least 3 times for 7 days post PBS incubation.
2.6. Cell Culture
MDA-MB-231 breast cancer cell line was purchased from Bioresource Collection and Research Center, Food Industry Research and Development Institute, Taiwan. The cells were cultured using Dulbecco’s Modified Eagle Medium (DMEM, Thermo Fisher Scientific, MA, USA) containing 10% fetal bovine serum (FBS, from Thermo Fisher Scientific, MA, USA) and 1% penicillin/streptomycin (Thermo Fisher Scientific, MA, USA). The 4T1 was purchased from ATCC (Manassas, Virginia, USA), and the culture method was identical to MDA-MB-231 cells.
2.7. In Vitro Antitumor Effects
MDA-MB-231 cells were treated with fucoidan or FuNPs at different concentrations and the cell viabilities at 24 h and 48 h were measured using CCK-8 assay. In brief, 5 × 103 cells were seeded in 96 well plates and cultured with culture medium (100 μL) at 37 °C overnight. Fucoidan and FuNPs with different concentrations were then added to the cells. After incubation for 24 h or 48 h, cells were washed with PBS, and CCK-8 solution was added to the medium and allowed reaction for 30 min. The plate was then subjected to Elisa reader (SpectraMax iD3, Molecular Devices, San Jose, CA, USA) and the absorbance was measured at 450 nm.
2.8. Monitoring Cell Internalization
We analyzed the cell internalization using flow cytometry (Attune Nxt flow cytometry, ThermoFisher, USA) and fluorescence microscopy (AxioImager. A1, Zeiss, Germany). To facilitate the observation, PLGA-Rhodamine was mixed with PLGA at 1:20 w/w ratio during the synthesis process to obtain the fluorescence-labeled FuNPs. After the incubation of FuNPs with 4T1 and MDA-MB-231 cells for 24 h and 48 h in a 6-well plate, the cells (1 × 105) were collected and measured using flow cytometry.
For fluorescence microscopy, 4T1 cells were seeded and allowed to grow on Millicell EZ SLID (Merck, Germany) overnight. After that, we incubated the cells with FuNPs for 24 h and washed the cells twice with PBS. To generate cellular permeability, triton X-100 (0.3% in PBS buffer) was added for 30 min. The cells were then blocked using FBS buffer (30 min), immobilized using anti-F-actin at 4 °C overnight, and further stained using a secondary antibody (1:250) for 1 h. After an 1 h incubation, the cells were washed twice with PBS and stained with DAPI (1 µg mL−1) for 5 min. The cells were then observed using a fluorescent microscope.
2.9. Animals
ICR mice were used in the safety study, in which the animal protocol has been reviewed and approved by the Institute of Animal Care and Use Committee (IACUC) of Agriculture Technology Research Institute, Taiwan, approval number of 109113. For therapeutic and safety studies using balc/c nude mice, the animal experiments were performed in compliance with the guidelines of the Animal Care and Use Committee (IACUC) of the China Medical University under the approval number of CMUIACUC-2021-299.
2.10. Safety of Fucoidan via IP Injection
A 14-day acute toxicity study of fucoidan via intraperitoneal (IP) injection was carried out to determine the safety and the adverse effect of fucoidan on 12 male and 12 female adult ICR mice (7–9 weeks old). Mice were randomly assigned to either Control, Low-dose, Mid-dose or High-dose groups in which they intraperitoneally (IP) received a 0, 50, 275 or 500 mg kg−1 of single dose fucoidan, respectively. During the 14-day observation period, clinical observation including body weight, mortality, clinical symptoms including exterior, behavior, breath, mouth and nose, eyes, skin, digestion, and metabolism were monitored and recorded. Following the monitoring for 14 days, the mice were sacrificed and were subjected to assessments including hematology, serum chemistry and histopathology. For hematology, the whole blood was collected from abdominal aorta through syringe aspiration and preserved in clean K2-EDTA centrifuge tubes at room temperature before the analysis of red blood cells (RBC), hemoglobin (HGB), white blood cells (WBC), hematocrit (HCT), blood platelet (PLT), lymphocyte composition, eosinophil composition, neutrophil composition, monocyte composition, and basophil composition.
For serum chemistry, the whole blood collected from the heart through syringe aspiration was preserved in clean centrifuge tubes at room temperature for 30 min and centrifuged to harvest the serum. Aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine, blood urea nitrogen (BUN), albumin, and glucose of the mice were measured using clinical chemistry analyzer.
For histopathology, the harvested organs include adrenal glands, heart, lungs with trachea, kidneys, spleen, liver with gallbladder, salivary glands, and mandibular lymph were preserved in 10% neutral buffered formalin (NBF) at room temperature for 72~96 h. After fixation, the tissues were trimmed, dehydrated with ethanol and infiltrated by paraffin. Thin sections (4–6 μm in thickness) were cut using a microtome and were subjected to hematoxylin and eosin (H&E) stain. Histopathological alterations were documented by a pathologist.
2.11. Therapeutic Effect of FuNPs
To evaluate the therapeutic effect, the MDA-MB-231 tumor-bearing mice were treated with saline (control) or FuNPs via intravenous (i.v, 100 mg kg
−1) injection (q3dx6) at 19 days after tumor inoculation (
n = 4). The tumor volumes were monitored using a digital caliper (Mitutoyo) three times a week using the following Equation (1):
in which W is the width of the tumor, and the L is the length of the tumor (W < L).
2.12. Safety of FuNPs
Following the monitoring of the tumor progression, the mice were later sacrificed on day 49 and were subjected to histopathological assessments. In brief, the harvested organs include adrenal glands, heart, lungs with trachea, kidneys, spleen, liver with gallbladder, salivary glands, mandibular lymph node, and inoculated neoplasm (human breast cancer model, MDA-MB-231 cell line) were preserved in 10% neutral buffered formalin (NBF) at room temperature for 72~96 h. After fixation, the tissues were trimmed, dehydrated with ethanol, and infiltrated by paraffin. The microtome was used to cut the sample with multiple sections with 4–6 μm in thickness These sections were subjected to hematoxylin and eosin (H&E) stains to observe histological alteration by the study pathologist.
2.13. Statistical Analysis
Results are expressed as mean ± s.d. unless otherwise noted. Non-parametric Kruskal–Wallis test was used to evaluate the significance of mean differences between the control and the fucoidan-treated group in body weights and clinical chemistry values. One-way ANOVA was used to evaluate the statistical differenceusing GraphPad Prism version 9.2.0, USA. p value < 0.05 was considered statistically significant. If a significant result was determined, post hoc tests were used to seek the differences with the control group.
4. Discussion
Fucoidan from fucus vesiculosus was demonstrated to be safe at the doses ranging from 50 to 500 mg kg
−1 and 50 to 275 mg kg
−1 via the parenteral IP administration route in male and female mice, respectively (
Figure 1 and
Supplementary Figure S1). While not every female mouse that received 500 mg kg
−1 fucoidan showed liver lesion, further studies involving more female mice are required to draw conclusions. Generally, our study provides solid evidence that fucoidan is a substance with a favorable safety profile for the applications associated with parenteral administration.
With the simple emulsion process, we synthesized the FuNPs with an inherently therapeutic effect on tumor inhibition. In
Figure 2a–d, we showed that larger FuNPs could be collected via centrifugation at a lower speed (i.e., 800×
g). By excluding the FuNPs with the particle size larger than 400 nm, the smaller ones presented a more efficient cell internalization efficacy in MDA-MB-231 and 4T1 breast cancer cell lines (
Figure 3). The storage of FuNPs in PBS at 4 °C was demonstrated to be stable for at least one week. In contrast, zeta potential and size of FuNPs significantly increased at 2- and 7-day post incubation in PBS at 37 °C (
Figure 4), indicating the structural instability took place within 48 h when subjecting the environment. The instability might be attributed to the hydrolytic degradation of PLGA in aqueous environment [
19], and lead to the detachment of fucoidan from the particle surface. The degradation of FuNPs at 37 °C (i.e., the biological temperature) addresses the potential of FuNP being a vesicle to encapsulate, deliver, and release active pharmaceutical ingredients to a tumor tissue, and such controlled drug release application is worth exploring in the future studies.
The anti-cancer activity and biological effects of fucoidan have been studied extensively. The regulation of cell cycle, inhibition for the receptors such as epidermal growth factor receptor (EGFR), transforming growth factor-beta receptors (TGFF), and Toll-like receptor 4 (TLR4) for triggering downstream apoptotic pathways [
2,
20] are among the common mechanisms that fucoidan activates to suppress the proliferation of cancer cells. Noteworthily, FuNPs we synthesized had a significantly stronger anti-cancer activity compared with the free form fucoidan (
Figure 5). The stronger anti-cancer activity could be attributed to the nanoparticle-related factors such as the enhanced cell uptake efficiency and the induction of higher-level reactive oxygen species (ROS) in cancer cells [
21].
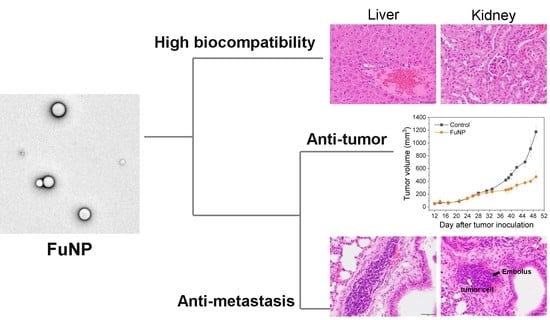
FuNPs significantly inhibited the tumor progress in MDA-MB-231-bearing mice, indicating the nanoscale formulation can successfully accumulate in the tumors after being IV injected into the bloodstream (
Figure 6a). Importantly, fucoidan has been demonstrated to inhibit metastasis in multiple cancer types including breast, lung, and liver cancer [
12,
20]. Encouragingly, FuNPs also demonstrated antimetastatic activities, where the tumor cells were found to lose the ability to invade into the lung after receiving the FuNPs treatments (
Figure 6c). In our study, multiple doses were administered in a 3-day treatment plan, and no adverse effect was observed in the histological assessments (
Figure 7). This indicates that FuNPs are a safe formulation with the dual effects of inhibiting tumor growth and metastasis under the current treatment regimen and dose. A further exploration of FuNPs dose escalation study is valuable since FuNPs have such a favorable therapeutic index and broad safety margin.
The aim of this study is to explore the possibility of forming fucoidan-based nanoparticles and evaluate their anti-cancer activity. Our results demonstrated that the stable FuNPs with appropriate size have a far superior tumor progress inhibition ability. The anti-metastasis function of FuNPs was also observed in the mouse cancer model. Noteworthily, under the intensive treatment regimen, FuNPs did not induce adverse effects as confirmed by histological analysis. However, future studies elucidating the mechanisms on tumor and metastasis inhibition are needed. While FuNPs with biodegradable ability can also act as drug carriers to achieve controlled drug release, exploring the mechanisms of FuNPs and discovering the ideal drugs to facilitate the synergistic combination for cancer therapy can open an avenue for a new class of therapeutic nanomedicines.