Green Nanotechnology in the Formulation of a Novel Solid Dispersed Multilayered Core-Sheath Raloxifene-Loaded Nanofibrous Buccal Film; In Vitro and In Vivo Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of RLX-Loaded Nanofibers
2.2.1. Preparation of RLX-Nanoemulsion
2.2.2. Preparation of Polymer Solutions
2.2.3. Preparation of RLX-Loaded Nanofibers
2.3. Characterization of RLX-Nanoemulsion
2.3.1. Determination of Particle Size (PS), Polydispersity Index (PDI), and Zeta Potential (ZP)
2.3.2. Drug Content
2.3.3. Spectroscopic Characterization of Percentage Transmission
2.4. Characterization of RLX-Loaded Nanofibers
2.4.1. Solid State Characterization of RLX-Loaded Nanofibers
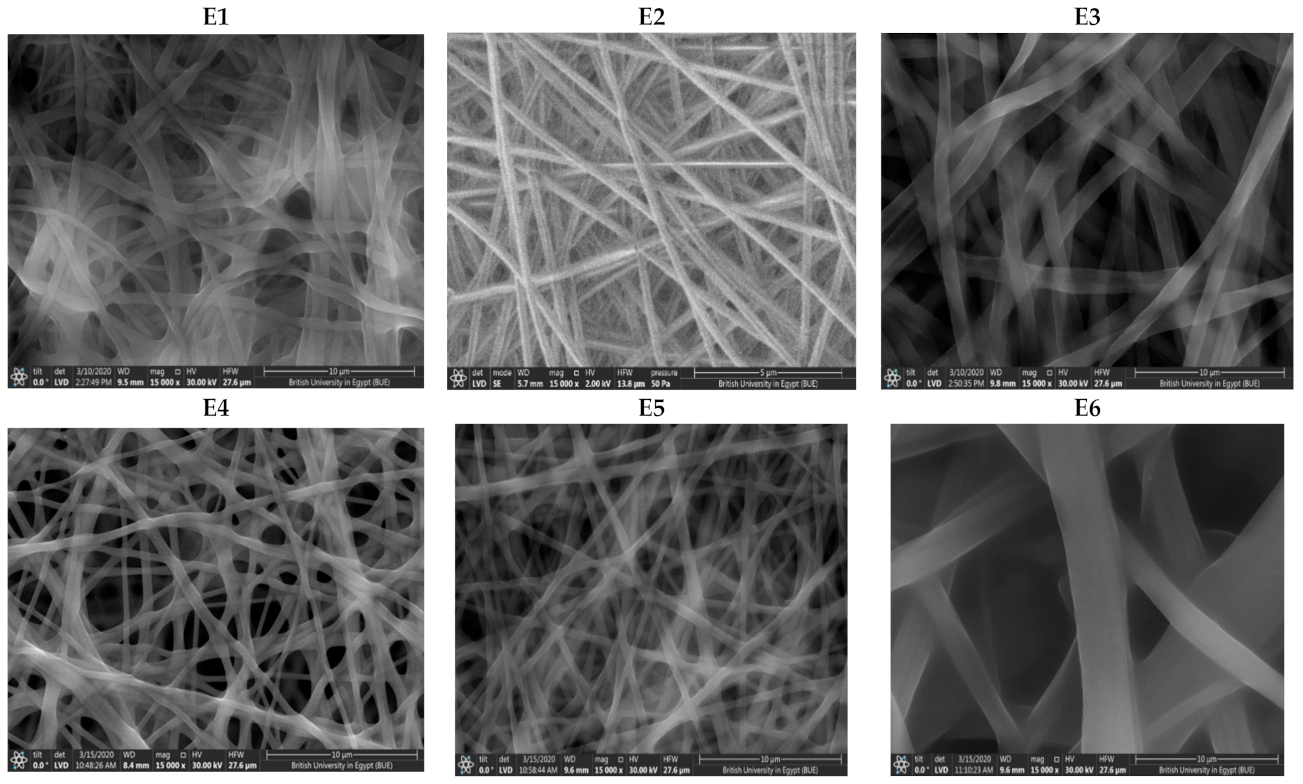
Scanning Electron Microscope (SEM)
Differential Scanning Calorimetry (DSC)
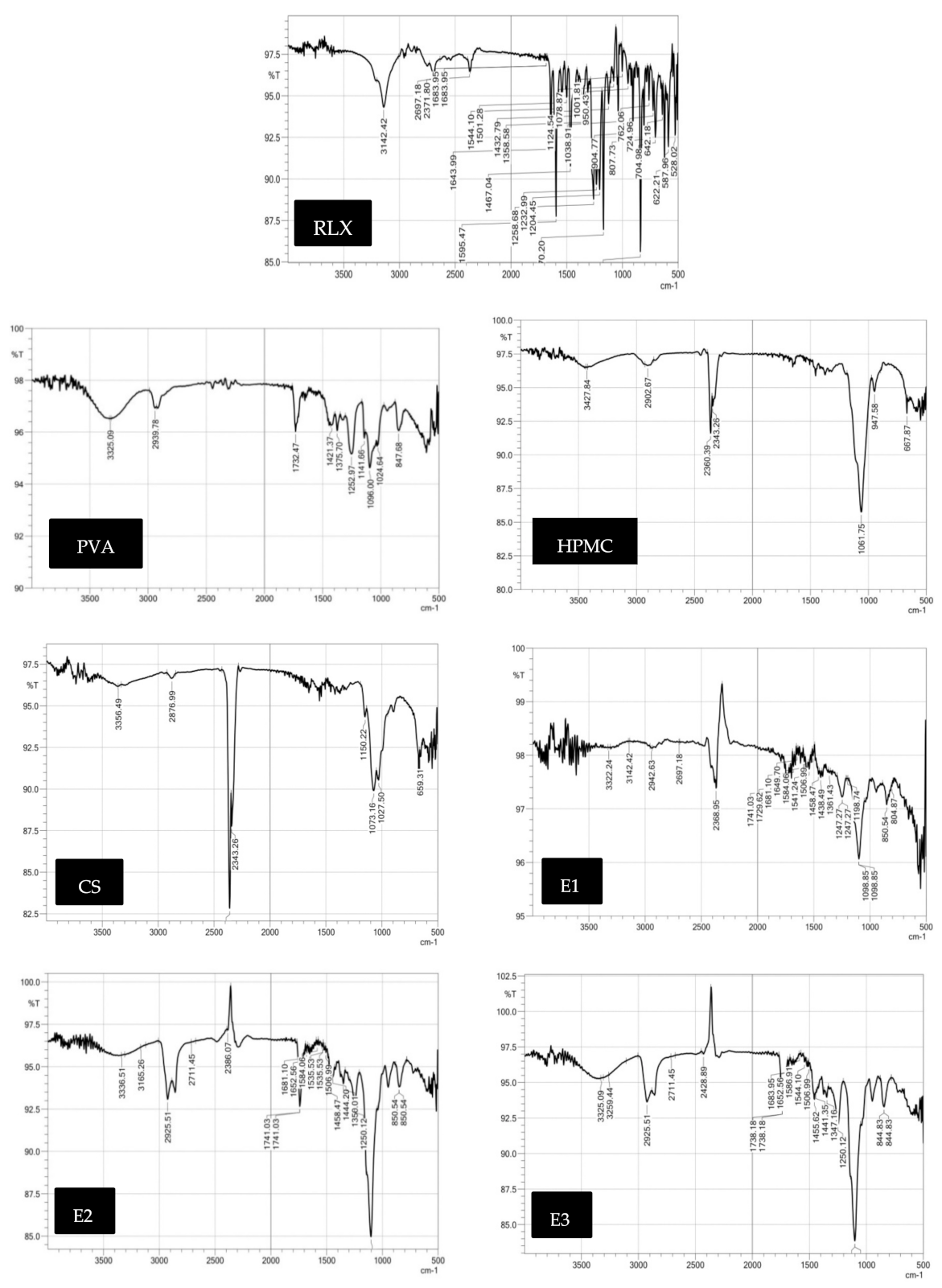
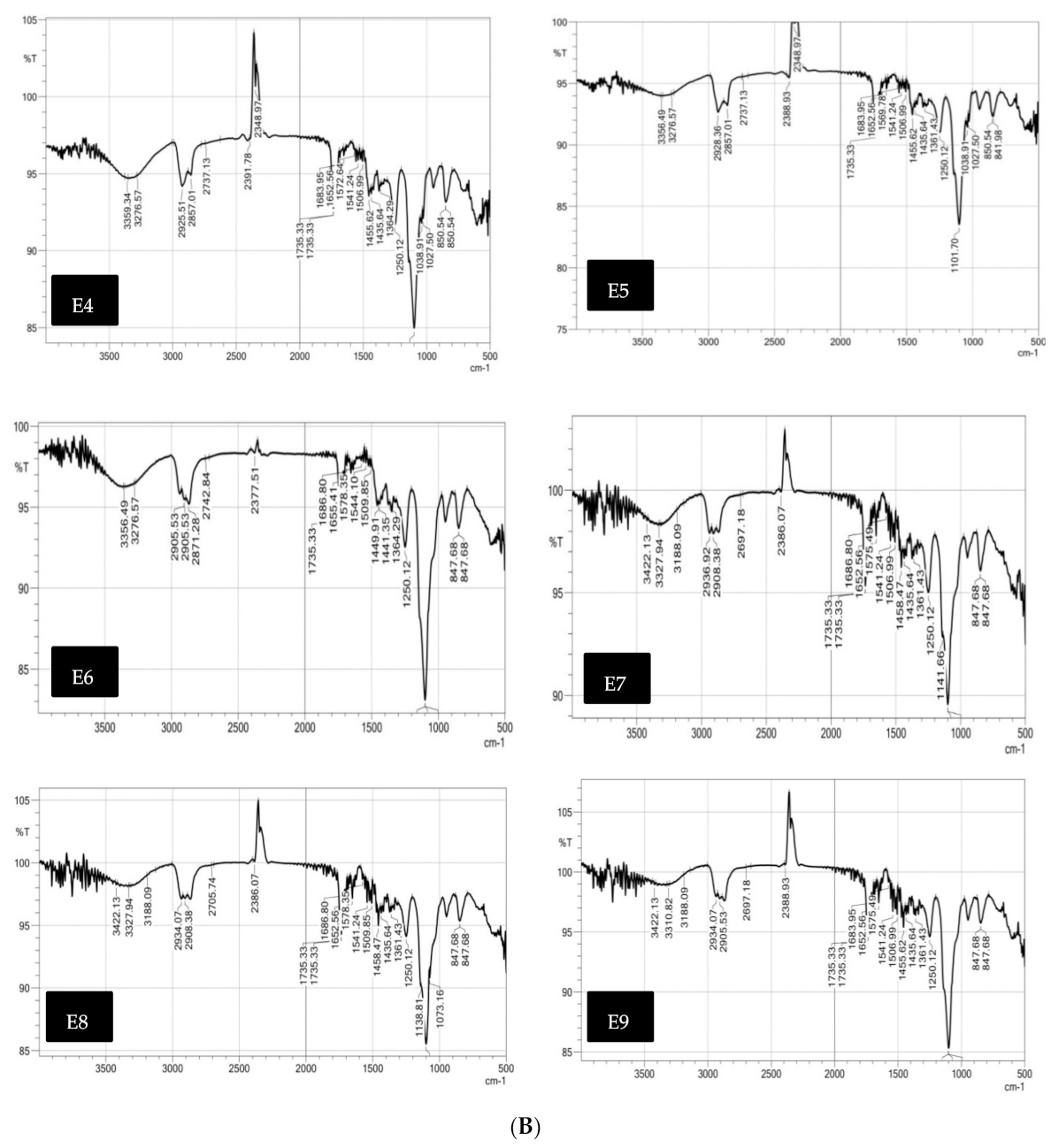
Fourier-Transform Infrared Spectra (FT-IR)
Powder X-ray Diffraction (PXRD)
2.4.2. Determination of Drug Content and Homogeneity of RLX-Loaded Nanofibers
2.4.3. In Vitro Release Studies
2.4.4. Bioadhesion Potential of RLX-Loaded Nanofibers
2.5. Optimization of RLX-Loaded Nanofibers via D-Optimal Response Surface Methodology
2.6. Characterization of the Optimum RLX-Loaded Nanofibers
2.6.1. High-Resolution Transmission Electron Microscope (HRTEM) of the Selected RLX-Loaded NFs
2.6.2. Ex Vivo Drug Permeation Studies
2.7. In Vivo Estimation of RLX Pharmacokinetic Parameters in Rabbits
2.7.1. Study Design
2.7.2. Animals
2.7.3. Administration of Treatments and Blood Collection
2.7.4. Estimation of RLX Concentration in Rabbit Plasma
2.7.5. Pharmacokinetics and Statistical Analyses
3. Results and Discussion
3.1. Preparation of RLX-Loaded Nanofibers
3.2. Characterization of RLX-Loaded Nanoemulsion
3.2.1. Determination of Particle Size (PS), Polydispersity Index (PDI), and Zeta Potential (ZP)
3.2.2. Drug Content
3.2.3. Spectroscopic Characterization of Percentage Transmission
3.3. Characterization of RLX-Loaded Nanofibers
Y2 (Q240) = 83.701 + 12.859 × A[1] − 21.58 × A[2] + 8.2778 × B − 2.753 × A[1]B + 6.6794 × A[2]B
Y3 (Fiber Size) = 647.96 − 115 × A[1] − 32.79 × [2] + 527.83 × B − 319.2 × A[1]B + 52.321 × A[2]B + 297.06 × B2
Y4 (Mucoadhesion Time) = 21.0542 − 0.02095 × A[1] + 0.02619 × A[2] − 11.7833 × B − 0.02857 × A[1]B + 0.03571 × A[2]B − 8.8375 × B2
3.3.1. Solid State Characterization of RLX-Loaded Nanofibers
Scanning Electron Microscope (SEM) and Fiber Size
Differential Scanning Calorimetry (DSC)
Fourier-Transform Infra-Red Spectra (FT-IR)
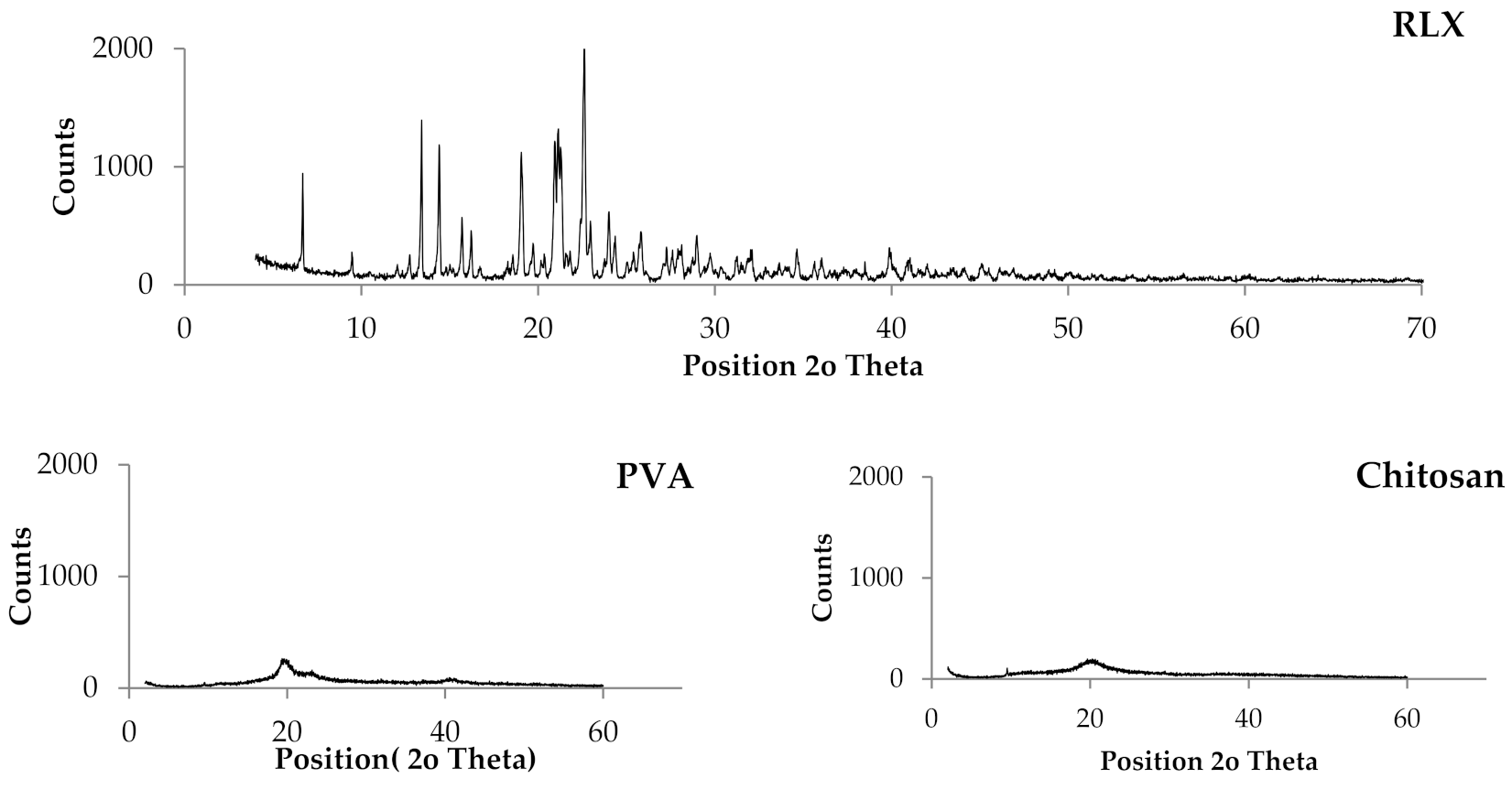
Powder X-ray Diffraction (PXRD) Studies
3.3.2. Determination of Drug Content and Homogeneity of RLX-Loaded Nanofibers
3.3.3. In Vitro Release Studies
3.3.4. Bioadhesion Potential of RLX-Loaded NFs
3.4. Elucidation of Optimum RLX-NFs Films
3.5. Characterization of the Optimum RLX-Loaded NFs
3.5.1. HRTEM of the Selected RLX-Loaded NFs Film
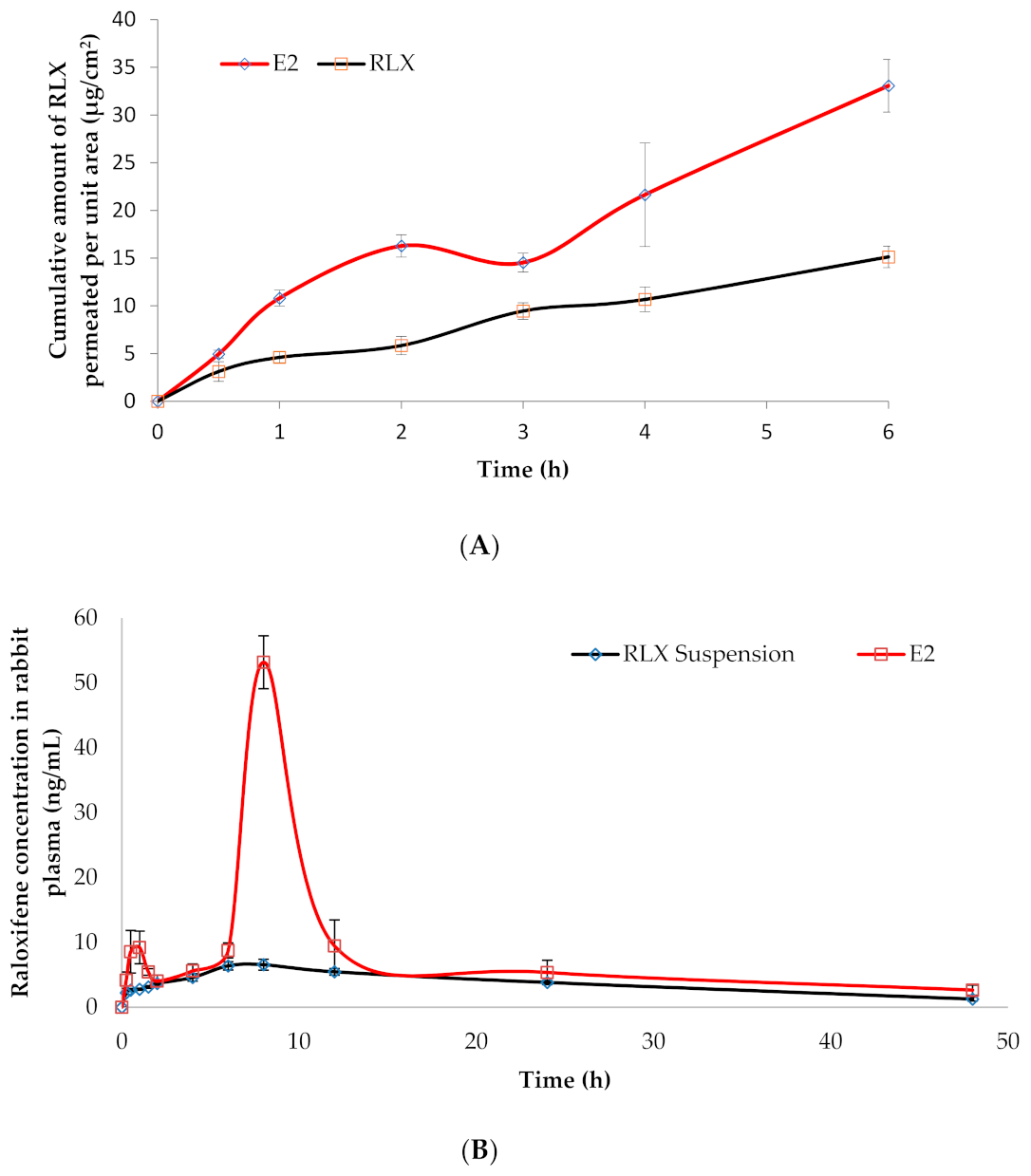
3.5.2. Ex Vivo Drug Permeation Studies
3.6. In Vivo Estimation of RLX Pharmacokinetics in Rabbits
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kannel, W.B.; Hjortland, M.C.; McNamara, P.M.; Gordon, T. Menopause and risk of cardiovascular disease. The framingham study. Obstet. Gynecol. Surv. 1977, 32, 239–242. [Google Scholar] [CrossRef]
- Parrish, H.M.; Carr, C.A.; Hall, D.G.; King, T.M. Time interval from castration in premenopausal women to development of excessive coronary atherosclerosis. Am. J. Obstet. Gynecol. 1967, 99, 155–162. [Google Scholar] [CrossRef]
- Aitken, J.M.; Hart, D.M.; Anderson, J.B.; Lindsay, R.; Smith, D.A.; Speirs, C.F. Osteoporosis after Oophorectomy for Non-malignant Disease in Premenopausal Women. BMJ 1973, 2, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Richelson, L.S.; Wahner, H.W.; Melton, L., III; Riggs, B.L. Relative contributions of aging and estrogen deficiency to postmenopausal bone loss. N. Engl. J. Med. 1984, 311, 1273–1275. [Google Scholar] [CrossRef] [PubMed]
- Delmas, P.D.; Bjarnason, N.H.; Mitlak, B.H.; Ravoux, A.-C.; Shah, A.S.; Huster, W.J.; Draper, M.; Christiansen, C. Effects of Raloxifene on Bone Mineral Density, Serum Cholesterol Concentrations, and Uterine Endometrium in Postmenopausal Women. N. Engl. J. Med. 1997, 337, 1641–1647. [Google Scholar] [CrossRef]
- Walsh, B.W.; Kuller, L.H.; Wild, R.A.; Paul, S.; Farmer, M.; Lawrence, J.B.; Shah, A.S.; Anderson, P.W. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA 1998, 279, 1445–1451. [Google Scholar] [CrossRef]
- Jordan, V.; Glusman, J.; Eckert, S.; Lippman, M.; Powles, T.; Costa, A.; Morrow, M.; Norton, L. Incident primary breast cancers are reduced by raloxifene: Integrated data from multicenter, double-blind, randomized trials in~ 12,000 postmenopausal women. Proc. Am. Soc. Clin. Oncol. 1998, 17, 122a. [Google Scholar]
- Cummings, S.R.; Eckert, S.; Krueger, K.A.; Grady, D.; Powles, T.J.; Cauley, J.A.; Norton, L.; Nickelsen, T.; Bjarnason, N.H.; Morrow, M.; et al. The Effect of Raloxifene on Risk of Breast Cancer in Postmenopausal Women: Results from the MORE Randomized Trial. Obstet. Gynecol. Surv. 2000, 55, 100. [Google Scholar] [CrossRef]
- Tu, Z.; Li, H.; Ma, Y.; Tang, B.; Tian, J.; Akers, W.; Achilefu, S.; Gu, Y. The enhanced antiproliferative response to combined treatment of trichostatin A with raloxifene in MCF-7 breast cancer cells and its relevance to estrogen receptor β expression. Mol. Cell. Biochem. 2012, 366, 111–122. [Google Scholar] [CrossRef]
- Hochner-Celnikier, D. Pharmacokinetics of raloxifene and its clinical application. Eur. J. Obstet. Gynecol. Reprod. Biol. 1999, 85, 23–29. [Google Scholar] [CrossRef]
- Vasconcelos, T.; Sarmento, B.; Costa, P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov. Today 2007, 12, 1068–1075. [Google Scholar] [CrossRef]
- Lam, P.-L.; Wong, W.-Y.; Bian, Z.; Chui, C.-H.; Gambari, R. Recent advances in green nanoparticulate systems for drug delivery: Efficient delivery and safety concern. Nanomedicine 2017, 12, 357–385. [Google Scholar] [CrossRef]
- Korany, M.A.; Mahgoub, H.; Haggag, R.S.; Ragab, M.A.A.; Elmallah, O.A. Green chemistry: Analytical and chromatography. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 839–852. [Google Scholar] [CrossRef]
- Shojaei, A.H.; Chang, R.K.; Guo, X.; Burnside, B.A.; Couch, R.A. Systemic drug delivery via the buccal mucosal route. Pharm. Technol. 2001, 25, 70–81. [Google Scholar]
- Modgill, V.; Garg, T.; Goyal, A.K.; Rath, G. Permeability study of ciprofloxacin from ultra-thin nanofibrous film through various mucosal membranes. Artif. Cells Nanomed. Biotechnol. 2014, 44, 122–127. [Google Scholar] [CrossRef]
- Weng, L.; Xie, J. Smart electrospun nanofibers for controlled drug release: Recent advances and new perspectives. Curr. Pharm. Des. 2015, 21, 1944–1959. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Mašek, J.; Mašková, E.; Lubasová, D.; Špánek, R.; Raška, M.; Turánek, J. Nanofibers in Mucosal Drug and Vaccine Delivery. In Nanofibers-from Preparation to Applications, Simona Clichici, Adriana Filip, Gutavo M. do Nascimento; IntechOpen: London, UK, 2018; pp. 171–198. [Google Scholar]
- Nikmaram, N.; Roohinejad, S.; Hashemi, S.; Koubaa, M.; Barba, F.J.; Abbaspourrad, A.; Greiner, R. Emulsion-based systems for fabrication of electrospun nanofibers: Food, pharmaceutical and biomedical applications. RSC Adv. 2017, 7, 28951–28964. [Google Scholar] [CrossRef]
- Qi, H.; Hu, P.; Xu, A.J.; Wang, A. Encapsulation of Drug Reservoirs in Fibers by Emulsion Electrospinning: Morphology Characterization and Preliminary Release Assessment. Biomacromolecules 2006, 7, 2327–2330. [Google Scholar] [CrossRef]
- Tian, L.; Prabhakaran, M.P.; Ding, X.; Ramakrishna, S. Biocompatibility evaluation of emulsion electrospun nanofibers using osteoblasts for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2013, 24, 1952–1968. [Google Scholar] [CrossRef]
- Murthy, A.; Ravi, P.R.; Kathuria, H.; Vats, R. Self-assembled lecithin-chitosan nanoparticles improve the oral bioavailability and alter the pharmacokinetics of raloxifene. Int. J. Pharm. 2020, 588, 119731. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.V.; Seth, A.K.; Balaraman, R.; Aundhia, C.J.; Maheshwari, R.A.; Parmar, G.R. Nanostructured lipid carriers for oral bioavailability enhancement of raloxifene: Design and in vivo study. J. Adv. Res. 2016, 7, 423–434. [Google Scholar] [CrossRef]
- Ağardan, N.B.M.; DeĞim, Z.; Yılmaz, Şükran; Altıntaş, L.; Topal, T. The Effectiveness of Raloxifene-Loaded Liposomes and Cochleates in Breast Cancer Therapy. AAPS PharmSciTech 2015, 17, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Pandya, B.R.; Chotai, N.P.; Suthar, R.M.; Patel, H.K. Formulation, In-vitro Evaluation and Optimization of Nanoemulsion of Raloxifene Hydrochloride. Int. J. Pharm. Sci. Res. 2017, 9, 38–48. [Google Scholar]
- Saini, D.; Fazil, M.; Ali, M.M.; Baboota, S.; Ali, J. Formulation, development and optimization of raloxifene-loaded chitosan nanoparticles for treatment of osteoporosis. Drug Deliv. 2014, 22, 1–14. [Google Scholar] [CrossRef]
- Abd-Elrasheed, E.; El-Helaly, S.N.; El-Ashmoony, M.M.; Salah, S. Brain Targeted Intranasal Zaleplon Nano-emulsion: In-Vitro Characterization and Assessment of Gamma Aminobutyric Acid Levels in Rabbits’ Brain and Plasma at Low and High Doses. Curr. Drug Deliv. 2018, 15, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-Q.; Cheng, Z.-Q.; Feng, Q.-J.; Li, H.-J.; Ye, S.-F.; Teng, B. Polycaprolactone nanofibres loaded with 20 (S)-protopanaxadiol for in vitro and in vivo anti-tumour activity study. R. Soc. Open Sci. 2018, 5, 180137. [Google Scholar] [CrossRef]
- Abd-Elbary, A.; Makky, A.M.; Tadros, M.I.; Alaa-Eldin, A.A. Laminated sponges as challenging solid hydrophilic matrices for the buccal delivery of carvedilol microemulsion systems: Development and proof of concept via mucoadhesion and pharmacokinetic assessments in healthy human volunteers. Eur. J. Pharm. Sci. 2016, 82, 31–44. [Google Scholar] [CrossRef]
- Yeap, S.P.; Lim, J.; Ngang, H.P.; Ooi, B.S.; Ahmad, A.L. Role of Particle–Particle Interaction Towards Effective Interpretation of Z-Average and Particle Size Distributions from Dynamic Light Scattering (DLS) Analysis. J. Nanosci. Nanotechnol. 2018, 18, 6957–6964. [Google Scholar] [CrossRef]
- Ammar, H.O.; Mohamed, M.I.; Tadros, M.I.; Fouly, A.A. Transdermal Delivery of Ondansetron Hydrochloride via Bilosomal Systems: In Vitro, Ex Vivo, and In Vivo Characterization Studies. AAPS PharmSciTech 2018, 19, 2276–2287. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, Y.S.R.; Abdallah, O.Y.; Gohar, E.Y.; Elsheikh, M.A. Nanoemulsion liquid preconcentrates for raloxifene hydrochloride: Optimization and in vivo appraisal. Int. J. Nanomed. 2012, 7, 3787–3802. [Google Scholar] [CrossRef]
- Nakamura, F.; Ohta, R.; Machida, Y.; Nagai, T. In vitro and in vivo nasal mucoadhesion of some water-soluble polymers. Int. J. Pharm. 1996, 134, 173–181. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Bawazir, A.O.; Alharbi, W.S.; Safo, M.K. Enhancement of Simvastatin ex vivo Permeation from Mucoadhesive Buccal Films Loaded with Dual Drug Release Carriers. Int. J. Nanomed. 2020, 15, 4001–4020. [Google Scholar] [CrossRef] [PubMed]
- Tayel, S.A.; Soliman, I.I.; Louis, D. Formulation of Ketotifen Fumarate Fast-Melt Granulation Sublingual Tablet. AAPS PharmSciTech 2010, 11, 679–685. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tawfik, M.A.; Tadros, M.I.; Mohamed, M.I.; El-Helaly, S.N. Low-Frequency versus High-Frequency Ultrasound-Mediated Transdermal Delivery of Agomelatine-Loaded Invasomes: Development, Optimization and in-vivo Pharmacokinetic Assessment. Int. J. Nanomed. 2020, 15, 8893–8910. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; Sayed, M.; Abdelnabi, M. A new protocol of anesthesia using thiopental, diazepam and xylazine in white New Zealand rabbits. Aust. J. Basic Appl. Sci. 2011, 5, 1296–1300. [Google Scholar]
- Shin, J.-W.; Seol, I.-C.; Son, C.-G. Interpretation of animal dose and human equivalent dose for drug development. J. Korean Med. 2010, 31, 1–7. [Google Scholar]
- Said, M.; Elsayed, I.; Aboelwafa, A.A.; Elshafeey, A.H. Transdermal agomelatine microemulsion gel: Pyramidal screening, statistical optimization and in vivo bioavailability. Drug Deliv. 2017, 24, 1159–1169. [Google Scholar] [CrossRef]
- Bhatt, P.; Madhav, S. A detailed review on nanoemulsion drug delivery system. Int. J. Pharm. Sci. Res. 2011, 2, 2482–2489. [Google Scholar]
- Reza, K.H. Nanoemulsion as a novel transdermal drug delivery system. Int. J. Pharm. Sci. Res. 2011, 2, 1938–1946. [Google Scholar]
- Nepal, P.R.; Han, H.-K.; Choi, H.-K. Preparation and in vitro–in vivo evaluation of Witepsol® H35 based self-nanoemulsifying drug delivery systems (SNEDDS) of coenzyme Q10. Eur. J. Pharm. Sci. 2010, 39, 224–232. [Google Scholar] [CrossRef]
- Singh, S.K.; Verma, P.R.P.; Razdan, B. Glibenclamide-loaded self-nanoemulsifying drug delivery system: Development and characterization. Drug Dev. Ind. Pharm. 2010, 36, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Q.; He, J.-H.; Xu, L. Non-ionic surfactants for enhancing electrospinability and for the preparation of electrospun nanofibers. Polym. Int. 2008, 57, 1079–1082. [Google Scholar] [CrossRef]
- Jia, Y.-T.; Gong, J.; Gu, X.-H.; Kim, H.-Y.; Dong, J.; Shen, X.-Y. Fabrication and characterization of poly (vinyl alcohol)/chitosan blend nanofibers produced by electrospinning method. Carbohydr. Polym. 2007, 67, 403–409. [Google Scholar] [CrossRef]
- Geng, X.; Kwon, O.-H.; Jang, J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterial 2005, 26, 5427–5432. [Google Scholar] [CrossRef]
- Sedghi, R.; Shaabani, A.; Mohammadi, Z.; Samadi, F.Y.; Isaei, E. Biocompatible electrospinning chitosan nanofibers: A novel delivery system with superior local cancer therapy. Carbohydr. Polym. 2017, 159, 1–10. [Google Scholar] [CrossRef]
- Desai, K.; Kit, K.; Li, J.; Zivanovic, S. Morphological and Surface Properties of Electrospun Chitosan Nanofibers. Biomacromolecules 2008, 9, 1000–1006. [Google Scholar] [CrossRef]
- Duan, B.; Dong, C.; Yuan, X.; Yao, K. Electrospinning of chitosan solutions in acetic acid with poly (ethylene oxide). J. Biomater. Sci. Polym. Ed. 2004, 15, 797–811. [Google Scholar] [CrossRef]
- Lu, J.-W.; Zhu, Y.-L.; Guo, Z.-X.; Hu, P.; Yu, J. Electrospinning of sodium alginate with poly (ethylene oxide). Polymer 2006, 47, 8026–8031. [Google Scholar] [CrossRef]
- Aydogdu, A.; Sumnu, G.; Sahin, S. A novel electrospun hydroxypropyl methylcellulose/polyethylene oxide blend nanofibers: Morphology and physicochemical properties. Carbohydr. Polym. 2018, 181, 234–246. [Google Scholar] [CrossRef]
- Neo, Y.P.; Ray, S.; Easteal, A.J.; Nikolaidis, M.G.; Quek, S.Y. Influence of solution and processing parameters towards the fabrication of electrospun zein fibers with sub-micron diameter. J. Food Eng. 2012, 109, 645–651. [Google Scholar] [CrossRef]
- Lin, T.; Fang, J.; Wang, H.; Cheng, T.; Wang, X. Using chitosan as a thickener for electrospinning dilute PVA solutions to improve fibre uniformity. Nanotechnology 2006, 17, 3718–3723. [Google Scholar] [CrossRef]
- Ignatova, M.; Starbova, K.; Markova, N.; Manolova, N.; Rashkov, I. Electrospun nano-fibre mats with antibacterial properties from quaternised chitosan and poly (vinyl alcohol). Carbohydr. Res. 2006, 341, 2098–2107. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, S.H.; Nouri, M. Chitosan-poly (vinyl alcohol) blend nanofibers: Morphology, biological and antimicrobial properties. e-Polymers 2009, 9, 1580–1591. [Google Scholar]
- Patil, P.H.; Belgamwar, V.S.; Patil, P.R.; Surana, S.J. Solubility enhancement of raloxifene using inclusion complexes and cogrinding method. J. Pharm. 2013, 2013. [Google Scholar] [CrossRef]
- Yalkowsky, S.H. Solubility and Partitioning V: Dependence of Solubility on Melting Point. J. Pharm. Sci. 1981, 70, 971–973. [Google Scholar] [CrossRef]
- Salah, S.; Mahmoud, A.A.; Kamel, A.O. Etodolac transdermal cubosomes for the treatment of rheumatoid arthritis: Ex vivo permeation and in vivo pharmacokinetic studies. Drug Deliv. 2017, 24, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, F.; Periolatto, M. Antimicrobial finish of textiles by chitosan UV-curing. J. Nanosci. Nanotechnol. 2012, 12, 4803–4810. [Google Scholar] [CrossRef] [PubMed]
- Demappa, T.; Ganesha, S.; Divakara, S.; Pattabi, M.; Somashekar, R. Physical and thermal properties of 8MeV electron beam irradiated HPMC polymer films. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2008, 266, 3975–3980. [Google Scholar]
- Tadros, M.I.; Fahmy, R.H. Controlled-release triple anti-inflammatory therapy based on novel gastroretentive sponges: Characterization and magnetic resonance imaging in healthy volunteers. Int. J. Pharm. 2014, 472, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Kenechukwu, F.C.; Ofokansi, K.C.; Ezugwu, R.O.; Attama, A.A. Improved dissolution and anti-inflammatory activity of ibuprofen-polyethylene glycol 8000 solid dispersion systems. Int. J. Pharm. Investig. 2016, 6, 139–147. [Google Scholar] [CrossRef]
- Jain, A. Solubilization of indomethacin using hydrotropes for aqueous injection. Eur. J. Pharm. Biopharm. 2008, 68, 701–714. [Google Scholar] [CrossRef]
- Damian, F.; Blaton, N.; Naesens, L.; Balzarini, J.; Kinget, R.; Augustijns, P.; Van den Mooter, G. Physicochemical characterization of solid dispersions of the antiviral agent UC-781 with polyethylene glycol 6000 and Gelucire 44/14. Eur. J. Pharm. Sci. 2000, 10, 311–322. [Google Scholar] [CrossRef]
- Hancock, B.C.; Zografi, G. Characteristics and Significance of the Amorphous State in Pharmaceutical Systems. J. Pharm. Sci. 1997, 86, 1–12. [Google Scholar] [CrossRef]
- Fini, A.; Moyano, J.R.; Ginés, J.M.; Perez-Martinez, J.I.; Rabasco, A.M. Diclofenac salts, II. Solid dispersions in PEG6000 and Gelucire 50/13. Eur. J. Pharm. Biopharm. 2005, 60, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, A.M.; Tadros, M.I.; Alaa-Eldin, A.A. Spray-Dried Silica Xerogel Nanoparticles as a Promising Gastroretentive Carrier System for the Management of Chemotherapy-Induced Nausea and Vomiting. Int. J. Nanomed. 2019, 14, 9619–9630. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, X.; Cui, W.; Zhou, S.; Tan, R.; Wang, C. Structural stability and release profiles of proteins from core-shell poly (DL-lactide) ultrafine fibers prepared by emulsion electrospinning. J. Biomed. Mater. Res. Part A 2008, 86, 374–385. [Google Scholar] [CrossRef]
- O’Donnell, K.L.; Oporto-Velásquez, G.S.; Comolli, N. Evaluation of Acetaminophen Release from Biodegradable Poly (Vinyl Alcohol) (PVA) and Nanocellulose Films Using a Multiphase Release Mechanism. Nanomaterials 2020, 10, 301. [Google Scholar] [CrossRef] [PubMed]
- Parameswara, P.; Demappa, T.; Mahadevaiah Prakash, Y.; Somashekarappa, H.; Byrappa, K.; Somashekar, R. Polymeric degradation of water soluble chitosan/HPMC films using WAXS data. Mater. Res. Innov. 2012, 16, 126–129. [Google Scholar] [CrossRef]
- Hassan, C.M.; Trakampan, P.; Peppas, N.A. Water Solubility Characteristics of Poly (vinyl alcohol) and Gels Prepared by Freezing/Thawing Processes. In Water Soluble Polymers; Metzler, J.B., Ed.; Springer: Boston, MA, USA, 2005; pp. 31–40. [Google Scholar]
- Varma, M.V.S.; Kaushal, A.M.; Garg, A.; Garg, S. Factors Affecting Mechanism and Kinetics of Drug Release from Matrix-Based Oral Controlled Drug Delivery Systems. Am. J. Drug Deliv. 2004, 2, 43–57. [Google Scholar] [CrossRef]
- Siepmann, J.; Siegel, R.A.; Rathbone, M.J. Fundamentals and Applications of Controlled Release Drug Delivery; Springer: New York, NY, USA, 2012; Volume 3, pp. 33–34. [Google Scholar]
- Mishra, S.K.; Garud, N.; Singh, R. Development and evaluation of mucoadhesive buccal patches of flurbiprofen. Acta Pol. Pharm. Drug Res. 2011, 68, 955–964. [Google Scholar]
- Peppas, N.A.; Buri, P.A. Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues. J. Control. Release 1985, 2, 257–275. [Google Scholar] [CrossRef]
- Ways, T.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef]
- Patel, H.; Srinatha, A.; Sridhar, B.K. External Cross-linked Mucoadhesive Microbeads for Prolonged Drug Release: Development and In vitro Characterization. Indian J. Pharm. Sci. 2014, 76, 437–444. [Google Scholar]
- Nafee, N.A.; Ismail, F.A.; Boraie, N.A.; Mortada, L.M. Mucoadhesive Delivery Systems. I. Evaluation of Mucoadhesive Polymers for Buccal Tablet Formulation. Drug Dev. Ind. Pharm. 2004, 30, 985–993. [Google Scholar] [CrossRef]
- Yu, J.H.; Fridrikh, S.V.; Rutledge, G.C. Production of Submicrometer Diameter Fibers by Two-Fluid Electrospinning. Adv. Mater. 2004, 16, 1562–1566. [Google Scholar] [CrossRef]
- Xu, X.; Zhuang, X.; Chen, X.; Wang, X.; Yang, L.; Jing, X. Preparation of Core-Sheath Composite Nanofibers by Emulsion Electrospinning. Macromol. Rapid Commun. 2006, 27, 1637–1642. [Google Scholar] [CrossRef]
- Helmy, S.A.; El-Bedaiwy, H.M.; El-Masry, S.M. Applying Biopharmaceutical Classification System criteria to predict the potential effect of Cremophor® RH 40 on fexofenadine bioavailability at higher doses. Ther. Deliv. 2020, 11, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Javadzadeh, Y.; Adibkia, K.; Hamishekar, H. Transcutol® (Diethylene Glycol Monoethyl Ether): A Potential Penetration Enhancer. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Metzler, J.B., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 195–205. [Google Scholar]
- Bhati, R.; Nagrajan, R.K. A detailed review on oral mucosal drug delivery system. Int. J. Pharm. Sci. Res. 2012, 3, 659–681. [Google Scholar]
- Wang, Z.; Li, Y. Raloxifene/SBE-β-CD Inclusion Complexes Formulated into Nanoparticles with Chitosan to Overcome the Absorption Barrier for Bioavailability Enhancement. Pharmaceutics 2018, 10, 76. [Google Scholar] [CrossRef]





| System | Composition (mL) | In Vitro Characterization Data of RLX-NFs | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RLX-NE (5 mg/mL) | PVA (10% w) | HPMC (1% w) | Chitosan (1.5% w/v) | Drug Content (%) | Mucoadhesion Time (h) | Fiber Size (nm) | Q60 * (%) | Q240 * (%) | |
| E1 | 1 | 9 | - | - | 99.90 ± 5.52 | 24 ± 0.00 | 555.82 ± 29.88 | 61.14 ± 7.5 | 91.10 ± 3.63 |
| E2 | 2 | 8 | - | - | 96.56 ± 0.62 | 24 ± 0.00 | 594.67 ± 26.63 | 56.04 ± 16.02 | 94.61 ± 8.16 |
| E3 | 4 | 6 | - | - | 102.45 ± 4.98 | 0.38 ± 0.01 | 1005.93 ± 3.52 | 83.36 ± 0.14 | 102.11 ± 2.61 |
| E4 | 1 | 7.2 | - | 1.8 | 105.33 ± 7.10 | 24 ± 0.00 | 374.54 ± 20.74 | 19.26 ± 0.65 | 45.20 ± 0.31 |
| E5 | 2 | 6.4 | - | 1.6 | 98.20 ± 14.62 | 24 ± 0.00 | 391.10 ± 15.42 | 33.91 ± 4.93 | 60.06 ± 2.60 |
| E6 | 4 | 4.8 | - | 1.2 | 97.31 ± 0.80 | 0.5 ± 0.07 | 1513.61 ± 46.23 | 68.87 ± 9.29 | 76.09 ± 15.59 |
| E7 | 1 | 7.2 | 1.8 | - | 102.33 ± 6.59 | 24 ± 0.00 | 321.20 ± 26.14 | 44.47 ± 7.53 | 87.76 ± 2.10 |
| E8 | 2 | 6.4 | 1.6 | - | 93.38 ± 7.03 | 24 ± 0.00 | 529.27 ± 79.49 | 66.92 ± 16.26 | 91.43 ± 1.77 |
| E9 | 4 | 4.8 | 1.2 | - | 92.38 ± 2.14 | 0.42 ± 0.05 | 1899 ± 2.19 | 80.08 ± 9.59 | 96.62 ± 0.57 |
| Treatments | Oral RLX Aqueous Dispersion | RLX-Loaded NF (E2) Buccal Film |
|---|---|---|
| Cmax (ng/mL) | 7.04 ± 0.26 | 53.18 ± 4.56 |
| Tmax (h) * | 8 | 8 |
| MRT0–∞ (h) | 17.81 ± 0.80 | 15.11 ± 1.70 |
| t1/2 (h) | 16.98 ± 3.31 | 24.93 ± 11.51 |
| AUC0–48 (ng·h/mL) | 177.92 ± 11.51 | 408.74 ± 59.21 |
| AUC0–∞ (ng·h/mL) | 209.61 ± 22.20 | 509.84 ± 71.72 |
| % relative bioavailability based on AUC (0–48) | 229.73 | |
| % relative bioavailability based on AUC (0–∞) | 243.22 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nageeb El-Helaly, S.; Abd-Elrasheed, E.; Salim, S.A.; Fahmy, R.H.; Salah, S.; EL-Ashmoony, M.M. Green Nanotechnology in the Formulation of a Novel Solid Dispersed Multilayered Core-Sheath Raloxifene-Loaded Nanofibrous Buccal Film; In Vitro and In Vivo Characterization. Pharmaceutics 2021, 13, 474. https://doi.org/10.3390/pharmaceutics13040474
Nageeb El-Helaly S, Abd-Elrasheed E, Salim SA, Fahmy RH, Salah S, EL-Ashmoony MM. Green Nanotechnology in the Formulation of a Novel Solid Dispersed Multilayered Core-Sheath Raloxifene-Loaded Nanofibrous Buccal Film; In Vitro and In Vivo Characterization. Pharmaceutics. 2021; 13(4):474. https://doi.org/10.3390/pharmaceutics13040474
Chicago/Turabian StyleNageeb El-Helaly, Sara, Eman Abd-Elrasheed, Samar A. Salim, Rania H. Fahmy, Salwa Salah, and Manal M. EL-Ashmoony. 2021. "Green Nanotechnology in the Formulation of a Novel Solid Dispersed Multilayered Core-Sheath Raloxifene-Loaded Nanofibrous Buccal Film; In Vitro and In Vivo Characterization" Pharmaceutics 13, no. 4: 474. https://doi.org/10.3390/pharmaceutics13040474
APA StyleNageeb El-Helaly, S., Abd-Elrasheed, E., Salim, S. A., Fahmy, R. H., Salah, S., & EL-Ashmoony, M. M. (2021). Green Nanotechnology in the Formulation of a Novel Solid Dispersed Multilayered Core-Sheath Raloxifene-Loaded Nanofibrous Buccal Film; In Vitro and In Vivo Characterization. Pharmaceutics, 13(4), 474. https://doi.org/10.3390/pharmaceutics13040474