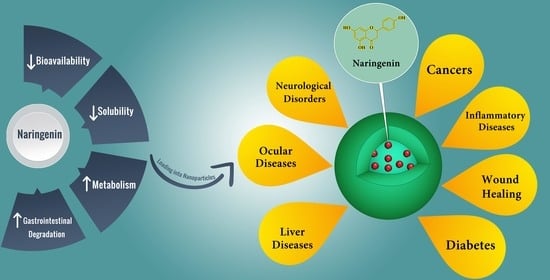
Naringenin Nano-Delivery Systems and Their Therapeutic Applications
Abstract
1. Introduction
2. Methods
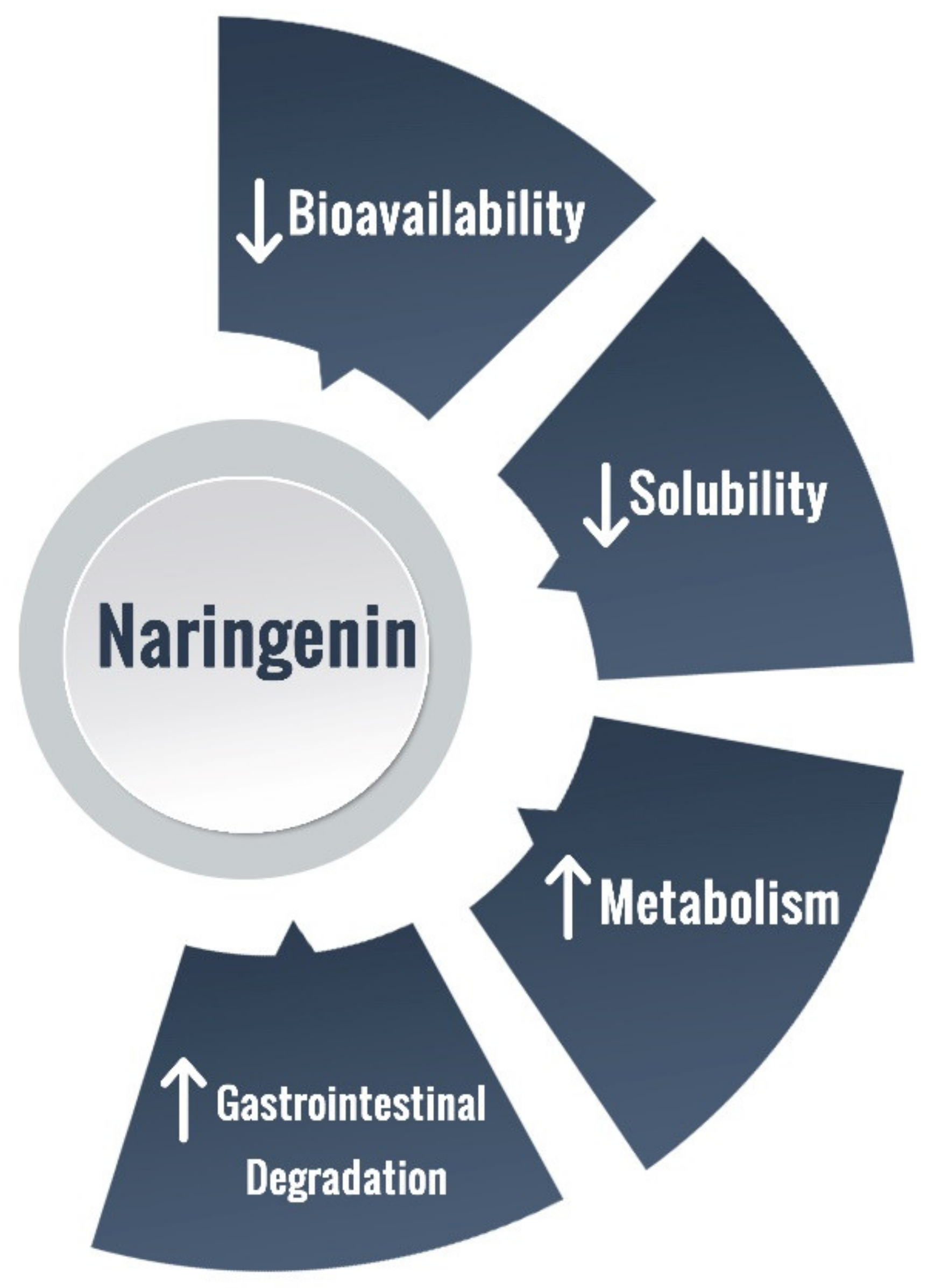
3. NRG Bioavailability and Delivery Challenges
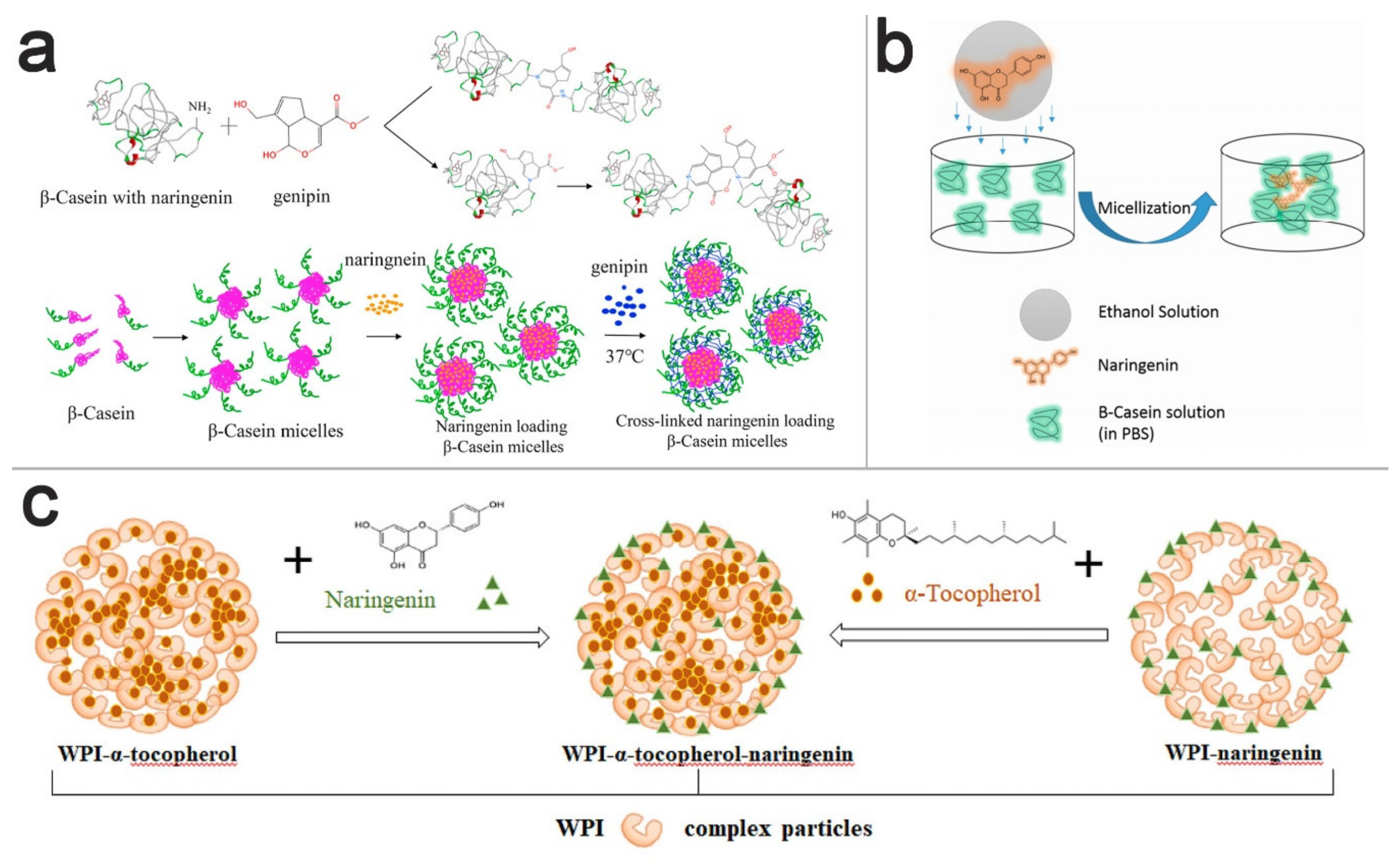
4. NRG Nano-Scaled Delivery Systems
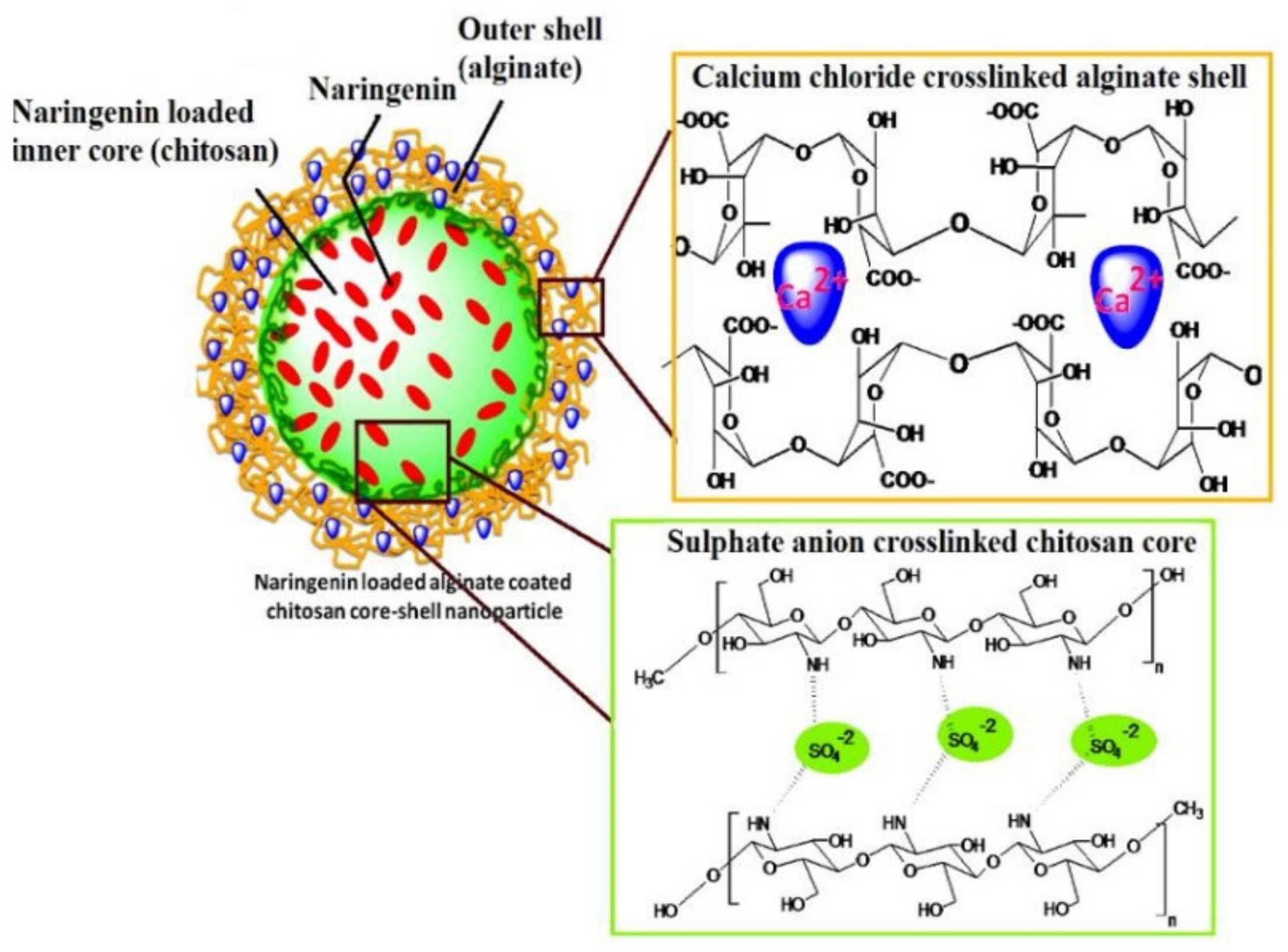
4.1. Polymeric Nanoparticles
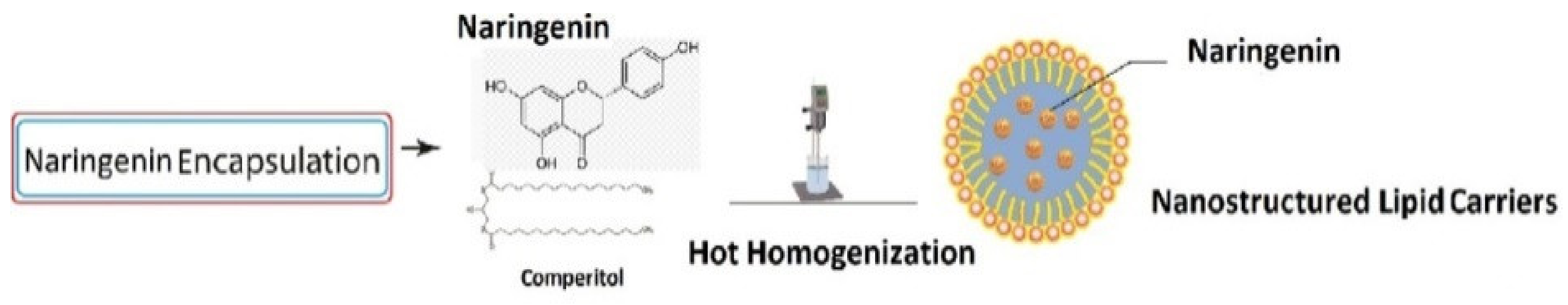
4.2. Lipid-Based Nanoparticles
4.3. Nanosuspensions
4.4. Nanoemulsions
4.5. Co-Delivery Systems
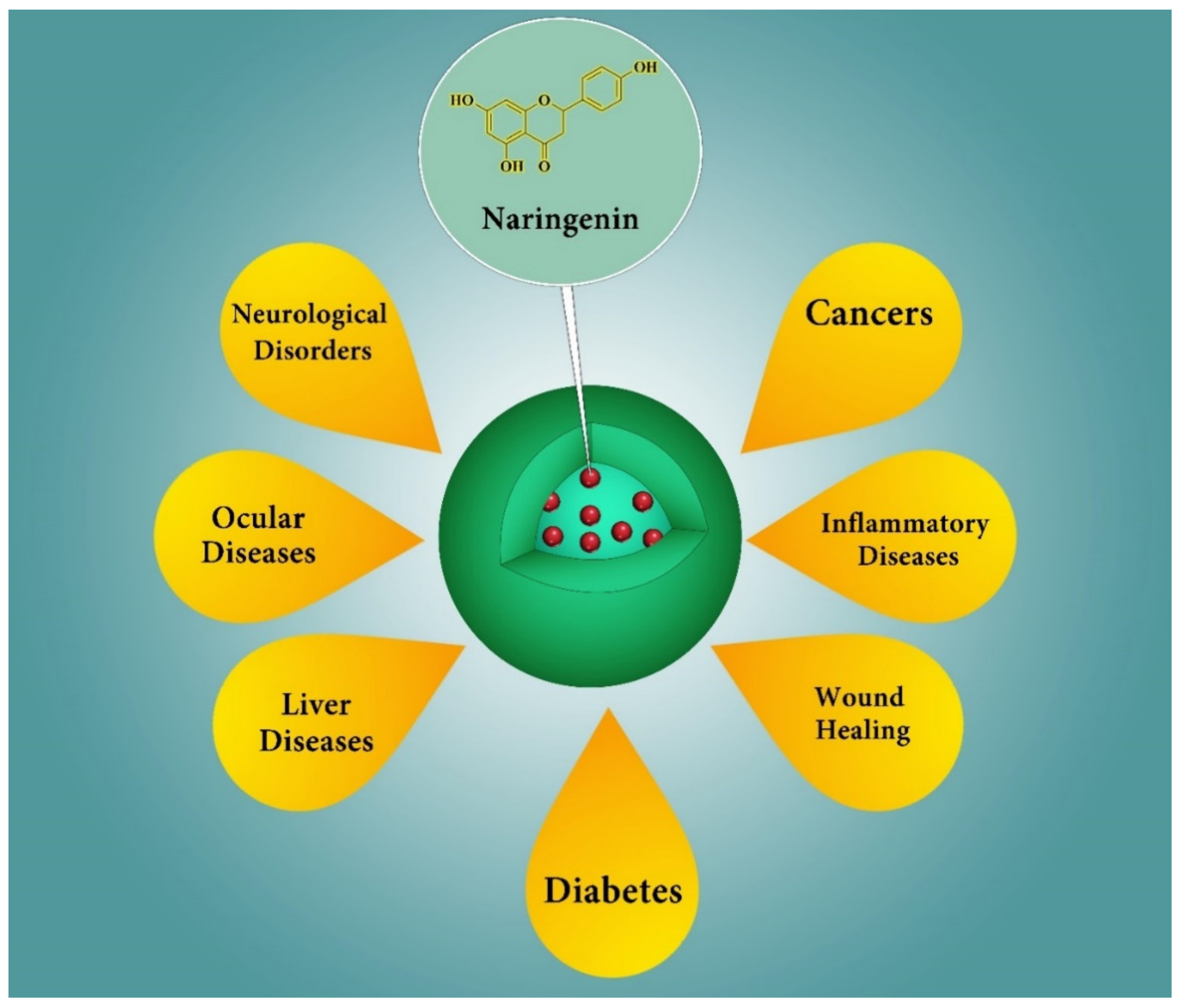
5. Potential Therapeutic Applications of NRG Nanoformulations
5.1. Cancer
5.2. Brain Diseases
5.3. Inflammatory Diseases
5.4. Topical Applications
5.5. Ocular Applications
5.6. Liver Diseases
5.7. Diabetes
6. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zobeiri, M.; Belwal, T.; Parvizi, F.; Naseri, R.; Farzaei, M.H.; Nabavi, S.F.; Sureda, A. Naringenin and its Nano-Formulations for Fatty Liver: Cellular Modes of Action and Clinical Perspective. Curr. Pharm. Biotechnol. 2018, 19, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Singh, G.K.; Patel, D.K. A Review on Pharmacological and Analytical Aspects of Naringenin. Chin. J. Integr. Med. 2014, 24, 551–560. [Google Scholar] [CrossRef]
- Nouri, Z.; Fakhri, S.; El-Senduny, F.F.; Sanadgol, N.; El Ghani, A.; Farzaei, M.H.; Chen, J.-T. On the Neuroprotective Effects of Naringenin: Pharmacological Targets, Signaling Pathways, Molecular Mechanisms, and Clinical Perspective. Biomolecules 2019, 9, 690. [Google Scholar] [CrossRef]
- Cavia-Saiz, M.; Busto, M.D.; Pilar-Izquierdo, M.C.; Ortega, N.; Perez-Mateos, M.; Muñiz, P. Antioxidant Properties, Radical Scavenging Activity and Biomolecule Protection Capacity of Flavonoid Naringenin and Its Glycoside Naringin: A Comparative Study. J. Sci. Food Agric. 2010, 90, 1238–1244. [Google Scholar] [CrossRef]
- NBSP; Tripoli, E.; Guardia, M.L.; Giammanco, S.; Majo, D.D.; Giammanco, M. Citrus Flavonoids: Molecular Structure, Biological Activity and Nutritional Properties: A Review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Nagula, R.L.; Wairkar, S. Recent Advances in Topical Delivery of Flavonoids: A Review. J. Control Release 2019, 296, 190–201. [Google Scholar] [CrossRef]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid Composition of Citrus Juices. Molecules 2007, 12, 1641–1673. [Google Scholar] [CrossRef]
- Llorach, R.; Martínez-Sánchez, A.; Tomás-Barberán, F.A.; Gil, M.I.; Ferreres, F. Characterisation of Polyphenols and Antioxidant Properties of Five Lettuce Varieties and Escarole. Food Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Lamuela-Raventós, R.M.; Elez-Martínez, P.; Martín-Belloso, O. Changes in the Polyphenol Profile of Tomato Juices Processed by Pulsed Electric Fields. J. Agric. Food Chem. 2012, 60, 9667–9672. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, Y.; Hou, Y.; Geng, Y.; Wu, X. Novel Self-Nanomicellizing Formulation Based on Rebaudioside A: A Potential Nanoplatform for Oral Delivery of Naringenin. Mater. Sci. Eng. C 2020, 112, 110926. [Google Scholar] [CrossRef]
- Joshi, R.; Kulkarni, Y.A.; Wairkar, S. Pharmacokinetic, Pharmacodynamic and Formulations Aspects of Naringenin: An Update. Life Sci. 2018, 215, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Venkateswara Rao, P.; Kiran, S.; Rohini, P.; Bhagyasree, P. Flavonoid: A Review on Naringenin. J. Pharmacogn. Phytochem. 2017, 6, 2778–2783. [Google Scholar]
- National Center for Biotechnology Information PubChem Compound Summary for CID 439246, Naringenin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Naringenin (accessed on 10 February 2021).
- Hernández-Aquino, E.; Muriel, P. Beneficial Effects of Naringenin in Liver Diseases: Molecular Mechanisms. World J. Gastroenterol. 2018, 24, 1679–1707. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, H.; Aschner, M.; Hasan, M.M.; Hassan, S.T. Therapeutic Potential of Naringin in Neurological Disorders. Food Chem. Toxicol. 2019, 132, 110646. [Google Scholar] [CrossRef] [PubMed]
- Dou, W.; Zhang, J.; Sun, A.; Zhang, E.; Ding, L.; Mukherjee, S.; Wei, X.; Chou, G.; Wang, Z.-T.; Mani, S. Protective Effect of Naringenin against Experimental Colitis via Suppression of Toll-like Receptor 4/NF-κB Signalling. Br. J. Nutr. 2013, 110, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceutics 2019, 12, 11. [Google Scholar] [CrossRef]
- Hartogh, D.J.D.; Tsiani, E. Antidiabetic Properties of Naringenin: A Citrus Fruit Polyphenol. Biomolecules 2019, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Jin, L.; Zhang, F.; Zhang, C.; Liang, W. Naringenin as a Potential Immunomodulator in Therapeutics. Pharmacol. Res. 2018, 135, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Zaidun, N.H.; Thent, Z.C.; Latiff, A.A. Combating Oxidative Stress Disorders with Citrus Flavonoid: Naringenin. Life Sci. 2018, 208, 111–122. [Google Scholar] [CrossRef]
- Orhan, E.I.; Nabavi, S.F.; Daglia, M.; Tenore, G.C.; Mansouri, K. Naringenin and Atherosclerosis: A Review of Literature. Curr. Pharm. Biotechnol. 2015, 16, 245–251. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Jung, H.A.; Paudel, P.; Seong, S.H.; Min, B.-S.; Choi, J.S. Structure-Related Protein Tyrosine Phosphatase 1b Inhibition by Naringenin Derivatives. Bioorganic Med. Chem. Lett. 2017, 27, 2274–2280. [Google Scholar] [CrossRef] [PubMed]
- Pannu, A.; Goyal, R.K.; Ojha, S.; Nandave, M. Naringenin: A Promising Flavonoid for Herbal Treatment of Rheumatoid Arthritis and Associated Inflammatory Disorders. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 343–354. [Google Scholar]
- Annadurai, T.; Thomas, P.A.; Geraldine, P. Ameliorative Effect of Naringenin on Hyperglycemia-Mediated Inflammation in Hepatic and Pancreatic Tissues of Wistar Rats with Streptozotocin-Nicotinamide-Induced Experimental Diabetes Mellitus. Free Radic. Res. 2013, 47, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Tiku, A.B. Naringenin Suppresses Chemically Induced Skin Cancer in Two-Stage Skin Carcinogenesis Mouse Model. Nutr. Cancer 2020, 72, 976–983. [Google Scholar] [CrossRef]
- Md, S.; Alhakamy, N.A.; Aldawsari, H.M.; Husain, M.; Kotta, S.; Abdullah, S.T.; Fahmy, U.A.; AlFaleh, M.A.; Asfour, H.Z. Formulation Design, Statistical Optimization, and In Vitro Evaluation of a Naringenin Nanoemulsion to Enhance Apoptotic Activity in A549 Lung Cancer Cells. Pharmaceutics 2020, 13, 152. [Google Scholar] [CrossRef]
- Noori, S.; Rezaei Tavirani, M.; Deravi, N.; Mahboobi Rabbani, M.I.; Zarghi, A. Naringenin Enhances the Anti-Cancer Effect of Cyclophosphamide against MDA-MB-231 Breast Cancer Cells Via Targeting the STAT3 Signaling Pathway. Iran. J. Pharm. Res. 2020, 19, 122–133. [Google Scholar] [CrossRef]
- Zhao, Z.; Jin, G.; Ge, Y.; Guo, Z. Naringenin Inhibits Migration of Breast Cancer Cells via Inflammatory and Apoptosis Cell Signaling Pathways. Inflammopharmacology 2019, 27, 1021–1036. [Google Scholar] [CrossRef]
- Lee, J.; Kim, D.-H.; Kim, J.H. Combined Administration of Naringenin and Hesperetin with Optimal Ratio Maximizes the Anti-cancer Effect in Human Pan-Creatic Cancer via down Regulation of FAK and p38 Signaling Pathway. Phytomedicine 2019, 58, 152762. [Google Scholar] [CrossRef]
- Han, K.-Y.; Chen, P.-N.; Hong, M.-C.; Hseu, Y.-C.; Chen, K.-M.; Hsu, L.-S.; Chen, W.-J. Naringenin Attenuated Prostate Cancer Invasion via Reversal of Epithelial–to–Mesenchymal Transition and Inhibited uPA Activity. Anticancer Res. 2018, 38, 6753–6758. [Google Scholar] [CrossRef]
- Lim, W.; Park, S.; Bazer, F.W.; Song, G. Naringenin-Induced Apoptotic Cell Death in Prostate Cancer Cells Is Mediated via the PI3K/AKT and MAPK Signaling Pathways. J. Cell. Biochem. 2017, 118, 1118–1131. [Google Scholar] [CrossRef]
- Bao, L.; Liu, F.; Guo, H.-B.; Li, Y.; Tan, B.-B.; Zhang, W.-X.; Peng, Y.-H. Naringenin Inhibits Proliferation, Migration, and Invasion as Well as Induces Apoptosis of Gastric Cancer SGC7901 Cell Line by Downregulation of AKT Pathway. Tumor Biol. 2016, 37, 11365–11374. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Qin, W.; Wu, F.; Wang, S.; Pan, C.; Wang, L.; Zeng, B.; Ma, S.; Liang, J. Apigenin and Naringenin Regulate Glucose and Lipid Metabolism, and Ameliorate Vascular Dysfunction in Type 2 Diabetic Rats. Eur. J. Pharmacol. 2016, 773, 13–23. [Google Scholar] [CrossRef]
- Karim, N.; Jia, Z.; Zheng, X.; Cui, S.; Chen, W. A Recent Review of Citrus Flavanone Naringenin on Metabolic Diseases and Its Potential Sources for High Yield-Production. Trends Food Sci. Technol. 2018, 79, 35–54. [Google Scholar] [CrossRef]
- Yoshida, H.; Takamura, N.; Shuto, T.; Ogata, K.; Tokunaga, J.; Kawai, K.; Kai, H. The Citrus Flavonoids Hesperetin and Naringenin Block the Lipolytic Actions of TNF-α in Mouse Adipocytes. Biochem. Biophys. Res. Commun. 2010, 394, 728–732. [Google Scholar] [CrossRef]
- Chen, S.; Ding, Y.; Tao, W.; Zhang, W.; Liang, T.; Liu, C. Naringenin Inhibits TNF-α Induced VSMC Proliferation and Migration via Induction of HO-1. Food Chem. Toxicol. 2012, 50, 3025–3031. [Google Scholar] [CrossRef]
- Stacks, N.M. Apigenin and Naringenin: Natural Sources, Pharmacology and Role in Cancer Prevention; Nova Science Publishers, Incorporated: Happauge, NY, USA, 2015; ISBN 1634639952. [Google Scholar]
- Al-Rejaie, S.S.; Abuohashish, H.M.; Al-Enazi, M.M.; Al-Assaf, A.H.; Parmar, M.Y.; Ahmed, M.M. Protective Effect of Naringenin on Acetic Acid-Induced Ulcerative Colitis in Rats. World J. Gastroenterol. WJG 2013, 19, 5633. [Google Scholar] [CrossRef]
- Song, H.-S.; Bhatia, S.K.; Gurav, R.; Choi, T.-R.; Kim, H.J.; Park, Y.-L.; Han, Y.-H.; Park, J.Y.; Lee, S.M.; Park, S.L. Naringenin as an Antibacterial Reagent Controlling of Biofilm Formation and Fatty Acid Metabolism in MRSA. bioRxiv 2020. [Google Scholar] [CrossRef]
- Martinez, S.E.; Lillico, R.; Lakowski, T.M.; Martinez, S.A.; Davies, N.M. Pharmacokinetic Analysis of an Oral Multicomponent Joint Dietary Supplement (Phycox®) in Dogs. Pharmaceutics 2017, 9, 30. [Google Scholar] [CrossRef]
- Yang, L.-J.; Ma, S.-X.; Zhou, S.-Y.; Chen, W.; Yuan, M.-W.; Yin, Y.-Q.; Yang, X.-D. Preparation and Characterization of Inclusion Complexes of Naringenin with β-Cyclodextrin or Its Derivative. Carbohydr. Polym. 2013, 98, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Shpigelman, A.; Shoham, Y.; Israeli-Lev, G.; Livney, Y.D. β-Lactoglobulin–Naringenin Complexes: Nano-Vehicles for the Delivery of a Hydrophobic Nutraceutical. Food Hydrocoll. 2014, 40, 214–224. [Google Scholar] [CrossRef]
- Kumar, R.P.; Abraham, A. PVP-Coated Naringenin Nanoparticles for Biomedical Applications—In Vivo Toxicological Evaluations. Chem. Interact. 2016, 257, 110–118. [Google Scholar] [CrossRef]
- Daeihamed, M.; Dadashzadeh, S.; Haeri, A.; Akhlaghi, M.F. Potential of Liposomes for Enhancement of Oral Drug Absorption. Curr. Drug Deliv. 2016, 13, 1. [Google Scholar] [CrossRef]
- Bayat, F.; Hosseinpour-Moghadam, R.; Mehryab, F.; Fatahi, Y.; Shakeri, N.; Dinarvand, R.; Hagen, T.L.T.; Haeri, A. Potential Application of Liposomal Nanodevices for Non-Cancer Diseases: An Update on Design, Characterization and Biophar-Maceutical Evaluation. Adv. Colloid Interface Sci. 2020, 277, 102121. [Google Scholar] [CrossRef]
- Karuppusamy, C.; Venkatesan, P. Role of Nanoparticles in Drug Delivery System: A Comprehensive Review. J. Pharm. Sci. Res. 2017, 9, 318. [Google Scholar]
- Zarepour, A.; Zarrabi, A.; Larsen, K.L. Fabricating Β-Cyclodextrin Based Ph-Responsive Nanotheranostics as a Programmable Polymeric Nanocapsule for Simultaneous Diagnosis and Therapy. Int. J. Nanomed. 2019, 14, 7017. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Shivakumar, A.; Ramachandran, S. Nano-Technology in Herbal Medicines: A Review. Int. J. Herb. Med. 2016, 4, 21–27. [Google Scholar] [CrossRef]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Nutraceutical Nanodelivery; An Insight into the Bioaccessibility/Bioavailability of Different Bioactive Compounds Loaded within Nanocarriers. Crit. Rev. Food Sci. Nutr. 2020, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioactive-Loaded Nanocarriers for Functional Foods: From Designing to Bioavailability. Curr. Opin. Food Sci. 2020, 33, 21–29. [Google Scholar] [CrossRef]
- Esfanjani, A.F.; Assadpour, E.; Jafari, S.M. Improving the Bioavailability of Phenolic Compounds by Loading Them within Lipid-Based Nanocarriers. Trends Food Sci. Technol. 2018, 76, 56–66. [Google Scholar] [CrossRef]
- Haeri, A.; Osouli, M.; Bayat, F.; Alavi, S.; Dadashzadeh, S. Nanomedicine Approaches for Sirolimus Delivery: A Review of Pharmaceutical Properties and Preclinical Studies. Artif. Cells Nanomed. Biotechnol. 2017, 46, 1–14. [Google Scholar] [CrossRef]
- Babadi, D.; Dadashzadeh, S.; Osouli, M.; Daryabari, M.S.; Haeri, A. Nanoformulation Strategies for Improving Intestinal Permeability of Drugs: A More Precise Look at Permeability Assessment Methods and Pharmacokinetic Properties Changes. J. Control. Release 2020, 321, 669–709. [Google Scholar] [CrossRef] [PubMed]
- Detsi, A.; Kavetsou, E.; Kostopoulou, I.; Pitterou, I.; Pontillo, A.R.N.; Tzani, A.; Christodoulou, P.; Siliachli, A.; Zoumpoulakis, P. Nanosystems for the Encapsulation of Natural Products: The Case of Chitosan Biopolymer as a Matrix. Pharmaceutics 2020, 12, 669. [Google Scholar] [CrossRef]
- Rahaiee, S.; Assadpour, E.; Esfanjani, A.F.; Silva, A.S.; Jafari, S.M. Application of Nano/Microencapsulated Phenolic Compounds against Cancer. Adv. Colloid Interface Sci. 2020, 279, 102153. [Google Scholar] [CrossRef]
- Qin, S.-Y.; Zhang, A.-Q.; Cheng, S.-X.; Rong, L.; Zhang, X.-Z. Drug Self-Delivery Systems for Cancer Therapy. Biomaterials 2017, 112, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, S.; Rabbani, S.; Alavi, S.; Alinaghi, A.; Radfar, F.; Dadashzadeh, S.; Haeri, A. Green Formulation of Curcumin Loaded Lipid-Based Nanoparticles as a Novel Carrier for Inhibition of Post-Angioplasty Restenosis. Mater. Sci. Eng. C 2019, 105, 110037. [Google Scholar] [CrossRef]
- Mohanty, S.; Sahoo, A.K.; Konkimalla, V.B.; Pal, A.; Si, S.C. Naringin in Combination with Isothiocyanates as Liposomal Formulations Potentiates the Anti-inflammatory Activity in Different Acute and Chronic Animal Models of Rheumatoid Arthritis. ACS Omega 2020, 5, 28319–28332. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, P.; Zhou, L.; Mo, F.; Jin, Z.; Ma, J.; Lin, R.; Liu, Y.; Zhang, J. Naringin-Loaded Polymeric Micelles as Buccal Tablets: Formulation, Characterization, in Vitro Release, Cytotoxicity and Histo-Pathology Studies. Pharm. Dev. Technol. 2020, 25, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Huang, Y.; Zhang, J.; Feng, Y.; Shen, L. Formulation, Preparation and Evaluation of Nanostructured Lipid Carrier Containing Naringin and Coix Seed Oil for Anti-Tumor Application Based on “Unification of Medicines and Excipients”. Drug Des. Dev. Ther. 2020, 14, 1481–1491. [Google Scholar] [CrossRef]
- Mohamed, E.A.; Abu Hashim, I.I.; Yusif, R.M.; Shaaban, A.A.A.; El-Sheakh, A.R.; Hamed, M.F.; Badria, F.A.E. Polymeric Micelles for Potentiated Antiulcer and Anticancer Activities of Naringin. Int. J. Nanomed. 2018, 13, 1009–1027. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.M. Formulation and Application of Nanoemulsions for Nutraceuticals and Phytochemicals. Curr. Med. Chem. 2020, 27, 3079–3095. [Google Scholar] [CrossRef]
- Maqsoudlou, A.; Assadpour, E.; Mohebodini, H.; Jafari, S.M. Improving the Efficiency of Natural Antioxidant Compounds via Different Nanocarriers. Adv. Colloid Interface Sci. 2020, 278, 102122. [Google Scholar] [CrossRef]
- Hosseini, H.; Jafari, S.M. Introducing Nano/Microencapsulated Bioactive Ingredients for Extending the Shelf-Life of Food Products. Adv. Colloid Interface Sci. 2020, 282, 102210. [Google Scholar] [CrossRef]
- Carissimi, G.; Montalbán, M.G.; Víllora, G.; Barth, A. Direct Quantification of Drug Loading Content in Polymeric Nanoparticles by Infrared Spectroscopy. Pharmacy 2020, 12, 912. [Google Scholar] [CrossRef] [PubMed]
- Gera, S.; Talluri, S.; Rangaraj, N.; Sampathi, S. Formulation and Evaluation of Naringenin Nanosuspensions for Bioavailability Enhancement. AAPS PharmSciTech 2017, 18, 3151–3162. [Google Scholar] [CrossRef]
- Akrawi, S.H.; Gorain, B.; Nair, A.B.; Choudhury, H.; Pandey, M.; Shah, J.N.; Venugopala, K.N. Development and Optimization of Naringenin-Loaded Chitosan-Coated Nanoemulsion for Topical Therapy in Wound Healing. Pharmacy 2020, 12, 893. [Google Scholar] [CrossRef]
- Ghazy, O.; Fouad, M.; Saleh, H.; Kholif, A.; Morsy, T. Ultrasound-Assisted Preparation of Anise Extract Nanoemulsion and Its Bioactivity against Different Pathogenic Bacteria. Food Chem. 2021, 341, 128259. [Google Scholar] [CrossRef] [PubMed]
- George, D.; Maheswari, P.U.; Begum, K.M.S. Chitosan-Cellulose Hydrogel Conjugated with L-Histidine and Zinc Oxide Nanoparticles for Sustained Drug Delivery: Kinetics and in-Vitro Biological Studies. Carbohydr. Polym. 2020, 236, 116101. [Google Scholar] [CrossRef]
- Wang, J.; Ding, Y.; Zhou, W. Albumin Self-Modified Liposomes for Hepatic Fibrosis Therapy via SPARC-Dependent Pathways. Int. J. Pharm. 2020, 574, 118940. [Google Scholar] [CrossRef] [PubMed]
- Hosny, K.M.; Alharbi, W.S.; Almehmady, A.M.; Bakhaidar, R.B.; Alkhalidi, H.M.; Sindi, A.M.; Hariri, A.H.; Shadab, M.; Zaki, R.M. Preparation and Optimization of Pravastatin-Naringenin Nanotransfersomes to Enhance Bioavailability and Reduce Hepatic Side Effects. J. Drug Deliv. Sci. Technol. 2020, 57, 101746. [Google Scholar] [CrossRef]
- Yang, F.; Hu, S.; Sheng, X.; Liu, Y. Naringenin Loaded Multifunctional Nanoparticles to Enhance the Chemotherapeutic Efficacy in Hepatic Fibrosis. Biomed. Microdevices 2020, 22, 1–9. [Google Scholar] [CrossRef]
- Padmanabhan, V.P.; Balakrishnan, S.; Kulandaivelu, R.; Narayanan, S.N.T.S.; Lakshmipathy, M.; Sagadevan, S.; Mohammad, F.; Al-Lohedan, H.A.; Paiman, S.; Oh, W.C. Nanoformulations of Core–Shell Type Hydroxyapatite-Coated Gum Acacia with Enhanced Bioactivity and Controlled Drug De-livery for Biomedical Applications. New J. Chem. 2020, 44, 7175–7185. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, X.; Chen, H.; Zhu, L. Naringenin-Loaded Dipalmitoylphosphatidylcholine Phytosome Dry Powders for Inhaled Treatment of Acute Lung Injury. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Shi, R.; Zheng, Y.; Zeng, X.; Fan, W.; Wang, Y.; Su, W. Characterization, in Vitro and in Vivo Evaluation of Naringenin-Hydroxypropyl-β-Cyclodextrin Inclusion for Pulmonary Delivery. Molecules 2020, 25, 554. [Google Scholar] [CrossRef]
- Fuster, M.G.; Carissimi, G.; Montalbán, M.G.; Víllora, G. Improving Anticancer Therapy with Naringenin-Loaded Silk Fibroin Nanoparticles. Nanomaterials 2020, 10, 718. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Huang, Y.-B.; Fang, J.-W.; Fu, Y.-S.; Wu, P.-C. Preparation and Characterization of Naringenin-Loaded Elastic Liposomes for Topical Application. PLoS ONE 2015, 10, e0131026. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, N.; Sulfikkarali, N.; Rajendraprasad, N.; Karthikeyan, S. Enhanced Anticancer Activity of Naringenin-Loaded Nanoparticles in Human Cervical (HeLa) Cancer Cells. Biomed. Prev. Nutr. 2011, 1, 223–231. [Google Scholar] [CrossRef]
- Wu, C.; Ji, P.; Yu, T.; Liu, Y.; Jiang, J.; Xu, J.; Zhao, Y.; Hao, Y.; Qiu, Y.; Zhao, W. Naringenin-Loaded Solid Lipid Nanoparticles: Preparation, Controlled Delivery, Cellular Uptake, and Pulmonary Pharmacokinetics. Drug Des. Dev. Ther. 2016, 10, 911–925. [Google Scholar] [CrossRef]
- Shadab, A.N.A.; Aldawsari, H.M.; Asfour, H.Z. Neuroprotective and Antioxidant Effect of Naringenin-Loaded Nanoparticles for Nose-to-Brain Delivery. Brain Sci. 2019, 9, 275. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Yang, H.; Wang, J.; Li, Q.; Qu, R.; Wu, X. Nanocomplexes Based Polyvinylpyrrolidone K-17PF for Ocular Drug Delivery of Naringenin. Int. J. Pharm. 2020, 578, 119133. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, X.; Hu, W.; Bai, Y.; Zhang, L. Preparation and Evaluation of Naringenin-Loaded Sulfobutylether-β-Cyclodextrin/Chitosan Nanoparticles for Ocular Drug Delivery. Carbohydr. Polym. 2016, 149, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Morais, R.P.; Novais, G.B.; Sangenito, L.S.; Santos, A.L.S.; Priefer, R.; Morsink, M.; Mendonça, M.C.; Souto, E.B.; Severino, P.; Cardoso, J.C. Naringenin-Functionalized Multi-Walled Carbon Nanotubes: A Potential Approach for Site-Specific Remote-Controlled Anticancer Delivery for the Treatment of Lung Cancer Cells. Int. J. Mol. Sci. 2020, 21, 4557. [Google Scholar] [CrossRef]
- Kanaze, I.F.; Bounartzi, I.M.; Georgarakis, M.; Niopas, I. Pharmacokinetics of the Citrus Flavanone Aglycones Hesperetin and Naringenin after Single Oral Administration in Human Subjects. Eur. J. Clin. Nutr. 2006, 61, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Najmanova, I.; Vopršalová, M.; Saso, L.; Mladěnka, P. The Pharmacokinetics of Flavanones. Crit. Rev. Food Sci. Nutr. 2020, 60, 3155–3171. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Peng, W.; Yang, C.; Zou, W.; Liu, M.; Wu, H.; Fan, L.; Li, P.; Zeng, X.; Su, W. Pharmacokinetics and Metabolism of Naringin and Active Metabolite Naringenin in Rats, Dogs, Humans, and the Differences Between Species. Front. Pharmacol. 2020, 11, 364. [Google Scholar] [CrossRef] [PubMed]
- Pimpão, R.C.; Ventura, M.R.; Ferreira, R.B.; Williamson, G.; Santos, C.N. Phenolic Sulfates as New and Highly Abundant Metabolites in Human Plasma after Ingestion of a Mixed Berry Fruit Purée. Br. J. Nutr. 2015, 113, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, R.P.; Boeres, A.; Massacessi, L.; Istas, G.; Ventura, M.R.; Dos Santos, C.N.; Heiss, C.; Rodriguez-Mateos, A. Identification and Quantification of Novel Cranberry-Derived Plasma and Urinary (Poly)Phenols. Arch. Biochem. Biophys. 2016, 599, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Venditti, I. Morphologies and Functionalities of Polymeric Nanocarriers as Chemical Tools for Drug Delivery: A Review. J. King Saud Univ. Sci. 2019, 31, 398–411. [Google Scholar] [CrossRef]
- Amoabediny, G.; Haghiralsadat, F.; Naderinezhad, S.; Helder, M.N.; Kharanaghi, E.A.; Arough, J.M.; Zandieh-Doulabi, B. Overview of Preparation Methods of Polymeric and Lipid-Based (Niosome, Solid Lipid, Liposome) Nanoparticles: A Comprehensive Review. Int. J. Polym. Mater. 2017, 67, 383–400. [Google Scholar] [CrossRef]
- George, A.; Shah, P.A.; Shrivastav, P.S. Natural Biodegradable Polymers Based Nano-Formulations for Drug Delivery: A Review. Int. J. Pharm. 2019, 561, 244–264. [Google Scholar] [CrossRef]
- Jahangiri, A.; Barghi, L. Polymeric Nanoparticles: Review of Synthesis Methods and Applications in Drug Delivery. J. Adv. Chem. Pharm. Mater. 2018, 1, 38–47. [Google Scholar]
- Rehman, A.; Jafari, S.M.; Aadil, R.M.; Assadpour, E.; Randhawa, M.A.; Mahmood, S. Development of Active Food Packaging via Incorporation of Biopolymeric Nanocarriers Containing Essential Oils. Trends Food Sci. Technol. 2020, 101, 106–121. [Google Scholar] [CrossRef]
- Rostami, M.R.; Yousefi, M.; Khezerlou, A.; Mohammadi, M.A.; Jafari, S.M. Application of Different Biopolymers for Nanoencapsulation of Antioxidants via Electrohydrodynamic Processes. Food Hydrocoll. 2019, 97, 105170. [Google Scholar] [CrossRef]
- Akbari-Alavijeh, S.; Shaddel, R.; Jafari, S.M. Encapsulation of Food Bioactives and Nutraceuticals by Various Chitosan-Based Nanocarriers. Food Hydrocoll. 2020, 105, 105774. [Google Scholar] [CrossRef]
- Yousefi, M.; Narmani, A.; Jafari, S.M. Dendrimers as Efficient Nanocarriers for the Protection and Delivery of Bioactive Phytochemicals. Adv. Colloid Interface Sci. 2020, 278, 102125. [Google Scholar] [CrossRef]
- Song, I.-S.; Cha, J.-S.; Choi, M.-K. Enhanced Oral Bioavailability of Naringenin Administered in a Mixed Micelle Formulation with Pluronic F127 and Tween 80 in Rats. J. Pharm. Investig. 2015, 45, 633–640. [Google Scholar] [CrossRef]
- Domínguez-Delgado, C.L.; Fuentes-Prado, E.; Escobar-Chávez, J.J.; Vidal-Romero, G.; Rodríguez Cruz, I.; Díaz-Torres, R. Chitosan and Pluronic® F-127: Pharmaceutical Applications. In Encyclopedia of Biomedical Polymers and Polymeric Biomaterials; Taylor and Francis: New York, NY, USA, 2016; pp. 1513–1535. [Google Scholar]
- Cheng, M.; Zeng, G.; Huang, D.; Yang, C.; Lai, C.; Zhang, C.; Liu, Y. Advantages and Challenges of Tween 80 Surfactant-Enhanced Technologies for the Remediation of Soils Contaminated with Hydrophobic Organic Compounds. Chem. Eng. J. 2017, 314, 98–113. [Google Scholar] [CrossRef]
- Li, M.; Wang, K.; Wang, Y.; Han, Q.; Ni, Y.; Wen, X. Effects of Genipin Concentration on Cross-Linked β-Casein Micelles as Nanocarrier of Naringenin: Colloidal Properties, Structural Characterization and Controlled Release. Food Hydrocoll. 2020, 108, 105989. [Google Scholar] [CrossRef]
- Chen, H.; Wooten, H.; Thompson, L.; Pan, K. Nanoparticles of Casein Micelles for Encapsulation of Food Ingredients. In Biopolymer Nanostructures for Food Encapsulation Purposes; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 39–68. [Google Scholar]
- Moeiniafshari, A.-A.; Zarrabi, A.; Bordbar, A.-K. Exploring the Interaction of Naringenin with Bovine Beta-Casein Nanoparticles Using Spectroscopy. Food Hydrocoll. 2015, 51, 1–6. [Google Scholar] [CrossRef]
- Yin, X.; Fu, X.; Cheng, H.; Wusigale, L.L. α-Tocopherol and Naringenin in Whey Protein Isolate Particles: Partition, Antioxidant Activity, Stability and Bioaccessibility. Food Hydrocoll. 2020, 106, 105895. [Google Scholar] [CrossRef]
- Wang, K.; Liu, T.; Lin, R.; Liu, B.; Yang, G.; Bu, X.; Wang, W.; Zhang, P.; Zhou, L.; Zhang, J. Preparation and in Vitro Release of Buccal Tablets of Naringenin-Loaded MPEG-PCL Nanoparticles. RSC Adv. 2014, 4, 33672–33679. [Google Scholar] [CrossRef]
- Muralidharan, S.; Shanmugam, K. Synthesis and Characterization of Naringenin-Loaded Chitosan-Dextran Sulfate Nanocarrier. J. Pharm. Innov. 2020, 1–10. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: A Review Emphasizing on Particle Structure and Drug Release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Samani, S.; Ghasemiyeh, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Novel Drug Delivery Systems: Applications, Advantages and Disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Falsafi, S.R.; Jafari, S.M. Nanoencapsulation of Carotenoids within Lipid-Based Nanocarriers. J. Control. Release 2019, 298, 38–67. [Google Scholar] [CrossRef]
- Mohammadi, M.; Assadpour, E.; Jafari, S.M. Encapsulation of Food Ingredients by Nanostructured Lipid Carriers (NLCs). In Lipid-Based Nanostructures for Food Encapsulation Purposes; Elsevier: Amsterdam, The Netherlends, 2019; pp. 217–270. [Google Scholar]
- Akhavan, S.; Assadpour, E.; Katouzian, I.; Jafari, S.M. Lipid Nano Scale Cargos for the Protection and Delivery of Food Bioactive Ingredients and Nutraceuticals. Trends Food Sci. Technol. 2018, 74, 132–146. [Google Scholar] [CrossRef]
- Katouzian, I.; Esfanjani, A.F.; Jafari, S.M.; Akhavan, S. Formulation and Application of a New Generation of Lipid Nano-Carriers for the Food Bioactive Ingredients. Trends Food Sci. Technol. 2017, 68, 14–25. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Firempong, C.K.; Zhang, H.; Wang, M.; Zhang, Y.; Zhu, Y.; Yuanwen, W.; Xu, X. Enhanced Solubility and Bioavailability of Naringenin via Liposomal Nanoformulation: Preparation and In Vitro and In Vivo Evaluations. AAPS Pharmscitech 2017, 18, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Raeisi, S.; Chavoshi, H.; Mohammadi, M.; Ghorbani, M.; Sabzichi, M.; Ramezani, F. Naringenin-Loaded Nano-Structured Lipid Carrier Fortifies Oxaliplatin-Dependent Apoptosis in HT-29 Cell Line. Process. Biochem. 2019, 83, 168–175. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, M.; Zhang, J.; Peng, W.; Firempong, C.K.; Deng, W.; Wang, Q.; Wang, S.; Shi, F.; Yu, J.; et al. Improved Oral Bioavailability of Capsaicin via Liposomal Nanoformulation: Preparation, in Vitro Drug Release and PharmaCo-Kinetics in Rats. Arch. Pharmacol. Res. 2015, 38, 512–521. [Google Scholar] [CrossRef]
- Haghiralsadat, F.; Amoabediny, G.; Sheikhha, M.H.; Zandieh-doulabi, B.; Naderinezhad, S.; Helder, M.N.; Forouzanfar, T. New Liposomal Doxorubicin Nanoformulation for Osteosarcoma: Drug Release Kinetic Study Based on Thermo and pH Sensitivity. Chem. Biol. Drug Des. 2017, 90, 368–379. [Google Scholar] [CrossRef]
- Jain, A.K.; Swarnakar, N.K.; Godugu, C.; Singh, R.P.; Jain, S. The Effect of the Oral Administration of Polymeric Nanoparticles on the Efficacy and Toxicity of Tamoxifen. Biomaterials 2011, 32, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Karadag, A.; Ozcelik, B.; Huang, Q. Quercetin Nanosuspensions Produced by High-Pressure Homogenization. J. Agric. Food Chem. 2014, 62, 1852–1859. [Google Scholar] [CrossRef]
- Mauludin, R.; Müller, R.H. Preparation and Storage Stability of Rutin Nanosuspensions. J. Pharm. Investig. 2013, 43, 395–404. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J. Emerging Role of Nanosuspensions in Drug Delivery Systems. Biomater. Res. 2020, 24, 1–16. [Google Scholar] [CrossRef]
- Singh, M.K.; Pooja, D.; Ravuri, H.G.; Gunukula, A.; Kulhari, H.; Sistla, R. Fabrication of Surfactant-Stabilized Nanosuspension of Naringenin to Surpass Its Poor Physiochemical Properties and Low Oral Bioavailability. Phytomedicine 2018, 40, 48–54. [Google Scholar] [CrossRef]
- Rajamani, S.; Radhakrishnan, A.; Sengodan, T.; Thangavelu, S. Augmented Anticancer Activity of Naringenin-Loaded TPGS Polymeric Nanosuspension for Drug Resistive MCF-7 Human Breast Cancer Cells. Drug Dev. Ind. Pharm. 2018, 44, 1752–1761. [Google Scholar] [CrossRef]
- Zhao, S.; Yang, X.; Garamus, V.M.; Handge, U.A.; Bérengère, L.; Zhao, L.; Salamon, G.; Willumeit, R.; Zou, A.; Fan, S. Mixture of Nonionic/Ionic Surfactants for the Formulation of Nanostructured Lipid Carriers: Effects on Physical Properties. Langmuir 2014, 30, 6920–6928. [Google Scholar] [CrossRef]
- Rajamani, S.; Kalyanasundaram, G.; Sengodan, T.; Thangavelu, S.; Shanmukhan, N.K.; Radhakrishnan, A. Hepato & Nephro Protective Effects of Naringenin-Loaded Tpgs Polymeric Nanosuspension against Cisplatin-Induced Toxicity. Int. J. Res. Pharm. Sci. 2019, 10, 2755–2764. [Google Scholar] [CrossRef]
- Sumathi, R.; Tamizharasi, S.; Sivakumar, T. Formulation and Evaluation of Polymeric Nanosuspension of Naringenin. Int. J. Appl. Pharm. 2017, 9, 60. [Google Scholar] [CrossRef]
- Khan, A.W.; Kotta, S.; Ansari, S.H.; Sharma, R.K.; Ali, J. Self-Nanoemulsifying Drug Delivery System (SNEDDS) of the Poorly Water-Soluble Grapefruit Flavonoid Naringenin: Design, Characterization, in Vitroandin Vivoevaluation. Drug Deliv. 2015, 22, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Fuior, E.V.; Mocanu, C.A.; Deleanu, M.; Voicu, G.; Anghelache, M.; Rebleanu, D.; Simionescu, M.; Calin, M. Evaluation of VCAM-1 Targeted Naringenin/Indocyanine Green-Loaded Lipid Nanoemulsions as Theranostic Nanoplatforms in Inflammation. Pharm. 2020, 12, 1066. [Google Scholar] [CrossRef] [PubMed]
- Fuior, E.V.; Deleanu, M.; Constantinescu, C.A.; Rebleanu, D.; Voicu, G.; Simionescu, M.; Calin, M. Functional Role of VCAM-1 Targeted Flavonoid-Loaded Lipid Nanoemulsions in Reducing Endothelium Inflammation. Pharmaceutics 2019, 11, 391. [Google Scholar] [CrossRef] [PubMed]
- Kanaze, F.; Kokkalou, E.; Niopas, I.; Barmpalexis, P.; Georgarakis, E.; Bikiaris, D. Dissolution Rate and Stability Study of Flavanone Aglycones, Naringenin and Hesperetin, by Drug Delivery Systems Based on Polyvinylpyrrolidone (PVP) Nanodispersions. Drug Dev. Ind. Pharm. 2010, 36, 292–301. [Google Scholar] [CrossRef]
- Kerdudo, A.; Dingas, A.; Fernandez, X.; Faure, C. Encapsulation of Rutin and Naringenin in Multilamellar Vesicles for Optimum Antioxidant Activity. Food Chem. 2014, 159, 12–19. [Google Scholar] [CrossRef]
- Sandhu, P.S.; Kumar, R.; Beg, S.; Jain, S.; Kushwah, V.; Katare, O.; Singh, B. Natural Lipids Enriched Self-Nano-Emulsifying Systems for Effective Co-Delivery of Tamoxifen and Naringenin: Systematic AP-Proach for Improved Breast Cancer Therapeutics. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1703–1713. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Ahmad, J.; Zafar, S.; Warsi, M.H.; Abdel-Wahab, B.A.; Akhter, S.; Alam, A. Omega-3 Fatty Acids as Adjunctive Therapeutics: Prospective of Nanoparticles in Its Formulation Development. Ther. Deliv. 2020, 11, 851–868. [Google Scholar] [CrossRef]
- Dasgupta, S.; Bhattacharyya, D.K. Dietary Effect of Eicosapentaenoic Acid (EPA) Containing Soyphospholipid. J. Oleo Sci. 2007, 56, 563–568. [Google Scholar] [CrossRef]
- Shirouchi, B.; Nagao, K.; Inoue, N.; Ohkubo, T.; Hibino, A.H.; Yanagita, T. Effect of Dietary Omega 3 Phosphatidylcholine on Obesity-Related Disorders in Obese Otsuka Long-Evans Tokushima Fatty Rats. J. Agric. Food Chem. 2007, 55, 7170–7176. [Google Scholar] [CrossRef] [PubMed]
- Parashar, P.; Rathor, M.; Dwivedi, M.; Saraf, S.A. Hyaluronic Acid Decorated Naringenin Nanoparticles: Appraisal of Chemopreventive and Curative Potential for Lung Cancer. Pharmaceutics 2018, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, S.; Patel, R.R.; Vure, P.; Mishra, B. Potential of Cationic-Polymeric Nanoparticles for Oral Delivery of Naringenin: In Vitro and In Vivo Investigations. J. Pharm. Sci. 2018, 107, 706–716. [Google Scholar] [CrossRef]
- Akhter, H.; Kumar, S.; Nomani, S. Sonication Tailored Enhance Cytotoxicity of Naringenin Nanoparticle in Pancreatic Cancer: Design, Optimization, and in Vitro Studies. Drug Dev. Ind. Pharm. 2020, 46, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Gaba, B.; Khan, T.; Haider, F.; Alam, T.; Baboota, S.; Parvez, S.; Ali, J. Vitamin E Loaded Naringenin Nanoemulsion via Intranasal Delivery for the Management of Oxidative Stress in a 6-OHDA Parkinson’s Disease Model. BioMed Res. Int. 2019, 2019, 1–20. [Google Scholar] [CrossRef]
- Abraham, R.P.K.A. Inhibition of LPS Induced Pro-Inflammatory Responses in RAW 264.7 Macrophage Cells by PVP-Coated Naringenin Nanoparticle via down Regulation of NF-κB/P38MAPK Mediated Stress Signaling. Pharmacol. Rep. 2017, 69, 908–915. [Google Scholar] [CrossRef]
- Chen, C.; Jie, X.; Ou, Y.; Cao, Y.; Xu, L.; Wang, Y.; Qi, R. Nanoliposome Improves Inhibitory Effects of Naringenin on Nonalcoholic Fatty Liver Disease in Mice. Nanomedicine 2017, 12, 1791–1800. [Google Scholar] [CrossRef]
- Maity, S.; Chakraborti, A.S. Formulation, PhysiCo-chemical Characterization and Antidiabetic Potential of Naringenin-Loaded Poly D, L Lactide-Co-Glycolide (N-PLGA) Nanoparticles. Eur. Polym. J. 2020, 134, 109818. [Google Scholar] [CrossRef]
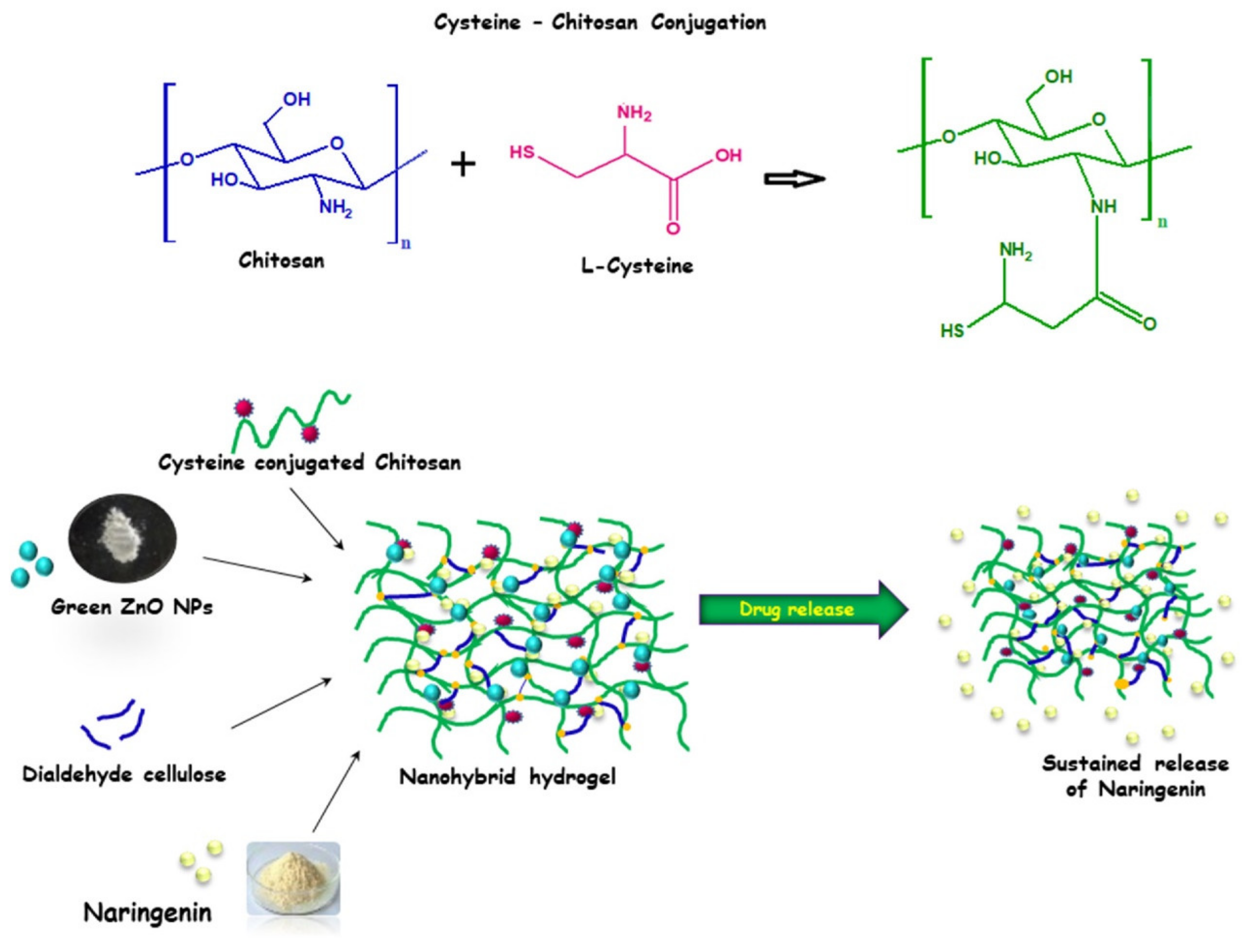
- George, D.; Maheswari, P.U.; Begum, K.M.S. Cysteine Conjugated Chitosan Based Green Nanohybrid Hydrogel Embedded with Zinc Oxide Nanoparticles Towards Enhanced Therapeutic Potential of Naringenin. React. Funct. Polym. 2020, 148, 104480. [Google Scholar] [CrossRef]
- Zhang, H.; Zhong, X.; Zhang, X.; Shang, D.; Zhou, Y.; Zhang, C. Enhanced Anticancer Effect of ABT-737 in Combination with Naringenin on Gastric Cancer Cells. Exp. Ther. Med. 2015, 11, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-L.; Chang, Y.-M.; Lai, S.-C.; Chen, K.-M.; Wang, K.-C.; Chiu, T.-T.; Chang, F.-H.; Hsu, L.-S. Naringenin Inhibits Migration of Lung Cancer Cells via the Inhibition of Matrix Metalloproteinases-2 and-9. Exp. Ther. Med. 2017, 13, 739–744. [Google Scholar] [CrossRef]
- Mir, I.A.; Tiku, A.B. Chemopreventive and Therapeutic Potential of “Naringenin,” a Flavanone Present in Citrus Fruits. Nutr. Cancer 2014, 67, 27–42. [Google Scholar] [CrossRef]
- Arafah, A.; Rehman, M.U.; Mir, T.M.; Wali, A.F.; Ali, R.; Qamar, W.; Khan, R.; Ahmad, A.; Aga, S.S.; Alqahtani, S.; et al. Multi-Therapeutic Potential of Naringenin (4′,5,7-Trihydroxyflavonone): Experimental Evidence and Mechanisms. Plants 2020, 9, 1784. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, S.; Patel, R.R.; Vure, P.; Mishra, B. Oral Naringenin Nanocarriers: Fabrication, Optimization, Pharmacokinetic and Chemotherapeutic Efficacy Assessments. Nanomedicine 2017, 12, 1243–1260. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.P.; Birundha, K.; Kaveri, K.; Devi, K.R. Antioxidant Studies of Chitosan Nanoparticles Containing Naringenin and Their Cytotoxicity Effects in Lung Cancer Cells. Int. J. Biol. Macromol. 2015, 78, 87–95. [Google Scholar] [CrossRef]
- Krishnakumar, N.; Sulfikkarali, N.K.; Manoharan, S.; Nirmal, R.M. Screening of Chemopreventive Effect of Naringenin-Loaded Nanoparticles in DMBA-Induced Hamster Buccal Pouch Carcino-Genesis by FT-IR Spectroscopy. Mol. Cell. Biochem. 2013, 382, 27–36. [Google Scholar] [CrossRef]
- Sulfikkarali, N.; Krishnakumar, N.; Manoharan, S.; Nirmal, R.M. Chemopreventive Efficacy of Naringenin-Loaded Nanoparticles in 7,12-Dimethylbenz(a)anthracene Induced Experimental Oral Carcinogenesis. Pathol. Oncol. Res. 2012, 19, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, N.; Sulfikkarali, N.; Manoharan, S.; Venkatachalam, P. Raman Spectroscopic Investigation of the Chemopreventive Response of Naringenin and Its Nanoparticles in DMBA-Induced Oral Carcinogenesis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 115, 648–653. [Google Scholar] [CrossRef]
- Sulfikkarali, N.K.; Krishnakumar, N. Evaluation of the Chemopreventive Response of Naringenin-Loaded Nanoparticles in Experimental Oral Carcinogenesis Using Laser-Induced Autofluorescence Spectroscopy. Laser Phys. 2013, 23, 154. [Google Scholar] [CrossRef]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, Regional, and National Burden of Neurological Disorders, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the Blood-Brain Barrier with Nanoparticles. J. Control. Release 2018, 270, 290–303. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Ahmad, F.J.; Ahmad, W.; Alam, A.; Amir, M.; Ali, A. Poloxamer-Chitosan-Based Naringenin Nanoformulation Used in Brain Targeting for the Treatment of Cerebral Ischemia. Saudi J. Biol. Sci. 2020, 27, 500–517. [Google Scholar] [CrossRef]
- Md, S.; Gan, S.Y.; Haw, Y.H.; Ho, C.L.; Wong, S.; Choudhury, H. In Vitro Neuroprotective Effects of Naringenin Nanoemulsion against β-Amyloid Toxicity through the Regulation of Amyloido-Genesis and Tau Phosphorylation. Int. J. Biol. Macromol. 2018, 118, 1211–1219. [Google Scholar] [CrossRef]
- Salehi, B.; Rescigno, A.; Dettori, T.; Calina, D.; Docea, A.O.; Singh, L.; Cebeci, F.; Özçelik, B.; Bhia, M.; Beirami, A.D.; et al. Avocado–Soybean Unsaponifiables: A Panoply of Potentialities to Be Exploited. Biomolecules 2020, 10, 130. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Sailo, B.L.; Banik, K.; Harsha, C.; Prasad, S.; Gupta, S.C.; Bharti, A.C.; Aggarwal, B.B. Chronic Diseases, Inflammation, and Spices: How Are They Linked? J. Transl. Med. 2018, 16, 1–25. [Google Scholar] [CrossRef]
- Roy, N.K.; Parama, D.; Banik, K.; Bordoloi, D.; Devi, A.K.; Thakur, K.K.; Padmavathi, G.; Shakibaei, M.; Fan, L.; Sethi, G.; et al. An Update on Pharmacological Potential of Boswellic Acids against Chronic Diseases. Int. J. Mol. Sci. 2019, 20, 4101. [Google Scholar] [CrossRef] [PubMed]
- TuTunchi, H.; Naeini, F.; Ostadrahimi, A.; Hosseinzadeh-Attar, M.J. Naringenin, a Flavanone with Antiviral and Anti-Inflammatory Effects: A Promising Treatment Strategy Against COVID-19. Phytother. Res. 2020, 34, 3137–3147. [Google Scholar] [CrossRef] [PubMed]
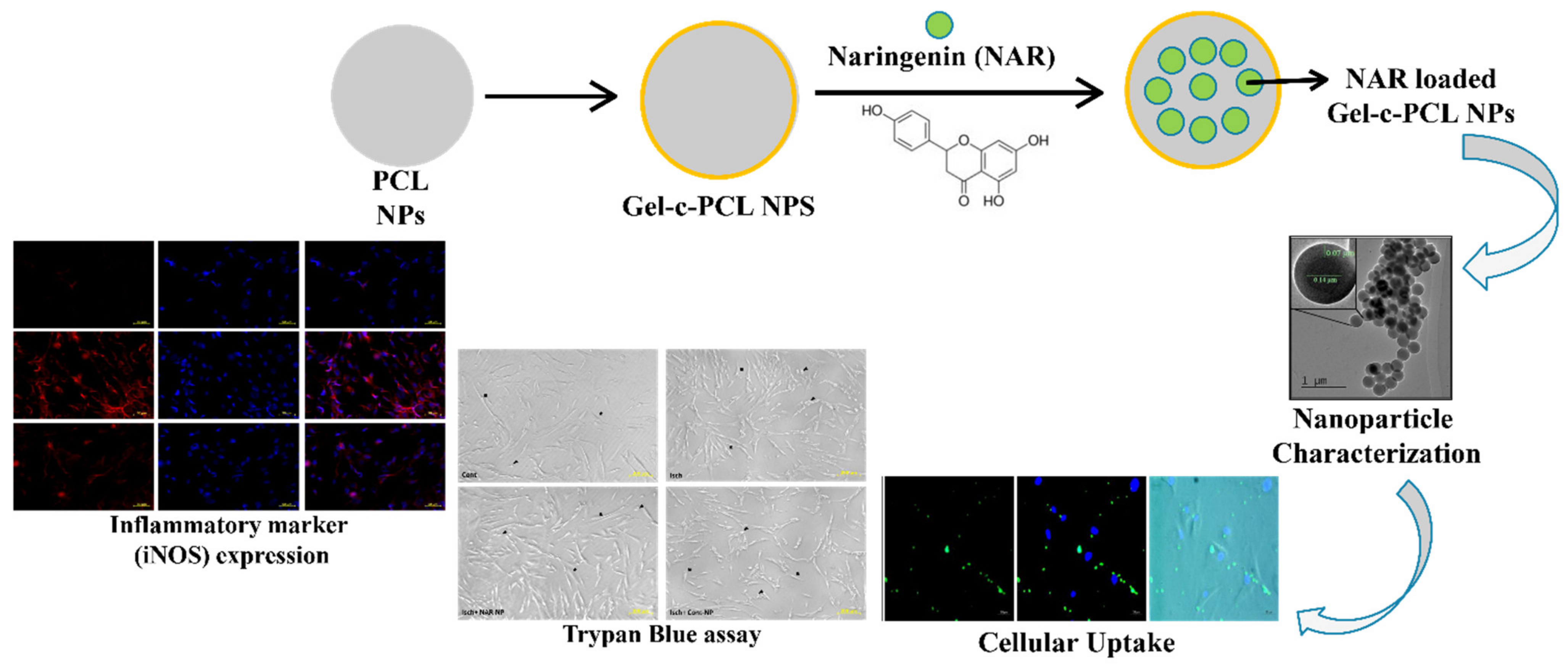
- Ahmad, A.; Fauzia, E.; Kumar, M.; Mishra, R.K.; Kumar, A.; Khan, M.A.; Raza, S.S.; Khan, R. Gelatin-Coated Polycaprolactone Nanoparticle-Mediated Naringenin Delivery Rescue Human Mesenchymal Stem Cells from Oxygen Glucose Deprivation-Induced Inflammatory Stress. ACS Biomater. Sci. Eng. 2018, 5, 683–695. [Google Scholar] [CrossRef]
- Mulyani, H.; Dewi, R.T.; Chaidir, C. ANTIBACTERIAL COMPOUND of Aspergillus Elegans SWEF9 Endophytic Fungi from Seaweed. Indones. J. Pharm. 2019, 30, 217. [Google Scholar] [CrossRef]
- Kim, T.-H.; Kim, G.-D.; Ahn, H.-J.; Cho, J.-J.; Park, Y.S.; Park, C.-S. The Inhibitory Effect of Naringenin on Atopic Dermatitis Induced by DNFB in NC/Nga Mice. Life Sci. 2013, 93, 516–524. [Google Scholar] [CrossRef]
- Gaggeri, R.; Rossi, D.; Hajikarimian, N.; Martino, E.; Bracco, F.; Grisoli, P.; Dacarro, C.; Leoni, F.; Mascheroni, G.; Collina, S.; et al. Preliminary Study on TNFα-Blocker Activity of Amygdalus lycioides Spach Extracts. Open Nat. Prod. J. 2010, 3, 20–25. [Google Scholar] [CrossRef]
- Gaggeri, R.; Rossi, D.; Christodoulou, M.S.; Passarella, D.; Leoni, F.; Azzolina, O.; Collina, S. Chiral Flavanones from Amygdalus Lycioides Spach: Structural Elucidation and Identification of TNFalpha Inhibitors by Bioactivity-guided Fractionation. Molecules 2012, 17, 1665–1674. [Google Scholar] [CrossRef]
- Chlapanidas, T.; Perteghella, S.; Leoni, F.; Faragò, S.; Marazzi, M.; Rossi, D.; Martino, E.; Gaggeri, R.; Collina, S. TNF-α Blocker Effect of Naringenin-Loaded Sericin Microparticles that Are Potentially Useful in the Treatment of Psoriasis. Int. J. Mol. Sci. 2014, 15, 13624–13636. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-J.; Huang, Y.-B.; Fang, J.-W.; Fu, Y.-S.; Jhih-Wun, F. Preparation and Evaluation of Submicron-Carriers for Naringenin Topical Application. Int. J. Pharm. 2015, 481, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Carreras, J.J.; Ramirez, E.T.W.; Sala, A.; Guillot, A.J.; Garrigues, T.M.; Melero, A. Ultraflexible Lipid Vesicles Allow Topical Absorption of Cyclosporin A. Drug Deliv. Transl. Res. 2019, 10, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Castoldi, A.; Herr, C.; Niederstraßer, J.; Labouta, H.I.; Melero, A.; Gordon, S.; Schneider-Daum, N.; Bals, R.; Lehr, C.-M. Calcifediol-Loaded Liposomes for Local Treatment of Pulmonary Bacterial Infections. Eur. J. Pharm. Biopharm. 2017, 118, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Dreier, J.; Sørensen, J.A.; Brewer, J.R. Superresolution and Fluorescence Dynamics Evidence Reveal That Intact Liposomes Do Not Cross the Human Skin Barrier. PLoS ONE 2016, 11, e0146514. [Google Scholar] [CrossRef]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Vignoli, J.A.; Barbosa, D.S.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A.; Casagrande, R. Naringenin Inhibits UVB Irradiation-Induced Inflammation and Oxidative Stress in the Skin of Hairless Mice. J. Nat. Prod. 2015, 78, 1647–1655. [Google Scholar] [CrossRef]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Silva, T.C.C.; Caviglione, C.V.; Bottura, C.; Fonseca, M.J.V.; Vicentini, F.T.M.C.; Vignoli, J.A.; Baracat, M.M.; et al. Topical Formulation Containing Naringenin: Efficacy against Ultraviolet B Irradiation-Induced Skin Inflammation and Oxidative Stress in Mice. PLoS ONE 2016, 11, e0146296. [Google Scholar] [CrossRef]
- Badea, G.; Badea, N.; Brasoveanu, L.I.; Mihaila, M.; Stan, R.; Istrati, D.; Balaci, T.; Lacatusu, I. Naringenin Improves the Sunscreen Performance of Vegetable Nanocarriers. New J. Chem. 2016, 41, 480–492. [Google Scholar] [CrossRef]
- Joshi, H.; Hegde, A.R.; Shetty, P.K.; Gollavilli, H.; Managuli, R.S.; Kalthur, G.; Mutalik, S. Sunscreen Creams Containing Naringenin Nanoparticles: Formulation Development and in Vitro and in Vivo Evaluations. Photodermatol. Photoimmunol. Photomed. 2018, 34, 69–81. [Google Scholar] [CrossRef]
- Al-Dosari, D.I.; Ahmed, M.M.; Al-Rejaie, S.S.; Alhomida, A.S.; Ola, M.S. Flavonoid Naringenin Attenuates Oxidative Stress, Apoptosis and Improves Neurotrophic Effects in the Diabetic Rat Retina. Nutrients 2017, 9, 1161. [Google Scholar] [CrossRef]
- Oguido, A.P.M.T.; Hohmann, M.S.N.; Pinho-Ribeiro, F.A.; Crespigio, J.; Domiciano, T.P.; Verri, W.A.; Casella, A.M.B. Naringenin Eye Drops Inhibit Corneal Neovascularization by Anti-Inflammatory and Antioxidant Mechanisms. Investig. Opthalmol. Vis. Sci. 2017, 58, 5764–5776. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, K.; Ohgami, K.; Ilieva, I.; Jin, X.-H.; Yoshida, K.; Kase, S.; Ohno, S. The Effects of Naringin and Naringenin on Endotoxin-Induced Uveitis in Rats. J. Ocul. Pharmacol. Ther. 2005, 21, 298–304. [Google Scholar] [CrossRef]
- Sahu, N.; Mishra, G.; Chandra, H.K.; Nirala, S.K.; Bhadauria, M. Naringenin Mitigates Antituberculosis Drugs Induced Hepatic and Renal Injury in Rats. J. Tradit. Complement. Med. 2020, 10, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Yadav, A.; Bozhkov, A.; Padalko, V.; Flora, S. Therapeutic Efficacy of Silymarin and Naringenin in Reducing Arsenic-Induced Hepatic Damage in Young Rats. Ecotoxicol. Environ. Saf. 2011, 74, 607–614. [Google Scholar] [CrossRef]
- Yen, F.-L.; Wu, T.-H.; Lin, L.-T.; Cham, T.-M.; Lin, C.-C. Naringenin-Loaded Nanoparticles Improve the Physicochemical Properties and the Hepatoprotective Effects of Naringenin in Orally-Administered Rats with CCl4-Induced Acute Liver Failure. Pharm. Res. 2008, 26, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Mukhopadhyay, P.; Kundu, P.P.; Chakraborti, A.S. Alginate Coated Chitosan Core-Shell Nanoparticles for Efficient Oral Delivery of Naringenin in Diabetic Animals—an in Vitro and in Vivo Approach. Carbohydr. Polym. 2017, 170, 124–132. [Google Scholar] [CrossRef] [PubMed]
| Potential Therapeutic Application | Platform | Dose (Model) | Effects on Biological Parameters | Key Results | Reference |
|---|---|---|---|---|---|
| Lung cancer | Polymeric nanoparticles | 50 mg/kg (urethane-induced lung cancer in rat albino wistar rat and A549 lung cancer cells and) | IC50 of 5.33 µMNRG-loaded nanoparticle compared with 25.1 µM for free NRG in A549 lung cancer cells. | NRG-loaded PCL nanoparticles decorated with hyaluronic acid were able to enhance the anticancer effect and cellular uptake of NRG in lung cancer cells nanoparticles and to suppress tumor growth in rats with urethane-induced lung cancer. | [135] |
| Colorectal cancer | Polymeric nanoparticles | 40 mg/kg (murine colon-26 tumor-bearing BALB/c mice) | Mice survival rates were 83.33% and 33.33% for NRG-loaded nanoparticles and free NRG, respectively. | NRG delivered with eudragit E-100 nanoparticles showed a significantly higher bioavailability, enhanced cytotoxicity in cancer cells, and significantly inhibited tumor growth and enhanced survival rate in mice-bearing colorectal tumors. | [136] |
| Pancreatic cancer | Polymeric nanoparticles | 0-60 µM (pancreatic cell line) | After 72 h, the IC50 was 32.08 µg/mL and 73.2 µg/mL for NRG-loaded nanoparticles and free NRG, respectively. | Treatment with NRG-loaded PLGA nanoparticles showed increased cytotoxicity in pancreatic cancer cells compared to NRG alone. | [137] |
| Parkinson’s disease | Nanoemulsion | 40 mg/mL (6-OHDA induced Parkinson’s disease in rats) | The antioxidant activity (DPPH test) of NRG nanoemulsion was 95.28 ± 0.64 % compared with 78.32 ± 0.81 % for free NRG | Intranasal administration of NRG nanoemulsion and levodopa lead to reversing Parkinson’s disease symptoms in rats. | [138] |
| Lipopolysaccharide-induced inflammation | Polymeric nanoparticles | 10 µg/mL to 200 µg/mL (LPS induced RAW264.7 cells) | Nitrite levels at a conceteration of 25 µg/mL of NRG were significantly lower when NRG-loaded nanoparticles were used compared with free NRG. | PVP-coated NRG nanoparticles showed anti-inflammatory effects on lipopolysaccharide-induced inflammation in RAW264.7 macrophage cells through the downregulation of iNOS and COX-2 expression after inhibiting MAPK and NF-κB pathways. | [139] |
| Wound healing | Nanoemulsion | 2 mg/mL once daily for 14 days (albino Wistar rats with abrasion wound) | NRG-loaded nanoemulsion showed mild to moderate penetration of inflammatory cells infiltration into the dermis, whereas the blank formulation showed acanthosis and infiltration of inflammatory cells into the dermis | Chitosan-coated NRG nanoemulsion showed a controlled release profile and significantly ameliorated the wound’s construction and stimulated the skin regeneration. | [68] |
| Ocular inflammation | Nanocomplexes | 5 mg/mL (New Zealand white rabbits) | conjunctiva swelling, congestion, and iris hyperemia were milder when NRG nanocomplexes were used compared with free NRG and control. | NRG PVP nanocomplex dispersion had better antioxidant properties and was well-tolerated with a significant improvement in terms of anti-inflammatory effects and intraocular permeation in the rabbit eyes. | [82] |
| Nonalcoholic fatty liver | Liposomes | 25 mg/ kg/day for NRG-loaded liposomes and 25, 50, 100 mg/ kg/day for free NRG (nonalcoholic fatty liver in male C57BL/6J mice) | NRG-loaded showed significantly better effects on AST, ALT, TG, and lipid accumulation compared with free NRG at the same dose; however, NRG-loaded liposomes showed comparable effects to free NRG at a dose of 100 mg/ kg/day. | Nanoliposomes loaded with NRG significantly improved the oral bioavailability of NRG and showed hepatoprotective effects in mice with nonalcoholic fatty liver disease with lower effective doses compared to NRG alone. | [140] |
| Diabetes | Polymeric nanoparticles | 25 mg/kg (streptozotocin-induced diabetic rats) | After treatment blood glucose levels were 83.3 ± 6.0 mg/dL and 126.4 ± 5.1 mg/dL for NRG-loaded nanoparticles and free NRG, respectively. | NRG-loaded PLGA nanoparticles normalized the blood glucose level in diabetic rats, increased insulin levels, reduced glycated hemoglobin level, ameliorated oxidative stress, hyperlipidemia, and hyperglycemia. | [141] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhia, M.; Motallebi, M.; Abadi, B.; Zarepour, A.; Pereira-Silva, M.; Saremnejad, F.; Santos, A.C.; Zarrabi, A.; Melero, A.; Jafari, S.M.; et al. Naringenin Nano-Delivery Systems and Their Therapeutic Applications. Pharmaceutics 2021, 13, 291. https://doi.org/10.3390/pharmaceutics13020291
Bhia M, Motallebi M, Abadi B, Zarepour A, Pereira-Silva M, Saremnejad F, Santos AC, Zarrabi A, Melero A, Jafari SM, et al. Naringenin Nano-Delivery Systems and Their Therapeutic Applications. Pharmaceutics. 2021; 13(2):291. https://doi.org/10.3390/pharmaceutics13020291
Chicago/Turabian StyleBhia, Mohammed, Mahzad Motallebi, Banafshe Abadi, Atefeh Zarepour, Miguel Pereira-Silva, Farinaz Saremnejad, Ana Cláudia Santos, Ali Zarrabi, Ana Melero, Seid Mahdi Jafari, and et al. 2021. "Naringenin Nano-Delivery Systems and Their Therapeutic Applications" Pharmaceutics 13, no. 2: 291. https://doi.org/10.3390/pharmaceutics13020291
APA StyleBhia, M., Motallebi, M., Abadi, B., Zarepour, A., Pereira-Silva, M., Saremnejad, F., Santos, A. C., Zarrabi, A., Melero, A., Jafari, S. M., & Shakibaei, M. (2021). Naringenin Nano-Delivery Systems and Their Therapeutic Applications. Pharmaceutics, 13(2), 291. https://doi.org/10.3390/pharmaceutics13020291