Antimicrobial Activities of Propolis in Poloxamer Based Topical Gels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurement of Propolis Solubility in Various Poloxamer Solutions
2.3. Preparation of Propolis-Poloxamer Formulations
2.4. Measurement of Release Profile, Viscosity, and Micelle Characterization
2.5. Measurement of Trypan Blue Diffusion Pattern
2.6. Bacterial Strains and Culture Conditions
2.7. Antimicrobial Susceptibility Test
2.8. Antimicrobial Kinetics Assay
2.9. Statistics
3. Results
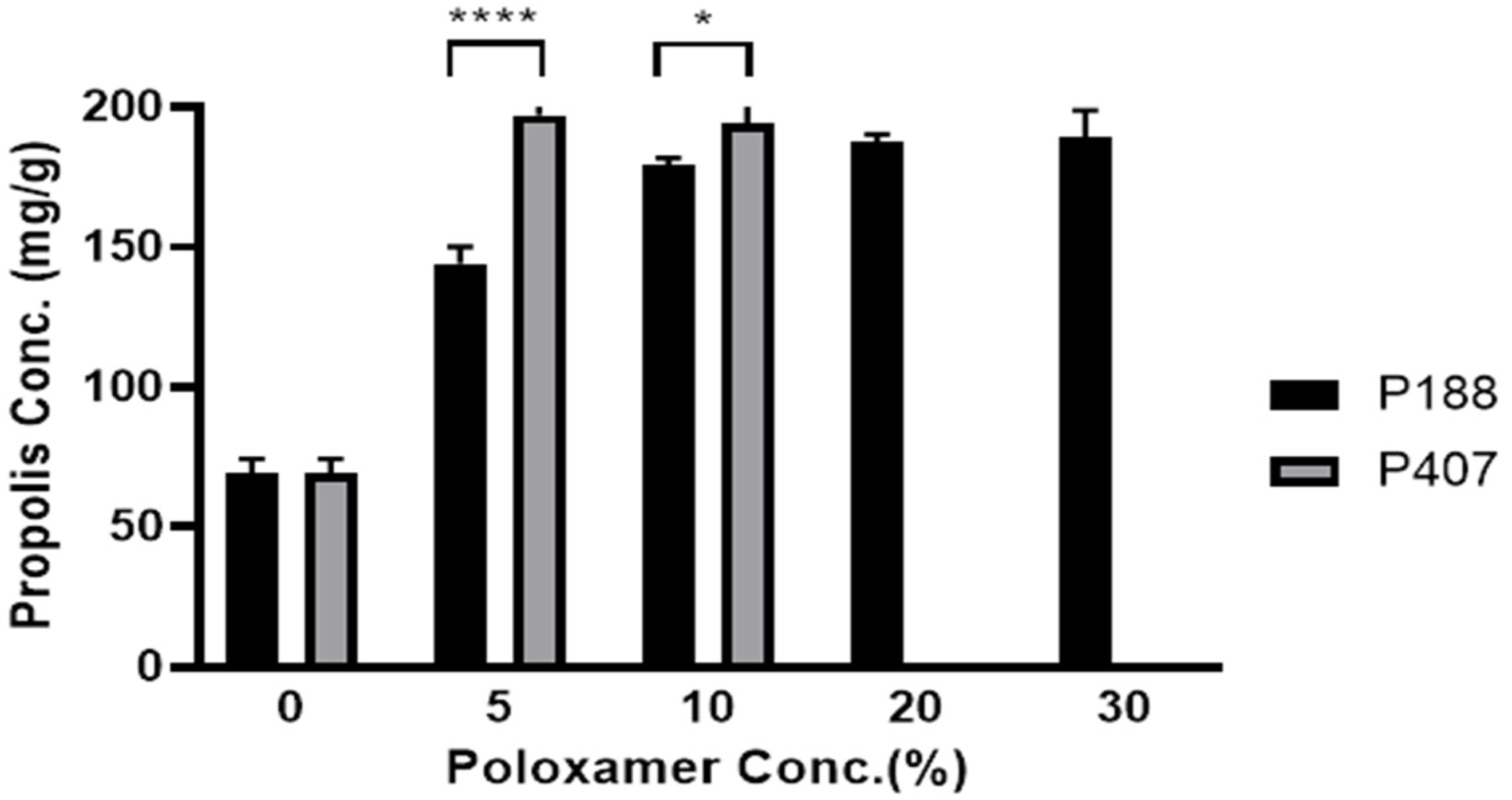
3.1. Effect of Poloxamer Type and Concentration on Propolis Solubility
3.2. Effect of Poloxamer Type and Concentration on Release of Propolis
3.3. Diffusion Patterns of Trypan Blue in P188, P407, and P188 with Added Carbopol Formulation
3.4. Antimicrobial Susceptibility Test of Propolis-Poloxamer Formulations
3.5. Time-Kill Kinetics Assay of Propolis-Poloxamer Formulations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Percival, S.L.; Emanuel, C.; Cutting, K.F.; Williams, D.W. Microbiology of the skin and the role of biofilms in infection. Int. Wound J. 2012, 9, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Howell-Jones, R.S.; Wilson, M.J.; Hill, K.E.; Howard, A.J.; Price, P.E.; Thomas, D.W. A review of the microbiology, antibiotic usage and resistance in chronic skin wounds. J. Antimicrob. Chemother. 2005, 55, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Sudbery, P. Candida albicans, a major human fungal pathogen. J. Microbiol. 2011, 49, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Godin, B.; Touitou, E.; Rubinstein, E.; Athamna, A.; Athamna, M. A new approach for treatment of deep skin infections by an ethosomal antibiotic preparation: An in vivo study. J. Antimicrob. Chemother. 2005, 55, 989–994. [Google Scholar] [CrossRef] [Green Version]
- Kyle, A.A.; Dahl, M.V. Topical therapy for fungal infections. Am. J. Clin. Derm. 2004, 5, 443–451. [Google Scholar] [CrossRef]
- Ivancajic, S.; Mileusnic, I.; Cenic-Milosevic, D. In vitro antibacterial activity of propolis extracts on 12 different bacteria in conditions of 3 various pH values. Arch. Biol. Sci. 2010, 62, 915–934. [Google Scholar] [CrossRef]
- Sforcin, J.M.; Fernandes, A.; Lopes, C.A.M.; Bankova, V.; Funari, S.R.C. Seasonal effect on Brazilian propolis antibacterial activity. J. Ethnopharmacol. 2000, 73, 243–249. [Google Scholar] [CrossRef]
- Sforcin, J.M.; Fernandes Júnior, A.; Lopes, C.A.M.; Funari, S.R.C.; Bankova, V. Seasonal effect of Brazilian propolis on Candida albicans and Candida tropicalis. J. Venom. Anim. Toxins 2001, 7, 139–144. [Google Scholar] [CrossRef]
- Jorge, R.; Furtado, N.A.J.C.; Sousa, J.P.B.; Da Silva Filho, A.A.; Gregório Junior, L.E.; Martins, C.H.G.; Soares, A.E.E.; Bastos, J.K.; Cunha, W.R.; Silva, M.L.A. Brazilian Propolis: Seasonal Variation of the Prenylatedp-Coumaric Acids and Antimicrobial Activity. Pharm. Biol. 2008, 46, 889–893. [Google Scholar] [CrossRef]
- Reis, C.M.F.; Carvalho, J.C.T.; Caputo, L.R.G.; Patricio, K.C.M.; Barbosa, M.V.J.; Chieff, A.L.; Bastos, J.K. Atividade antiinflamatória, antiúlcera gástrica e toxicidade subcrônica do extrato etanólico de própolis. Rev. Bras. Farmacogn. 2000, 9–10, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Adachi, T.; Yoshikawa, S.; Tezuka, H.; Tsuji, N.M.; Ohteki, T.; Karasuyama, H.; Kumazawa, T. Propolis induces Ca2+ signaling in immune cells. Biosci. Microbiota Food Health 2019, 38, 141–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krol, W.; Czuba, Z.; Scheller, S.; Gabrys, J.; Grabiec, S.; Shani, J. Anti-oxidant property of ethanolic extract of propolis (EEP) as evaluated by inhibiting the chemiluminescence oxidation of luminol. Biochem. Int. 1990, 21, 593–597. [Google Scholar] [PubMed]
- Sameni, H.R.; Ramhormozi, P.; Bandegi, A.R.; Taherian, A.A.; Mirmohammadkhani, M.; Safari, M. Effects of ethanol extract of propolis on histopathological changes and anti-oxidant defense of kidney in a rat model for type 1 diabetes mellitus. J. Diabetes Investig. 2016, 7, 506–513. [Google Scholar] [CrossRef]
- Khacha-ananda, S.; Tragoolpua, K.; Chantawannakul, P.; Tragoolpua, Y. Antioxidant and anti-cancer cell proliferation activity of propolis extracts from two extraction methods. Asian Pac. J. Cancer Prev. 2013, 14, 6991–6995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, H.; Nguyen, B.C.Q.; Uto, Y.; Shahinozzaman, M.; Tawata, S.; Maruta, H. 1,2,3-Triazolyl esterization of PAK1-blocking propolis ingredients, artepillin C (ARC) and caffeic acid (CA), for boosting their anti-cancer/anti-PAK1 activities along with cell-permeability. Drug Discov. 2017, 11, 104–109. [Google Scholar] [CrossRef] [Green Version]
- Bankova, V.S.; De Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Hegazi, A.G.; Abd El Hady, F.K.; Abd Allah, F.A.M. Chemical Composition and Antimicrobial Activity of European Propolis. Z. Nat. C 2000, 55, 70–75. [Google Scholar] [CrossRef]
- Grecka, K.; Kuś, P.M.; Okińczyc, P.; Worobo, R.W.; Walkusz, J.; Szweda, P. The Anti-Staphylococcal Potential of Ethanolic Polish Propolis Extracts. Molecules 2019, 24, 1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, J.F.; Santos, U.P.D.; Rocha, P.D.S.D.; Damião, M.J.; Balestieri, J.B.P.; Cardoso, C.A.L.; Paredes-Gamero, E.J.; Estevinho, L.M.; De Picoli Souza, K.; Santos, E.L.D. Antimicrobial, Antioxidant, Anti-Inflammatory, and Cytotoxic Activities of Propolis from the Stingless BeeTetragonisca fiebrigi(Jataí). Evid. Based Complementary Altern. Med. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bhargava, P.; Mahanta, D.; Kaul, A.; Ishida, Y.; Terao, K.; Wadhwa, R.; Kaul, S.C. Experimental Evidence for Therapeutic Potentials of Propolis. Nutrients 2021, 13, 2528. [Google Scholar] [CrossRef]
- Nichitoi, M.M.; Josceanu, A.M.; Isopescu, R.D.; Isopencu, G.O.; Geana, E.-I.; Ciucure, C.T.; Lavric, V. Polyphenolics profile effects upon the antioxidant and antimicrobial activity of propolis extracts. Sci. Rep. 2021, 11. [Google Scholar] [CrossRef]
- Thaís Alberti, D.C.; Ana, V.; Roniele, I.; Leticia, M.; Marcelo, M.; Beatriz, V. Effect of Propolis Nanoparticles on Early-Stage Wound Healing in a Diabetic Noncontractile Wound Model. Nanotechnol. Adv. Mater. Sci. 2019, 2, 1–10. [Google Scholar] [CrossRef]
- Petar, D.; Petrov, G.G.; Valeria, G.; Boryana, T.; Vassya, B.; Christo, B.T. Development of propolis-loaded block copolymer micelles of superior structural stability and high loading capacity. Polymer 2017, 125, 102–109. [Google Scholar] [CrossRef]
- Mendez-Pfeiffer, P.; Juarez, J.; Hernández, J.; Taboada, P.; Virues, C.; Valencia, D.; Velazquez, C. Nanocarriers as drug delivery systems for propolis: A therapeutic approach. J. Drug Deliv. Sci. Technol. 2021, 102762. [Google Scholar] [CrossRef]
- McKenzie, M.; Betts, D.; Suh, A.; Bui, K.; Kim, L.D.; Cho, H. Hydrogel-Based Drug Delivery Systems for Poorly Water-Soluble Drugs. Molecules 2015, 20, 20397–20408. [Google Scholar] [CrossRef] [Green Version]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Ramsey, J.D.; Samadi, A.; Atoufi, Z.; Yazdi, M.K.; Ganjali, M.R.; Amirabad, L.M.; Zangene, E.; Farokhi, M.; Formela, K.; et al. Poloxamer: A versatile tri-block copolymer for biomedical applications. Acta Biomater. 2020, 110, 37–67. [Google Scholar] [CrossRef]
- Ban, E.; Park, M.; Jeong, S.; Kwon, T.; Kim, E.H.; Jung, K.; Kim, A. Poloxamer-Based Thermoreversible Gel for Topical Delivery of Emodin: Influence of P407 and P188 on Solubility of Emodin and Its Application in Cellular Activity Screening. Molecules 2017, 22, 246. [Google Scholar] [CrossRef] [Green Version]
- Leung, B.; Dharmaratne, P.; Yan, W.; Chan, B.C.L.; Lau, C.B.S.; Fung, K.P.; Ip, M.; Leung, S.S.Y. Development of thermosensitive hydrogel containing methylene blue for topical antimicrobial photodynamic therapy. J. Photochem. Photobiol. B 2020, 203, 111776. [Google Scholar] [CrossRef]
- Yanai, R.; Ueda, K.; Nishida, T.; Toyohara, M.; Mori, O. Effects of ionic and surfactant agents on the antimicrobial activity of polyhexamethylene biguanide. Eye Contact Lens 2011, 37, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Nnamani, P.; Kenechukwu, F.; Anugwolu, C.; Attama, A. Evaluation of Hydrogels Based on Poloxamer 407 and Polyacrylic Acids for Enhanced Topical Activity of Gentamicin against Susceptible Infections. Trop. J. Pharm. Res. 2014, 13, 1385. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, M.; Holden, B.; Taylor, M.; Wilson, J.; Coburn, J.; Hilton, B.; Nance, T.; Gubler, S.; Genberg, C.; Deng, S.; et al. Antibacterial and Antifungal Activities of Poloxamer Micelles Containing Ceragenin CSA-131 on Ciliated Tissues. Molecules 2018, 23, 596. [Google Scholar] [CrossRef] [Green Version]
- Dantas Silva, R.P.; Machado, B.A.; Barreto, G.A.; Costa, S.S.; Andrade, L.N.; Amaral, R.G.; Carvalho, A.A.; Padilha, F.F.; Barbosa, J.D.; Umsza-Guez, M.A. Antioxidant, antimicrobial, antiparasitic, and cytotoxic properties of various Brazilian propolis extracts. PLoS ONE 2017, 12, e0172585. [Google Scholar] [CrossRef]
- Garedew, A.; Schmolz, E.; Lamprecht, I. Microbiological and calorimetric investigations on the antimicrobial actions of different propolis extracts: An in vitro approach. Thermochim. Acta 2004, 422, 115–124. [Google Scholar] [CrossRef]
- Park, Y.K.; Paredes-Guzman, J.F.; Aguiar, C.L.; Alencar, S.M.; Fujiwara, F.Y. Chemical Constituents inBaccharis dracunculifoliaas the Main Botanical Origin of Southeastern Brazilian Propolis. J. Agric. Food Chem. 2004, 52, 1100–1103. [Google Scholar] [CrossRef]
- Jeong, S.; Jeong, S.; Chung, S.; Kim, A. Revisiting in vitro release test for topical gel formulations: The effect of osmotic pressure explored for better bio-relevance. Eur. J. Pharm. Sci. 2018, 112, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Lozina, L.A.; Peichoto, M.E.; Boehringer, S.I.; Koscinczuk, P.; Granero, G.E.; Acosta, O.C. Efficacy of Argentine propolis formulation for topical treatment of canine otitis externa. Arq. Bras. Med. Vet. E Zootec. 2010, 62, 1359–1366. [Google Scholar] [CrossRef]
- Berretta, A.A.; De Castro, P.A.; Cavalheiro, A.H.; Fortes, V.S.; Bom, V.P.; Nascimento, A.P.; Marquele-Oliveira, F.; Pedrazzi, V.; Ramalho, L.N.Z.; Goldman, G.H. Evaluation of Mucoadhesive Gels with Propolis (EPP-AF) in Preclinical Treatment of Candidiasis Vulvovaginal Infection. Evid. Based Complementary Altern. Med. 2013, 2013, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Srichana, C.C.T. Efficiency of sildenafil encapsulation in poloxamer micelles. J. Disper. Sci. Technol. 2019, 40, 1461–1468. [Google Scholar] [CrossRef]
- Desai, P.R.; J, N.J.; Sharma, R.K.; Bahadur, P. Effect of additives on the micellization of PEO/PPO/PEO block copolymer F127 in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2001, 178, 57–69. [Google Scholar] [CrossRef]
- Wilke, C.R.; Chang, P. Correlation of diffusion coefficients in dilute solutions. AIChE J. 1955, 1, 264–270. [Google Scholar] [CrossRef]
- Hayes, S. Remington: The Science and Practice of Pharmacy, volume I and volume II. Twenty-second edition. J. Med. Libr. Assoc. JMLA 2014, 102, 220–221. [Google Scholar] [CrossRef] [Green Version]
- Popova, M.; Trusheva, B.; Antonova, D.; Cutajar, S.; Mifsud, D.; Farrugia, C.; Tsvetkova, I.; Najdenski, H.; Bankova, V. The specific chemical profile of Mediterranean propolis from Malta. Food Chem. 2011, 126, 1431–1435. [Google Scholar] [CrossRef] [Green Version]
- Prytzyk, E.; Dantas, A.P.; Salomao, K.; Pereira, A.S.; Bankova, V.S.; De Castro, S.L.; Neto, F.R. Flavonoids and trypanocidal activity of Bulgarian propolis. J. Ethnopharmacol. 2003, 88, 189–193. [Google Scholar] [CrossRef]
- Machado, B.A.S.; Silva, R.P.D.; Barreto, G.D.A.; Costa, S.S.; Silva, D.F.D.; Brandão, H.N.; Rocha, J.L.C.D.; Dellagostin, O.A.; Henriques, J.A.P.; Umsza-Guez, M.A.; et al. Chemical Composition and Biological Activity of Extracts Obtained by Supercritical Extraction and Ethanolic Extraction of Brown, Green and Red Propolis Derived from Different Geographic Regions in Brazil. PLoS ONE 2016, 11, e0145954. [Google Scholar] [CrossRef] [PubMed]
- Kolayli, S.; Palabiyik, I.; Atik, D.S.; Keskin, M.; Bozdeveci, A.; Karaoglu, S.-H.A. Comparison of Antibacterial and Antifungal Effects of Different Varieties of Honey and Propolis Samples. Acta Aliment. 2020, 49, 515–523. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Alsherbiny, M.A.; Low, M.N.; Zhou, X.; Kaur, K.; Li, G.; Li, C.G. Broad-spectrum pharmacological activity of Australian propolis and metabolomic-driven identification of marker metabolites of propolis samples from three continents. Food Funct. 2021, 12, 2498–2519. [Google Scholar] [CrossRef]








| Composition No. | P188 (wt%) | P407 (wt%) | Carbopol (wt%) | Propolis Sol. (wt%) | DW (wt%) |
|---|---|---|---|---|---|
| C1 | 5 | 0 | 0 | 66.6 (10 as propolis propylene glycol-extract) | 28.4 |
| C2 | 10 | 0 | 0 | 23.4 | |
| C3 | 20 | 0 | 0 | 13.4 | |
| C4 | 30 | 0 | 0 | 3.4 | |
| C5 | 0 | 5 | 0 | 28.4 | |
| C6 | 0 | 10 | 0 | 23.4 | |
| C2 + Carbopol | 10 | 0 | 0.3 | 23.1 |
| Formulation No. | P188 (wt%) | P407 (wt%) | Carbopol (wt%) | Propolis Sol. (wt%) | DW (wt%) |
|---|---|---|---|---|---|
| F1 | 5 | 0 | 0 | 33.3 (10 as propolis extract) | 61.7 |
| F2 | 10 | 0 | 0 | 56.7 | |
| F3 | 20 | 0 | 0 | 46.7 | |
| F4 | 30 | 0 | 0 | 36.7 | |
| F5 | 0 | 5 | 0 | 61.7 | |
| F6 | 0 | 10 | 0 | 56.7 | |
| F2 + Carbopol | 10 | 0 | 0.3 | 46.4 |
| Dilution Factor | P188 10% | P407 10% | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No Propolis Extract | 10% Propolis Extract | No Propolis Extract | 10% Propolis Extract | ||||||
| Z-Aver | PDI | Z-Aver | PDI | Z-Aver | PDI | Z-Aver | PDI | ||
| Poloxamer sol. | 1 | 5.45 ± 0.21 | 0.25 ± 0.01 | N/A | 15.17 ± 0.26 | 0.42 ± 0.01 | N/A | ||
| 10 | 70.09 ± 50.87 | 0.25 ± 0.02 | 24.58 ± 0.50 | 0.06 ± 0.01 | |||||
| 40 | 402.83 ± 109.78 | 0.40 ± 0.06 | 25.75 ± 0.21 | 0.13 ± 0.00 | |||||
| Poloxamer sol. + PG | 1 | 8.87 ± 0.21 | 0.31 ± 0.01 | N.D. | 21.40 ± 0.17 | 0.46 ± 0.00 | N.D. | ||
| 10 | 6.52 ± 0.34 | 0.29 ± 0.03 | 42.36 ± 0.45 | 0.39 ± 0.00 | 25.12 ± 0.25 | 0.04 ± 0.01 | 29.92 ± 0.43 | 0.08 ± 0.01 | |
| 40 | 1392.67 ± 219.61 | 0.26 ± 0.10 | 33.70 ± 0.72 | 0.23 ± 0.00 | 26.08 ± 0.17 | 0.12 ± 0.01 | 28.35 ± 0.24 | 0.07 ± 0.01 | |
| Poloxamer sol. + EtOH | 1 | 6.96 ± 0.30 | 0.20 ± 0.03 | Precipitated | 12.54 ± 0.46 | 0.38 ± 0.02 | N.D. | ||
| 10 | 6.93 ± 0.20 | 0.27 ± 0.01 | 24.72 ± 0.66 | 0.06 ± 0.01 | 28.86 ± 0.52 | 0.05 ± 0.00 | |||
| 40 | 212.90 ± 0.26 | 17.16 ± 0.01 | 26.35 ± 0.71 | 0.13 ± 0.03 | 29.10 ± 0.84 | 0.06 ± 0.00 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, S.-H.; Ban, E.; Chung, I.-Y.; Cho, Y.-H.; Kim, A. Antimicrobial Activities of Propolis in Poloxamer Based Topical Gels. Pharmaceutics 2021, 13, 2021. https://doi.org/10.3390/pharmaceutics13122021
An S-H, Ban E, Chung I-Y, Cho Y-H, Kim A. Antimicrobial Activities of Propolis in Poloxamer Based Topical Gels. Pharmaceutics. 2021; 13(12):2021. https://doi.org/10.3390/pharmaceutics13122021
Chicago/Turabian StyleAn, Seong-Hyeon, Eunmi Ban, In-Young Chung, You-Hee Cho, and Aeri Kim. 2021. "Antimicrobial Activities of Propolis in Poloxamer Based Topical Gels" Pharmaceutics 13, no. 12: 2021. https://doi.org/10.3390/pharmaceutics13122021
APA StyleAn, S.-H., Ban, E., Chung, I.-Y., Cho, Y.-H., & Kim, A. (2021). Antimicrobial Activities of Propolis in Poloxamer Based Topical Gels. Pharmaceutics, 13(12), 2021. https://doi.org/10.3390/pharmaceutics13122021






