Nanoencapsulation of Pomegranate Extract to Increase Stability and Potential Dermatological Protection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cauliflower Inflorescences Vesicles (CI-Vesicles)
2.3. Particle Size, Zeta Potential, and Polydispersity Index Analysis of Cauliflower Inflorescences Vesicles (CI-Vesicles)
2.4. Pomegranate Extract Encapsulation and Entrapment Efficiency (EE)
2.5. Protein Content
2.6. Color
2.7. Antioxidant Capacity
2.8. HaCaT Cells Culture
2.9. Applied Treatments and Stresses (Heavy Metals and UV-B Radiation)
2.10. Cell Viability (MTT Assay)
2.11. Lipid Peroxidation Levels
2.12. DNA Extraction and Analysis and Quantitative PCR
2.13. Statistical Analyses
3. Results
3.1. Physicochemical and Morphological Characterization
3.2. Pomegranate Extract Entrapment Efficiency (EE)
3.3. Stability of CI-Vesicles with Encapsulated PG-E
3.4. Antioxidant Activity
3.5. Cytotoxic Effects in HaCaT Cells of PG-E Encapsulated in CI-Vesicles
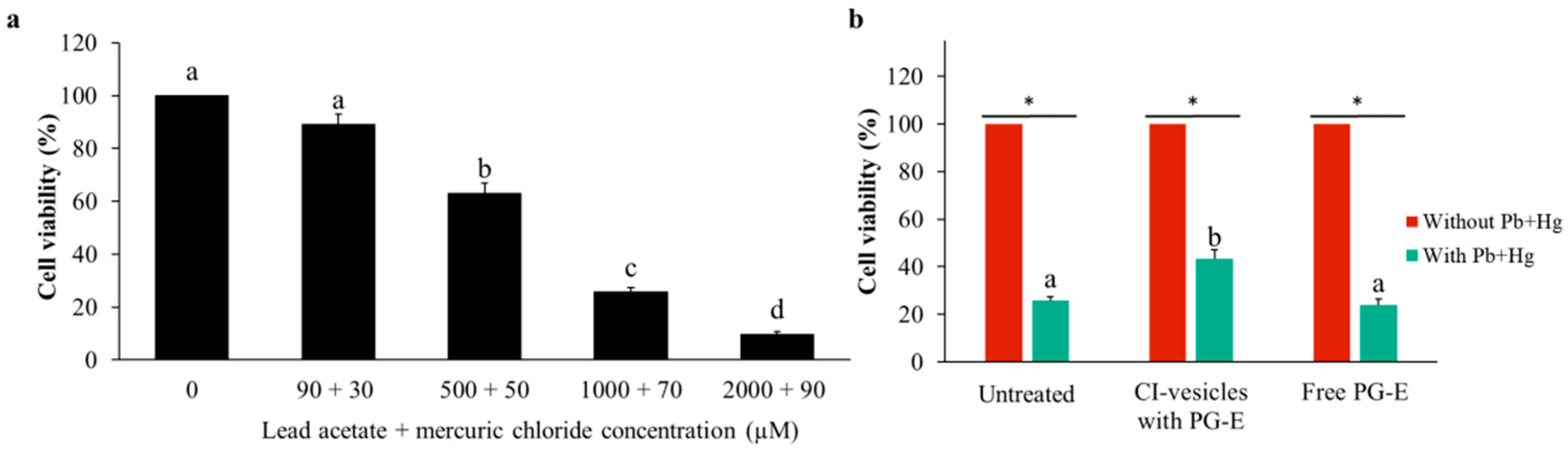
3.6. Effect of CI-Vesicles Containing PG-E Against Heavy Metals in HaCaT Cells
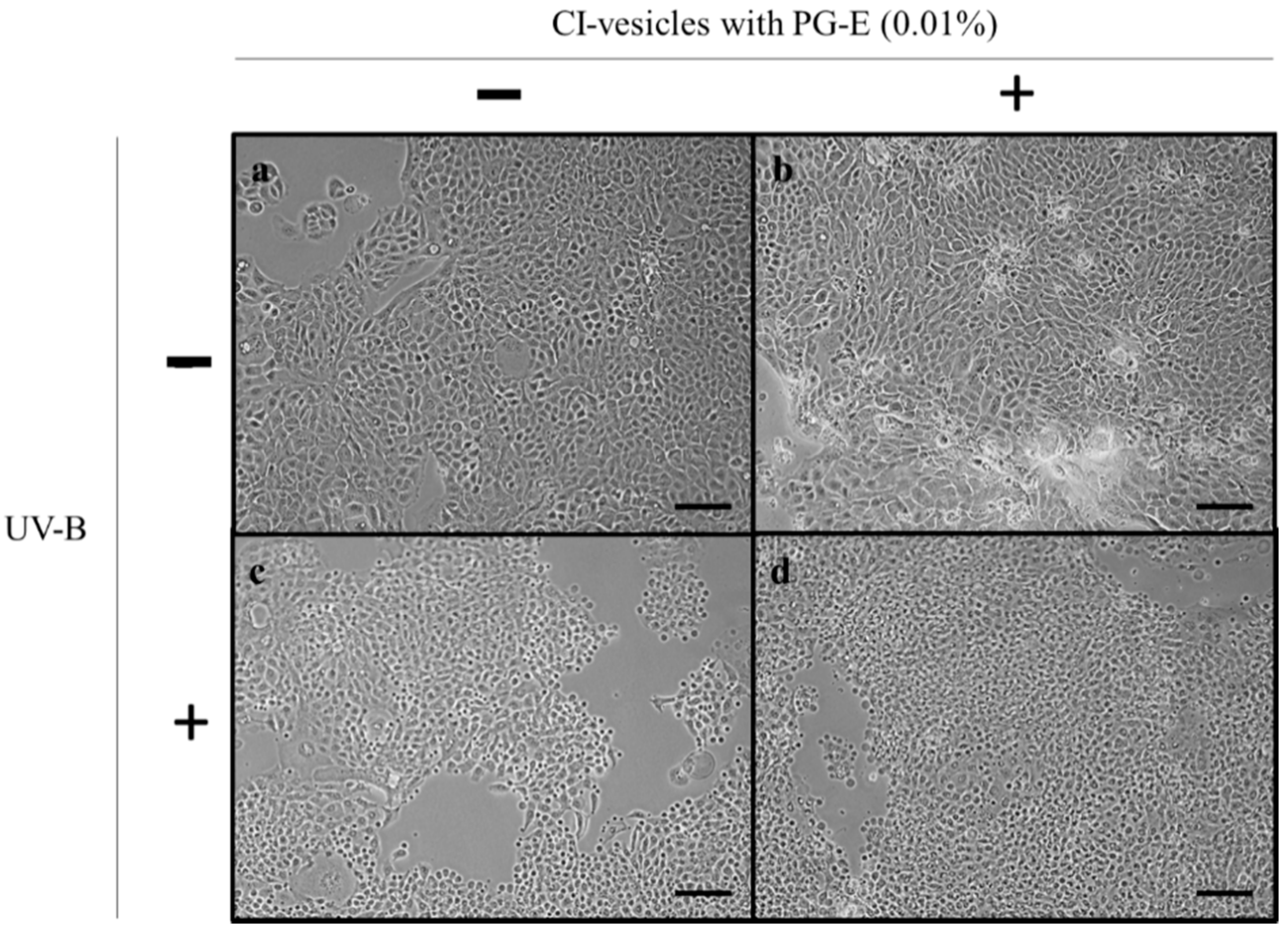
3.7. Effect of CI-Vesicles Containing PG-E in UV-Irradiated HaCaT Cells
3.8. Effect of CI-Vesicles with PG-E on MtDNA Common Deletion in UV-Irradiated HaCaT Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jurenka, J. Therapeutic Applications of Pomegranate (Punica granatum L.): A Review. Altern. Med. Rev. 2008, 13, 128–144. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomas-Barberan, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant Activity of Pomegranate Juice and Its Relationship with Phenolic Composition and Processing. J. Agric. Food Chem. 2000, 48, 4581–4598. [Google Scholar] [CrossRef]
- Russo, M.; Fanali, C.; Tripodo, G.; Dugo, P.; Muleo, R.; Dugo, L.; de Gara, L.; Mondello, L. Analysis of Phenolic Compounds in Different Parts of Pomegranate (Punica granatum) Fruit by HPLC-PDA-ESI/MS and Evaluation of Their Antioxidant Activity: Application to Different Italian Varieties. Anal. Bioanal. Chem. 2018, 410, 3507–3520. [Google Scholar] [CrossRef]
- Liu, G.; Xu, X.; Hao, Q.; Gao, Y. Supercritical CO2 Extraction Optimization of Pomegranate (Punica granatum L.) Seed Oil Using Response Surface Methodology. LWT Food Sci. Technol. 2009, 42, 1491–1495. [Google Scholar] [CrossRef]
- Hernández, F.; Melgarejo, P.; Tomás-Barberán, F.A.; Artés, F. Evolution of Juice Anthocyanins during Ripening of New Selected Pomegranate (Punica granatum) Clones. Eur. Food Res. Technol. 1999, 210, 39–42. [Google Scholar] [CrossRef]
- Espín, J.C.; García-Conesa, M.T.; Tomás-Barberán, F.A. Nutraceuticals: Facts and Fiction. Phytochemistry 2007, 68, 2986–3008. [Google Scholar] [CrossRef]
- Katz, S.R.; Newman, R.A.; Lansky, E.P. Punica granatum: Heuristic Treatment for Diabetes Mellitus. J. Med. Food 2007, 10, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Lansky, E.P.; Newman, R.A. Punica granatum (pomegranate) and Its Potential for Prevention and Treatment of Inflammation and Cancer. J. Ethnopharmacol. 2007, 109, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Sohrab, G.; Nasrollahzadeh, J.; Zand, H.; Amiri, Z.; Tohidi, M.; Kimiagar, M. Effects of Pomegranate Juice Consumption on Inflammatory Markers in Patients with Type 2 Diabetes: A Randomized, Placebo-Controlled Trial. J. Res. Med. Sci. 2014, 19, 215–220. [Google Scholar] [PubMed]
- Hosseini, B.; Saedisomeolia, A.; Wood, L.G.; Yaseri, M.; Tavasoli, S. Effects of Pomegranate Extract Supplementation on Inflammation in Overweight and Obese Individuals: A Randomized Controlled Clinical Trial. Complementary Ther. Clin. Pract. 2016, 22, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Hossin, F.L.A. Effect of Pomegranate (Punica granatum) Peels and It’s Extract on Obese Hypercholesterolemic Rats. Pak. J. Nutr. 2009, 8, 1251–1257. [Google Scholar] [CrossRef] [Green Version]
- Afaq, F.; Zaid, M.A.; Khan, N.; Dreher, M.; Mukhtar, H. Protective Effect of Pomegranate-Derived Products on UVB-Mediated Damage in Human Reconstituted skin. Exp. Dermatol. 2009, 18, 553–561. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Guo, H.; DaSilva, N.A.; Li, D.; Zhang, K.; Wan, Y.; Gao, X.-H.; Chen, H.-D.; Seeram, N.P.; Ma, H. Pomegranate (Punica granatum) Phenolics Ameliorate Hydrogen Peroxide-Induced Oxidative Stress and Cytotoxicity in Human Keratinocytes. J. Funct. Foods 2019, 54, 559–567. [Google Scholar] [CrossRef]
- Baroni, A.; Buommino, E.; de Gregorio, V.; Ruocco, E.; Ruocco, V.; Wolf, R. Structure and Function of the Epidermis Related to Barrier Properties. Clin. Dermatol. 2012, 30, 257–262. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kappus, H.; Reinhold, C. Heavy Metal-Induced Cytotoxicity to Cultured Human Epidermal Keratinocytes and Effects of Antioxidants. Toxicol. Lett. 1994, 71, 105–109. [Google Scholar] [CrossRef]
- Aitken, G.R.; Henderson, J.R.; Chang, S.C.; McNeil, C.J.; Birch-Machin, M.A. Direct Monitoring of UV-Induced Free Radical Generation in HaCaT Keratinocytes. Clin. Exp. Dermatol. 2007, 32, 722–727. [Google Scholar] [CrossRef]
- Nzengue, Y.; Steiman, R.; Garrel, C.; Lefèbvre, E.; Guiraud, P. Oxidative Stress and DNA Damage Induced by Cadmium in the Human Keratinocyte HaCaT Cell Line: Role of Glutathione in the Resistance to Cadmium. Toxicology 2008, 243, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA Damage: Mechanisms, Mutation, and Disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakes, F.M.; Chen, Y.; van Houten, B. PCR-Based Assays for the Detection and Quantitation of DNA Damage and Repair. In BT-Technologies for Detection of DNA Damage and Mutations; Pfeifer, G.P., Ed.; Springer: Boston, MA, USA, 1996; pp. 171–184. ISBN 978-1-4899-0301-3. [Google Scholar]
- Shoffner, J.M.; Lott, M.T.; Voljavec, A.S.; Soueidan, S.A.; Costigan, D.A.; Wallace, D.C. Spontaneous Kearns-Sayre/Chronic External Ophthalmoplegia Plus Syndrome Associated with a Mitochondrial DNA Deletion: A Slip-Replication Model and Metabolic Therapy. Proc. Natl. Acad. Sci. USA 1989, 86, 7952–7956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaeger, A.; Weiss, D.G.; Jonas, L.; Kriehuber, R. Oxidative Stress-Induced Cytotoxic and Genotoxic Effects of Nano-Sized Titanium Dioxide Particles in Human HaCaT Keratinocytes. Toxicology 2012, 296, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Palencia, L.A.; Noratto, G.; Hingorani, L.; Talcott, S.T.; Mertens-Talcott, S.U. Protective Effects of Standardized Pomegranate (Punica granatum L.) Polyphenolic Extract in Ultraviolet-Irradiated Human Skin Fibroblasts. J. Agric. Food Chem. 2008, 56, 8434–8441. [Google Scholar] [CrossRef] [PubMed]
- Ventura, J.; Alarcón-Aguilar, F.; Roman-Ramos, R.; Campos-Sepulveda, E.; Reyes-Vega, M.L.; Daniel Boone-Villa, V.; Jasso-Villagómez, E.I.; Aguilar, C.N. Quality and Antioxidant Properties of a Reduced-Sugar Pomegranate Juice Jelly with an Aqueous Extract of Pomegranate Peels. Food Chem. 2013, 136, 109–115. [Google Scholar] [CrossRef]
- Çam, M.; Içyer, N.C.; Erdoǧan, F. Pomegranate Peel Phenolics: Microencapsulation, Storage Stability and Potential Ingredient for Functional Food Development. LWT Food Sci. Technol. 2014, 55, 117–123. [Google Scholar] [CrossRef]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.B.; Wang, B.; Zhang, L.; et al. Grape Exosome-Like Nanoparticles Induce Intestinal Stem Cells and Protect Mice from DSS-Induced Colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- György, B.; Hung, M.E.; Breakefield, X.O.; Leonard, J.N. Therapeutic Applications of Extracellular Vesicles: Clinical Promise and Open Questions. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 439–464. [Google Scholar] [CrossRef] [Green Version]
- Yepes-Molina, L.; Martínez-Ballesta, M.C.; Carvajal, M. Plant Plasma Membrane Vesicles Interaction with Keratinocytes Reveals Their Potential as Carriers. J. Adv. Res. 2020, 23, 101–111. [Google Scholar] [CrossRef]
- Peng, L.-H.; Wang, M.-Z.; Chu, Y.; Zhang, L.; Niu, J.; Shao, H.-T.; Yuan, T.-J.; Jiang, Z.-H.; Gao, J.-Q.; Ning, X.-H. Engineering Bacterial Outer Membrane Vesicles as Transdermal Nanoplatforms for Photo-TRAIL—Programmed Therapy Against Melanoma. Sci. Adv. 2020, 6, eaba2735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Viennois, E.; Xu, C.; Merlin, D. Plant Derived Edible Nanoparticles as a New Therapeutic Approach Against Diseases. Tissue Barriers 2016, 4, e1134415. [Google Scholar] [CrossRef] [PubMed]
- Martínez Ballesta, M.C.; Pérez-Sánchez, H.; Moreno, D.A.; Carvajal, M. Plant Plasma Membrane Aquaporins in Natural Vesicles as Potential Stabilizers and Carriers of Glucosinolates. Colloids Surf. B Biointerfaces 2016, 143, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Martínez Ballesta, M.C.; García-Gomez, P.; Yepes-Molina, L.; Guarnizo, A.L.; Teruel, J.A.; Carvajal, M. Plasma Membrane Aquaporins Mediates Vesicle Stability in Broccoli. PLoS ONE 2018, 13, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Ibañez, P.; Nicolas-Espinosa, J.; Carvajal, M. Plasma Membrane Vesicles from Cauliflower Meristematic Tissue and Their Role in Water Passage. BMC Plant Biol. 2021, 21, 30. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rios, J.J.; Garcia-Ibañez, P.; Carvajal, M. The Use of Biovesicles to Improve the Efficiency of Zn Foliar Fertilization. Colloids Surf. B Biointerfaces 2019, 173, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.J.; Yepes-Molina, L.; Martinez-Alonso, A.; Carvajal, M. Nanobiofertilization as a Novel Technology for Highly Efficient Foliar Application of Fe and B in Almond Trees. R. Soc. Open Sci. 2020, 7, 200905. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Karsch-Bluman, A.; Avraham, S.; Assayag, M.; Schwob, O.; Benny, O. Encapsulated Carbenoxolone Reduces Lung Metastases. Cancers 2019, 11, 1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirai, Y.; Terashima, T.; Takenaka, M.; Sawamoto, M. Precision Self-Assembly of Amphiphilic Random Copolymers into Uniform and Self-Sorting Nanocompartments in Water. Macromolecules 2016, 49, 5084–5091. [Google Scholar] [CrossRef]
- Ben Yehuda Greenwald, M.; Ben Sasson, S.; Bianco-Peled, H. A New Method for Encapsulating Hydrophobic Compounds within Cationic Polymeric Nanoparticles. J. Microencapsul. 2013, 30, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Yepes-Molina, L.; Carvajal, M.; Martínez-Ballesta, M.C. Detergent Resistant Membrane Domains in Broccoli Plasma Membrane Associated to the Response to Salinity Stress. Int. J. Mol. Sci. 2020, 21, 7694. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and Mechanisms of Antioxidant Activity Using the DPPH· Free Radical Method. LWT Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal Keratinization in a Spontaneously Immortalized Aneuploid Human Keratinocyte Cell Line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Fraga, C.G.; Leibovitz, B.E.; Tappel, A.L. Lipid Peroxidation Measured as Thiobarbituric Acid-Reactive Substances in Tissue Slices: Characterization and Comparison with Homogenates and Microsomes. Free Radic. Biol. Med. 1988, 4, 155–161. [Google Scholar] [CrossRef]
- Koch, H.; Wittern, K.P.; Bergemann, J. In Human Keratinocytes the Common Deletion Reflects Donor Variabilities Rather Than Chronologic Aging and Can Be Induced by Ultraviolet a Irradiation. J. Invest. Dermatol. 2001, 117. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yan, Y.; Xu, H.; Qu, T.; Wang, B. Selection of Reference Genes for Gene Expression Studies in Ultraviolet B-Irradiated Human Skin Fibroblasts Using Quantitative Real-Time PCR. BMC Mol. Biol. 2011. [Google Scholar] [CrossRef] [Green Version]
- Powers, J.M.; Murphy, G.; Ralph, N.; O’Gorman, S.M.; Murphy, J.E.J. Mitochondrial DNA Deletion Percentage in Sun Exposed and Non Sun Exposed Skin. J. Photochem. Photobiol. B Biol. 2016, 165, 277–282. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; p. 201. [Google Scholar]
- Vallar, S.; Houivet, D.; El Fallah, J.; Kervadec, D.; Haussonne, J.M. Oxide Slurries Stability and Powders Dispersion: Optimization with Zeta Potential and Rheological Measurements. J. Eur. Ceram. Soc. 1999, 19, 1017–1021. [Google Scholar] [CrossRef]
- Standard, I. Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity. Geneve Switz. Int. Organ. Stand. 2009, 3, 10993–10995. [Google Scholar]
- D’Angelo, S.; Ingrosso, D.; Migliardi, V.; Sorrentino, A.; Donnarumma, G.; Baroni, A.; Masella, L.; Tufano, M.A.; Zappia, M.; Galletti, P. Hydroxytyrosol, a Natural Antioxidant from Olive Oil, Prevents PROTEin Damage Induced by Long-Wave Ultraviolet Radiation in Melanoma Cells. Free Radic. Biol. Med. 2005, 38, 908–919. [Google Scholar] [CrossRef]
- Sorrenti, V.; Randazzo, C.L.; Caggia, C.; Ballistreri, G.; Romeo, F.V.; Fabroni, S.; Timpanaro, N.; Raffaele, M.; Vanella, L. Beneficial Effects of Pomegranate Peel Extract and Probiotics on Pre-Adipocyte Differentiation. Front. Microbiol. 2019, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Marabini, L.; Melzi, G.; Lolli, F.; Dell’Agli, M.; Piazza, S.; Sangiovanni, E.; Marinovich, M. Effects of Vitis Vinifera L. Leaves Extract on UV Radiation Damage in Human Keratinocytes (HaCaT). J. Photochem. Photobiol. B Biol. 2020, 204, 111810. [Google Scholar] [CrossRef] [PubMed]
- Rajnochová Svobodová, A.; Gabrielová, E.; Ulrichová, J.; Zálešák, B.; Biedermann, D.; Vostálová, J. A Pilot Study of the UVA-Photoprotective Potential of Dehydrosilybin, Isosilybin, Silychristin, and Silydianin on Human Dermal Fibroblasts. Arch. Dermatol. Res. 2019, 311, 477–490. [Google Scholar] [CrossRef]
- Lohani, A.; Verma, A. Vesicles: Potential Nano Carriers for the Delivery of Skin Cosmetics. J. Cosmet. Laser Ther. 2017, 19, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Voronin, D.; Vikulina, A.; Voronin, D.; Fakhrullin, R.; Vinokurov, V.; Volodkin, D. Naturally Derived Nano- and Micro-Drug Delivery Vehicles: Halloysite, Vaterite and Nanocellulose. New J. Chem. 2020, 44, 5638–5655. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Xue, J.; Lou, X.; Abbas, S.; Guan, Y.; Feng, B.; Zhang, X.; Xia, S. Liposomes as Delivery Systems for Carotenoids: Comparative Studies of Loading Ability, Storage Stability and in Vitro Release. Food Funct. 2014, 5, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Marín, D.; Alemán, A.; Sánchez-Faure, A.; Montero, P.; Gómez-Guillén, M.C. Freeze-Dried Phosphatidylcholine Liposomes Encapsulating Various Antioxidant Extracts from Natural Waste as Functional Ingredients in Surimi Gels. Food Chem. 2018, 245, 525–535. [Google Scholar] [CrossRef] [Green Version]
- Pawlikowska-Pawlȩga, B.; Dziubińska, H.; Król, E.; Trȩbacz, K.; Jarosz-Wilkołazka, A.; Paduch, R.; Gawron, A.; Gruszecki, W.I. Characteristics of Quercetin Interactions with Liposomal and Vacuolar Membranes. Biochim. Biophys. Acta Biomembr. 2014, 1838, 254–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gekko, K.; Timasheff, S.N. Mechanism of Protein Stabilization by Glycerol: Preferential Hydration in Glycerol-Water Mixtures. Biochemistry 1981, 20, 4667–4676. [Google Scholar] [CrossRef] [PubMed]
- Robert, P.; Gorena, T.; Romero, N.; Sepulveda, E.; Chavez, J.; Saenz, C. Encapsulation of Polyphenols and Anthocyanins from Pomegranate (Punica granatum) by Spray Drying. Int. J. Food Sci. Technol. 2010, 45, 1386–1394. [Google Scholar] [CrossRef]
- Mali, A.B.; Khedkar, K.; Lele, S.S. Effect of Gamma Irradiation on Total Phenolic Content and in Vitro Antioxidant Activity of Pomegranate (Punica Granatum L.) Peels. Food Nutr. Sci. 2011, 2, 5756. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, M.; Werner, S. The Cornified Envelope: A First Line of Defense against Reactive Oxygen Species. J. Invest. Dermatol. 2011, 131, 1409–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, A.; Suresh, P.K. Enhanced Induction of Apoptosis in HaCaT Cells by Luteolin Encapsulated in PEGylated Liposomes—Role of Caspase-3/Caspase-14. Appl. Biochem. Biotechnol. 2019, 188, 147–164. [Google Scholar] [CrossRef]
- Díaz, C.; Vargas, E.; Gätjens-Boniche, O. Cytotoxic effect induced by retinoic acid loaded into galactosyl-sphingosine containing liposomes on human hepatoma cell lines. Int. J. Pharm. 2006, 325, 108–115. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Health Risks of Heavy Metals from Long-Range Transboundary Air Pollution; WHO: Copenhagen, Denmark, 2007. [Google Scholar]
- Hwang, T.-L.; Chen, H.-Y.; Changchien, T.-T.; Wang, C.-C.; Wu, C.-M. The Cytotoxicity of Mercury Chloride to the Keratinocytes is Associated with Metallothionein Expression. Biomed. Rep. 2013, 1, 379–382. [Google Scholar] [CrossRef] [Green Version]
- Bae, D.S.; Gennings, C.; Carter, W.H.; Yang, R.S.H.; Campain, J.A. Toxicological Interactions among Arsenic, Cadmium, Chromium, and Lead in Human Keratinocytes. Toxicol. Sci. 2001, 63, 132–142. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.; Mahmood, R. Mercury Chloride Toxicity in Human Erythrocytes: Enhanced Generation of ROS and RNS, Hemoglobin Oxidation, Impaired Antioxidant Power, and Inhibition of Plasma Membrane Redox System. Environ. Sci. Pollut. Res. 2019, 26, 5645–5657. [Google Scholar] [CrossRef] [PubMed]
- Patra, R.C.; Rautray, A.K.; Swarup, D. Oxidative Stress in Lead and Cadmium Toxicity and Its Amelioration. Vet. Med. Int. 2011, 2011, 457327. [Google Scholar] [CrossRef] [Green Version]
- Anna, B.; Blazej, Z.; Jacqueline, G.; Andrew, C.J.; Jeffrey, R.; Andrzej, S. Mechanism of UV-Related Carcinogenesis and Its Contribution to Nevi/Melanoma. Expert Rev. Dermatol. 2007, 2, 451–469. [Google Scholar] [PubMed]
- Halliday, G.M. Inflammation, Gene Mutation and Photoimmunosuppression in Response to UVR-Induced Oxidative Damage Contributes to Photocarcinogenesis. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2005, 571, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Zaid, M.A.; Afaq, F.; Syed, D.N.; Dreher, M.; Mukhtar, H. Inhibition of UVB-Mediated Oxidative Stress and Markers of Photoaging in Immortalized HaCaT Keratinocytes by Pomegranate Polyphenol Extract POMx. Photochem. Photobiol. 2007, 83, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Malik, A.; Syed, D.; Maes, D.; Matsui, M.S.; Mukhtar, H. Pomegranate Fruit Extract Modulates UVB-Mediated Phosphorylation of Mitogen Activated Protein Kinases and Activation of Nuclear Factor Kappa B in Normal Human Epidermal Keratinocytes. Photochem. Photobiol. 2005, 81, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Birch-Machin, M.A.; Tindall, M.; Turner, R.; Haldane, F.; Rees, J.L. Mitochondrial DNA Deletions in Human Skin Reflect Photo- Rather Than Chronologic Aging. J. Invest. Dermatol. 1998, 110, 149–152. [Google Scholar] [CrossRef] [Green Version]
- Jou, M.J.; Peng, T.I.; Yu, P.Z.; Jou, S.B.; Reiter, R.J.; Chen, J.Y.; Wu, H.Y.; Chen, C.C.; Hsu, L.F. Melatonin Protects Against Common Deletion of Mitochondrial DNA-Augmented Mitochondrial Oxidative Stress and Apoptosis. J. Pineal Res. 2007, 43, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Tavanai, E.; Mohammadkhani, G. Role of Antioxidants in Prevention of Age-Related Hearing Loss: A Review of Literature. Eur. Arch. Oto Rhino Laryngol. 2017, 274, 1821–1834. [Google Scholar] [CrossRef] [PubMed]
- Dunaway, S.; Odin, R.; Zhou, L.; Ji, L.; Zhang, Y.; Kadekaro, A.L. Natural Antioxidants: Multiple Mechanisms to Protect Skin from Solar Radiation. Front. Pharmacol. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Target Name | Product Size | Forward Primer (5′ → 3′) | Reverse Primer (5′ → 3′) |
|---|---|---|---|
| Beta-actin a | 205 bp | GGCGGCAACACCATGTACCCT | AGGGGCCGGACTCGTCATACT |
| Total mtDNA b | 83 bp | GATTTGGGTACCACCCAAGTATT | AATATTCATGGTGGCTGGCAGTA |
| Common deletion mtDNA4977 c | 107 bp | ACCCCCATACTCCTTACACTATTCC | AAGGTATTCCTGCTAATGCTAGGCT |
| CI-Vesicles | CI-Vesicles with PG-E | PG-E | |
|---|---|---|---|
| Z-average (nm) | 620.72 25.17 a | 797.50 38.93 b | - |
| Polydispersity index (0–1) | 0.70 0.03 a | 0.76 0.12 a | - |
| Z-potential (mV) | −21.56 0.38 a | −21.65 0.24 a | −15.04 0.40 b |
| Free PG-E | CI-Vesicles with PG-E | |
|---|---|---|
| The total area under the curve (a.u.) | 3160 ± 33.20 | 3357 ± 161.40 |
| Encapsulated area (a.u.) | - | 1561 ± 234.15 |
| Entrapment efficiency (%) | - | 46.50 ± 1.62 |
| Protein before column (mg) | 0 | 0.22 ± 0.02 |
| Total protein collected (mg) | 0 | 0.21 ± 0.01 |
| Antioxidant Activity (µM TE) | |
|---|---|
| CI-vesicles | 0 |
| PG-E, free | 5830.25 ± 169.00 |
| CI-vesicles with PG-E | 5786.29 ± 148.00 |
| CI-Vesicles with PG-E | Free PG-E | |||
|---|---|---|---|---|
| Cell Viability (%) | Untreated | CI-vesicles with PG-E | Untreated | Free PG-E |
| 25.74 ± 1.71 | 43.22 ± 3.97 | 25.74 ± 1.71 | 23.89 ± 2.47 | |
| Viability improvement (%) | 74.40 ± 22.14 | −5.11 ± 11.94 | ||
| Mortality protection (%) | 27.13 ± 6.31 | −2.80 ± 4.24 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yepes-Molina, L.; Hernández, J.A.; Carvajal, M. Nanoencapsulation of Pomegranate Extract to Increase Stability and Potential Dermatological Protection. Pharmaceutics 2021, 13, 271. https://doi.org/10.3390/pharmaceutics13020271
Yepes-Molina L, Hernández JA, Carvajal M. Nanoencapsulation of Pomegranate Extract to Increase Stability and Potential Dermatological Protection. Pharmaceutics. 2021; 13(2):271. https://doi.org/10.3390/pharmaceutics13020271
Chicago/Turabian StyleYepes-Molina, Lucía, José A. Hernández, and Micaela Carvajal. 2021. "Nanoencapsulation of Pomegranate Extract to Increase Stability and Potential Dermatological Protection" Pharmaceutics 13, no. 2: 271. https://doi.org/10.3390/pharmaceutics13020271
APA StyleYepes-Molina, L., Hernández, J. A., & Carvajal, M. (2021). Nanoencapsulation of Pomegranate Extract to Increase Stability and Potential Dermatological Protection. Pharmaceutics, 13(2), 271. https://doi.org/10.3390/pharmaceutics13020271








