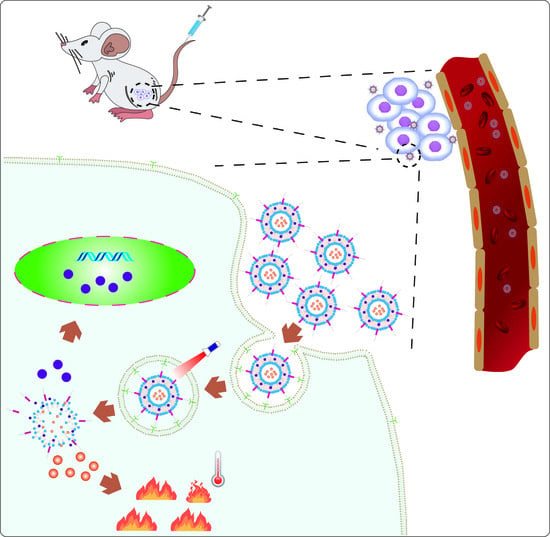
Design and Application of Near-Infrared Nanomaterial-Liposome Hybrid Nanocarriers for Cancer Photothermal Therapy
Abstract
1. Introduction
2. Types of NIRN-Lips
2.1. Liposomes Loading Carbon-Based Nanomaterials
2.2. Liposomes Loading Gold-Based Nanomaterials
2.3. Liposomes Loading Semiconductor Quantum Dots
3. Synthesis of NIRN-Lips
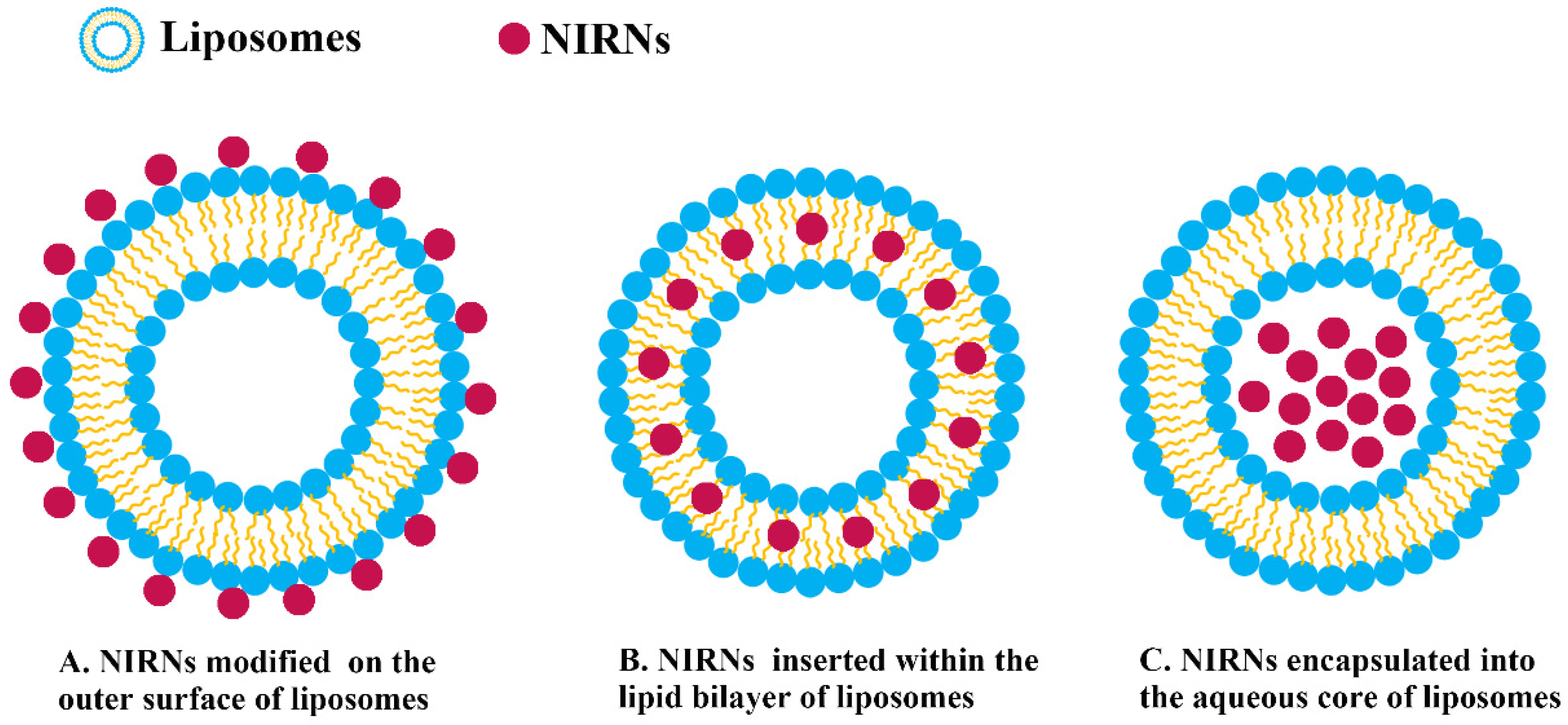
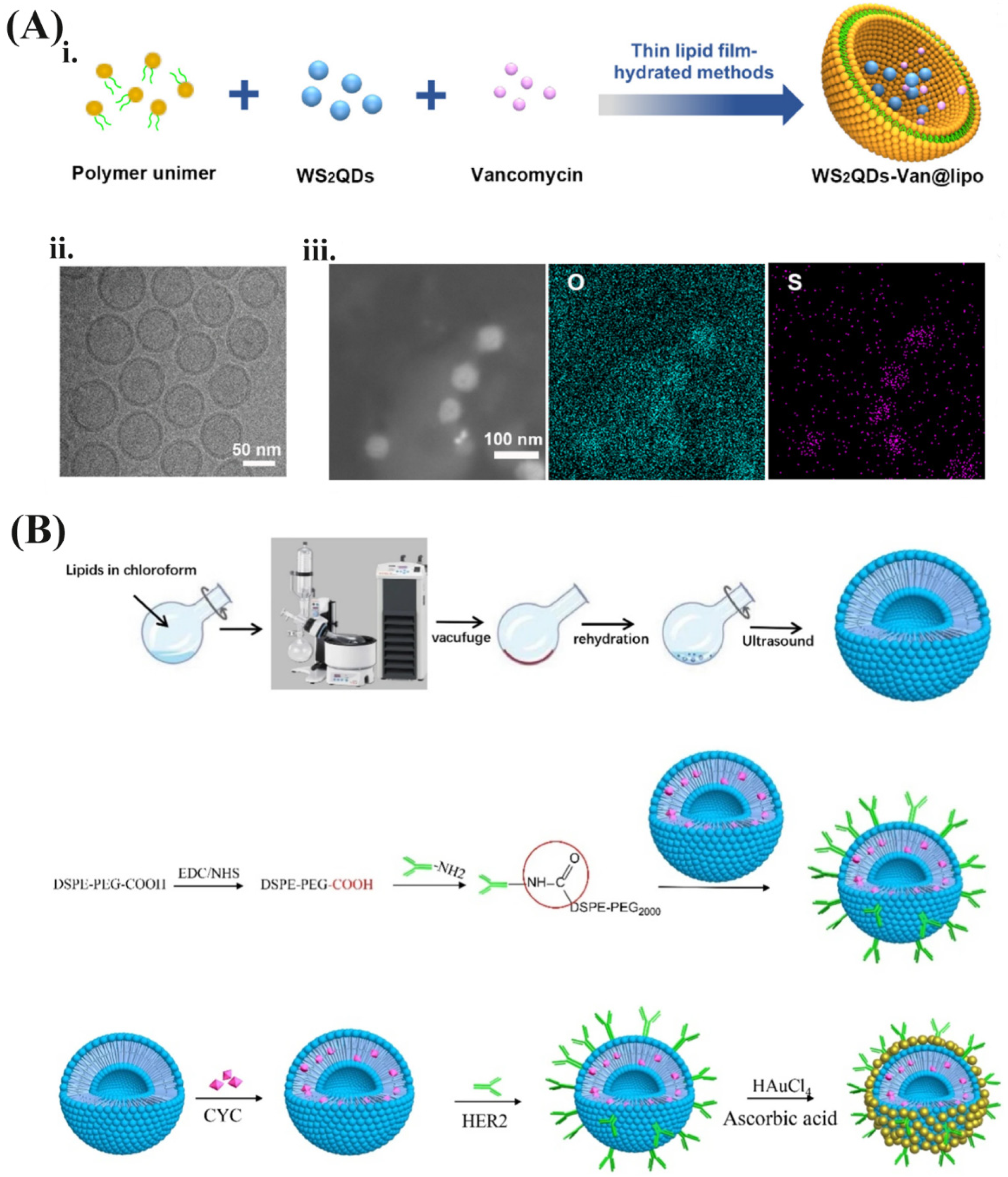
3.1. Preparation Method and Encapsulation Strategy of NIRN-Lips
3.2. Design Consideration and Surface Modification of NIRN-Lips
4. NIR Light-Triggered Drug Release Mechanism
5. Application
5.1. Cell Death Mechanism Induced by PTT
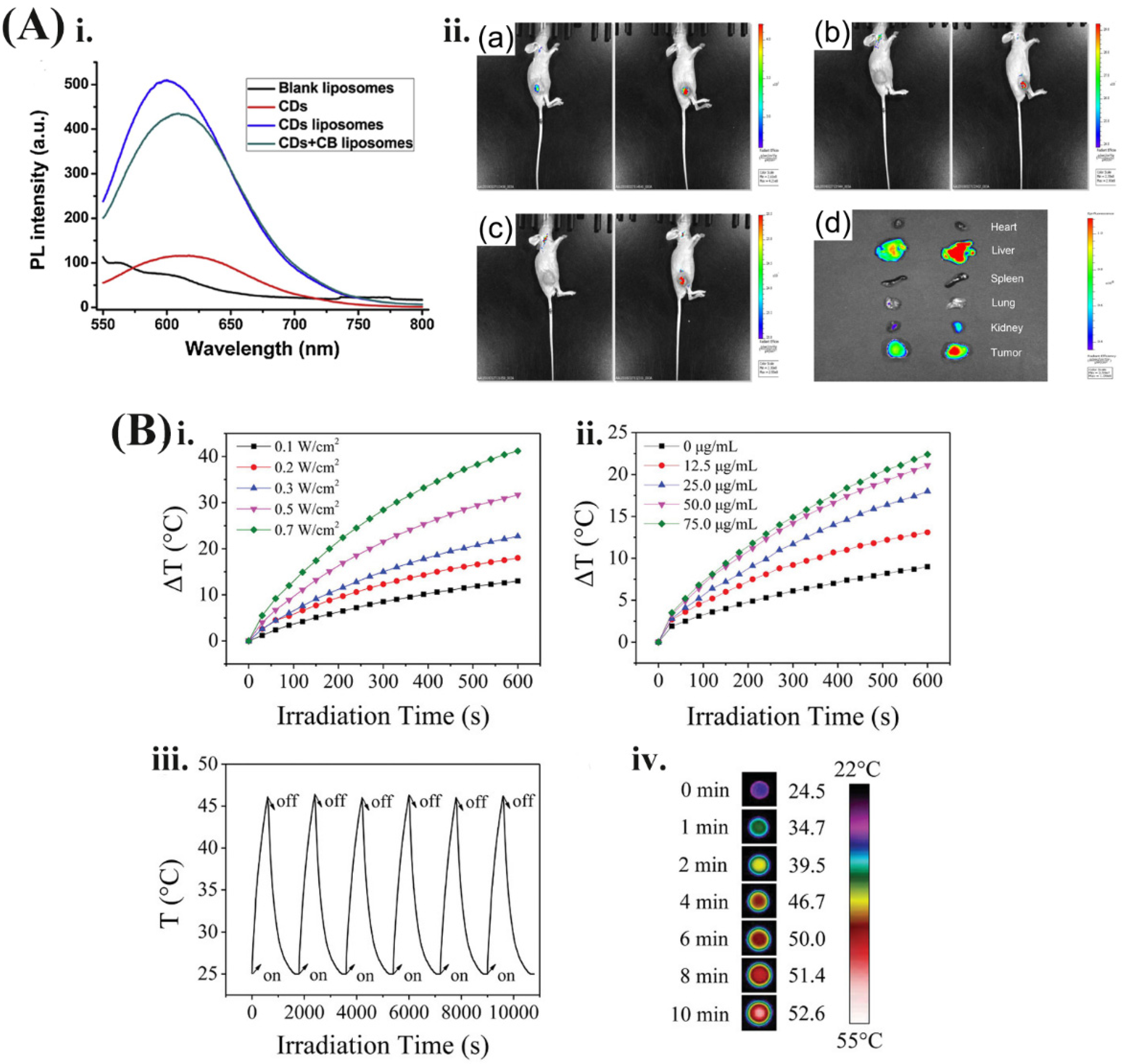
5.2. NIRN-Lips Mediated Cancer PTT Treatment
6. Challenges and Futures of NIRN-Lips
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Akamatsu, H.; Ninomiya, K.; Kenmotsu, H.; Morise, M.; Daga, H.; Goto, Y.; Kozuki, T.; Miura, S.; Sasaki, T.; Tamiya, A.; et al. The Japanese Lung Cancer Society Guideline for non-small cell lung cancer, stage IV. Int. J. Clin. Oncol. 2019, 24, 731–770. [Google Scholar] [CrossRef]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef]
- Thakur, V.; Kutty, R.V. Recent advances in nanotheranostics for triple negative breast cancer treatment. J. Exp. Clin. Cancer Res. 2019, 38, 430. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Pang, L.; Cong, H.; Shen, Y.; Yu, B. Application and design of esterase-responsive nanoparticles for cancer therapy. Drug Deliv. 2019, 26, 416–432. [Google Scholar] [CrossRef] [PubMed]
- Goyal, P.; Goyal, K.; Vijaya Kumar, S.G.; Singh, A.; Katare, O.P.; Mishra, D.N. Liposomal drug delivery systems-clinical applications. Acta Pharm 2005, 55, 1–25. [Google Scholar]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Mazur, F.; Bally, M.; Stadler, B.; Chandrawati, R. Liposomes and lipid bilayers in biosensors. Adv. Colloid Interface Sci. 2017, 249, 88–99. [Google Scholar] [CrossRef]
- Theresa, A.; Allen, M.; Pieter, B.; Cullis, R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar]
- Luo, L.; Bian, Y.; Liu, Y.; Zhang, X.; Wang, M.; Xing, S.; Li, L.; Gao, D. Combined Near Infrared Photothermal Therapy and Chemotherapy Using Gold Nanoshells Coated Liposomes to Enhance Antitumor Effect. Small 2016, 12, 4103–4112. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Gao, C.; Cui, L.; Wang, S.; Wang, J.; Dai, Z. Self-Assembly of an Amphiphilic Janus Camptothecin-Floxuridine Conjugate into Liposome-Like Nanocapsules for More Efficacious Combination Chemotherapy in Cancer. Adv. Mater. 2017, 29, 1703135. [Google Scholar] [CrossRef]
- Mathiyazhakan, M.; Wiraja, C.; Xu, C. A Concise Review of Gold Nanoparticles-Based Photo-Responsive Liposomes for Controlled Drug Delivery. Nanomicro Lett. 2018, 10, 85–94. [Google Scholar] [CrossRef]
- Zhao, L.P.; Xiong, H.; Peng, H.; Wang, Q.; Dan, H.; Bai, C.Q.; Liu, Y.Z.; Shi, S.H.; Deng, B. PEG-coated lyophilized proliposomes: Preparation, characterizations and in vitro release evaluation of vitamin E. Eur. Food Res. Technol. 2011, 232, 647–654. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Zhang, L.; Song, H.; Zhang, L. Targeted delivery system based on magnetic mesoporous silica nanocomposites with light-controlled release character. ACS Appl. Mater. Interfaces 2013, 5, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Chan, H.F.; Shi, B.; Li, M.; Leong, K.W. Light: A Magical Tool for Controlled Drug Delivery. Adv. Funct. Mater. 2020, 30, 2005029. [Google Scholar] [CrossRef]
- Bouchaala, R.; Anton, N.; Anton, H.; Vandamme, T.; Vermot, J.; Smail, D.; Mély, Y.; Klymchenko, A.S. Light-triggered release from dye-loaded fluorescent lipid nanocarriers in vitro and in vivo. J Colloids Surf. B Biointerfaces 2017, 156, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Yi, G.; Yoo, J.; Park, C.; Koo, H.; Choi, H.S. Light-responsive nanomedicine for biophotonic imaging and targeted therapy. Adv. Drug Deliv. Rev. 2019, 138, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Li, J.; Liu, F.; Yeow, E.K.L.; Xing, B. Near-Infrared Light-Mediated Photoactivation of a Platinum Antitumor Prodrug and Simultaneous Cellular Apoptosis Imaging by Upconversion-Luminescent Nanoparticles. Angew. Chem. 2013, 126, 1030–1034. [Google Scholar] [CrossRef]
- Karimi, M.; Zangabad, P.S.; Baghaee-Ravari, S.; Ghazadeh, M.; Mirshekari, H.; Hamblin, M.R. Smart nanostructures for cargo delivery: Uncaging and activating by light. J. Am. Chem. Soc. 2017, 139, 4584–4610. [Google Scholar] [CrossRef]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef]
- Dariva, C.G.; Coelho, J.F.J.; Serra, A.C. Near infrared light-triggered nanoparticles using singlet oxygen photocleavage for drug delivery systems. J. Control Release 2019, 294, 337–354. [Google Scholar] [CrossRef]
- Vankayala, R.; Hwang, K.C. Near-Infrared-Light-Activatable Nanomaterial-Mediated Phototheranostic Nanomedicines: An Emerging Paradigm for Cancer Treatment. Adv. Mater. 2018, 30, e1706320. [Google Scholar] [CrossRef] [PubMed]
- Jaque, D.; Maestro, L.M.; Rosal, B.D.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Rodríguez, E.M. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef]
- Zhang, P.; Hu, C.; Ran, W.; Meng, J.; Yin, Q.; Li, Y. Recent Progress in Light-Triggered Nanotheranostics for Cancer Treatment. Theranostics 2016, 6, 948–968. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Xu, P.; Liang, F. Nanomaterial-Based Tumor Photothermal Immunotherapy. Int. J. Nanomed. 2020, 15, 9159–9180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, X.; Wang, X.; Guan, X.; Zhang, W.; Ma, J. Recent advances in selective photothermal therapy of tumor. J. Nanobiotechnol. 2021, 19, 335. [Google Scholar] [CrossRef]
- Lv, S.; Miao, Y.; Liu, D.; Song, F. Recent Development of Photothermal Agents (PTAs) Based on Small Organic Molecular Dyes. ChemBioChem 2020, 21, 2098–2110. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Meng, H.M.; Li, Z. Near-infrared inorganic nanomaterial-based nanosystems for photothermal therapy. Nanoscale 2021, 13, 8751–8772. [Google Scholar] [CrossRef]
- Lee, E.H.; Lee, M.K.; Lim, S.J. Enhanced Stability of Indocyanine Green by Encapsulation in Zein-Phosphatidylcholine Hybrid Nanoparticles for Use in the Phototherapy of Cancer. Pharmaceutics 2021, 13, 305. [Google Scholar] [CrossRef]
- Huang, T.Y.; Huang, G.L.; Zhang, C.Y.; Zhuang, B.W.; Liu, B.X.; Su, L.Y.; Ye, J.Y.; Xu, M.; Kuang, M.; Xie, X.Y. Supramolecular Photothermal Nanomedicine Mediated Distant Tumor Inhibition via PD-1 and TIM-3 Blockage. Front. Chem. 2020, 8, 1. [Google Scholar] [CrossRef]
- Cheung, C.C.L.; Ma, G.; Karatasos, K.; Seitsonen, J.; Ruokolainen, J.; Koffi, C.-R.; Hassan, H.A.F.M.; Al-Jamal, W.T. Liposome-Templated Indocyanine Green J- Aggregates for In Vivo Near-Infrared Imaging and Stable Photothermal Heating. Nanotheranostics 2020, 4, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Giulia, D.P.; Andrea, D.L.; Marina, S.F.; Nina, K. Cyanine Dyes for Photo-Thermal Therapy: A Comparison of Synthetic Liposomes and Natural Erythrocyte-Based Carriers. Int. J. Mol. Sci. 2021, 22, 6914. [Google Scholar]
- Ling, C.; Wang, X.; Shen, Y. Advances in Hollow Inorganic Nanomedicines for Photothermal-Based Therapies. Int. J. Nanomed. 2021, 16, 493–513. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chao, Y.M. Multifunctional quantum dots and liposome complexes in drug delivery. J. Biomed. Res. 2018, 32, 91–106. [Google Scholar]
- Li, Y.; Song, W.; Hu, Y.; Xia, Y.; Li, Z.; Lu, Y.; Shen, Y. Petal-like size-tunable gold wrapped immunoliposome to enhance tumor deep penetration for multimodal guided two-step strategy. J. Nanobiotechnol. 2021, 19, 293. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, T.; Cao, Y.; Sun, J.; Zhou, Q.; Chen, H.; Guo, S.; Wang, Y.; Zhen, Y.; Liang, X.J.; et al. Temperature-Sensitive Lipid-Coated Carbon Nanotubes for Synergistic Photothermal Therapy and Gene Therapy. ACS Nano 2021, 15, 6517–6529. [Google Scholar] [CrossRef]
- Jia, X.; Xu, W.; Ye, Z.; Wang, Y.; Dong, Q.; Wang, E.; Li, D.; Wang, J. Functionalized Graphene@Gold Nanostar/Lipid for Pancreatic Cancer Gene and Photothermal Synergistic Therapy under Photoacoustic/Photothermal Imaging Dual-Modal Guidance. Small 2020, 16, e2003707. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Su, Y.L.; Hu, S.H.; Chen, S.Y. Functionalized graphene nanocomposites for enhancing photothermal therapy in tumor treatment. Adv. Drug Deliv. Rev. 2016, 105, 190–204. [Google Scholar] [CrossRef]
- Markovic, Z.M.; Harhaji-Trajkovic, L.M.; Todorovic-Markovic, B.M.; Kepic, D.P.; Arsikin, K.M.; Jovanovic, S.P.; Pantovic, A.C.; Dramicanin, M.D.; Trajkovic, V.S. In vitro comparison of the photothermal anticancer activity of graphene nanoparticles and carbon nanotubes. Biomaterials 2011, 32, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Hoseini-Ghahfarokhi, M.; Mirkiani, S.; Mozaffari, N.; Amin, M.; Karimi, M. Applications of Graphene and Graphene Oxide in Smart Drug/Gene Delivery: Is the World Still Flat? Int. J. Nanomed. 2020, 15, 9469–9496. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, L.; Raffa, S.; Prato, M.; Bianco, A.; Kostarelos, K. Cell-penetrating CNTs for delivery of therapeutics. Nanotoday 2007, 2, 38–43. [Google Scholar] [CrossRef]
- Fanarraga, M.L.; Villegas, J.C.; Fernandez-Luna, J.L.; Gonzalez, J.; Valiente, R.; Garcia-Hevia, L. Anti-Cancer Cytotoxic Effects of Multiwalled Carbon Nanotubes. J. Curr. Pharm. Des. 2015, 21, 1920–1929. [Google Scholar]
- Shen, S.; Ren, J.; Chen, J.; Lu, X.; Deng, C.; Jiang, X. Development of magnetic multiwalled carbon nanotubes combined with near-infrared radiation-assisted desorption for the determination of tissue distribution of doxorubicin liposome injects in rats. J. Chromatogr. A 2011, 1218, 4619–4626. [Google Scholar] [CrossRef]
- Liang, C.; Diao, S.; Wang, C.; Gong, H.; Liu, T.; Hong, G.; Shi, X.; Dai, H.; Liu, Z. Tumor metastasis inhibition by imaging-guided photothermal therapy with single-walled carbon nanotubes. Adv. Mater. 2014, 26, 5646–5652. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.R.; Ghosn, E.E.; Rallapalli, H.; Prescher, J.A.; Larson, T.; Herzenberg, L.A.; Gambhir, S.S. Selective uptake of single-walled carbon nanotubes by circulating monocytes for enhanced tumour delivery. Nat. Nanotechnol. 2014, 9, 481–487. [Google Scholar] [CrossRef]
- Montané, X.; Bajek, A.; Roszkowski, K.; Montornés, J.M.; Giamberini, M.; Roszkowski, S.; Kowalczyk, O.; Garcia-Valls, R.; Tylkowski, B. Encapsulation for Cancer Therapy. Molecules 2020, 25, 1605. [Google Scholar] [CrossRef]
- Karousis, N.; Suarez-Martinez, I.; Ewels, C.P.; Tagmatarchis, N. Structure, Properties, Functionalization, and Applications of Carbon Nanohorns. Chem. Rev. 2016, 116, 4850–4883. [Google Scholar] [CrossRef]
- Yang, J.; Su, H.; Sun, W.; Cai, J.; Liu, S.; Chai, Y.; Zhang, C. Dual Chemodrug-Loaded Single-Walled Carbon Nanohorns for Multimodal Imaging-Guided Chemo-Photothermal Therapy of Tumors and Lung Metastases. Theranostics 2018, 8, 1966–1984. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Dong, P.; Lin, Z.; Guo, X.; Jiang, B.P.; Ji, S.; Liang, H.; Shen, X.C. Near-Infrared Light Responsive Imaging-Guided Photothermal and Photodynamic Synergistic Therapy Nanoplatform Based on Carbon Nanohorns for Efficient Cancer Treatment. Chemistry 2018, 24, 12827–12837. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, C.; Jiang, F.; Liu, Z.; Shu, C.; Wan, L.J. In vitro and in vivo photothermally enhanced chemotherapy by single-walled carbon nanohorns as a drug delivery system. J. Mater. Chem. B 2014, 2, 4726–4732. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hou, M.; Sun, W.; Wu, Q.; Xu, J.; Xiong, L.; Chai, Y.; Liu, Y.; Yu, M.; Wang, H.; et al. Sequential PDT and PTT Using Dual-Modal Single-Walled Carbon Nanohorns Synergistically Promote Systemic Immune Responses against Tumor Metastasis and Relapse. Adv. Sci. 2020, 7, 2001088. [Google Scholar] [CrossRef]
- Stergiou, A.; Tagmatarchis, N. Functionalized Carbon Nanohorns as Drug Delivery Platforms. Methods Mol. Biol. 2021, 2207, 13–24. [Google Scholar] [PubMed]
- Kumari, S.; Sharma, N.; Sahi, S.V. Advances in Cancer Therapeutics: Conventional Thermal Therapy to Nanotechnology-Based Photothermal Therapy. Pharmaceutics 2021, 13, 1174. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Zhao, Y.; Hou, Y.; Shan, G.; Yan, D.; Liu, Y. Glycosylated liposomes loading carbon dots for targeted recognition to HepG2 cells. Talanta 2018, 182, 314–323. [Google Scholar] [CrossRef]
- Wang, F.; Hao, Q.; Zhang, Y.; Xu, Y.; Lei, W. Fluorescence quenchometric method for determination of ferric ion using boron-doped carbon dots. Microchim. Acta 2016, 183, 273–279. [Google Scholar] [CrossRef]
- Hashemi, M.; Omidi, M.; Muralidharan, B.; Tayebi, L.; Herpin, M.J.; Mohagheghi, M.A.; Mohammadi, J.; Smyth, H.D.C.; Milner, T.E. Layer-by-layer assembly of graphene oxide on thermosensitive liposomes for photo-chemotherapy. Acta Biomater. 2018, 65, 376–392. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Chen, S.; Liao, Y.; Li, S.; Ge, J.; Tao, F.; Huo, Q.; Zhang, Y.; Zhao, Z. Near-infrared fluorescent carbon dots encapsulated liposomes as multifunctional nano-carrier and tracer of the anticancer agent cinobufagin in vivo and in vitro. Colloids Surf. B Biointerfaces 2019, 174, 384–392. [Google Scholar] [CrossRef]
- Li, J.; Wu, X.; Peng, Q.; Wang, C.X.; Yang, F. Liposome Coated Mesoporous Carbon Nanotube with Resveratrol Loading for Targeted and Near-infrared Laser-triggered Chemo/Photothermal Synergistic Cancer Therapy. Acta Laser Biol. Sin. 2020, 29, 550–560. [Google Scholar]
- Yan, S.; Zeng, X.; Tang, Y.; Liu, B.F.; Wang, Y.; Liu, X. Activating Antitumor Immunity and Antimetastatic Effect Through Polydopamine-Encapsulated Core-Shell Upconversion Nanoparticles. Adv. Mater. 2019, 31, e1905825. [Google Scholar] [CrossRef]
- Xuan, Y.; Yang, M.; Bo, P.; Vara, M.; Xia, Y. Gold nanomaterials at work in biomedicine. Chem. Rev. 2015, 115, 10410–10488. [Google Scholar]
- Wei, W.; Zhang, X.; Zhang, S.; Wei, G.; Su, Z. Biomedical and bioactive engineered nanomaterials for targeted tumor photothermal therapy: A review. J. Mater. Sci. Eng. C 2019, 104, 109891. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.Y.; Zhang, J.W.; Li, R.F.; Wang, Z.X.; Wang, W.J.; Wang, W. Unique Roles of Gold Nanoparticles in Drug Delivery, Targeting and Imaging Applications. Molecules 2017, 22, 1445. [Google Scholar] [CrossRef]
- Kwon, H.J.; Byeon, Y.; Jeon, H.N.; Cho, S.H.; Han, H.D.; Shin, B.C. Gold cluster-labeled thermosensitive liposmes enhance triggered drug release in the tumor microenvironment by a photothermal effect. J. Control Release 2015, 216, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Sailor, M.J.; Park, J.H. Hybrid nanoparticles for detection and treatment of cancer. Adv. Mater. 2012, 24, 3779–3802. [Google Scholar] [CrossRef]
- Zhao, Y.; He, Z.; Wang, R.; Cai, P.; Zhang, X.; Yuan, Q.; Zhang, J.; Gao, F.; Gao, X. Comparison of the Therapeutic Effects of Gold Nanoclusters and Gold Nanoparticles on Rheumatoid Arthritis. J. Biomed. Nanotechnol. 2019, 15, 2281–2290. [Google Scholar] [CrossRef] [PubMed]
- Alvi, S.B.; Appidi, T.; Deepak, B.P.; Rajalakshmi, P.S.; Minhas, G.; Singh, S.P.; Begum, A.; Bantal, V.; Srivastava, R.; Khan, N.; et al. The “nano to micro” transition of hydrophobic curcumin crystals leading to in situ adjuvant depots for Au-liposome nanoparticle mediated enhanced photothermal therapy. Biomater. Sci. 2019, 7, 3866–3875. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, J.; Shan, G.; Yan, D.; Chen, Y.; Liu, Y. SERS-active liposome Ag/Au nanocomposite for NIR light-driven drug release. Colloids Surf. B Biointerfaces 2017, 154, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; Yuan, M.; Li, Z.; Hu, G.; Chen, J.; Cun, X.; Zhang, Q.; Yang, Y.; Qin, H.; Gao, H. Tumor microenvironment sensitive doxorubicin delivery and release to glioma using angiopep-2 decorated gold nanoparticles. Biomaterials 2015, 37, 425–435. [Google Scholar] [CrossRef]
- Wang, W.; Wang, W.; Liu, L.; Xu, L.; Kuang, H.; Zhu, J.; Xu, C. Nanoshell-Enhanced Raman Spectroscopy on a Microplate for Staphylococcal Enterotoxin B Sensing. ACS Appl. Mater. Interfaces 2016, 8, 15591–15597. [Google Scholar] [CrossRef] [PubMed]
- Al-Jamal, W.T.; Kostarelos, K. Liposomes: From a Clinically Established Drug Delivery System to a Nanoparticle Platform for Theranostic Nanomedicine. Acc. Chem. Res. 2011, 44, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Zuo, W.; Li, N.; Hou, Y.; Song, Z.; Gou, G.; Yang, J. Design of multifunctional liposome-quantum dot hybrid nanocarriers and their biomedical application. J. Drug Target 2017, 25, 661–672. [Google Scholar] [CrossRef]
- Yang, C.; Ding, N.; Xu, Y.; Qu, X.; Zhang, J.; Zhao, C.; Hong, L.; Lu, Y.; Xiang, G. Folate receptor-targeted quantum dot liposomes as fluorescence probes. J. Drug Target 2009, 17, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Wlodek, M.; Kolasinska-Sojka, M.; Szuwarzynski, M.; Kereiche, S.; Kovacik, L.; Zhou, L.; Islas, L.; Warszynski, P.; Briscoe, W.H. Supported lipid bilayers with encapsulated quantum dots (QDs) via liposome fusion: Effect of QD size on bilayer formation and structure. Nanoscale 2018, 10, 17965–17974. [Google Scholar] [CrossRef] [PubMed]
- Ortega, G.A.; Del Sol-Fernandez, S.; Portilla, Y.; Cedeno, E.; Reguera, E.; Srinivasan, S.; Barber, D.F.; Marin, E.; Rajabzadeh, A.R. Rodlike Particles of Polydopamine-CdTe Quantum Dots: An Actuator As a Photothermal Agent and Reactive Oxygen Species-Generating Nanoplatform for Cancer Therapy. ACS Appl. Mater. Interfaces 2021, 13, 42357–42369. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Mei, Y.; Zhao, Q.; Zhang, A.; Tang, L.; Gao, H.; Wang, W. Black Phosphorus, an Emerging Versatile Nanoplatform for Cancer Immunotherapy. Pharmaceutics 2021, 13, 1344. [Google Scholar] [CrossRef]
- Mcelroy, N.; Page, R.C.; Espinbarro-Valazquez, D.; Lewis, E.; Haigh, S.; O’Brien, P.; Binks, D.J. Comparison of solar cells sensitised by CdTe/CdSe and CdSe/CdTe core/shell colloidal quantum dots with and without a CdS outer layer. Thin Solid Films 2014, 560, 65–70. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, F.; Yang, P.; Chen, B.; Aguilar, Z.P.; Fu, F.; Xu, H. Effects of QDs exposure on the reproductive and embryonic developmental toxicity in mice at various pregnancy stages. Toxicol. Res. 2020, 9, 371–378. [Google Scholar] [CrossRef]
- Karakoti, A.S.; Shukla, R.; Shanker, R.; Singh, S. Surface functionalization of quantum dots for biological applications. Adv. Colloid Interface Sci. 2015, 215, 28–45. [Google Scholar] [CrossRef]
- Wegner, K.D.; Hildebrandt, N. Quantum dots: Bright and versatile in vitro and in vivo fluorescence imaging biosensors. Chem. Soc. Rev. 2015, 44, 4792–4834. [Google Scholar] [CrossRef]
- Xu, H.L.; Yang, J.J.; Zhu-Ge, D.L.; Lin, M.T.; Zhu, Q.Y.; Jin, B.H.; Tong, M.Q.; Shen, B.X.; Xiao, J.; Zhao, Y.Z. Glioma-Targeted Delivery of a Theranostic Liposome Integrated with Quantum Dots, Superparamagnetic Iron Oxide, and Cilengitide for Dual-Imaging Guiding Cancer Surgery. Adv. Healthc. Mater. 2018, 7, e1701130. [Google Scholar] [CrossRef] [PubMed]
- Tahara, K.; Fujimoto, S.; Fujii, F.; Tozuka, Y.; Jin, T.; Takeuchi, H. Quantum Dot-Loaded Liposomes to Evaluate the Behavior of Drug Carriers after Oral Administration. J. Pharm. 2013, 2013, 848275. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Critchley, K.; Matsunaga, T.; Evans, S.D.; Staniland, S.S. Fabrication of lipid tubules with embedded quantum dots by membrane tubulation protein. Small 2012, 8, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Mao, K.L.; Tian, F.R.; Yang, J.J.; Chen, P.P.; Xu, J.; Fan, Z.L.; Zhao, Y.P.; Li, W.F.; Zheng, L.; et al. Brain tumor-targeted delivery and therapy by focused ultrasound introduced doxorubicin-loaded cationic liposomes. Cancer Chemother. Pharmacol. 2016, 77, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Samadikhah, H.R.; Nikkhah, M.; Hosseinkhani, S. Enhancement of cell internalization and photostability of red and green emitter quantum dots upon entrapment in novel cationic nanoliposomes. Luminescence 2017, 32, 517–528. [Google Scholar] [CrossRef]
- Souza, S.O.; Lira, R.B.; Cunha, C.R.A.; Santos, B.S.; Fontes, A.; Pereira, G. Methods for Intracellular Delivery of Quantum Dots. Top. Curr. Chem. 2021, 379, 1. [Google Scholar] [CrossRef]
- Aizik, G.; Waiskopf, N.; Agbaria, M.; Levi-Kalisman, Y.; Banin, U.; Golomb, G. Delivery of Liposomal Quantum Dots via Monocytes for Imaging of Inflamed Tissue. ACS Nano 2017, 11, 3038–3051. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xin, J.; Zhang, L.; Zhou, Y.; Yao, C.; Wang, B.; Wang, J.; Zhang, Z. Cantharidin-encapsulated thermal-sensitive liposomes coated with gold nanoparticles for enhanced photothermal therapy on A431 cells. Int. J. Nanomed. 2018, 13, 2143–2160. [Google Scholar] [CrossRef]
- Sonkar, R.; Jha, A.; Viswanadh, M.K.; Burande, A.S.; Pawde, D.M.; Patel, K.K.; Singh, M.; Koch, B.; Muthu, M.S. Gold liposomes for brain-targeted drug delivery: Formulation and brain distribution kinetics. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111652. [Google Scholar] [CrossRef]
- Tao, Y.; Li, M.; Kim, B.; Auguste, D.T. Incorporating gold nanoclusters and target-directed liposomes as a synergistic amplified colorimetric sensor for HER2-positive breast cancer cell detection. Theranostics 2017, 7, 899–911. [Google Scholar] [CrossRef]
- Chen, T.H.; Chang, H.T. Stable and Photoswitchable Carbon-Dot Liposome. ACS Appl. Mater. Interfaces 2017, 9, 44259–44263. [Google Scholar] [CrossRef]
- Miyako, E.; Kono, K.; Yuba, E.; Hosokawa, C.; Nagai, H.; Hagihara, Y. Carbon nanotube-liposome supramolecular nanotrains for intelligent molecular-transport systems. Nat. Commun. 2012, 3, 1226. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cong, H.; Wang, S.; Yu, B.; Shen, Y. Liposomes modified with bio-substances for cancer treatment. Biomater. Sci. 2020, 8, 6442–6468. [Google Scholar] [CrossRef]
- Pippa, N.; Stangel, C.; Kastanas, I.; Triantafyllopoulou, E.; Naziris, N.; Stellas, D.; Zhang, M.; Yudasaka, M.; Demetzos, C.; Tagmatarchis, N. Carbon nanohorn/liposome systems: Preformulation, design and in vitro toxicity studies. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110114. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Hu, Y.; Xiao, Y.; Zhang, Y.; Sun, K.; Wu, T.; Lv, N.; Wang, W.; Ding, W.; Li, F.; et al. Near-Infrared-Controlled Nanoplatform Exploiting Photothermal Promotion of Peroxidase-like and OXD-like Activities for Potent Antibacterial and Anti-biofilm Therapies. ACS Appl. Mater. Interfaces 2020, 12, 50260–50274. [Google Scholar] [CrossRef]
- Wen, C.J.; Zhang, L.W.; Al-Suwayeh, S.A.; Yen, T.C.; Fang, J.Y. Theranostic liposomes loaded with quantum dots and apomorphine for brain targeting and bioimaging. Int. J. Nanomed. 2012, 7, 1599–1611. [Google Scholar]
- Bao, Q.Y.; Zhang, N.; Geng, D.D.; Xue, J.W.; Merritt, M.; Zhang, C.; Ding, Y. The enhanced longevity and liver targetability of Paclitaxel by hybrid liposomes encapsulating Paclitaxel-conjugated gold nanoparticles. Int. J. Pharm. 2014, 477, 408–415. [Google Scholar] [CrossRef]
- Lozano, N.; Al-Jamal, W.T.; Taruttis, A.; Beziere, N.; Burton, N.C.; Van den Bossche, J.; Mazza, M.; Herzog, E.; Ntziachristos, V.; Kostarelos, K. Liposome-gold nanorod hybrids for high-resolution visualization deep in tissues. J. Am. Chem. Soc. 2012, 134, 13256–13258. [Google Scholar] [CrossRef] [PubMed]
- Wiraja, C.; Mathiyazhakan, M.; Movahedi, F.; Upputuri, P.K.; Cheng, Y.; Pramanik, M.; Yang, L.; Becker, D.L.; Xu, C. Near-infrared light-sensitive liposomes for enhanced plasmid DNA transfection. Bioeng. Transl. Med. 2016, 1, 357–364. [Google Scholar] [CrossRef]
- Zhu, H.; Han, W.; Gan, Y.; Li, Q.; Li, X.; Shao, L.; Zhu, D.; Guo, H. Combined Modality Therapy Based on Hybrid Gold Nanostars Coated with Temperature Sensitive Liposomes to Overcome Paclitaxel-Resistance in Hepatic Carcinoma. Pharmaceutics 2019, 11, 683. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Zhang, X.; Luo, L.; Cao, W.; Li, L.; He, Y.; An, J.; Gao, D. Doxorubicin/gold nanoparticles coated with liposomes for chemo-photothermal synergetic antitumor therapy. Nanotechnology 2018, 29, 405101. [Google Scholar] [CrossRef]
- Lee, J.; Shin, Y.; Lee, W.; Whang, K.; Kim, D.; Lee, L.; Choi, J.; Kang, T. General and programmable synthesis of hybrid liposome/metal nanoparticles. Sci. Adv. 2016, 2, e1601838. [Google Scholar] [CrossRef]
- Momper, R.; Steinbrecher, J.; Dorn, M.; Rorich, I.; Bretschneider, S.; Tonigold, M.; Ramanan, C.; Ritz, S.; Mailander, V.; Landfester, K.; et al. Enhanced photoluminescence properties of a carbon dot system through surface interaction with polymeric nanoparticles. J. Colloid Interface Sci. 2018, 518, 11–20. [Google Scholar] [CrossRef]
- Liu, C.W.; Lin, T.N.; Chang, L.Y.; Jiang, Z.C.; Shen, J.L.; Chen, P.W.; Wang, J.S.; Yuan, C.T. Carbon Nanodots with Sub-Nanosecond Spontaneous Emission Lifetime. Chemphyschem 2017, 18, 42–46. [Google Scholar] [CrossRef]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; Bahadur, D.; Srivastava, R. Protein-Poly (amino acid) Nanocore-Shell Mediated Synthesis of Branched Gold Nanostructures for Computed Tomographic Imaging and Photothermal Therapy of Cancer. ACS Appl. Mater. Interfaces 2016, 8, 15889–15903. [Google Scholar] [CrossRef]
- Wang, M.; Liu, Y.; Zhang, X.; Luo, L.; Li, L.; Xing, S.; He, Y.; Cao, W.; Zhu, R.; Gao, D. Gold nanoshell coated thermo-pH dual responsive liposomes for resveratrol delivery and chemo-photothermal synergistic cancer therapy. J. Mater. Chem. B 2017, 5, 2161–2171. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Chen, D.; Meng, X.; Liu, T.; Fu, C.; Hao, N.; Zhang, Y.; Wu, X.; Ren, J. Multifunctional Fe3O4@P(St/MAA)@Chitosan@Au Core/Shell Nanoparticles for Dual Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2013, 5, 4966–4971. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Alvi, S.B.; Pemmaraju, D.B.; Singh, A.D.; Manda, S.V.; Srivastava, R.; Rengan, A.K. NIR triggered liposome gold nanoparticles entrapping curcumin as in situ adjuvant for photothermal treatment of skin cancer. Int. J. Biol. Macromol. 2018, 110, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Chauhan, D.S.; Yadav, A.S.; Devrukhkar, J.; Singh, B.; Gorain, M.; Temgire, M.; Bellare, J.; Kundu, G.C.; Srivastava, R. Biodegradable fluorescent nanohybrid for photo-driven tumor diagnosis and tumor growth inhibition. Nanoscale 2018, 10, 19082–19091. [Google Scholar] [CrossRef]
- Wang, C.J.; Wang, H.Z.; Li, W. A novel conjunction of folate-targeted carbon nanotubes containing protohemin and oridonin-liposome loaded microbubbles for cancer chemo-sonodynamic therapy. J. Drug Target 2019, 27, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Roy, E.; Madhuri, R.; Sharma, P.K. The next generation cell-penetrating peptide and carbon dot conjugated nano-liposome for transdermal delivery of curcumin. Biomater. Sci. 2016, 4, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.W.; Jeong, H.Y.; Kang, S.J.; Jeong, I.H.; Choi, M.J.; You, Y.M.; Im, C.S.; Song, I.H.; Lee, T.S.; Lee, J.S.; et al. Anti-EGF Receptor Aptamer-Guided Co-Delivery of Anti-Cancer siRNAs and Quantum Dots for Theranostics of Triple-Negative Breast Cancer. Theranostics 2019, 9, 837–852. [Google Scholar] [CrossRef]
- Ye, C.; Wang, Y.; Li, C.; Yu, J.; Hu, Y. Preparation of liposomes loaded with quantum dots, fluorescence resonance energy transfer studies, and near-infrared in-vivo imaging of mouse tissue. Microchim. Acta 2012, 180, 117–125. [Google Scholar] [CrossRef]
- Shao, D.; Li, J.; Pan, Y.; Zhang, X.; Zheng, X.; Wang, Z.; Zhang, M.; Zhang, H.; Chen, L. Noninvasive theranostic imaging of HSV-TK/GCV suicide gene therapy in liver cancer by folate-targeted quantum dot-based liposomes. Biomater. Sci. 2015, 3, 833–841. [Google Scholar] [CrossRef]
- Geng, S.; Wu, L.; Cui, H.; Tan, W.; Chen, T.; Chu, P.K.; Yu, X.F. Synthesis of lipid-black phosphorus quantum dot bilayer vesicles for near-infrared-controlled drug release. Chem. Commun. 2018, 54, 6060–6063. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Mohammadi, J.; Omidi, M.; Smyth, H.D.C.; Muralidharan, B.; Milner, T.E.; Yadegari, A.; Ahmadvand, D.; Shalbaf, M.; Tayebi, L. Self-assembling of graphene oxide on carbon quantum dot loaded liposomes. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109860. [Google Scholar] [CrossRef]
- Xue, X.; Fang, T.; Yin, L.; Jiang, J.; He, Y.; Dai, Y.; Wang, D. Multistage delivery of CDs-DOX/ICG-loaded liposome for highly penetration and effective chemo-photothermal combination therapy. Drug Deliv. 2018, 25, 1826–1839. [Google Scholar] [CrossRef]
- Chen, W.; Li, J.; Xing, Y.; Wang, X.; Zhang, H.; Xia, M.; Wang, D. Dual-pH Sensitive Charge-Reversal Drug Delivery System for Highly Precise and Penetrative Chemotherapy. Pharm. Res. 2020, 37, 134. [Google Scholar] [CrossRef]
- Wo, F.; Xu, R.; Shao, Y.; Zhang, Z.; Chu, M.; Shi, D.; Liu, S. A Multimodal System with Synergistic Effects of Magneto-Mechanical, Photothermal, Photodynamic and Chemo Therapies of Cancer in Graphene-Quantum Dot-Coated Hollow Magnetic Nanospheres. Theranostics 2016, 6, 485–500. [Google Scholar] [CrossRef]
- Tajvar, S.; Mohammadi, S.; Askari, A.; Janfaza, S.; Nikkhah, M.; Tamjid, E.; Hosseinkhani, S. Preparation of liposomal doxorubicin-graphene nanosheet and evaluation of its in vitro anti-cancer effects. J. Liposome Res. 2019, 29, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Jain, N.K.; Yadav, A.S.; Chauhan, D.S.; Devrukhkar, J.; Kumawat, M.K.; Shinde, S.; Gorain, M.; Thakor, A.S.; Kundu, G.C.; et al. Liposomal nanotheranostics for multimode targeted in vivo bioimaging and near-infrared light mediated cancer therapy. Commun. Biol. 2020, 3, 284. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, M.; Niu, M.; Zhao, Y.; Zhu, Y.; Li, Z.; Feng, N. Delivery of vincristine sulfate-conjugated gold nanoparticles using liposomes: A light-responsive nanocarrier with enhanced antitumor efficiency. Int. J. Nanomed. 2015, 10, 3081–3095. [Google Scholar]
- Hamzawy, M.A.; Abo-Youssef, A.M.; Salem, H.F.; Mohammed, S.A. Antitumor activity of intratracheal inhalation of temozolomide (TMZ) loaded into gold nanoparticles and/or liposomes against urethane-induced lung cancer in BALB/c mice. Drug Deliv. 2017, 24, 599–607. [Google Scholar] [CrossRef]
- Li, Y.; He, D.; Tu, J.; Wang, R.; Zu, C.; Chen, Y.; Yang, W.; Shi, D.; Webster, T.J.; Shen, Y. Comparative effect of wrapping solid gold nanoparticles and hollow gold nanoparticles with doxorubicin-loaded thermosensitive liposomes for cancer thermo-chemotherapy. Nanoscale 2018, 10, 8628–8641. [Google Scholar] [CrossRef]
- Gu, X.; Shen, C.; Li, H.; Goldys, E.M.; Deng, W. X-ray induced photodynamic therapy (PDT) with a mitochondria-targeted liposome delivery system. J. Nanobiotechnol. 2020, 18, 87. [Google Scholar] [CrossRef]
- Hua, H.; Zhang, N.; Liu, D.; Song, L.; Liu, T.; Li, S.; Zhao, Y. Multifunctional gold nanorods and docetaxel-encapsulated liposomes for combined thermo- and chemotherapy. Int. J. Nanomed. 2017, 12, 7869–7884. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, H.; Chen, M.; Chen, G.; Zou, W.; Zhao, Y.; Zhao, Q. Comprehensive Effects of Near-Infrared Multifunctional Liposomes on Cancer Cells. Molecules 2020, 25, 1098. [Google Scholar] [CrossRef]
- Bruun, K.; Hille, C. Study on intracellular delivery of liposome encapsulated quantum dots using advanced fluorescence microscopy. Sci. Rep. 2019, 9, 10504. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, H.; Liu, A.Y.; Shen, J.J.; Shah, V.; Zhang, C.; Hong, J.; Ding, Y. Gold conjugate-based liposomes with hybrid cluster bomb structure for liver cancer therapy. Biomaterials 2016, 74, 280–291. [Google Scholar] [CrossRef]
- Mady, M.M.; Fathy, M.M.; Youssef, T.; Khalil, W.M. Biophysical characterization of gold nanoparticles-loaded liposomes. Phys. Med. 2012, 28, 288–295. [Google Scholar] [CrossRef]
- You, J.; Zhang, P.; Hu, F.; Du, Y.; Yuan, H.; Zhu, J.; Wang, Z.; Zhou, J.; Li, C. Near-infrared light-sensitive liposomes for the enhanced photothermal tumor treatment by the combination with chemotherapy. Pharm. Res. 2014, 31, 554–565. [Google Scholar] [CrossRef]
- Mathiyazhakan, M.; Yang, Y.; Liu, Y.; Zhu, C.; Liu, Q.; Ohl, C.D.; Tam, K.C.; Gao, Y.; Xu, C. Non-invasive controlled release from gold nanoparticle integrated photo-responsive liposomes through pulse laser induced microbubble cavitation. Colloids Surf. B Biointerfaces 2015, 126, 569–574. [Google Scholar] [CrossRef]
- Aizik, G.; Waiskopf, N.; Agbaria, M.; Ben-David-Naim, M.; Levi-Kalisman, Y.; Shahar, A.; Banin, U.; Golomb, G. Liposomes of Quantum Dots Configured for Passive and Active Delivery to Tumor Tissue. Nano Lett. 2019, 19, 5844–5852. [Google Scholar] [CrossRef]
- Mukthavaram, R.; Wrasidlo, W.; Hall, D.; Kesari, S.; Makale, M. Assembly and targeting of liposomal nanoparticles encapsulating quantum dots. Bioconjug. Chem. 2011, 22, 1638–1644. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rengan, A.K.; Jagtap, M.; De, A.; Banerjee, R.; Srivastava, R. Multifunctional gold coated thermo-sensitive liposomes for multimodal imaging and photo-thermal therapy of breast cancer cells. Nanoscale 2014, 6, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.; Kumari, A.; Srivastava, R.; Panda, D. Quercetin Encapsulated Biodegradable Plasmonic Nanoparticles for Photothermal Therapy of Hepatocellular Carcinoma Cells. ACS Appl. Bio Mater. 2019, 2, 5727–5738. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Liu, Z.; Wang, L.; Luo, L.; Wang, M.; Wang, Q.; Gao, D. Gold nanoshell-based betulinic acid liposomes for synergistic chemo-photothermal therapy. Nanomedicine 2017, 13, 1891–1900. [Google Scholar] [CrossRef]
- Rodrigues, A.R.O.; Matos, J.O.G.; Nova Dias, A.M.; Almeida, B.G.; Pires, A.; Pereira, A.M.; Araujo, J.P.; Queiroz, M.R.P.; Castanheira, E.M.S.; Coutinho, P.J.G. Development of Multifunctional Liposomes Containing Magnetic/Plasmonic MnFe2O4/Au Core/Shell Nanoparticles. Pharmaceutics 2018, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Zappacosta, R.; Di Giulio, M.; Ettorre, V.; Bosco, D.; Hadad, C.; Siani, G.; Di Bartolomeo, S.; Cataldi, A.; Cellini, L.; Fontana, A. Liposome-induced exfoliation of graphite to few-layer graphene dispersion with antibacterial activity. J. Mater. Chem. B 2015, 3, 6520–6527. [Google Scholar] [CrossRef]
- Hai, L.; He, D.; He, X.; Wang, K.; Yang, X.; Liu, J.; Cheng, H.; Huang, X.; Shangguan, J. Facile fabrication of a resveratrol loaded phospholipid@reduced graphene oxide nanoassembly for targeted and near-infrared laser-triggered chemo/photothermal synergistic therapy of cancer in vivo. J. Mater. Chem. B 2017, 5, 5783–5792. [Google Scholar] [CrossRef]
- Liang, R.; Xie, J.; Li, J.; Wang, K.; Liu, L.; Gao, Y.; Hussain, M.; Shen, G.; Zhu, J.; Tao, J. Liposomes-coated gold nanocages with antigens and adjuvants targeted delivery to dendritic cells for enhancing antitumor immune response. Biomaterials 2017, 149, 41–50. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Liang, X.; Mei, L.; Wu, X. In vitro study of black phosphorus quantum dot-loaded liposomes for photothermal therapy of cervical cancer. Acta Pharm. Sin. 2019, 54, 729–736. [Google Scholar]
- Amstad, E.; Kohlbrecher, J.; Müller, E.; Schweizer, T.; Reimhult, E. Triggered Release from Liposomes through Magnetic Actuation of Iron Oxide Nanoparticle Containing Membranes. Nano Lett. 2011, 11, 1664–1670. [Google Scholar] [CrossRef]
- Mauro, N.; Utzeri, M.A.; Varvara, P.; Cavallaro, G. Functionalization of Metal and Carbon Nanoparticles with Potential in Cancer Theranostics. Molecules 2021, 26, 3085. [Google Scholar] [CrossRef]
- Garrell, R.L. Surface-Enhanced Raman Spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601. [Google Scholar] [CrossRef]
- Lyon, L.A.; Peña, D.J.; Natan, M.J. Surface Plasmon Resonance of Au Colloid-Modified Au Films: Particle Size Dependence. J. Phys. Chem. B 1999, 103, 5826–5831. [Google Scholar] [CrossRef]
- Faried, M.; Suga, K.; Okamoto, Y.; Shameli, K.; Miyake, M.; Umakoshi, H. Membrane Surface-Enhanced Raman Spectroscopy for Cholesterol-Modified Lipid Systems: Effect of Gold Nanoparticle Size. ACS Omega 2019, 4, 13687–13695. [Google Scholar] [CrossRef]
- Wi, H.S.; Lee, K.; Pak, H.K. Interfacial energy consideration in the organization of a quantum dot–lipid mixed system. J. Phys. Condens. Matter 2008, 20, 494211. [Google Scholar]
- Ginzburg, V.; Balijepalli, S. Modeling the thermodynamics of the interaction of nanoparticles with cell membranes. Nano Lett. 2007, 7, 3716–3722. [Google Scholar] [CrossRef] [PubMed]
- Quadros, M.; Momin, M.; Verma, G. Design strategies and evolving role of biomaterial assisted treatment of osteosarcoma. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111875. [Google Scholar] [CrossRef] [PubMed]
- Geng, B.; Qin, H.; Shen, W.; Li, P.; Fang, F.; Li, X.; Pan, D.; Shen, L. Carbon dot/WS2 heterojunctions for NIR-II enhanced photothermal therapy of osteosarcoma and bone regeneration. Chem. Eng. J. 2020, 383, 123102. [Google Scholar] [CrossRef]
- Park, J.H.; Maltzahn, G.V.; Xu, M.J.; Fogal, V.; Kotamraju, V.R.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Cooperative nanomaterial system to sensitize, target, and treat tumors. Proc. Natl. Acad. Sci. USA 2010, 107, 981–986. [Google Scholar] [CrossRef]
- Chauhan, V.P.; Jain, R.K. Strategies for advancing cancer nanomedicine. Nat. Mater. 2013, 12, 958–962. [Google Scholar] [CrossRef]
- Chatterjee, H.; Rahman, D.S.; Sengupta, M.; Gosh, S.K. Gold Nanostars in Plasmonic Photothermal Therapy: The Role of Tip Heads in the Thermoplasmonic Landscape. J. Phys. Chem. C 2018, 122, 13082–13094. [Google Scholar] [CrossRef]
- Pu, Y.; Zhao, Y.; Zheng, P.; Li, M. Elucidating the growth mechanism of plasmonic gold nanostars with tunable optical and photothermal properties. Inorg. Chem. 2018, 57, 8599–8607. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Thai, D.; Schwartzberg, A.M.; Zhang, J.Z. Key Factors Affecting the Reproducibility of Synthesis and Growth Mechanism of Near-Infrared Absorbing Hollow Gold Nanospheres. Chem. Mater. 2014, 26, 6805–6810. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, L.; Wang, X.; Hu, Z. From Carbon-Based Nanotubes to Nanocages for Advanced Energy Conversion and Storage. Acc. Chem. Res. 2017, 50, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Niyomtham, N.; Apiratikul, N.; Suksen, K.; Opanasopit, P.; Yingyongnarongkul, B.E. Synthesis and in vitro transfection efficiency of spermine-based cationic lipids with different central core structures and lipophilic tails. Bioorg. Med. Chem. Lett. 2015, 25, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Al-Jamal, W.T.; Al-Jamal, K.T.; Tian, B.; Cakebread, A.; Halket, J.M.; Kostarelos, K. Tumor Targeting of Functionalized Quantum Dot?Liposome Hybrids by Intravenous Administration. Mol. Pharm. 2009, 6, 520–530. [Google Scholar] [CrossRef]
- Al-Jamal, W.T.; Al-Jamal, K.T.; Tian, B.; Lacerda, L.; Bomans, P.H.; Frederik, P.M.; Kostarelos, K. Lipid-quantum dot bilayer vesicles enhance tumor cell uptake and retention in vitro and in vivo. ACS Nano 2008, 2, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Sambamoorthy, U.; Venkataraju, P.; Manjappa, S.; Bhanoji, M.E. Gemcitabine-loaded Folic Acid Tagged Liposomes: Improved Pharmacokinetic and Biodistribution Profile. Curr. Drug Deliv. 2018, 16, 111–122. [Google Scholar]
- Sheng, W.; Dong, Z.; Jiao, N.; Wang, H.; Zhang, L. Development of an efficient transdermal drug delivery system with TAT-conjugated cationic polymeric lipid vesicles. J. Mater. Chem. B 2014, 2, 877–884. [Google Scholar]
- Gan, C.W.; Feng, S.S. Transferrin-conjugated nanoparticles of poly(lactide)-D-alpha-tocopheryl polyethylene glycol succinate diblock copolymer for targeted drug delivery across the blood-brain barrier. Biomaterials 2010, 31, 7748–7757. [Google Scholar] [CrossRef]
- Sawant, R.R.; Jhaveri, A.M.; Koshkaryev, A.; Zhu, L.; Qureshi, F.; Torchilin, V.P. Targeted transferrin-modified polymeric micelles: Enhanced efficacy in vitro and in vivo in ovarian carcinoma. Mol. Pharm. 2014, 11, 375–381. [Google Scholar] [CrossRef]
- Muthu, M.S.; Kutty, R.V.; Luo, Z.; Xie, J.; Feng, S.S. Theranostic vitamin E TPGS micelles of transferrin conjugation for targeted co-delivery of docetaxel and ultra bright gold nanoclusters. Biomaterials 2015, 39, 234–248. [Google Scholar] [CrossRef]
- Valeria, S.; Donna, J.; Arndt, J.; Thomas, M.J. Targeted Cellular Delivery of Quantum Dots Loaded on and in Biotinylated__Liposomes. Bioconjug. Chem. 2010, 21, 1465–1472. [Google Scholar]
- Yuan, Z.; Das, S.; Lazenby, R.A.; White, R.J.; Park, Y.C. Repetitive drug releases from light-activatable micron-sized liposomes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126778. [Google Scholar] [CrossRef]
- Fomina, N.; Sankaranarayanan, J.; Almutairi, A. Photochemical mechanisms of light-triggered release from nanocarriers. Adv. Drug Deliv. Rev. 2012, 64, 1005–1020. [Google Scholar] [CrossRef]
- Xu, D.; Li, Z.; Li, L.; Wang, J. Insights into the Photothermal Conversion of 2D MXene Nanomaterials: Synthesis, Mechanism, and Applications. Adv. Funct. Mater. 2020, 30, 2000712. [Google Scholar] [CrossRef]
- Kim, M.; Lin, M.; Son, J.; Xu, H.; Nam, J.M. Hot-Electron-Mediated Photochemical Reactions: Principles, Recent Advances, and Challenges. Adv. Opt. Mater. 2017, 5, 1700004. [Google Scholar] [CrossRef]
- Brongersma, M.L.; Halas, N.J.; Nordlander, P.J.N.N. Plasmon-induced hot carrier science and technology. Nat. Nanotechnol. 2015, 10, 25–34. [Google Scholar] [CrossRef]
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1449. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Deng, L.; Wei, N.; Weng, Y.; Dong, S.; Qi, D.; Qiu, J.; Chen, X.; Wu, T. High-Performance Photothermal Conversion of Narrow-Bandgap Ti2O3 Nanoparticles. Adv. Mater. 2017, 29, 1603730. [Google Scholar] [CrossRef]
- Zou, Q.; Abbas, M.; Zhao, L.; Li, S.; Shen, G.; Yan, X. Biological Photothermal Nanodots Based on Self-Assembly of Peptide-Porphyrin Conjugates for Antitumor Therapy. J. Am. Chem. Soc. 2017, 139, 1921–1927. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Goldys, E.M.; Deng, W. Light-induced liposomes for cancer therapeutics. Prog. Lipid Res. 2020, 79, 101052. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Li, G.G.; Zhou, F.; Wu, R.; Zhu, J.J.; Wang, H. Gold-Nanosponge-Based Multistimuli-Responsive Drug Vehicles for Targeted Chemo-Photothermal Therapy. Adv. Mater. 2016, 28, 8218–8226. [Google Scholar] [CrossRef]
- An, X.; Zhan, F.; Zhu, Y. Smart photothermal-triggered bilayer phase transition in AuNPs-liposomes to release drug. Langmuir 2013, 29, 1061–1068. [Google Scholar] [CrossRef]
- Natassa, P.; Demetrios, D.C.; Dimitris, S.; Rodrigo, F.; Raul, A.; Costas, D.; Nikos, T. Design and development of multi-walled carbon nanotube-liposome drug delivery platforms. Int. J. Pharm. 2017, 528, 429–439. [Google Scholar]
- Ayala-Orozc, C.; Urban, C.; Knight, M.W.; Urban, A.S.; Neumann, O.; Bishnoi, S.W.; Mukherjee, S. Au Nanomatryoshkas as Efficient Near-Infrared Photothermal Transducers for Cancer Treatment Benchmarking against Nanoshells. ACS Nano 2014, 8, 6372–6381. [Google Scholar] [CrossRef]
- Minai, L.; Zeidan, A.; Yeheskely-Hayon, D.; Yudovich, S.; Kviatkovsky, I.; Yelin, D. Experimental Proof for the Role of Nonlinear Photoionization in Plasmonic Phototherapy. Nano Lett. 2016, 16, 4601–4607. [Google Scholar] [CrossRef]
- Beere, H.M. The stress of dying: The role of heat shock proteins in the regulation of apoptosis. J. Cell Sci. 2004, 117, 2641–2652. [Google Scholar] [CrossRef]
- Liao, S.; Yue, W.; Cai, S.; Tang, Q.; Lu, W.; Huang, L.; Qi, T.; Liao, J. Improvement of Gold Nanorods in Photothermal Therapy: Recent Progress and Perspective. Front. Pharmacol. 2021, 12, 664123. [Google Scholar] [CrossRef]
- Day, E.S.; Thompson, P.A.; Zhang, L.; Lewinski, N.A.; Ahmed, N.; Drezek, R.A.; Blaney, S.M.; West, J.L. Nanoshell-mediated photothermal therapy improves survival in a murine glioma model. J. Neurooncol. 2011, 104, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Jilian, R.M.; Rachel, S.E.; Emily, S.D. Elucidating the Fundamental Mechanisms of Cell Death Triggered by Photothermal Therapy. ACS Nano 2015, 9, 6–11. [Google Scholar]
- Zhang, W.; Ding, X.; Cheng, H.; Yin, C.; Yan, J.; Mou, Z.; Wang, W.; Cui, D.; Fan, C.; Sun, D. Dual-Targeted Gold Nanoprism for Recognition of Early Apoptosis, Dual-Model Imaging and Precise Cancer Photothermal Therapy. Theranostics 2019, 9, 5610–5625. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Liu, J.; Shi, J.L. Intranuclear Photosensitizer Delivery and Photosensitization for Enhanced Photodynamic Therapy with Ultralow Irradiance. Adv. Funct. Mater. 2014, 24, 7318–7327. [Google Scholar] [CrossRef]
- Ge, J.; Jia, Q.; Liu, W.; Guo, L.; Liu, Q.; Lan, M.; Zhang, H.; Meng, X.; Wang, P. Red-Emissive Carbon Dots for Fluorescent, Photoacoustic, and Thermal Theranostics in Living Mice. Adv. Mater. 2015, 27, 4169–4177. [Google Scholar] [CrossRef]
- Ali, M.R.K.; Rahman, M.A.; Wu, Y.; Han, T.; Peng, X.; Mackey, M.A.; Wang, D.; Shin, H.J.; Chen, Z.G.; Xiao, H.; et al. Efficacy, long-term toxicity, and mechanistic studies of gold nanorods photothermal therapy of cancer in xenograft mice. Proc. Natl. Acad. Sci. USA 2017, 114, E3110. [Google Scholar] [CrossRef]
- Dhumale, S.S.; Waghela, B.N.; Pathak, C. Quercetin protects necrotic insult and promotes apoptosis by attenuating the expression of RAGE and its ligand HMGB1 in human breast adenocarcinoma cells. IUBMB Life 2015, 67, 361–373. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Guo, G.; Wang, L.; Liu, D.; Wei, Z.; Zhou, J. Novel Cs-Based Upconversion Nanoparticles as Dual-Modal CT and UCL Imaging Agents for Chemo-Photothermal Synergistic Therapy. Theranostics 2016, 6, 1491–1505. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Chen, Y.; Wang, Y.; Li, H.; Han, H.; Chen, T.; Jin, Q.; Ji, J. pH- and NIR Light-Responsive Polymeric Prodrug Micelles for Hyperthermia-Assisted Site-Specific Chemotherapy to Reverse Drug Resistance in Cancer Treatment. Small 2016, 12, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Ca, H.; Tzeng, S.Y.; Young, N.P.; Abutaleb, A.O.; Quinones-Hinojosa, A.; Green, J.J. Biodegradable Polymeric Nanoparticles Show High Efficacy and Specificity at DNA Delivery to Human Glioblastoma in Vitro and in Vivo. ACS Nano 2014, 8, 5141–5153. [Google Scholar] [CrossRef]
- Setten, R.L.; Rossi, J.J.; Han, S.P. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 2019, 18, 421–446. [Google Scholar] [CrossRef]
- Lyu, Y.; Cui, D.; Sun, H.; Miao, Y.; Duan, H.; Pu, K. Dendronized Semiconducting Polymer as Photothermal Nanocarrier for Remote Activation of Gene Expression. Angew. Chem. Int. Ed. Engl. 2017, 56, 9155–9159. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.; Jeong, C.; Kim, W.J. Synergistic nanomedicine by combined gene and photothermal therapy. Adv. Drug Deliv. Rev. 2016, 98, 99–112. [Google Scholar] [CrossRef]
- Zhang, X.D.; Wu, D.; Shen, X.; Liu, P.X.; Fan, F.Y.; Fan, S.J. In vivo renal clearance, biodistribution, toxicity of gold nanoclusters. Biomaterials 2012, 33, 4628–4638. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Itty Ipe, B.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef]
- Hu, J.J.; Cheng, Y.J.; Zhang, X.Z. Recent advances in nanomaterials for enhanced photothermal therapy of tumors. Nanoscale 2018, 10, 22657–22672. [Google Scholar] [CrossRef]
- Zhu, H.; Cheng, P.; Chen, P.; Pu, K. Recent progress in the development of near-infrared organic photothermal and photodynamic nanotherapeutics. Biomater. Sci. 2018, 6, 746–765. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Tan, D.; Lou, C.; Guo, S.; Jin, X.; Qu, H.; Jing, L.; Li, S. A tumor-penetrable drug nanococktail made from human histones for interventional nucleus-targeted chemophotothermal therapy of drug-resistant tumors. Bioact. Mater 2021, 9, 554–565. [Google Scholar] [CrossRef] [PubMed]




| Preparation Method | Encapsulation Strategy | Type of NIRNs | Lipid Composition | Drug Load | Average Diameter (nm) | Zeta Potential (mV) | Ref. |
|---|---|---|---|---|---|---|---|
| Thin-film hydration | Within the lipid bilayer | CNHs | DMPC, DSPC, Chol | / | 80.0–100.0 | / | [94] |
| Within the lipid bilayer | CDs | DPHHP, DSHHP, Chol | CUR | 128.0 | 29.9 | [112] | |
| Within the lipid bilayer | AuNPs | SPC, Chol | PTX | 281.1 | 45.3 | [97] | |
| Within the lipid bilayer | QDs | EPC, Chol, DSPE-PEG2000 | CGT | 100.0 | −17.1 | [81] | |
| Within the lipid bilayer | QDs | PC, Chol, DSPE-PEG2000 | APO | 142.0 | 50.3 | [96] | |
| Within the lipid bilayer | QDs | Chol, DSPE-PEG2000 | siRNA | 171.7 | −2.7 | [113] | |
| within the lipid bilayer | QDs | PC, Chol, PEG-6000 | / | 270.0 | / | [114] | |
| Within the lipid bilayer | QDs | DPPC, DC-Chol, DSPE-PEG2000 | / | 89.7 | 20.1 | [115] | |
| Within the lipid bilayer | QDs | L-α-lysolecithin, Chol | DOX | 105.6 | 0.5 | [116] | |
| Within the lipid bilayer | GO, CDs | DPPC, Brij 78, Chol | DOX | 129.6 | −7.3 | [117] | |
| On the outer surface | AuNPs | SPC, Chol | DOX | 100.0 | −14.7 | [68] | |
| On the outer surface | AuNRs | DOTAP, DOPE, Chol | NIR-797 | 89.0 | 46.4 | [98] | |
| Encapsulated into the aqueous core | CDs | DSPE-mPEG2000, EPG, SPC, Chol | DOX | 87.4 | −12.9 | [118] | |
| Encapsulated into the aqueous core | CDs | SPC, Chol, cephalin | / | 80.0 | −15.4 | [55] | |
| Encapsulated into the aqueous core | CDs | DSPE-mPEG2000, DSPE-mPEG2000-FA, DOPE, DSPC, Chol | DOX | 108.9 | −31.4 | [119] | |
| Encapsulated into the aqueous core | CDs | DSPE-mPEG2000, DPPC, Chol | CB | 60.0–80.0 | −2.6 | [58] | |
| Encapsulated into the aqueous core | GO | SPC | DOX | 391.3 | / | [120] | |
| Encapsulated into the aqueous core | GNs | DPPC, Chol, DSPE-mPEG2000 | DOX | 141.0 | −1.3 | [121] | |
| Encapsulated into the aqueous core | AuNSs | P90G, Chol | calcein | 170.0 | −70 | [99] | |
| Encapsulated into the aqueous core | AuNSs | DPPC, MSPC, DSPE-PEG-SH, Chol | PTX | 293.9 | 2.5 | [100] | |
| Encapsulated into the aqueous core | GQDs, AuNPs | DSPC, Chol | DOX | 167.0 | 13.0 | [122] | |
| Encapsulated into the aqueous core | AuNPs | EYPC, DSPE-PEG2000 | VCR | 113.4 | −11.3 | [123] | |
| Encapsulated into the aqueous core | AuNPs | SPC, Chol | TMZ | 89.0 | −69 | [124] | |
| Encapsulated into the aqueous core | AuNPs | DSPE-PEG2000, DPPC | DOX | 196.8 | −29.5 | [125] | |
| Encapsulated into the aqueous core | AuNPs | SPC, Chol, PEG2000 | DOX | 182.2 | / | [101] | |
| Encapsulated into the aqueous core | AuNPs | DOPC, DOTAP, DSPE-PEG2000 | VP | 170.0 | 45.0 | [126] | |
| Encapsulated into the aqueous core | AuNRs | SPC, HSPC, DSPE-PEG2000 | DOC | 163.1 | −32.8 | [127] | |
| Encapsulated into the aqueous core | QDs | DPPC, DSPG, DSPE-PEG2000 | PTX | 102.5 | −19.8 | [128] | |
| Encapsulated into the aqueous core | QDs | DSPC, DOTAP, Chol | / | 114.0 | 24.8 | [87] | |
| Encapsulated into the aqueous core | QDs | DOPC, DOPE | / | 103.0 | −13.2 | [129] | |
| Encapsulated into the aqueous core/within the lipid layer | AuNPs | SPC, Chol, DSPE-PEG2000 | PTX | 149.2 | −2.5 | [130] | |
| Encapsulated into the aqueous core/within the lipid layer | AuNPs | DPPC | / | 160.0 | −6.2 | [131] | |
| Encapsulated into the aqueous core/on the outer surface | AuNPs | DPPC, HSPC, EPC, | DOX | 154.8 | −38.0 | [132] | |
| Encapsulated into the aqueous core/on the outer surface | AuNPs | DPPC, MPPC, DSPE-PEG2000 | Calcein | 118.0–146.0 | −9.6 | [133] | |
| Encapsulated into the aqueous core/on the outer surface | QDs | DSPC, DOTAP, DSPE-PEG2000 | / | 107.4 | −0.8 | [134] | |
| Encapsulated into the aqueous core/on the outer surface | QDs | DOPE, DSPC, Chol, DSPE-PEG2000 | / | 100.0 | 0 | [135] | |
| On the outer surface | AuNPs | DPPC, HSPC, DSPE-PEG2000, Chol | CTD | 96.4 | 28.7 | [88] | |
| On the outer surface | GNS | SPC, Chol | RES | 141.7 | 22.7 | [107] | |
| On the outer surface | AuNPs | HSPC | CUR | 100.0 | 22.0 | [109] | |
| On the outer surface | AuNPs | DSPC, Chol | / | 200.0 | / | [136] | |
| On the outer surface | AuNPs | DSPC, Chol | QUE | 120.0 | 11.8 | [137] | |
| On the outer surface | AuNPs | SPC, CS | OA | 172.0 | 22.7 | [9] | |
| On the outer surface | AuNPs | SPC, Chol | BA | 149.4 | / | [138] | |
| On the outer surface | AuNPs | SPC | CUR | 100.0–120.0 | / | [67] | |
| Solvent injection | encapsulated into the aqueous core | AuNPs | EPC, TPGS-COOH, Chol | DOC | 217.1 | −14.5 | [89] |
| Encapsulated into the aqueous core | AuNPs | EPC, DOPG | / | 140.0–150.0 | / | [139] | |
| Ultrasonication | On the outer surface/encapsulated into the aqueous core | CDs | DSPC, Chol | / | 230.0 | 20.0 | [110] |
| Within the lipid bilayer | GO | POPC | / | 238.0 | −15.2 | [140] | |
| Within the lipid bilayer | GO | FA-PEG-DSPE, biotin-PEG-DSPE, DMPG | RES | 148.0 | −23.6 | [141] | |
| Encapsulated into the aqueous core | AuNCs | DOPC, DSPE-PEG2000, Chol | TRP2 | 64.5 | −10.0 | [142] | |
| Hydrothermal method | On the outer surface | CDs | triolein | / | 103.0 | / | [91] |
| Covalent attachment | On the outer surface | CNTs | SPC, DSPE-PEG2000, Chol | Oridonin | / | / | [111] |
| On the outer surface | CNTs | biotin-PEG2000-PL, HSPC, PE | Calcein | 10.0 | −16.3 | [92] | |
| Ionic interaction assembly method | On the outer surface | GO | DPPC, Brij 78, DOTAP, Chol | DOX | 153.9 | −32.6 | [57] |
| Plasmon resonance coating method | On the outer surface | AuCLs | DPPC, MPPC, DSPE-PEG2000 | DOX | 171.5 | −1.0 | [64] |
| Extrusion method | Encapsulated into the aqueous core | GNC | DOPC, N-dod-PE | / | 175.0 | −37.7 | [90] |
| Within the lipid bilayer | QDs | L-α-lysolecithin, Chol, PEG-Chol, DOPE | BP | 104.2 | −11.3 | [143] |
| Type of NIRN-Lips | Drug Load | Surface Modification | Targeted Tumor Cells | Surface Engineering Techniques Used | Characterization | Ref. |
|---|---|---|---|---|---|---|
| FA-MWNTs-Lips | Oridonin | FA | HepG2 cells | FA-conjugated chitosan attached onto MWNTs-COOH using a non-covalent bond method; liposome containing oridonin covalently attached to MWNTs-COOH to form MWNTs-Lips. | FTIR, DLS, TEM, TGA | [111] |
| FA-CDs-Lips | / | FA | 4T1 cells | Terminal amino functional group of CDs-Lips reacted with the carboxyl groups of FA. | DLS, TEM, FTIR | [110] |
| FA-GQDs/AuNPs-Lips | DOX | FA | 4T1 cells | PEGylated FA (1 mg/mL) as targeting ligand was attached on the surface of AuNPs/QODs-Lips (5 mg/mL) through incubation process at room temperature. | FTIR, DLS, TEM, AFM, EDAX, X-ray, CT | [122] |
| FL/QDs-TK | / | FA | BEL-7402, Hep3B and SMMC-7721 cells | DSPE-PEG2000-folate were modified on liposomes by thin film hydration method. | DLS, TEM, FESEM, UV-vis, Bio-Rad imaging system | [115] |
| FA-DOX@CDs-Lips | DOX | FA | 4T1 cells | DSPE-MPEG2000-FA was noncovalently inserted into the lipid bilayer. | DLS, FTIR, TEM, XPS, 1HNMR spectra | [119] |
| FA-PEG-Lip@rGO/RES | RES | FA, PEG | A549 and MCF-7 cells | 0.1 μmol FA-PEG-DSPE was added to stabilize and modify liposome system. | DLS, TEM, AFM | [141] |
| CPP-CDs-Lips | CUR | CPP | MCF-7 cells | Carboxylic groups of CPP reacted with cholesterol to form conjugate. | DLS, TEM, FTIR | [112] |
| Man-CDs-Lips | / | D-mannose | HepG2 cells | D-mannose was non-covalently attached to the liposome surface. | TEM, AFM, XRD | [55] |
| SPACE-AuNSs-Lips | Calcein | SPACE peptides | NIH-3T3 cells | 5 mg/mL POPE-NHS and 5 mg/mL SPACE peptide (pH = 8) were added into the mixture following a 2 h preincubation at room temperature. | DLS, TEM, DSC | [99] |
| DOC-AuGSH-TPGS-Tf | DOC | TPGS, Tf | glioma cells | TPGS-COOH on the liposome surface were activated and then incubated with 1 mL Tf solution (10 mg/mL) at room temperature for 30 min and kept overnight at 4 °C. | DLS, TEM, AFM, NMR | [89] |
| TPP-Lips-VP-10AuNPs | VP | TPP | mitochondria of HCT116 cells | DSPE-PEG2000-NH2 were inserted into the pre-formed liposomes, and then the PEGylated and TPP-coupled liposomes were prepared by the EDC-NHS coupling method. | DLS, TEM, spectrophotometer | [126] |
| Aptamo-QDs-Lips | siRNA | Anti-EGFR aptamer | MDA-MB-231 cells | DSPE-mPEG2000-aptamer were added to the prepared QDs-Lips and incubated for 4 h at 37 °C | DLS, TEM | [113] |
| Biotin-QDs-Lips | / | Biotin | A431 cells | Biotin-DSPE (0.012 μmol/mL) were added to prepare liposomes. | DLS, TEM, spectrofluorometer | [167] |
| Type of NIRN-Lips | NIR Laser | Temperature Reached | Drug Load | Antitumor Mechanism | Cancer Treated | Ref. |
|---|---|---|---|---|---|---|
| GNS-BA-Lips | 808 nm | 43 °C in 10 min | BA | (1) Local heat generated from NIR light cause PTT; (2) enhance intracellular BA accumulation | Cervical cancer | [138] |
| CDs-CB-Lips | 500 nm | / | CB | (1) Increase cytotoxicity and cellular uptake of CB | Breast cancer | [58] |
| CTD-TSL@GNS | 808 nm | 44 °C in 20 min | CTD | (1) Block the heat shock response and inhibit the expression of HSP70 and BAG3, thus enhance therapeutic effect of CTD | Cervical cancer | [88] |
| Au Lips Cur NPs | 780 nm | 50 °C in 5 min | CUR | (1) Exert cytotoxic effect; inhibit cell proliferation and migration; (2) NIR light irradiation on Au Lips Cur NPs trigger the release of CUR | Melanoma | [67,109] |
| CDs-DOX/ICG-Lips | 808 nm | 56.8 °C in 5 min | DOX | (1) Induce cell apoptosis; (2) inhibit cell proliferation; (3) generate heat to kill cells | Liver cancer | [118] |
| FA-DOX@CDs-Lips | 480 nm | / | DOX | (1) Induce cell apoptosis; (2) increase cytotoxicity and intracellular uptake of DOX | Breast cancer | [119] |
| HMNS/SiO2/GQD-DOX-Lips | 808 nm | 56.8 °C in 20 min | DOX | (1) Induce ROS generation and heat produced by NIR irradiation to kill cells | Esophagus cancer | [120] |
| FA-GQDs/AuNPs-Lips | 750 nm | 55 °C in10 min | DOX | (1) Generate ROS and heat to kill cells; (2) Increase cytotoxicity; (3) generate heat to kill cells | Breast cancer | [122] |
| GNPs and DOX-TSL; HGNPs and DOX-TSL | 808 nm | 45 °C in 5 min | DOX | (1) Increase cytotoxicity and cellular uptake of DOX; (2) transfer NIR light to heat | Breast cancer | [125] |
| DOX and HAuNS-TSL | 808 nm | 49.9 °C in 5 min | DOX | (1) Enhance cytotoxicity; increase intracellular DOX concentration | Liver cancer | [132] |
| DOX/AuCLs-TSL | 808 nm | / | DOX | (1) AuCLs on the TSL absorb the NIR light to cause membrane destabilization; (2) increase cell cytotoxicity | Triple-negative breast cancer | [64] |
| AuNRs/DOCL-R | 748 nm | 60 °C in 10 min | DOC | (1) Enhance intracellular entrance; (2) increase DOC accumulation in tumor site; (3) induce ROS generation | Prostate cancer | [127] |
| QE-LipoAu | 750 nm | 48 °C in 7 min | QE | (1) After PTT, increase photothermal cytotoxicity, induce cell apoptosis; (2) depolymerize microtubules, suppress HSP70 expression, and cause DNA damage | Hepatocellular carcinoma | [137] |
| GNS-CS-OA-Lips | 808 nm | / | OA | (1) After NIR light irradiation, a local temperature increase caused by AuNPs to kill cells; (2) hyperthermia promotes phase conversion from gel-to-liquid crystalline of cells membrane, and dramatically enhances intracellular uptake of OA, leading to the tumor cells apoptosis. | Osteosarcoma | [9] |
| FA-PEG-Lip@rGO/RES | 780 nm | 59.6 °C in 5 min | RES | (1) Enhance cellular uptake of RES; protect stability of resveratrol; (2) generate heat to kill cells | Breast cancer | [141] |
| GNS@CTS@RES-Lips | 808 nm | 66 °C in 10 min | RES | (1) Convert NIR light to heat to enhance the release and intracellular accumulation of RES | Cervical cancer | [107] |
| FA-CDs-Lips | 808 nm | 57–62 °C in 5 min | / | (1) Induce ROS generation; (2) increase cellular uptake | Breast cancer | [110] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, P.; Mao, L.; Dong, Y.; Zhao, Z.; Sun, Q.; Mazhar, M.; Ma, Y.; Yang, S.; Ren, W. Design and Application of Near-Infrared Nanomaterial-Liposome Hybrid Nanocarriers for Cancer Photothermal Therapy. Pharmaceutics 2021, 13, 2070. https://doi.org/10.3390/pharmaceutics13122070
Liang P, Mao L, Dong Y, Zhao Z, Sun Q, Mazhar M, Ma Y, Yang S, Ren W. Design and Application of Near-Infrared Nanomaterial-Liposome Hybrid Nanocarriers for Cancer Photothermal Therapy. Pharmaceutics. 2021; 13(12):2070. https://doi.org/10.3390/pharmaceutics13122070
Chicago/Turabian StyleLiang, Pan, Linshen Mao, Yanli Dong, Zhenwen Zhao, Qin Sun, Maryam Mazhar, Yining Ma, Sijin Yang, and Wei Ren. 2021. "Design and Application of Near-Infrared Nanomaterial-Liposome Hybrid Nanocarriers for Cancer Photothermal Therapy" Pharmaceutics 13, no. 12: 2070. https://doi.org/10.3390/pharmaceutics13122070
APA StyleLiang, P., Mao, L., Dong, Y., Zhao, Z., Sun, Q., Mazhar, M., Ma, Y., Yang, S., & Ren, W. (2021). Design and Application of Near-Infrared Nanomaterial-Liposome Hybrid Nanocarriers for Cancer Photothermal Therapy. Pharmaceutics, 13(12), 2070. https://doi.org/10.3390/pharmaceutics13122070