Intra-Articular Drug Delivery for Osteoarthritis Treatment
Abstract
:1. Introduction
2. Synovial Joints
3. Medication Therapies for OA
3.1. Drugs for the Treatment of OA
3.2. Routes of Drug Administration
4. Intra-Articular (IA) Drug Delivery Systems
4.1. Microparticles (MPs)
4.2. Nanoparticles (NPs)
4.3. Liposomes
4.4. Hydrogels
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jahn, S.; Seror, J.; Klein, J. Lubrication of Articular Cartilage. Annu. Rev. Biomed. Eng. 2016, 18, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Forster, H.; Fisher, J. The Influence of Loading Time and Lubricant on the Friction of Articular Cartilage. Proc. Inst. Mech. Eng. H 1996, 210, 109–119. [Google Scholar] [CrossRef]
- Cornelis, F.M.F.; Monteagudo, S.; Guns, L.K.A.; den Hollander, W.; Nelissen, R.; Storms, L.; Peeters, T.; Jonkers, I.; Meulenbelt, I.; Lories, R.J. ANP32A regulates ATM expression and prevents oxidative stress in cartilage, brain, and bone. Sci. Transl. Med. 2018, 10, eaar8426. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Batt, M. An update on the pathophysiology of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, C.; Nguyen, C.; Lefevre-Colau, M.M.; Rannou, F.; Poiraudeau, S. Risk factors and burden of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Riyaz, B.; Patil, A.; Kaur, A.; Kapoor, B.; Mishra, V. Novel drug delivery systems for NSAIDs in management of rheumatoid arthritis: An overview. Biomed. Pharmacother. 2018, 106, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Lopez de Figueroa, P.; Blanco, F.J.; Mendes, A.F.; Carames, B. Insulin decreases autophagy and leads to cartilage degradation. Osteoarthr. Cartil. 2016, 24, 731–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Hawker, G.A.; Laporte, A.; Croxford, R.; Coyte, P.C. The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology 2005, 44, 1531–1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arden, N.K.; Perry, T.A.; Bannuru, R.R.; Bruyère, O.; Cooper, C.; Haugen, I.K.; Hochberg, M.C.; McAlindon, T.E.; Mobasheri, A.; Reginster, J.-Y. Non-surgical management of knee osteoarthritis: Comparison of ESCEO and OARSI 2019 guidelines. Nat. Rev. Rheumatol. 2021, 17, 59–66. [Google Scholar] [CrossRef]
- Beswick, A.D.; Wylde, V.; Gooberman-Hill, R.; Blom, A.; Dieppe, P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open 2012, 2, e000435. [Google Scholar] [CrossRef]
- Walker, L.C.; Clement, N.D.; Deehan, D.J. Predicting the Outcome of Total Knee Arthroplasty Using the WOMAC Score: A Review of the Literature. J. Knee Surg. 2019, 32, 736–741. [Google Scholar] [CrossRef]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Schmidt, T.A.; Gastelum, N.S.; Nguyen, Q.T.; Schumacher, B.L.; Sah, R.L. Boundary lubrication of articular cartilage: Role of synovial fluid constituents. Arthritis Rheum. 2007, 56, 882–891. [Google Scholar] [CrossRef]
- Sluzalska, K.D.; Liebisch, G.; Lochnit, G.; Ishaque, B.; Hackstein, H.; Schmitz, G.; Rickert, M.; Steinmeyer, J. Interleukin-1beta affects the phospholipid biosynthesis of fibroblast-like synoviocytes from human osteoarthritic knee joints. Osteoarthr. Cartil. 2017, 25, 1890–1899. [Google Scholar] [CrossRef] [Green Version]
- Adams, D.; Swanson, S.A. Direct measurement of local pressures in the cadaveric human hip joint during simulated level walking. Ann. Rheum. Dis. 1985, 44, 658–666. [Google Scholar] [CrossRef] [Green Version]
- Hodge, W.A.; Fijan, R.S.; Carlson, K.L.; Burgess, R.G.; Harris, W.H.; Mann, R.W. Contact pressures in the human hip joint measured in vivo. Proc. Natl. Acad. Sci. USA 1986, 83, 2879–2883. [Google Scholar] [CrossRef] [Green Version]
- Dowson, D. Bio-tribology. Faraday Discuss. 2012, 156, 9–30. [Google Scholar] [CrossRef]
- Seror, J.; Zhu, L.; Goldberg, R.; Day, A.J.; Klein, J. Supramolecular synergy in the boundary lubrication of synovial joints. Nat. Commun. 2015, 6, 6497. [Google Scholar] [CrossRef]
- Klein, J. Hydration lubrication. Friction 2013, 1, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Kampf, N.; Lin, W.; Klein, J. Normal and shear forces between boundary sphingomyelin layers under aqueous conditions. Soft Matter 2020, 16, 3973–3980. [Google Scholar] [CrossRef]
- Cao, Y.; Kampf, N.; Klein, J. Boundary Lubrication, Hemifusion, and Self-Healing of Binary Saturated and Monounsaturated Phosphatidylcholine Mixtures. Langmuir 2019, 35, 15459–15468. [Google Scholar] [CrossRef]
- Lin, W.; Klein, J. Recent Progress in Cartilage Lubrication. Adv. Mater. 2021, 33, e2005513. [Google Scholar] [CrossRef]
- Costa, B.R.d.; Hari, R.; Jüni, P. Intra-articular Corticosteroids for Osteoarthritis of the Knee. JAMA Clin. Evid. Synop. 2016, 316, 2671–2672. [Google Scholar] [CrossRef]
- Kompel, A.J.; Roemer, F.W.; Murakami, A.M.; Diaz, L.E.; Crema, M.D.; Guermazi, A. Intra-articular Corticosteroid Injections in the Hip and Knee: Perhaps Not as Safe as We Thought? Radiology 2019, 293, 656–663. [Google Scholar] [CrossRef] [PubMed]
- McAlindon, T.E.; Harkey, M.S.; Ward, R.J.; Hochberg, M.C.; Driban, J.B. Intra-articular Corticosteroid Injections in the Hip and Knee: Perhaps Not as Dangerous as They Want You to Believe? Radiology 2020, 295, 249–250. [Google Scholar] [CrossRef] [PubMed]
- Kawata, M.; Okamoto, A.; Endo, T.; Tsukamoto, Y. Viscoelasticity of synovial fluids and additive effect of hyaluronate. Hydrocolloids 2000, 2, 343–348. [Google Scholar] [CrossRef]
- Evans, C.H.; Kraus, V.B.; Setton, L.A. Progress in intra-articular therapy. Nat. Rev. Rheumatol. 2014, 10, 11–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Lin, W.; Fan, Y.; Kampf, N.; Wang, Y.; Klein, J. Effects of Hyaluronan Molecular Weight on the Lubrication of Cartilage-Emulating Boundary Layers. Biomacromolecules 2020, 21, 4345–4354. [Google Scholar] [CrossRef]
- Hilšer, P.; Suchánková, A.; Mendová, K.; Filipič, K.E.; Daniel, M.; Vrbka, M. A new insight into more effective viscosupplementation based on the synergy of hyaluronic acid and phospholipids for cartilage friction reduction. Biotribology 2021, 25, 100166. [Google Scholar] [CrossRef]
- Lin, W.; Liu, Z.; Kampf, N.; Klein, J. The Role of Hyaluronic Acid in Cartilage Boundary Lubrication. Cells 2020, 9, 1606. [Google Scholar] [CrossRef]
- Lin, W.; Mashiah, R.; Seror, J.; Kadar, A.; Dolkart, O.; Pritsch, T.; Goldberg, R.; Klein, J. Lipid-hyaluronan synergy strongly reduces intrasynovial tissue boundary friction. Acta Biomater. 2019, 83, 314–321. [Google Scholar] [CrossRef]
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Barnett, R. Osteoarthritis. Lancet 2018, 391, 1985. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Orlowsky, E.W.; Kraus, V.B. The role of innate immunity in osteoarthritis: When our first line of defense goes on the offensive. J. Rheumatol. 2015, 42, 363–371. [Google Scholar] [CrossRef] [Green Version]
- Haseeb, A.; Haqqi, T.M. Immunopathogenesis of osteoarthritis. Clin. Immunol. 2013, 146, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, C.; Pesesse, L.; Gabay, O.; Delcour, J.P.; Msika, P.; Baudouin, C.; Henrotin, Y.E. Regulation of subchondral bone osteoblast metabolism by cyclic compression. Arthritis Rheum. 2012, 64, 1193–1203. [Google Scholar] [CrossRef]
- Bennett, J.M.; Reeves, G.; Billman, G.E.; Sturmberg, J.P. Inflammation-Nature’s Way to Efficiently Respond to All Types of Challenges: Implications for Understanding and Managing “the Epidemic” of Chronic Diseases. Front. Med. 2018, 5, 316. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Albani, S.; Koffeman, E.C.; Prakken, B. Induction of immune tolerance in the treatment of rheumatoid arthritis. Nat. Rev. Rheumatol. 2011, 7, 272–281. [Google Scholar] [CrossRef]
- Farooq, S.M.; Ashour, H.M. Type II Collagen-Specific B Cells Induce Immune Tolerance in Th1-Skewed, Th2-Skewed, and Arthritis-Prone Strains of Mice. Cells 2021, 10, 870. [Google Scholar] [CrossRef]
- Elshabrawy, H.A.; Volin, M.V.; Essani, A.B.; Chen, Z.; McInnes, I.B.; Van Raemdonck, K.; Palasiewicz, K.; Arami, S.; Gonzalez, M.; Ashour, H.M.; et al. IL-11 facilitates a novel connection between RA joint fibroblasts and endothelial cells. Angiogenesis 2018, 21, 215–228. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Zhang, W.; Jones, A.; Doherty, M. Efficacy of topical non-steroidal anti-inflammatory drugs in the treatment of osteoarthritis: Meta-analysis of randomised controlled trials. BMJ 2004, 329, 324. [Google Scholar] [CrossRef] [Green Version]
- Bannuru, R.R.; Osani, M.; Vaysbrot, E.E.; McAlindon, T.E. Comparative safety profile of hyaluronic acid products for knee osteoarthritis: A systematic review and network meta-analysis. Osteoarthr. Cartil. 2016, 24, 2022–2041. [Google Scholar] [CrossRef]
- He, Z.; Wang, B.; Hu, C.; Zhao, J. An overview of hydrogel-based intra-articular drug delivery for the treatment of osteoarthritis. Colloids Surf. B Biointerfaces 2017, 154, 33–39. [Google Scholar] [CrossRef]
- Oo, W.M.; Little, C.; Duong, V.; Hunter, D.J. The Development of Disease-Modifying Therapies for Osteoarthritis (DMOADs): The Evidence to Date. Drug Des. Devel. Ther. 2021, 15, 2921–2945. [Google Scholar] [CrossRef]
- Charlier, E.; Relic, B.; Deroyer, C.; Malaise, O.; Neuville, S.; Collee, J.; Malaise, M.G.; De Seny, D. Insights on Molecular Mechanisms of Chondrocytes Death in Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 2146. [Google Scholar] [CrossRef] [Green Version]
- Chevalier, X.; Eymard, F. Anti-IL-1 for the treatment of OA: Dead or alive? Nat. Rev. Rheumatol. 2019, 15, 191–192. [Google Scholar] [CrossRef]
- Sasaki, H.; Takayama, K.; Matsushita, T.; Ishida, K.; Kubo, S.; Matsumoto, T.; Fujita, N.; Oka, S.; Kurosaka, M.; Kuroda, R. Autophagy modulates osteoarthritis-related gene expression in human chondrocytes. Arthritis Rheum. 2012, 64, 1920–1928. [Google Scholar] [CrossRef]
- Roy, S.G. Regulation of autophagy by miRNAs in human diseases. Nucleus 2021, 64, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Siebuhr, A.S.; Werkmann, D.; Bay-Jensen, A.-C.; Thudium, C.S.; Karsdal, M.A.; Serruys, B.; Ladel, C.; Michaelis, M.; Lindemann, S. The Anti-ADAMTS-5 Nanobody® M6495 Protects Cartilage Degradation Ex Vivo. Int. J. Mol. Sci. 2020, 21, 5992. [Google Scholar] [CrossRef] [PubMed]
- Gigout, A.; Guehring, H.; Froemel, D.; Meurer, A.; Ladel, C.; Reker, D.; Bay-Jensen, A.C.; Karsdal, M.A.; Lindemann, S. Sprifermin (rhFGF18) enables proliferation of chondrocytes producing a hyaline cartilage matrix. Osteoarthr. Cartil. 2017, 25, 1858–1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortier, L.A.; Barker, J.U.; Strauss, E.J.; McCarrel, T.M.; Cole, B.J. The role of growth factors in cartilage repair. Clin. Orthop. Relat. Res. 2011, 469, 2706–2715. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.; Zhu, S.; Tremblay, M.S.; Payette, J.N.; Wang, J.; Bouchez, L.C.; Meeusen, S.; Althage, A.; Cho, C.Y.; Wu, X.; et al. A Stem Cell–Based Approach to Cartilage Repair. Science 2012, 336, 717–721. [Google Scholar] [CrossRef] [Green Version]
- Cook, C.S.; Smith, P.A. Clinical Update: Why PRP Should Be Your First Choice for Injection Therapy in Treating Osteoarthritis of the Knee. Curr. Rev. Musculoskelet. Med. 2018, 11, 583–592. [Google Scholar] [CrossRef]
- Jevotovsky, D.S.; Alfonso, A.R.; Einhorn, T.A.; Chiu, E.S. Osteoarthritis and stem cell therapy in humans: A systematic review. Osteoarthr. Cartil. 2018, 26, 711–729. [Google Scholar] [CrossRef] [Green Version]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2020, 72, 149–162. [Google Scholar] [CrossRef]
- Primorac, D.; Molnar, V.; Matisic, V.; Hudetz, D.; Jelec, Z.; Rod, E.; Cukelj, F.; Vidovic, D.; Vrdoljak, T.; Dobricic, B.; et al. Comprehensive Review of Knee Osteoarthritis Pharmacological Treatment and the Latest Professional Societies’ Guidelines. Pharmaceuticals 2021, 14, 205. [Google Scholar] [CrossRef]
- Fleischmann, R.M.; Bliddal, H.; Blanco, F.J.; Schnitzer, T.J.; Peterfy, C.; Chen, S.; Wang, L.; Feng, S.; Conaghan, P.G.; Berenbaum, F.; et al. A Phase II Trial of Lutikizumab, an Anti-Interleukin-1alpha/beta Dual Variable Domain Immunoglobulin, in Knee Osteoarthritis Patients with Synovitis. Arthritis Rheumatol. 2019, 71, 1056–1069. [Google Scholar] [CrossRef]
- Iannitti, T.; Elhensheri, M.; Bingol, A.O.; Palmieri, B. Preliminary histopathological study of intra-articular injection of a novel highly cross-linked hyaluronic acid in a rabbit model of knee osteoarthritis. J. Mol. Histol. 2013, 44, 191–201. [Google Scholar] [CrossRef] [Green Version]
- Raza, K.; Kumar, M.; Kumar, P.; Malik, R.; Sharma, G.; Kaur, M.; Katare, O.P. Topical Delivery of Aceclofenac: Challenges and Promises of Novel Drug Delivery Systems. Biomed. Res. Int. 2014, 2014, 406731. [Google Scholar] [CrossRef]
- Li, Y.-T.; Jiao, J.; Zhang, Y.; Huang, C.-B.; Wang, H.-D.; Wang, B.; Su, X.; Song, H.; Zhao, M.-S.; Jiang, D.-X.; et al. Clinical Efficacy of Cortex Daphnes (Zushima) Patch in Patients with Symptomatic Knee Osteoarthritis: A Multicenter Non-Inferiority Randomized Controlled Clinical Trial. Front. Pharmacol. 2021, 12, 646310. [Google Scholar] [CrossRef]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef]
- Krishnan, Y.; Rees, H.A.; Rossitto, C.P.; Kim, S.E.; Hung, H.K.; Frank, E.H.; Olsen, B.D.; Liu, D.R.; Hammond, P.T.; Grodzinsky, A.J. Green fluorescent proteins engineered for cartilage-targeted drug delivery: Insights for transport into highly charged avascular tissues. Biomaterials 2018, 183, 218–233. [Google Scholar] [CrossRef]
- Bajpayee, A.G.; Scheu, M.; Grodzinsky, A.J.; Porter, R.M. Electrostatic interactions enable rapid penetration, enhanced uptake and retention of intra-articular injected avidin in rat knee joints. J. Orthop. Res. 2014, 32, 1044–1051. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, Y.; Zhang, Z.; Yang, Z.; Huang, G. Intra-articular delivery of tetramethylpyrazine microspheres with enhanced articular cavity retention for treating osteoarthritis. Asian J. Pharm. Sci. 2018, 13, 229–238. [Google Scholar] [CrossRef]
- Ju, X.D.; Deng, M.; Ao, Y.F.; Yu, C.L.; Wang, J.Q.; Yu, J.K.; Cui, G.Q.; Hu, Y.L. The protective effect of tetramethylpyrazine on cartilage explants and chondrocytes. J. Ethnopharmacol. 2010, 132, 414–420. [Google Scholar] [CrossRef]
- Li, X.H.; Peng, J.; Xu, Y.F.; Wu, M.X.; Ye, H.Z.; Zheng, C.S.; Wu, G.W.; Xu, H.F.; Chen, X.Z.; Liu, X.X. Tetramethylpyrazine (TMP) promotes chondrocyte proliferation via pushing the progression of cell cycle. J. Med. Plants Res. 2011, 5, 3896–3903. [Google Scholar]
- Pawar, V.A.; Manjappa, A.S.; Murumkar, P.R.; Gajaria, T.K.; Devkar, R.V.; Mishra, A.K.; Yadav, M.R. Drug-fortified liposomes as carriers for sustained release of NSAIDs: The concept and its validation in the animal model for the treatment of arthritis. Eur. J. Pharm. Sci. 2018, 125, 11–22. [Google Scholar] [CrossRef]
- Park, J.W.; Yun, Y.P.; Park, K.; Lee, J.Y.; Kim, H.J.; Kim, S.E.; Song, H.R. Ibuprofen-loaded porous microspheres suppressed the progression of monosodium iodoacetate-induced osteoarthritis in a rat model. Colloids Surf. B Biointerfaces 2016, 147, 265–273. [Google Scholar] [CrossRef]
- Williams, G.W.; Hubbard, R.C.; Yu, S.S.; Zhao, W.; Geis, G.S. Comparison of once-daily and twice-daily administration of celecoxib for the treatment of osteoarthritis of the knee. Clin. Ther. 2001, 23, 213–227. [Google Scholar] [CrossRef]
- Janssen, M.; Timur, U.T.; Woike, N.; Welting, T.J.; Draaisma, G.; Gijbels, M.; van Rhijn, L.W.; Mihov, G.; Thies, J.; Emans, P.J. Celecoxib-loaded PEA microspheres as an auto regulatory drug-delivery system after intra-articular injection. J. Control. Release 2016, 244, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Rudnik-Jansen, I.; Schrijver, K.; Woike, N.; Tellegen, A.; Versteeg, S.; Emans, P.; Mihov, G.; Thies, J.; Eijkelkamp, N.; Tryfonidou, M.; et al. Intra-articular injection of triamcinolone acetonide releasing biomaterial microspheres inhibits pain and inflammation in an acute arthritis model. Drug Deliv. 2019, 26, 226–236. [Google Scholar] [CrossRef] [Green Version]
- Kraus, V.B.; Conaghan, P.G.; Aazami, H.A.; Mehra, P.; Kivitz, A.J.; Lufkin, J.; Hauben, J.; Johnson, J.R.; Bodick, N. Synovial and systemic pharmacokinetics (PK) of triamcinolone acetonide (TA) following intra-articular (IA) injection of an extended-release microsphere-based formulation (FX006) or standard crystalline suspension in patients with knee osteoarthritis (OA). Osteoarthr. Cartil. 2018, 26, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Musumeci, T.; Bonaccorso, A.; Carbone, C.; Impallomeni, G.; Ballistreri, A.; Duskey, J.T.; Puglisi, G.; Pignatello, R. Development and biocompatibility assessments of poly(3-hydroxybutyrate-co-ε-caprolactone) microparticles for diclofenac sodium delivery. J. Drug Deliv. Sci. Technol. 2020, 60, 102081. [Google Scholar] [CrossRef]
- Ahmed, A.Y.; Gad, A.M.; El-Raouf, O.M.A. Curcumin ameliorates diclofenac sodium-induced nephrotoxicity in male albino rats. J. Biochem. Mol. Toxicol. 2017, 31, e21951. [Google Scholar] [CrossRef]
- Ratanavaraporn, J.; Soontornvipart, K.; Shuangshoti, S.; Shuangshoti, S.; Damrongsakkul, S. Localized delivery of curcumin from injectable gelatin/Thai silk fibroin microspheres for anti-inflammatory treatment of osteoarthritis in a rat model. Inflammopharmacology 2017, 25, 211–221. [Google Scholar] [CrossRef]
- Abd-Allah, H.; Kamel, A.O.; Sammour, O.A. Injectable long acting chitosan/tripolyphosphate microspheres for the intra-articular delivery of lornoxicam: Optimization and in vivo evaluation. Carbohydr. Polym. 2016, 149, 263–273. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Bi, X.L.; Li, H.; Huang, G.H. Enhanced targeting efficiency of PLGA microspheres loaded with Lornoxicam for intra-articular administration. Drug Deliv. 2011, 18, 536–544. [Google Scholar] [CrossRef]
- Tellier, L.E.; Trevino, E.A.; Brimeyer, A.L.; Reece, D.S.; Willett, N.J.; Guldberg, R.E.; Temenoff, J.S. Intra-articular TSG-6 delivery from heparin-based microparticles reduces cartilage damage in a rat model of osteoarthritis. Biomater. Sci. 2018, 6, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Drummond, S.P.; Anand, S.; Bartnik, E.; Milner, C.M. A Novel Chondroprotective Property of Tsg-6 Has Therapeutic Potential for Oa. Osteoarthr. Cartil. 2016, 24, S19–S20. [Google Scholar] [CrossRef]
- Lengert, E.; Saveleva, M.; Verkhovskii, R.; Abramova, A.; Goryacheva, I. Novel formulation of glucocorticoid based on silver alginate microcapsules for intraarticular drug delivery. Mater. Lett. 2021, 288, 129339. [Google Scholar] [CrossRef]
- Marin, E.; Tapeinos, C.; Lauciello, S.; Ciofani, G.; Sarasua, J.R.; Larranaga, A. Encapsulation of manganese dioxide nanoparticles into layer-by-layer polymer capsules for the fabrication of antioxidant microreactors. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 117, 111349. [Google Scholar] [CrossRef]
- Li, W.; Liu, Z.; Liu, C.Q.; Guan, Y.J.; Ren, J.S.; Qu, X.G. Manganese Dioxide Nanozymes as Responsive Cytoprotective Shells for Individual Living Cell Encapsulation. Angew. Chem. Int. Edit. 2017, 56, 13661–13665. [Google Scholar] [CrossRef]
- Agarwal, R.; Volkmer, T.M.; Wang, P.; Lee, L.A.; Wang, Q.; Garcia, A.J. Synthesis of self-assembled IL-1Ra-presenting nanoparticles for the treatment of osteoarthritis. J. Biomed. Mater. Res. A 2016, 104, 595–599. [Google Scholar] [CrossRef] [Green Version]
- Kraus, V.B.; Birmingham, J.; Stabler, T.V.; Feng, S.; Taylor, D.C.; Moorman, C.T.; Garrett, W.E.; Toth, A.P. Effects of intraarticular IL1-Ra for acute anterior cruciate ligament knee injury: A randomized controlled pilot trial (NCT00332254). Osteoarthr. Cartil. 2012, 20, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, Y.; Chubinskaya, S.; Schoeberl, B.; Florine, E.; Kopesky, P.; Grodzinsky, A.J. Effects of insulin-like growth factor-1 and dexamethasone on cytokine-challenged cartilage: Relevance to post-traumatic osteoarthritis. Osteoarthr. Cartil. 2015, 23, 266–274. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.B.; Chen, E.H.; Lynch, S.E. A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthr. Cartil. 2006, 14, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Geiger, B.C.; Wang, S.; Padera, R.F.; Grodzinsky, A.J.; Hammond, P.T. Cartilage-penetrating nanocarriers improve delivery and efficacy of growth factor treatment of osteoarthritis. Sci. Transl. Med. 2018, 10, eaat8800. [Google Scholar] [CrossRef] [Green Version]
- Jin, T.; Wu, D.; Liu, X.M.; Xu, J.T.; Ma, B.J.; Ji, Y.; Jin, Y.Y.; Wu, S.Y.; Wu, T.; Ma, K. Intra-articular delivery of celastrol by hollow mesoporous silica nanoparticles for pH-sensitive anti-inflammatory therapy against knee osteoarthritis. J. Nanobiotechnol. 2020, 18, 94. [Google Scholar] [CrossRef]
- Cascao, R.; Vidal, B.; Raquel, H.; Neves-Costa, A.; Figueiredo, N.; Gupta, V.; Fonseca, J.E.; Moita, L.F. Effective treatment of rat adjuvant-induced arthritis by celastrol. Autoimmun. Rev. 2012, 11, 856–862. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Sun, T.; Yan, Y.; Ji, X.; Sun, Y.; Zhao, X.; Qi, J.; Cui, W.; Deng, L.; Zhang, H. Cartilage matrix-inspired biomimetic superlubricated nanospheres for treatment of osteoarthritis. Biomaterials 2020, 242, 119931. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, H.; Wang, H.; Han, Y.; Zheng, Z.; Liu, X.; Feng, B.; Zhang, H. Light-responsive dual-functional biodegradable mesoporous silica nanoparticles with drug delivery and lubrication enhancement for the treatment of osteoarthritis. Nanoscale 2021, 13, 6394–6399. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.A.; Ahmed, H.M.S.; El-Nekeety, A.A.; Sharaf, H.A.; Abdel-Aziem, S.H.; Abdel-Wahhab, M.A. Biosynthesis of gold nanoparticles for the treatment of osteoarthritis alone or in combination with Diacerein((R)) in a rat model. Inflammopharmacology 2021, 29, 705–719. [Google Scholar] [CrossRef]
- Mishima, S.; Kashiwakura, J.I.; Toyoshima, S.; Sasaki-Sakamoto, T.; Sano, Y.; Nakanishi, K.; Matsumoto, K.; Okayama, Y. Higher PGD2 production by synovial mast cells from rheumatoid arthritis patients compared with osteoarthritis patients via miR-199a-3p/prostaglandin synthetase 2 axis. Sci. Rep. 2021, 11, 5738. [Google Scholar] [CrossRef]
- Pucino, V.; Certo, M.; Varricchi, G.; Marone, G.; Ursini, F.; Rossi, F.W.; De Paulis, A.; Mauro, C.; Raza, K.; Buckley, C.D. Metabolic Checkpoints in Rheumatoid Arthritis. Front. Physiol. 2020, 11, 347. [Google Scholar] [CrossRef] [Green Version]
- Min, H.K.; Kim, K.W.; Lee, S.H.; Kim, H.R. Roles of mast cells in rheumatoid arthritis. Korean J. Intern. Med. 2020, 35, 12–24. [Google Scholar] [CrossRef]
- Maudens, P.; Seemayer, C.A.; Thauvin, C.; Gabay, C.; Jordan, O.; Allemann, E. Nanocrystal-Polymer Particles: Extended Delivery Carriers for Osteoarthritis Treatment. Small 2018, 14, 1703108. [Google Scholar] [CrossRef] [Green Version]
- Zeng, W.N.; Zhang, Y.; Wang, D.; Zeng, Y.P.; Yang, H.; Li, J.; Zhou, C.P.; Liu, J.L.; Yang, Q.J.; Deng, Z.L.; et al. Intra-articular Injection of Kartogenin-Enhanced Bone Marrow-Derived Mesenchymal Stem Cells in the Treatment of Knee Osteoarthritis in a Rat Model. Am. J. Sports Med. 2021, 49, 2795–2809. [Google Scholar] [CrossRef]
- Xiong, F.; Qin, Z.; Chen, H.; Lan, Q.; Wang, Z.; Lan, N.; Yang, Y.; Zheng, L.; Zhao, J.; Kai, D. pH-responsive and hyaluronic acid-functionalized metal-organic frameworks for therapy of osteoarthritis. J. Nanobiotechnol. 2020, 18, 139. [Google Scholar] [CrossRef]
- Chen, C.H.; Kuo, S.M.; Tien, Y.C.; Shen, P.C.; Kuo, Y.W.; Huang, H.H. Steady Augmentation of Anti-Osteoarthritic Actions of Rapamycin by Liposome-Encapsulation in Collaboration with Low-Intensity Pulsed Ultrasound. Int. J. Nanomed. 2020, 15, 3771–3790. [Google Scholar] [CrossRef]
- Pal, B.; Endisha, H.; Zhang, Y.; Kapoor, M. mTOR: A potential therapeutic target in osteoarthritis? Drugs R D 2015, 15, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Vasheghani, F.; Li, Y.H.; Blati, M.; Simeone, K.; Fahmi, H.; Lussier, B.; Roughley, P.; Lagares, D.; Pelletier, J.P.; et al. Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis. Ann. Rheum. Dis. 2015, 74, 1432–1440. [Google Scholar] [CrossRef]
- Chen, P.; Xia, C.; Mei, S.; Wang, J.; Shan, Z.; Lin, X.; Fan, S. Intra-articular delivery of sinomenium encapsulated by chitosan microspheres and photo-crosslinked GelMA hydrogel ameliorates osteoarthritis by effectively regulating autophagy. Biomaterials 2016, 81, 1–13. [Google Scholar] [CrossRef]
- Sun, Y.; Yao, Y.; Ding, C.Z. A combination of sinomenine and methotrexate reduces joint damage of collagen induced arthritis in rats by modulating osteoclast-related cytokines. Int. Immunopharmacol. 2014, 18, 135–141. [Google Scholar] [CrossRef]
- Zhou, Y.R.; Zhao, Y.; Bao, B.H.; Li, J.X. SND-117, a sinomenine bivalent alleviates type II collagen-induced arthritis in mice. Int. Immunopharmacol. 2015, 26, 423–431. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, M.; Wang, W.; Lang, Y.; Tong, Z.; Wang, K.; Zhang, H.; Chen, G.; Liu, M.; Yao, Y.; et al. Sinomenine hydrochloride protects against polymicrobial sepsis via autophagy. Int. J. Mol. Sci. 2015, 16, 2559–2573. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Chen, C.; Liu, W.; Fu, Q.; Han, Z.; Li, Y.; Feng, S.; Li, X.; Qi, C.; Wu, J.; et al. Injectable microcryogels reinforced alginate encapsulation of mesenchymal stromal cells for leak-proof delivery and alleviation of canine disc degeneration. Biomaterials 2015, 59, 53–65. [Google Scholar] [CrossRef]
- Xing, D.; Liu, W.; Wang, B.; Li, J.J.; Zhao, Y.; Li, H.; Liu, A.; Du, Y.; Lin, J. Intra-articular Injection of Cell-laden 3D Microcryogels Empower Low-dose Cell Therapy for Osteoarthritis in a Rat Model. Cell Transplant. 2020, 29, 963689720932142. [Google Scholar] [CrossRef]
- Hanafy, A.S.; El-Ganainy, S.O. Thermoresponsive Hyalomer intra-articular hydrogels improve monoiodoacetate-induced osteoarthritis in rats. Int. J. Pharm. 2020, 573, 118859. [Google Scholar] [CrossRef] [PubMed]
- Stefani, R.M.; Lee, A.J.; Tan, A.R.; Halder, S.S.; Hu, Y.; Guo, X.E.; Stoker, A.M.; Ateshian, G.A.; Marra, K.G.; Cook, J.L.; et al. Sustained low-dose dexamethasone delivery via a PLGA microsphere-embedded agarose implant for enhanced osteochondral repair. Acta Biomater. 2020, 102, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.M.M.; Sequeira, A.; Garcia, A.J.; Guldberg, R.E. Articular Cartilage- and Synoviocyte-Binding Poly(ethylene glycol) Nanocomposite Microgels as Intra-Articular Drug Delivery Vehicles for the Treatment of Osteoarthritis. ACS Biomater. Sci. Eng. 2020, 6, 5084–5095. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhu, Y.; Wang, F.; Deng, L.; Xu, X.; Cui, W. Microfluidic liposomes-anchored microgels as extended delivery platform for treatment of osteoarthritis. Chem. Eng. J. 2020, 400, 126004. [Google Scholar] [CrossRef]
- Qi, X.; Qin, X.; Yang, R.; Qin, J.; Li, W.; Luan, K.; Wu, Z.; Song, L. Intra-articular Administration of Chitosan Thermosensitive In Situ Hydrogels Combined With Diclofenac Sodium-Loaded Alginate Microspheres. J. Pharm. Sci. 2016, 105, 122–130. [Google Scholar] [CrossRef]
- Mwangi, T.K.; Bowles, R.D.; Tainter, D.M.; Bell, R.D.; Kaplan, D.L.; Setton, L.A. Synthesis and characterization of silk fibroin microparticles for intra-articular drug delivery. Int. J. Pharm. 2015, 485, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Pereira, D.R.; Tapeinos, C.; Rebelo, A.L.; Oliveira, J.M.; Reis, R.L.; Pandit, A. Scavenging Nanoreactors that Modulate Inflammation. Adv. Biosyst. 2018, 2, 1800086. [Google Scholar] [CrossRef]
- Horisawa, E.; Kubota, K.; Tuboi, I.; Sato, K.; Yamamoto, H.; Takeuchi, H.; Kawashima, Y. Size-dependency of DL-lactide glycolide copolymer particulates for Intra-Articular Delivery System on Phagocytosis in Rat Synovium. Pharm. Res. 2002, 19, 132–139. [Google Scholar] [CrossRef]
- Kang, M.L.; Ko, J.Y.; Kim, J.E.; Im, G.I. Intra-articular delivery of kartogenin-conjugated chitosan nano/microparticles for cartilage regeneration. Biomaterials 2014, 35, 9984–9994. [Google Scholar] [CrossRef]
- Cheng, H.; Chewla, A.; Yang, Y.F.; Li, Y.X.; Zhang, J.; Jang, H.L.; Khademhosseini, A. Development of nanomaterials for bone-targeted drug delivery. Drug Discov. Today 2017, 22, 1336–1350. [Google Scholar] [CrossRef]
- Cheng, Y.Y.; Xu, T.W. The effect of dendrimers on the pharmacodynamic and pharmacokinetic behaviors of non-covalently or covalently attached drugs. Eur. J. Med. Chem. 2008, 43, 2291–2297. [Google Scholar] [CrossRef]
- Yang, W.J.; Li, Y.W.; Cheng, Y.Y.; Wu, Q.L.; Wen, L.P.; Xu, T.W. Evaluation of Phenylbutazone and Poly(amidoamine) Dendrimers Interactions by a Combination of Solubility, 2D-NOESY NMR, and Isothermal Titration Calorimetry Studies. J. Pharm. Sci. 2009, 98, 1075–1085. [Google Scholar] [CrossRef]
- Cheng, Y.Y.; Wu, Q.L.; Li, Y.W.; Hu, J.J.; Xu, T.W. New Insights into the Interactions between Dendrimers and Surfactants: 2. Design of New Drug Formulations Based on Dendrimer-Surfactant Aggregates. J. Phys. Chem. B 2009, 113, 8339–8346. [Google Scholar] [CrossRef]
- Zhao, L.B.; Cheng, Y.Y.; Hu, J.J.; Wu, Q.L.; Xu, T.W. Host-Guest Chemistry of Dendrimer-Drug Complexes. 3. Competitive Binding of Multiple Drugs by a Single Dendrimer for Combination Therapy. J. Phys. Chem. B 2009, 113, 14172–14179. [Google Scholar] [CrossRef]
- Yang, K.; Weng, L.A.; Cheng, Y.Y.; Zhang, H.F.; Zhang, J.H.; Wu, Q.L.; Xu, T.W. Host-Guest Chemistry of Dendrimer-Drug Complexes. 6. Fully Acetylated Dendrimers as Biocompatible Drug Vehicles Using Dexamethasone 21-Phosphate as a Model Drug. J. Phys. Chem. B 2011, 115, 2185–2195. [Google Scholar] [CrossRef]
- Bajpayee, A.G.; Quadir, M.A.; Hammond, P.T.; Grodzinsky, A.J. Charge based intra-cartilage delivery of single dose dexamethasone using Avidin nano-carriers suppresses cytokine-induced catabolism long term. Osteoarthr. Cartil. 2016, 24, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Hubbell, J.A.; Thomas, S.N.; Swartz, M.A. Materials engineering for immunomodulation. Nature 2009, 462, 449–460. [Google Scholar] [CrossRef] [Green Version]
- Ding, Q.H.; Cheng, Y.; Chen, W.P.; Zhong, H.M.; Wang, X.H. Celastrol, an inhibitor of heat shock protein 90beta potently suppresses the expression of matrix metalloproteinases, inducible nitric oxide synthase and cyclooxygenase-2 in primary human osteoarthritic chondrocytes. Eur. J. Pharmacol. 2013, 708, 1–7. [Google Scholar] [CrossRef]
- Sani, A.; Cao, C.; Cui, D. Toxicity of gold nanoparticles (AuNPs): A review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef]
- Carnovale, C.; Bryant, G.; Shukla, R.; Bansal, V. Identifying trends in gold nanoparticle toxicity and uptake: Size, shape, capping ligand, and biological corona. ACS Omega 2019, 4, 242–256. [Google Scholar] [CrossRef] [Green Version]
- Soenen, S.J.; Manshian, B.; Montenegro, J.M.; Amin, F.; Meermann, B.; Thiron, T.; Cornelissen, M.; Vanhaecke, F.; Doak, S.; Parak, W.J.; et al. Cytotoxic effects of gold nanoparticles: A multiparametric study. ACS Nano 2012, 6, 5767–5783. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Dou, Y.; Xie, L.H.; Rutledge, W.; Li, J.R.; Zhou, H.C. Zr-based metal-organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zheng, M.; Xie, Z. Nanoscale metal-organic frameworks for drug delivery: A conventional platform with new promise. J. Mater. Chem. B 2018, 6, 707–717. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Liu, L.; Lin, L.; Liu, F.; Xie, Z.; Tian, H.; Chen, X. Engineering Metal-Organic Frameworks for Photoacoustic Imaging-Guided Chemo-/Photothermal Combinational Tumor Therapy. ACS Appl. Mater. Interfaces 2018, 10, 41035–41045. [Google Scholar] [CrossRef]
- Cherkasov, V.R.; Mochalova, E.N.; Babenyshev, A.V.; Rozenberg, J.M.; Sokolov, I.L.; Nikitin, M.P. Antibody-directed metal-organic framework nanoparticles for targeted drug delivery. Acta Biomater. 2020, 103, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Lin, L.; Zhang, Y.; Wang, Y.; Sheng, S.; Xu, C.; Tian, H.; Chen, X. A Tumor-Microenvironment-Activated Nanozyme-Mediated Theranostic Nanoreactor for Imaging-Guided Combined Tumor Therapy. Adv. Mater. 2019, 31, e1902885. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, L.; Liu, L.; Liu, F.; Sheng, S.; Tian, H.; Chen, X. Positive feedback nanoamplifier responded to tumor microenvironments for self-enhanced tumor imaging and therapy. Biomaterials 2019, 216, 119255. [Google Scholar] [CrossRef]
- Mazaleuskaya, L.L.; Muzykantov, V.R.; FitzGerald, G.A. Nanotherapeutic-directed approaches to analgesia. Trends Pharmacol. Sci. 2021, 42, 527–550. [Google Scholar] [CrossRef]
- Kosinska, M.K.; Liebisch, G.; Lochnit, G.; Wilhelm, J.; Klein, H.; Kaesser, U.; Lasczkowski, G.; Rickert, M.; Schmitz, G.; Steinmeyer, J. A lipidomic study of phospholipid classes and species in human synovial fluid. Arthritis Rheum. 2013, 65, 2323–2333. [Google Scholar] [CrossRef]
- Sarma, A.V.; Powell, G.L.; LaBerge, M. Phospholipid composition of articular cartilage boundary lubricant. J. Orthop. Res. 2001, 19, 671–676. [Google Scholar] [CrossRef]
- Anderson, R.; Franch, A.; Castell, M.; Perez-Cano, F.J.; Bräuer, R.; Pohlers, D.; Gajda, M.; Siskos, A.P.; Katsila, T.; Tamvakopoulos, C.; et al. Liposomal encapsulation enhances and prolongs the anti-inflammatory effects of water-soluble dexamethasone phosphate in experimental adjuvant arthritis. Arthritis Res. Ther. 2010, 12, R147. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Kampf, N.; Goldberg, R.; Driver, M.J.; Klein, J. Poly-phosphocholinated Liposomes Form Stable Superlubrication Vectors. Langmuir 2019, 35, 6048–6054. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [Green Version]
- Cipollaro, L.; Trucillo, P.; Bragazzi, N.L.; Della Porta, G.; Reverchon, E.; Maffulli, N. Liposomes for Intra-Articular Analgesic Drug Delivery in Orthopedics: State-of-Art and Future Perspectives. Insights from a Systematic Mini-Review of the Literature. Medicina 2020, 56, 423. [Google Scholar] [CrossRef]
- Al-Jamal, W.T.; Kostarelos, K. Liposomes from a clinically established drug delivery system to a nanoparticle platform for theranostic nanomedicine. Acc. Chem. Res. 2011, 44, 1094–1104. [Google Scholar] [CrossRef]
- Zucker, D.; Marcus, D.; Barenholz, Y.; Goldblum, A. Liposome drugs’ loading efficiency: A working model based on loading conditions and drug’s physicochemical properties. J. Control. Release 2009, 139, 73–80. [Google Scholar] [CrossRef]
- Bonanomi, M.H.; Velvart, M.; Stimpel, M.; Roos, K.M.; Fehr, K.; Weder, H.G. Studies of pharmacokinetics and therapeutic effects of glucocorticoids entrapped in liposomes after intraarticular application in healthy rabbits and in rabbits with antigen-induced arthritis. Rheumatol. Int. 1987, 7, 203–212. [Google Scholar] [CrossRef]
- Zhu, Q.; Sun, Y.H.; Zhu, J.; Fang, T.; Zhang, W.; Li, J.X. Antinociceptive effects of sinomenine in a rat model of neuropathic pain. Sci. Rep. 2014, 4, 7270. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef] [Green Version]
- An, I.; Van Den Bulcke, B.B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and Rheological Properties of Methacrylamide Modified Gelatin Hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef]
- Benton, J.A.; DeForest, C.A.; Vivekanandan, V.; Anseth, K.S. Photocrosslinking of Gelatin Macromers to Synthesize Porous Hydrogels That Promote Valvular Interstitial Cell Function. Tissue Eng. Part A 2009, 15, 3221–3230. [Google Scholar] [CrossRef] [PubMed]
- Mero, A.; Campisi, M. Hyaluronic Acid Bioconjugates for the Delivery of Bioactive Molecules. Polymers 2014, 6, 346–369. [Google Scholar] [CrossRef] [Green Version]
- Mero, A.; Pasqualin, M.; Campisi, M.; Renier, D.; Pasut, G. Conjugation of hyaluronan to proteins. Carbohydr. Polym. 2013, 92, 2163–2170. [Google Scholar] [CrossRef] [PubMed]
- Schante, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohyd. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Springer, J.; Kooy, F.K.; van den Broek, L.A.M.; Eggink, G. Production Methods for Hyaluronan. Int. J. Carbohydr. Chem. 2013, 2013, 1–14. [Google Scholar] [CrossRef] [Green Version]



| Carrier/Materials | Size | Drug/Active Compound | Therapeutic Mechanism of Drug | Advantages of the Formulation | Ref. |
|---|---|---|---|---|---|
| Microparticles | |||||
| Poly(lactic-co-glycolic acid) (PLGA) | About 10 µm | Tetramethylpyrazine (TMP) | Alleviating IL-1-induced destruction of cartilage and chondrocytes, reducing the degradation of glycosaminoglycan, increasing chondrocyte activity, and inhibiting chondrocyte apoptosis; promoting chondrocyte proliferation by promoting cell cycle progression [67,68,69] | Prolong the retention time of TMP to 10 days | [67] |
| PLGA | ca. 250 µm | Ibuprofen | Inhibitor of cycloxygenase-2 enzyme [70,71] | Sustained drug release for 63 days, porous microspheres with light texture, strong bearing capacity, and fast diffusion rate | [71] |
| Polyester amide (PEA) | 5, 15–20 µm (PDI 1.6) | Celecoxib | An anti-inflammatory drug that has been shown to be an effective analgesic for OA-related pain [72] | Auto-regulatory behavior, slow release for > 80 days, good intra-articular biocompatibility | [73] |
| PLGA, PEA | 39.4 µm, PDI 1.30 (PLGA MPs) 23.8 µm, PDI 1.30 (PEA MPs) | Triamcinolone acetonide (TAA) | Inhibiting pain and inflammation for prolonged periods in OA knee joints [74,75] | Reduced synovitis and alleviating pain for at least 42 days | [74] |
| Poly(3-hydroxybutyrate-co-ε-caprolactone) (PHBCL) copolymers | 0.5 and 4.5 µm | Diclofenac sodium | An anti-inflammatory drug that inhibits various enzymes and blocks the synthesis of prostaglandin [76,77] | Slow release | [76] |
| Gelatin, gelatin/silk fibroin | 100–300 μm | Curcumin | Reduced the level of IL-6 in serum, delayed the cellular destruction in the articular joint and synovial tissue [78] | Lasting anti-inflammatory effect | [78] |
| Chitosan, tripolyphosphate (TPP) | 3.57 to 6.12 µm | Lornoxicam | Inhibiting cyclo-oxygenase (COX), key enzyme of arachidonic acid pathway, thus inhibiting prostaglandin synthesis [79,80] | Long-term anti-inflammatory effects | [79] |
| Heparin | 80 ± 60 µm | Hep-N tumor necrosis factor-alpha stimulated gene-6 (TSG-6) | Suppressing the response of chondrocytes to inflammatory factors, such as IL-1 and TNF-α [81,82] | Heparin sulfation significantly enhanced anti-plasmin activity of TSG-6, reducing cartilage injury, and protecting the bone and joint | [81] |
| Silver alginate microcapsules | 1.3 ± 0.2 µm | Betamethasone dipropionate (Bm) | Inhibiting inflammation [83] | Improve the bioavailability and effectiveness compared to free Bm | [83] |
| Poly(sodium-p-styrene sulfonate)/poly(allylamine hydrochloride) (PSS-PAH) | ca. 50 nm | MnO2 | MnO2 nanoparticles can be used as reactive oxygen scavengers that mimic catalase and superoxide dismutase (SOD) activity simultaneously [84,85] | Robust capsules capable of multiple re-use and resisting ethanol sterilization | [84] |
| Nanoparticles | |||||
| Poly(2-hydroxyethyl methacrylate)-pyridine | 300–700 nm | IL-1Ra | Binding to the IL-1 receptor (IL-1R) without triggering an agonist response, and thus functioning as a receptor antagonist [86,87] | Good biocompatibility and stability | [86] |
| PEGylated cationic polyamidoamine (PAMAM) dendrimer | <15 nm | Insulin-like growth factor 1 (IGF-1) | Promotes chondrocyte survival, proliferation, and biosynthesis of cartilage matrix macromolecules; anti-inflammatory effects [88,89] | Targeting and improved residence time | [90] |
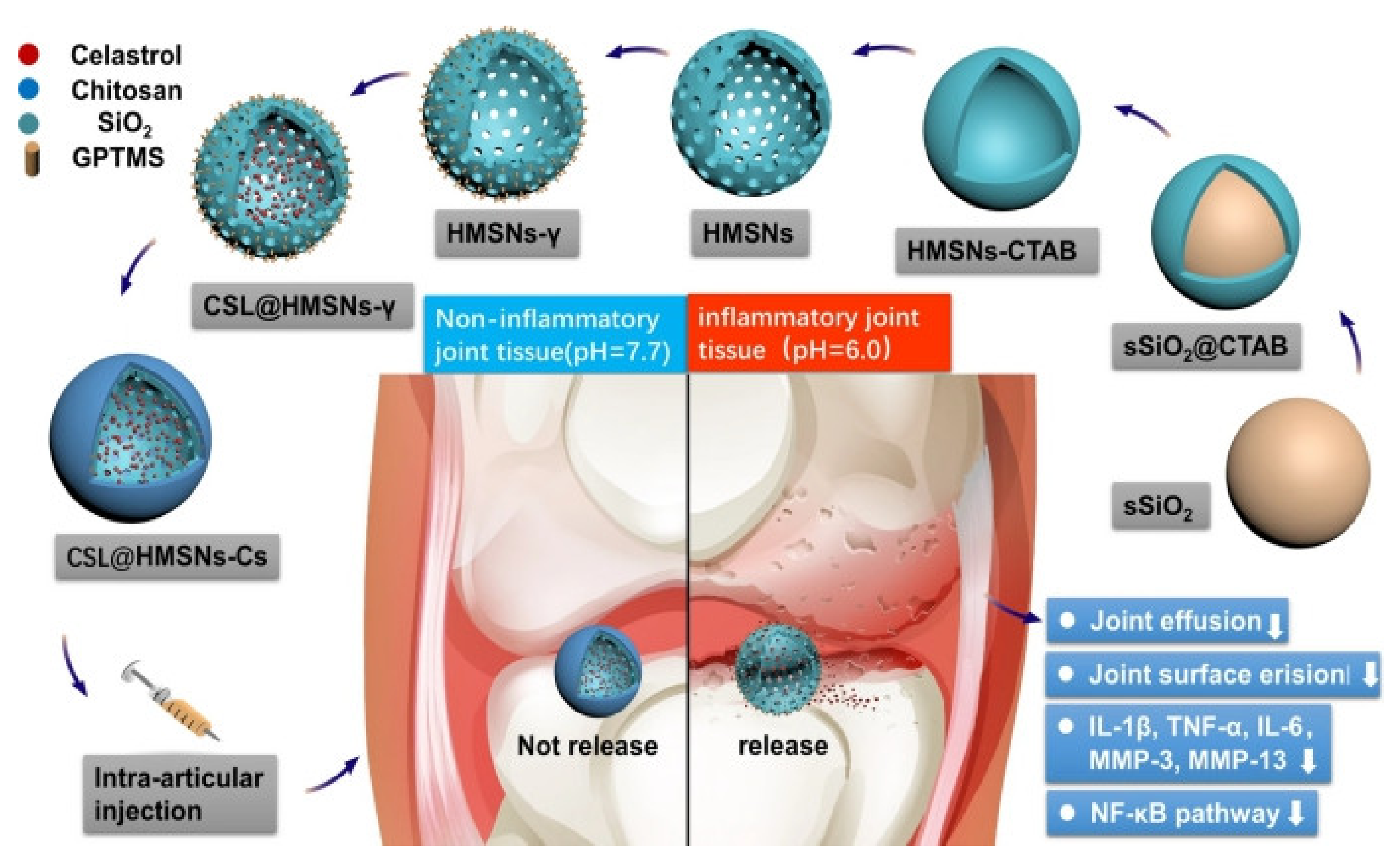
| Hollow mesoporous silica nanoparticles capped with chitosan | 260.76–290.17 nm | Celastrol | Celastrol can block the secretion of IL-1β and TNF in OA animals, celastrol can eliminate the infiltration and proliferation of immune cells and prevent cartilage and bone damage [91,92] | pH-responsive, huge loading capacity, and good biocompatibility | [91] |
| Poly(2-methacryloyloxyethyl phosphorylcholine)-grafted mesoporous silica nanospheres (MSNs-NH2@PMPC) | 180–260 nm | Diclofenac sodium | Analgesic and anti-inflammatory effects by inhibiting various enzymes and blocking the synthesis of prostaglandin. [77] | Super lubricating effect, high drug load, and slow release | [93] |
| Azobenzene-modified mesoporous silica nanoparticles with β-cyclodextrin-modified poly(2-methacryloy- loxyethyl phosphorylcholine) (bMSNs-AZO/DS/CD-PMPC) | Ca. 100 nm | Diclofenac sodium | Analgesic and anti-inflammatory effects by inhibiting various enzymes and blocking the synthesis of prostaglandin. [93] | Light-responsive drug release and super lubrication property | [94] |
| Gold nanoparticles | 20 nm | Gold (Au) compounds | Suppression of lysosomal enzyme release by the phagocytic cells, modulation of prostaglandins, the suppression of synovial cells proliferation, and collagen synthesis [95,96,97,98] | Better therapeutic effect | [95] |
| PLA-Cy7 | 10–25 µm | KGN | Enhance cartilage regeneration of bone marrow mesenchymal stem cells (BMSCs) [99,100] | Cartilage protective effect and a high drug loading | [99] |
| MIL-100 (Fe) | Around 100 nm | Protocatechuic acid (PCA) | Anti-inflammatory and antibacterial, downregulate the indicators of inflammatory factors, including inducible nitric oxide synthase (iNOS), cyclo-oxygenase-2 (COX2), and metalloproteinase with thrombospondin motifs (ADAMTSs) [101] | High loading, good biocompatibility, and pH responsiveness | [101] |
| Liposomes | |||||
| HSPC and DOTAP | 500–900 nm | 6-methoxy-2-naphthylacetic acid (6-MNA) and its double salt with DSPE | A nonsteroidal anti-inflammatory drug, a potential and selective inhibitor of cyclo-oxygenase-2 enzyme [70] | Good biocompatibility, a long half-life (21–27 h), and tendency to penetrate well into synovial fluid | [70] |
| DSPC, cholesterol, and octadecylamine | 135.17 ± 28.3 nm | Rapamycin | The potential therapeutic effect of rapamycin is the mammalian target of rapamycin (mTOR), which regulates many cellular processes, such as growth, proliferation, and protein synthesis to reduce the severity of osteoarthritis [102,103,104] | Together with low-intensity pulsed ultrasound (LIPUS), reduced drug dose and administration frequency | [102] |
| Gels | |||||
| Chitosan microspheres (CMS) in hydrogel of photo-crosslinked gelatin methacrylate (GelMA) | ca. 100 µm | Sinomeniumis | The level of matrix metallopeptidase 13 (MMP-13) protein, a marker of cartilage degradation in rats, was decreased through the NF-κB signaling pathway, the pathogenesis of collagen-induced arthritis was blocked, and the expression of MMP13 was down-regulated; regulating autophagy, and attenuating the release of inflammatory cytokines [105,106,107,108] | Low degradation rate and high swelling ratio of GelMA hydrogel, controlled release of drugs | [105] |
| Three-dimensional (3D) gelatin-based microcyrogel | - | Mesenchymal stem cells (MSCs) | Anti-inflammatory and pro-regenerative paracrine functions of the MSCs within the lesion site [109,110] | Minimized cell dose while retaining therapeutic effects | [110] |
| HA, poloxamer 407 | - | HA, and diclofenac potassium (DK) | Joint lubrication, inhibiting various enzymes and blocking the synthesis of prostaglandin [77,111] | Extended release time of drug, thermo-responsive | [111] |
| PLGA MPs in acellular agarose hydrogel | 46 ± 17 µm | Dexamethasone (DEX) | Anticatabolic and pro-anabolic effects on cartilage [112] | Targeted, low DEX dose carrier with improved effects | [112] |
| PEG-4MAL microgel, PLGA NPs, synoviocyte- or cartilage-targeting peptides | 50.4–51.1 µm | Model small molecules | - | Long retention time (>3 weeks) in the joint, synoviocyte- or cartilage-targeting | [113] |
| Lectin-cholesterol liposome in cross-linked gelatin methacryloyl (GelMA) microgel | 75–145 µm | KGN | Enhancing cartilage regeneration of bone marrow mesenchymal stem cells (BMSCs) [114] | Slow release of KGN | [114] |
| Alginate microspheres in thermosensitive chitosan and β-glycerophosphate hydrogel | 10.744 ± 1.246 µm (alginate microparticles) | Diclofenac sodium | Inhibiting various enzymes and blocking the synthesis of prostaglandin [77,115] | Thermo-sensitive, sustained drug release (5 days), biocompatible, superior anti-inflammatory effect | [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Ma, Y.; Tao, Y.; Lin, W.; Wang, P. Intra-Articular Drug Delivery for Osteoarthritis Treatment. Pharmaceutics 2021, 13, 2166. https://doi.org/10.3390/pharmaceutics13122166
Cao Y, Ma Y, Tao Y, Lin W, Wang P. Intra-Articular Drug Delivery for Osteoarthritis Treatment. Pharmaceutics. 2021; 13(12):2166. https://doi.org/10.3390/pharmaceutics13122166
Chicago/Turabian StyleCao, Yifeng, Yifeng Ma, Yi Tao, Weifeng Lin, and Ping Wang. 2021. "Intra-Articular Drug Delivery for Osteoarthritis Treatment" Pharmaceutics 13, no. 12: 2166. https://doi.org/10.3390/pharmaceutics13122166
APA StyleCao, Y., Ma, Y., Tao, Y., Lin, W., & Wang, P. (2021). Intra-Articular Drug Delivery for Osteoarthritis Treatment. Pharmaceutics, 13(12), 2166. https://doi.org/10.3390/pharmaceutics13122166






