Evaluation of Ruthenium-Based Assemblies as Carriers of Photosensitizers to Treat Rheumatoid Arthritis by Photodynamic Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Compounds
2.2. Preparation of Human Synovial Cells
2.3. Culture of Human RA FLS and Treatment
2.4. Antiproliferative Assays
2.5. Protein Extraction and Western-Blot Analysis
2.6. Assay of COX-2 Activity
2.7. Assay of IL-1β Production
2.8. Statistical Analysis
3. Results and Discussion
3.1. Phototoxicity Tests
3.2. Inflammatory Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Roux, C.H.; Saraux, A.; Le Bihan, E.; Fardellone, P.; Guggenbuhl, P.; Fautrel, B.; Masson, C.; Chary-Valckenaere, I.; Cantagrel, A.; Juvin, R.; et al. Rheumatoid arthritis and spondyloarthropathies: Geographical variations in prevalence in France. J. Rheumatol. 2007, 34, 117–122. [Google Scholar]
- Scherer, H.U.; Häupl, T.; Burmester, G.R. The etiology of rheumatoid arthritis. J. Autoimmun. 2020, 110, 102400. [Google Scholar] [CrossRef] [PubMed]
- Kerkman, P.F.; Fabre, E.; van der Voort, E.I.; Zaldumbide, A.; Rombouts, Y.; Rispens, T.; Wolbink, G.; Hoeben, R.C.; Spits, H.; Baeten, D.L.; et al. Identification and characterization of citrullinated antigen-specific B cells in peripheral blood of patients with rheumatoid arthritis. Ann. Rheum. Dis. 2016, 75, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Alasti, F.; Smolen, J.S. Rheumatoid factor, not antibodies against citrullinated proteins, is associated with baseline disease activity in rheumatoid arthritis clinical trials. Arthritis Res. Ther. 2015, 17, 229. [Google Scholar] [CrossRef] [Green Version]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [Green Version]
- Smolen, J.S.; Landewé, R.B.; Bijlsma, J.W.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; de Wit, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020, 79, 685–699. [Google Scholar] [CrossRef] [Green Version]
- Smolen, J.S.; van der Heijde, D.; Machold, K.P.; Aletaha, D.; Landewé, R. Proposal for a new nomenclature of disease-modifying antirheumatic drugs. Ann. Rheum. Dis. 2014, 73, 3–5. [Google Scholar] [CrossRef]
- Ma, X.; Xu, S. TNF inhibitor therapy for rheumatoid arthritis. Biomed. Rep. 2013, 1, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Dowty, M.E.; Lin, T.H.; Jesson, M.I.; Hegen, M.; Martin, D.A.; Katkade, V.; Menon, S.; Telliez, J.B. Janus kinase inhibitors for the treatment of rheumatoid arthritis demonstrate similar profiles of in vitro cytokine receptor inhibition. Pharmacol. Res. Perspect. 2019, 7, e00537. [Google Scholar] [CrossRef] [Green Version]
- Gallardo-Villagrán, M.; Leger, D.Y.; Liagre, B.; Therrien, B. Photosensitizers used in the photodynamic therapy of rheumatoid arthritis. Int. J. Mol. Sci. 2019, 20, 3339. [Google Scholar] [CrossRef] [Green Version]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [Green Version]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer, J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Hopper, C. Photodynamic therapy: A clinical reality in the treatment of cancer. Lancet Oncol. 2000, 1, 212–219. [Google Scholar] [CrossRef]
- Trauner, K.B.; Gandour-Edwards, R.; Bamberg, M.; Shortkroff, S.; Sledge, C.; Hasan, T. Photodynamic synovectomy using benzoporphyrin derivative in an antigen-induced arthritis model for rheumatoid arthritis. Photochem. Photobiol. 1998, 67, 133–139. [Google Scholar] [CrossRef]
- Miyazawa, S.; Nishida, K.; Komiyama, T.; Nakae, Y.; Takeda, K.; Yorimitsu, M.; Kitamura, A.; Kunisada, T.; Ohtsuka, A.; Inoue, H. Novel transdermal photodynamic therapy using ATX-S10· Na (II) induces apoptosis of synovial fibroblasts and ameliorates collagen antibody-induced arthritis in mice. Rheumatol. Int. 2006, 26, 717. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, L.; Lin, Z.; Wang, L.; Lin, L.; Li, M.; Zhang, Y.; Yin, Q.; Li, Q.; Xia, H. A new treatment modality for rheumatoid arthritis: Combined photothermal and photodynamic therapy using Cu7. 2S4 nanoparticles. Adv. Healthc. Mater. 2018, 7, 1800013. [Google Scholar] [CrossRef] [PubMed]
- Koderhold, G.; Jindra, R.; Koren, H.; Alth, G.; Schenk, G. Experiences of photodynamic therapy in dermatology. J. Photochem. Photobiol. Biol. 1996, 36, 221–223. [Google Scholar] [CrossRef]
- Búzová, D.; Kasák, P.; Miškovský, P.; Jancura, D. Solubilization of poorly soluble photosensitizer hypericin by polymeric micelles and polyethylene glycol. Gen. Physiol. Biophys. 2013, 32, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Bai, J.; Qian, Y. The investigation of unique water-soluble heptamethine cyanine dye for use as NIR photosensitizer in photodynamic therapy of cancer cells. Spectrochim. Acta A 2020, 228, 117702. [Google Scholar] [CrossRef]
- Schmitt, F.; Freudenreich, J.; Barry, N.P.; Juillerat-Jeanneret, L.; Süss-Fink, G.; Therrien, B. Organometallic cages as vehicles for intracellular release of photosensitizers. J. Am. Chem. Soc. 2012, 134, 754–757. [Google Scholar] [CrossRef] [Green Version]
- Barry, N.P.E.; Zava, O.; Dyson, P.J.; Therrien, B. Excellent Correlation between Drug Release and Portal Size in Metalla-Cage Drug-Delivery Systems. Chem. Eur. J. 2011, 17, 9669–9677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barry, N.P.; Edafe, F.; Therrien, B. Anticancer activity of tetracationic arene ruthenium metalla-cycles. Dalton Trans. 2011, 40, 7172–7180. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Zhu, X.H.; Shen, Q.; Lu, J.; Li, J.Q. Catalytic cyclotrimerization of arylnitriles using the novel samarium (II) complexes as catalysts. Chin. J. Chem. 2002, 20, 1334–1339. [Google Scholar] [CrossRef]
- Amoroso, A.J.; Thompson, A.M.C.; Maher, J.P.; McCleverty, J.A.; Ward, M.D. Di-, tri-, and tetranucleating pyridyl ligands which Facilitate multicenter magnetic exchange between paramagnetic molybdenum centers. Inorg. Chem. 1995, 34, 4828–4835. [Google Scholar] [CrossRef]
- Wang, L.; Tao, X.T.; Yang, J.X.; Yu, W.T.; Ren, Y.; Xin, Q.; Liu, Z.; Jiang, M.H. Synthesis, structure and two-photon absorption properties of a new multi-branched compound, 1, 2, 4, 5-tetrakis (4-pyridylvinyl) benzene. J. Solid State Chem. 2004, 177, 4293–4299. [Google Scholar] [CrossRef]
- Freudenreich, J.; Dalvit, C.; Süss-Fink, G.; Therrien, B. Encapsulation of photosensitizers in hexa-and octanuclear organometallic cages: Synthesis and characterization of carceplex and host–guest systems in solution. Organometallics 2013, 32, 3018–3033. [Google Scholar] [CrossRef]
- Dogutan, D.K.; Ptaszek, M.; Lindsey, J.S. Direct synthesis of magnesium porphine via 1-formyldipyrromethane. J. Org. Chem. 2007, 72, 5008–5011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; Mcshane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S.; et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheumatol. 1988, 31, 315–324. [Google Scholar] [CrossRef]
- Liagre, B.; Vergne-Salle, P.; Corbiere, C.; Charissoux, J.L.; Beneytout, J.L. Diosgenin, a plant steroid, induces apoptosis in human rheumatoid arthritis synoviocytes with cyclooxygenase-2 overexpression. Arthritis Res. Ther. 2004, 6, R373. [Google Scholar] [CrossRef] [Green Version]
- Glant, T.T.; Jacobs, J.J.; Molnár, G.; Shanbhag, A.S.; Valyon, M.; Galante, J.O. Bone resorption activity of particulate-stimulated macrophages. J. Bone Miner. Res. 1993, 8, 1071–1079. [Google Scholar] [CrossRef]
- Bonnet, C.; Bertin, P.; Cook-Moreau, J.; Chable-Rabinovitch, H.; Treves, R.; Rigaud, M. Lipoxygenase products and expression of 5-lipoxygenase and 5-lipoxygenase-activating protein in human cultured synovial cells. Prostaglandins 1995, 50, 127–135. [Google Scholar] [CrossRef]
- Semaan, J.; Pinon, A.; Rioux, B.; Hassan, L.; Limami, Y.; Pouget, C.; Fagnère, C.; Sol, V.; Diab-Assaf, M.; Simon, A.; et al. Resistance to 3-HTMC-induced apoptosis through activation of PI3K/Akt, MEK/ERK, and p38/COX-2/PGE2 pathways in human HT-29 and HCT116 colorectal cancer cells. J. Cell. Biochem. 2016, 117, 2875–2885. [Google Scholar] [CrossRef] [PubMed]
- Kamkaew, A.; Lim, S.H.; Lee, H.B.; Kiew, L.V.; Chung, L.Y.; Burgess, K. BODIPY dyes in photodynamic therapy. Chem. Soc. Rev. 2013, 42, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Lutton, J.D.; Abraham, N.G.; Drummond, G.S.; Levere, R.D.; Kappas, A. Zinc porphyrins: Potent inhibitors of hematopoieses in animal and human bone marrow. Proc. Natl. Acad. Sci. USA 1997, 94, 1432–1436. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Nguyen, X.; Ou, J.; Rekulapelli, P.; Stevenson, D.K.; Dennery, P.A. Unique effects of zinc protoporphyrin on HO-1 induction and apoptosis. Am. J. Hematol. 2001, 97, 1306–1313. [Google Scholar] [CrossRef] [Green Version]
- Bartok, B.; Firestein, G.S. Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunol. Rev. 2010, 233, 233–255. [Google Scholar] [CrossRef]
- Park, C.; Moon, D.O.; Choi, I.W.; Choi, B.T.; Nam, T.J.; Rhu, C.H.; Kwon, T.K.; Lee, W.H.; Kim, G.Y.; Choi, Y.H. Curcumin induces apoptosis and inhibits prostaglandin E2 production in synovial fibroblasts of patients with rheumatoid arthritis. Int. J. Mol. Med. 2007, 20, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Nasry, W.H.S.; Rodriguez-Lecompte, J.C.; Martin, C.K. Role of COX-2/PGE2 mediated inflammation in oral squamous cell carcinoma. Cancers 2018, 10, 348. [Google Scholar] [CrossRef] [Green Version]
- Sung, M.S.; Lee, E.G.; Jeon, H.S.; Chae, H.J.; Park, S.J.; Lee, Y.C.; Yoo, W.H. Quercetin inhibits IL-1β-induced proliferation and production of MMPs, COX-2, and PGE2 by rheumatoid synovial fibroblast. Inflammation 2012, 35, 1585–1594.3. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Breyssens, H.; Buytaert, E.; Hendrickx, N. Regulatory pathways in photodynamic therapy induced apoptosis. Photochem. Photobiol. Sci. 2004, 3, 721–729. [Google Scholar] [CrossRef]
- Downey, G.P.; Gumbay, R.S.; Doherty, D.E.; LaBrecque, J.F.; Henson, J.E.; Henson, P.M.; Worthen, G.S. Enhancement of pulmonary inflammation by PGE2: Evidence for a vasodilator effect. J. Appl. Physiol. 1988, 64, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Fidanzi-Dugas, C.; Liagre, B.; Chemin, G.; Perraud, A.; Carrion, C.; Couquet, C.Y.; Granet, R.; Sol, V.; Léger, D.Y. Analysis of the in vitro and in vivo effects of photodynamic therapy on prostate cancer by using new photosensitizers, protoporphyrin IX-polyamine derivatives. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1676–1690. [Google Scholar] [CrossRef]
- Luna, M.; Wong, S.; Ferrario, A.; Gomer, C.J. Cyclooxygenase-2 Expression Induced by Photofrin Photodynamic Therapy Involves the p38 MAPK Pathway. Photochem. Photobiol. 2008, 84, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Rayar, A.M.; Lagarde, N.; Martin, F.; Blanchard, F.; Liagre, B.; Ferroud, C.; Zagury, J.F.; Montes, M.; Veitía, M.S.I. New selective cyclooxygenase-2 inhibitors from cyclocoumarol: Synthesis, characterization, biological evaluation and molecular modeling. Eur. J. Med. Chem. 2018, 146, 577–587. [Google Scholar] [CrossRef]
- Hendrickx, N.; Volanti, C.; Moens, U.; Seternes, O.M.; De Witte, P.; Vandenheede, J.R.; Piette, J.; Agostinis, P. Up-regulation of cyclooxygenase-2 and apoptosis resistance by p38 MAPK in hypericin-mediated photodynamic therapy of human cancer cells. J. Biol. Chem. 2003, 278, 52231–52239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrario, A.; Von Tiehl, K.; Wong, S.; Luna, M.; Gomer, C.J. Cyclooxygenase-2 inhibitor treatment enhances photodynamic therapy-mediated tumor response. Cancer Res. 2002, 62, 3956–3961. [Google Scholar] [PubMed]
- Williams, C.S.; Mann, M.; DuBois, R.N. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 1999, 18, 7908–7916. [Google Scholar] [CrossRef] [Green Version]
- van Dalen, S.C.M.; Blom, A.B.; Slöetjes, A.W.; Helsen, M.M.A.; Roth, J.; Vogl, T.; van de Loo, F.A.; Koenders, M.I.; van der Kraan, P.M.; van den Berg, W.B.; et al. Interleukin-1 is not involved in synovial inflammation and cartilage destruction in collagenase-induced osteoarthritis. Osteoarthr. Cartil. 2017, 25, 385–396. [Google Scholar] [CrossRef] [Green Version]
- Nasi, S.; Ea, H.K.; So, A.; Busso, N. Revisiting the role of interleukin-1 pathway in osteoarthritis: Interleukin-1α and-1β, and NLRP3 inflammasome are not involved in the pathological features of the murine menisectomy model of osteoarthritis. Front. Pharmacol. 2017, 8, 282. [Google Scholar] [CrossRef]
- Shimomura, K.; Kanamoto, T.; Kita, K.; Akamine, Y.; Nakamura, N.; Mae, T.; Yoshikawa, H.; Nakata, K. Cyclic compressive loading on 3D tissue of human synovial fibroblasts upregulates prostaglandin E2 via COX-2 production without IL-1β and TNF-α. Bone Jt. Res. 2014, 3, 280–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]




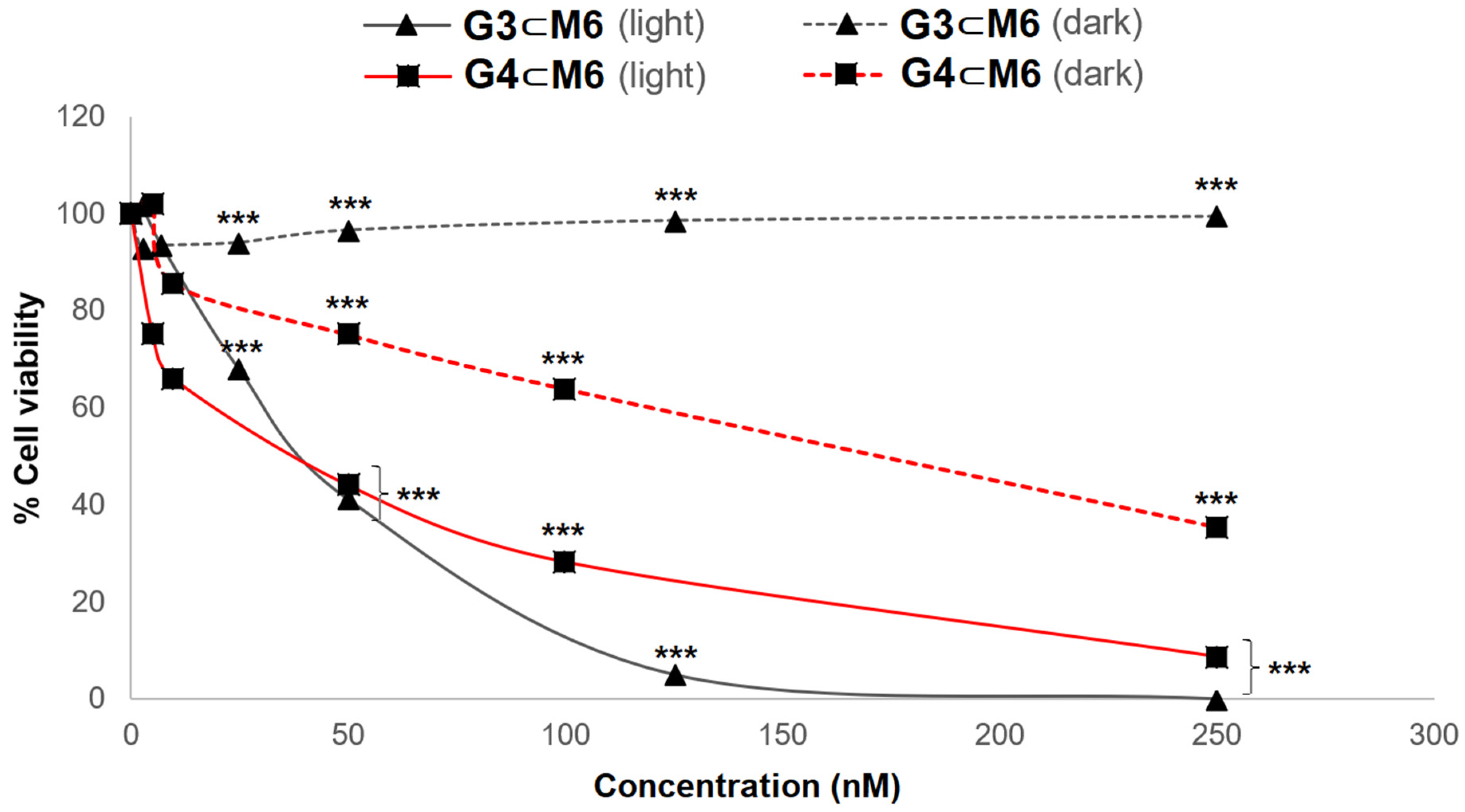

| Entry | G ⊂ M | IC50 (nM) Light | IC50 (nM) Dark | ΦF (%) |
|---|---|---|---|---|
| 1 | G1 ⊂ M1 | 211.7 ± 5.8 | >1500 | - |
| 2 | G1 ⊂ M2 | 95.0 ± 5.9 | >1500 | - |
| 3 | G1 ⊂ M3 | 53.6 ± 4.3 | >1500 | - |
| 4 | G1 ⊂ M4 | 48.1 ± 9.7 | >1500 | - |
| 5 | G1 ⊂ M5 | 35.4 ± 4.7 | >1500 | 0.8 |
| 6 | G1 ⊂ M6 | 31.7 ± 6.6 | >1500 | 1.1 |
| 7 | G2 ⊂ M1 | 302.6 ± 5.2 | >1500 | - |
| 8 | G2 ⊂ M4 | 100.7 ± 5.8 | >1500 | - |
| 9 | G2 ⊂ M6 | 91.8 ± 8.3 | >1500 | 2.0 |
| 10 | G3 ⊂ M4 | >1500 | >1500 | - |
| 11 | G3 ⊂ M5 | 53.4 ± 4.5 | >1500 | 0.11 |
| 12 | G3 ⊂ M6 | 47.4 ± 6.3 | >1500 | - |
| 13 | G4 ⊂ M4 | >1500 | >1500 | - |
| 14 | G4 ⊂ M5 | 66.0 ± 2.6 | 103.8 ± 2.9 | 1.6 |
| 15 | G4 ⊂ M6 | 64.4 ± 4.4 | 163.8 ± 17.1 | - |
| Entry | G ⊂ M | PGE2 (pg/mL) | IL-1β (pg/mL) |
|---|---|---|---|
| Ctrl | - | 286.6 ± 0.1 | 1.8 ± 0.7 |
| 1 | G1 ⊂ M1 | 460.8 ± 4.3 | 2.3 ± 1.2 |
| 2 | G1 ⊂ M2 | 471.2 ± 3.4 | 1.9 ± 1.0 |
| 3 | G1 ⊂ M3 | 445.1 ± 4.7 | 2.8 ± 0.1 |
| 4 | G1 ⊂ M4 | 378.3 ± 14.2 | 3.2 ± 0.4 |
| 5 | G1 ⊂ M5 | 407.4 ± 14.5 | 2.1 ± 0.2 |
| 6 | G1 ⊂ M6 | 439.2 ± 10.1 | 1.6 ± 0.1 |
| 7 | G2 ⊂ M1 | 476.8 ± 3.4 | 1.9 ± 0.6 |
| 8 | G2 ⊂ M4 | 473.6 ± 7.5 | 1.4 ± 0.2 |
| 9 | G2 ⊂ M6 | 430.6 ± 1.4 | 2.2 ± 0.2 |
| 10 | G3 ⊂ M5 | 368.2 ± 26.5 | 2.4 ± 0.4 |
| 11 | G3 ⊂ M6 | 425.2 ± 2.7 | 0.1 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallardo-Villagrán, M.; Paulus, L.; Charissoux, J.-L.; Sutour, S.; Vergne-Salle, P.; Leger, D.Y.; Liagre, B.; Therrien, B. Evaluation of Ruthenium-Based Assemblies as Carriers of Photosensitizers to Treat Rheumatoid Arthritis by Photodynamic Therapy. Pharmaceutics 2021, 13, 2104. https://doi.org/10.3390/pharmaceutics13122104
Gallardo-Villagrán M, Paulus L, Charissoux J-L, Sutour S, Vergne-Salle P, Leger DY, Liagre B, Therrien B. Evaluation of Ruthenium-Based Assemblies as Carriers of Photosensitizers to Treat Rheumatoid Arthritis by Photodynamic Therapy. Pharmaceutics. 2021; 13(12):2104. https://doi.org/10.3390/pharmaceutics13122104
Chicago/Turabian StyleGallardo-Villagrán, Manuel, Lucie Paulus, Jean-Louis Charissoux, Sylvain Sutour, Pascale Vergne-Salle, David Yannick Leger, Bertrand Liagre, and Bruno Therrien. 2021. "Evaluation of Ruthenium-Based Assemblies as Carriers of Photosensitizers to Treat Rheumatoid Arthritis by Photodynamic Therapy" Pharmaceutics 13, no. 12: 2104. https://doi.org/10.3390/pharmaceutics13122104
APA StyleGallardo-Villagrán, M., Paulus, L., Charissoux, J.-L., Sutour, S., Vergne-Salle, P., Leger, D. Y., Liagre, B., & Therrien, B. (2021). Evaluation of Ruthenium-Based Assemblies as Carriers of Photosensitizers to Treat Rheumatoid Arthritis by Photodynamic Therapy. Pharmaceutics, 13(12), 2104. https://doi.org/10.3390/pharmaceutics13122104








