Effect of Curcumin Nanoemulsions Stabilized with MAG and DAG-MCFAs in a Fructose-Induced Hepatic Steatosis Rat Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MAG and DAG Emulsifier Enriched with Medium Chain Fatty Acids
2.3. Quantification of Acylglycerides
2.4. Preparation of Nanoemulsions (NE)
2.5. Characterization of Curcumin NE
2.6. Determination of Concentration and Entrapping Efficiency of Curcumin
2.7. NE Stability
2.8. NAFLD Induction in a Wistar Rat Model
2.9. Serum Parameters
2.10. Histological Analysis
2.11. Statistical Analysis
3. Results
3.1. Obtaining MAG and DAG Emulsifier from MCFAs
3.2. Curcumin NE with MAG and DAG
3.3. NE Stability
3.4. Entrapment Efficiency of Curcumin in NE
3.5. Experimental Animal Model
3.6. Serological Parameters
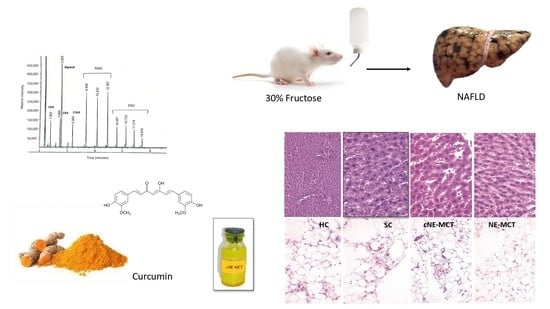
3.7. Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernández Bermejo, M. Caracterización del Papel de la Ácido Graso Translocasa CD36 en la Enfermedad Hepática Grasa no Alcohólica y en la Hepatitis Crónica por Virus C. Ph.D. Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 2014. [Google Scholar]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [Green Version]
- Quintana-Castro, R.; Aguirre-Maldonado, I.; Soto-Rodríguez, I.; Deschamps-Lago, R.A.; Gruber-Pagola, P.; de Larrea, Y.K.U.; Juárez-Rivera, V.E.; Ramos-Manuel, L.E.; Alexander-Aguilera, A. Cd36 gene expression in adipose and hepatic tissue mediates the lipids accumulation in liver of obese rats with sucrose-induced hepatic steatosis. Prostaglandins Other Lipid Mediat. 2020, 147, 106404. [Google Scholar] [CrossRef]
- Caballeria, L.; Torán, P. The fatty liver epidemic: An analysis from the primary care. Aten. Primaria 2019, 51, 525–526. [Google Scholar] [CrossRef]
- Idilman, I.S.; Ozdeniz, I.; Karcaaltincaba, M. Hepatic Steatosis: Etiology, Patterns, and Quantification. Semin. Ultrasound CT MRI 2016, 37, 501–510. [Google Scholar] [CrossRef]
- Chalasani, N.; Szabo, G. Pathogenesis of NAFLD and NASH. In Alcoholic and Non-Alcoholic Fatty Liver Disease, 1st ed.; Springer: Cham, Switzerland, 2016; pp. 71–102. ISBN 9783319205373. [Google Scholar]
- Vos, M.B.; LaVine, J.E. Dietary fructose in nonalcoholic fatty liver disease. Hepatology 2013, 57, 2525–2531. [Google Scholar] [CrossRef] [PubMed]
- Sulis, P.M.; Motta, K.; Barbosa, A.M.; Besen, M.H.; da Silva, J.S.; Nunes, E.A.; Rafacho, A. Impact of Fish Oil Supplementation and Interruption of Fructose Ingestion on Glucose and Lipid Homeostasis of Rats Drinking Different Concentrations of Fructose. BioMed Res. Int. 2017, 2017, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Lambertz, J.; Weiskirchen, S.; Landert, S.; Weiskirchen, R. Fructose: A Dietary Sugar in Crosstalk with Microbiota Contributing to the Development and Progression of Non-Alcoholic Liver Disease. Front. Immunol. 2017, 8, 1159. [Google Scholar] [CrossRef] [Green Version]
- Cascales, M. Lipogénesis “de novo” Y Termogénesis. Monogr. Real Acad. Nac. Farm. 2015, 6, 186–213. [Google Scholar]
- Carvallo, P.; Carvallo, E.; Barbosa-Da-Silva, S.; Mandarim-De-Lacerda, C.A.; Hernández, A.; Del-Sol, M. Efectos Metabólicos del Consumo Excesivo de Fructosa Añadida. Int. J. Morphol. 2019, 37, 1058–1066. [Google Scholar] [CrossRef] [Green Version]
- Farkhondeh, T.; Samini, F.; Samarghandian, S. Antidotal effects of curcumin against neurotoxic agents: An updated review. Asian Pac. J. Trop. Med. 2016, 9, 947–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teong, B.; Lin, C.-Y.; Chang, S.-J.; Niu, G.C.-C.; Yao, C.-H.; Chen, I.-F.; Kuo, S.-M. Enhanced anti-cancer activity by curcumin-loaded hydrogel nanoparticle derived aggregates on A549 lung adenocarcinoma cells. J. Mater. Sci. Mater. Electron. 2015, 26, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, Y.; Wang, Y.-W.; Huang, M.-T.; Ho, C.-T.; Huang, Q. Enhancing anti-inflammation activity of curcumin through O/W nanoemulsions. Food Chem. 2008, 108, 419–424. [Google Scholar] [CrossRef]
- Nayak, A.P.; Mills, T.; Norton, I. Lipid Based Nanosystems for Curcumin: Past, Present and Future. Curr. Pharm. Des. 2016, 22, 4247–4256. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.; Mukherjee, T.K. Structure-Function Elucidation of Antioxidative and Prooxidative Activities of the Polyphenolic Compound Curcumin. Chin. J. Biol. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Wang, T.; Li, J.; Wang, S.; Qiu, F.; Yu, H.; Zhang, Y.; Wang, T. Effects of Natural Products on Fructose-Induced Nonalcoholic Fatty Liver Disease (NAFLD). Nutrients 2017, 9, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Lv, Y.; Yao, H.; Yin, L.; Shang, J. Curcumin prevents the non-alcoholic fatty hepatitis via mitochondria protection and apoptosis reduction. Int. J. Clin. Exp. Pathol. 2015, 8, 11503–11509. [Google Scholar] [PubMed]
- Graham, A. Curcumin adds spice to the debate: Lipid metabolism in liver disease. Br. J. Pharmacol. 2009, 157, 1352–1353. [Google Scholar] [CrossRef]
- Inzaugarat, M.E.; De Matteo, E.; Baz, P.; Lucero, D.; García, C.C.; Ballerga, E.G.; Daruich, J.; Sorda, J.A.; Wald, M.R.; Cherñavsky, A.C. New evidence for the therapeutic potential of curcumin to treat nonalcoholic fatty liver disease in humans. PLoS ONE 2017, 12, e0172900. [Google Scholar] [CrossRef]
- Shapiro, H.; Bruck, R. Therapeutic potential of curcumin in non-alcoholic steatohepatitis. Nutr. Res. Rev. 2005, 18, 212–221. [Google Scholar] [CrossRef] [Green Version]
- Dalgleish, D.G. Food emulsions—Their structures and structure-forming properties. Food Hydrocoll. 2006, 20, 415–422. [Google Scholar] [CrossRef]
- Naseema, A.; Kovooru, L.; Behera, A.K.; Kumar, K.P.P.; Srivastava, P. A critical review of synthesis procedures, applications and future potential of nanoemulsions. Adv. Colloid Interface Sci. 2021, 287, 102318. [Google Scholar] [CrossRef]
- Pucek, A.; Tokarek, B.; Waglewska, E.; Bazylińska, U. Recent Advances in the Structural Design of Photosensitive Agent Formulations Using “Soft” Colloidal Nanocarriers. Pharmaceutics 2020, 12, 587. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anton, N.; Vandamme, T.F. Nano-emulsions and Micro-emulsions: Clarifications of the Critical Differences. Pharm. Res. 2011, 28, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Esperón-Rojas, A.A.; Baeza-Jiménez, R.; García, H.S. Preparation of curcumin-carrying nanoemulsions using mono- and diacylglycerides enzymatically structured with bioactive fatty acids. Biocatal. Biotransform. 2020, 38, 93–103. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9781498726696. [Google Scholar]
- Esperón-Rojas, A.A.; Baeza-Jiménez, R.; Cano-Sarmiento, C.; García, H.S. Structured Mono- and Diacylglycerols with a High Content of Medium Chain Fatty Acids. J. Oleo Sci. 2017, 66, 991–996. [Google Scholar] [CrossRef] [Green Version]
- Ochoa-Flores, A.A.; Hernández-Becerra, J.A.; Cavazos-Garduño, A.; Soto-Rodriguez, I.; Sanchez-Otero, M.G.; Vernon-Carter, E.J.; García, H.S. Enhanced Bioavailability of Curcumin Nanoemulsions Stabilized with Phosphatidylcholine Modified with Medium Chain Fatty Acids. Curr. Drug Deliv. 2017, 14, 377–385. [Google Scholar] [CrossRef]
- Guerrero, O.A.; Aguilera, A.A.; Castro, R.Q.; Rodriguez, I.S.; Otero, G.S.; Ros, R.M.O. CD36 Gene Expression Induced by Fish Oil in Abdominal Adipose Tissue of Rats with Metabolic Syndrome. J. Food Nutr. Disord. 2017, 6, 6. [Google Scholar] [CrossRef]
- Lê, K.-A.; Ith, M.; Kreis, R.; Faeh, D.; Bortolotti, M.; Tran, C.; Boesch, C.; Tappy, L. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am. J. Clin. Nutr. 2009, 89, 1760–1765. [Google Scholar] [CrossRef] [Green Version]
- Bengmark, S.; Mesa, M.D.; Gil, A. Plant-derived health: The effects of turmeric and curcuminoids. Nutr. Hosp. 2009, 24, 273–281. [Google Scholar]
- Vera-Ramirez, L.; Pérez-Lopez, P.; Varela-Lopez, A.; Ramirez-Tortosa, M.; Battino, M.; Quiles, J.L. Curcumin and liver disease. BioFactors 2013, 39, 88–100. [Google Scholar] [CrossRef]
- Mohammed, N.K.; Muhialdin, B.J.; Hussin, A.S.M. Characterization of nanoemulsion of Nigella sativa oil and its application in ice cream. Food Sci. Nutr. 2020, 8, 2608–2618. [Google Scholar] [CrossRef] [Green Version]
- Malvajerd, S.S.; Azadi, A.; Izadi, Z.; Kurd, M.; Dara, T.; Dibaei, M.; Zadeh, M.S.; Javar, H.A.; Hamidi, M. Brain Delivery of Curcumin Using Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Preparation, Optimization, and Pharmacokinetic Evaluation. ACS Chem. Neurosci. 2019, 10, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Igarashi, K.; Koeda, T.; Sugimoto, K.; Nakagawa, K.; Hayashi, S.; Yamaji, R.; Inui, H.; Fukusato, T.; Yamanouchi, T. Rats Fed Fructose-Enriched Diets Have Characteristics of Nonalcoholic Hepatic Steatosis. J. Nutr. 2009, 139, 2067–2071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelany, M.E.; Hakami, T.M.; Omar, A.H. Curcumin improves the metabolic syndrome in high-fructose-diet-fed rats: Role of TNF-α, NF-κB, and oxidative stress. Can. J. Physiol. Pharmacol. 2017, 95, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Um, M.Y.; Hwang, K.H.; Ahn, J.; Ha, T.Y. Curcumin Attenuates Diet-Induced Hepatic Steatosis by Activating AMP-Activated Protein Kinase. Basic Clin. Pharmacol. Toxicol. 2013, 113, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, J.; Bargut, T.C.L.; Mandarim-De-Lacerda, C.A.; Aguila, M.B. Medium-chain triglyceride reinforce the hepatic damage caused by fructose intake in mice. Prostaglandins Leukot. Essent. Fat. Acids 2019, 140, 64–71. [Google Scholar] [CrossRef]
- Mamikutty, N.; Thent, Z.C.; Sapri, S.R.; Sahruddin, N.N.; Yusof, M.R.M.; Suhaimi, F.H. The Establishment of Metabolic Syndrome Model by Induction of Fructose Drinking Water in Male Wistar Rats. BioMed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Jin, T.; Song, Z.; Weng, J.; Fantus, I.G. Curcumin and other dietary polyphenols: Potential mechanisms of metabolic actions and therapy for diabetes and obesity. Am. J. Physiol. Metab. 2018, 314, E201–E205. [Google Scholar] [CrossRef]
- Francisqueti, F.V.; Santos, K.C.; Ferron, A.J.; Lo, A.T.; Minatel, I.O.; Campos, D.H.; Ferreira, A.L.A.; Corrêa, C.R. Fructose: Toxic effect on cardiorenal risk factors and redox state. SAGE Open Med. 2016, 4, 205031211668429. [Google Scholar] [CrossRef]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendía, L.E.; Sahebkar, A. Curcumin Lowers Serum Lipids and Uric Acid in Subjects with Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial. J. Cardiovasc. Pharmacol. 2016, 68, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Deters, M.; Klabunde, T.; Meyer, H.; Resch, K.; Kaever, V. Effects of Curcumin on Cyclosporine-Induced Cholestasis and Hypercholesterolemia and on Cyclosporine Metabolism in the Rat. Planta Med. 2003, 69, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Man, S.; Li, J.; Liu, J.; Zhang, L.; Yu, P.; Gao, W. Overdose Intake of Curcumin Initiates the Unbalanced State of Bodies. J. Agric. Food Chem. 2016, 64, 2765–2771. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.-H.; Liao, F.-H.; Chien, Y.-W. Medium-Chain Triglycerides Lower Blood Lipids and Body Weight in Streptozotocin-Induced Type 2 Diabetes Rats. Nutrients 2018, 10, 963. [Google Scholar] [CrossRef] [Green Version]
- Sachdev, M.S.; A Riely, C.; Madan, A.K. Nonalcoholic Fatty Liver Disease of Obesity. Obes. Surg. 2006, 16, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Ganjali, S.; Blesso, C.N.; Banach, M.; Pirro, M.; Majeed, M.; Sahebkar, A. Effects of curcumin on HDL functionality. Pharmacol. Res. 2017, 119, 208–218. [Google Scholar] [CrossRef]
- Sohaei, S.; Amani, R.; Tarrahi, M.J.; Ghasemi-Tehrani, H. The effects of curcumin supplementation on glycemic status, lipid profile and hs-CRP levels in overweight/obese women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled clinical trial. Complement. Ther. Med. 2019, 47, 102201. [Google Scholar] [CrossRef]
- Ghelani, H.; Razmovski-Naumovski, V.; Chang, D.; Nammi, S. Chronic treatment of curcumin improves hepatic lipid metabolism and alleviates the renal damage in adenine-induced chronic kidney disease in Sprague-Dawley rats. BMC Nephrol. 2019, 20, 431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cheng, X.; Gao, Y.; Zhang, C.; Bao, J.; Guan, H.; Yu, H.; Lu, R.; Xu, Q.; Sun, Y. Curcumin inhibits metastasis in human papillary thyroid carcinoma BCPAP cells via down-regulation of the TGF-β/Smad2/3 signaling pathway. Exp. Cell Res. 2016, 341, 157–165. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Zhang, R.; Zhang, Y.; Xu, Q.; Zhang, J.; Zhang, Y.; Zheng, Z.; Yu, X.; Jing, H.; et al. A good response to oil with medium- and long-chain fatty acids in body fat and blood lipid profiles of male hypertriglyceridemic subjects. Asia Pac. J. Clin. Nutr. 2009, 18, 351–358. [Google Scholar]
- Zhang, X.; Zhang, Y.; Liu, Y.; Wang, J.; Xu, Q.; Yu, X.; Yang, X.; Liu, Z.; Xue, C. Medium-chain triglycerides promote macrophage reverse cholesterol transport and improve atherosclerosis in ApoE-deficient mice fed a high-fat diet. Nutr. Res. 2016, 36, 964–973. [Google Scholar] [CrossRef]
- Acevedo, M.; Krämer, V.; Tagle, R.; Corbalán, R.; Arnaiz, P.; Berríos, X.; Navarrete, C. Relación colesterol total a HDL y colesterol no HDL: Los mejores indicadores lipídicos de aumento de grosor de la íntima media carotidea. Rev. Méd. Chile 2012, 140, 969–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Hadi, H.; Di Vincenzo, A.; Vettor, R.; Rossato, M. Food Ingredients Involved in White-to-Brown Adipose Tissue Conversion and in Calorie Burning. Front. Physiol. 2019, 9, 1954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Qi, J.; Li, L. Phytochemicals as potential candidates to combat obesity via adipose non-shivering thermogenesis. Pharmacol. Res. 2019, 147, 104393. [Google Scholar] [CrossRef]
- Silvester, A.J.; Aseer, K.R.; Yun, J.W. Dietary polyphenols and their roles in fat browning. J. Nutr. Biochem. 2019, 64, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Revelo, X.; Shao, W.; Tian, L.; Zeng, K.; Lei, H.; Sun, H.-S.; Woo, M.; Winer, D.; Jin, T. Dietary Curcumin Intervention Targets Mouse White Adipose Tissue Inflammation and Brown Adipose Tissue UCP1 Expression. Obesity 2018, 26, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Aceñero, M.J.; Medina, L.O.; Maroto, M. Herbal Drugs: Friend or Foe? J. Clin. Exp. Hepatol. 2019, 9, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri-Tehrani, S.A.; Rezayat, S.M.; Mansouri, S.; Qorbani, M.; Alavian, S.M.; Daneshi-Maskooni, M.; Hosseinzadeh-Attar, M.-J. Nano-curcumin improves glucose indices, lipids, inflammation, and Nesfatin in overweight and obese patients with non-alcoholic fatty liver disease (NAFLD): A double-blind randomized placebo-controlled clinical trial. Nutr. Metab. 2019, 16, 1–13. [Google Scholar] [CrossRef]
- Lee, E.J.; Hwang, J.S.; Kang, E.S.; Lee, S.B.; Hur, J.; Lee, W.J.; Choi, M.-J.; Kim, J.T.; Seo, H.G. Nanoemulsions improve the efficacy of turmeric in palmitate- and high fat diet-induced cellular and animal models. Biomed. Pharmacother. 2019, 110, 181–189. [Google Scholar] [CrossRef]




| Time | Acetonitrile (%) | 2.8% Acetic Acid Solution (%) | Methanol (%) |
|---|---|---|---|
| 0 | 35 | 55 | 10 |
| 4 | 45 | 25 | 30 |
| 6 | 40 | 30 | 30 |
| 8 | 35 | 55 | 10 |
| Author | Emulsifier | Mean Size (nm) | PDI |
|---|---|---|---|
| Esperón-Rojas et al., 2020 | MAG and DAG MCFAs | 184.4 ± 0.02 | 0.091 ± 0.002 |
| Ochoa-Flores et al., 2017 | PC-MCFAs | 29.6 ± 2.3 | 0.24 ± 0.08 |
| This research | MAG and DAG MCFAs | 174.8 ± 1.11 | 0.195 ± 0.076 |
| Weight (g) | Healthy Control Group | Sick Control Group | NE-MCFA | cNE-MCFA |
|---|---|---|---|---|
| Starting weight | 149.23 ± 9.40 a | 147.80 ± 7.44 a | 145.26 ± 1.68 a | 137.80 ± 2.67 a |
| Final weight | 331.16 ± 16.04 a | 240.43 ± 3.75 b | 256.2 ± 18.8 b | 204.1 ± 22.3 c |
| Body weight gain | 181.93 ± 13.95 a | 92.63 ± 7.79 b | 113.1 ± 17.4 b | 65.85 ± 12.76 c |
| Liquid and food consumption | ||||
| Starting liquid intake (mL/day) | 49.50 ± 6.54 a | 78.83 ± 2.25 b | 76.00 ± 7.94 b | 80.00 ± 6.54 b |
| Final liquid intake (mL/day) | 106.75 ± 2.25 a | 75.8 ± 18.5 b | 77.74 ± 4.98 b | 69.54 ± 8.96 b |
| Liquid intake (mL/day/100 g bw) | 31.23 ± 5.67 a | 43.50 ± 12.36 b | 38.06 ± 10.18 ab | 43.54 ± 11.38 b |
| Kcal equivalent | 0.00 a | 52.20 ± 14.83 b | 45.67 ± 12.22 b | 52.25 ± 13.66 b |
| Food consumption (g/day/100 g bw) | 19.13 ± 4.89 a | 13.60 ± 2.87 b | 14.029 ± 3.34 b | 10.97 ± 2.40 b |
| Kcal equivalent | 65.63 ± 16.77 a | 46.63 ± 9.85 b | 48.12 ± 11.47 b | 37.63 ± 8.24 b |
| Total Kcal/day/100 g bw | 65.63 ± 16.77 a | 98.83 ± 22.7 b | 93.79 ± 21.92 b | 89.88 ± 17.54 b |
| Liver | ||||
| Liver weight (g) | 8.55 ± 0.015 b | 11.52 ± 1.49 a | 6.99 ± 1.00 bc | 4.99 ± 0.84 c |
| Hepatosomatic Index | 2.58 | 4.79 | 2.73 | 2.45 |
| Healthy Control Group | Sick Control Group | NE-MCFA | cNE-MCFA | |
|---|---|---|---|---|
| Glucose (mg/dL) | 93.49 ± 6.91 a | 85.87 ± 17.39 a | 78.12 ± 15.66 a | 110.421 ± 30.6 a |
| Cholesterol (mg/dL) | 62.84 ± 3.07 ab | 79.24 ± 6.56 a | 33.44 ± 5.01 c | 55.47 ± 9.96 b |
| Triacylglycerols (mg/dL) | 87.36 ± 8.43 b | 116.77 ± 10.55 a | 80.07 ± 0.38 b | 122.69 ± 13.67 a |
| HDL (mg/dL) | 20.3 ± 0.75 a | 17.3 ± 0.720 b | 21.8 ± 0.755 a | 19.6 ± 1.51 ab |
| LDL (mg/dL) | 48.8 ± 3.96 ab | 54.2 ± 5.85 a | 34.3 ± 2.64 c | 42.2 ± 4.57 bc |
| HDL/LDL | 0.417 ± 0.018 bc | 0.321 ± 0.025 c | 0.638 ± 0.027 a | 0.469 ± 0.079 b |
| LDL/HDL | 2.40 ± 0.106 b | 3.13 ± 0.246 a | 1.57 ± 0.066 c | 2.18 ± 0.399 bc |
| Cholesterol/HDL | 3.099 ± 0.266 b | 4.578 ± 0.201 a | 1.539 ± 0.283 c | 2.826 ± 0.394 b |
| Aspartate aminotransferase (AST) (U/L) | 91.06 ± 6.87 b | 121.92 ± 10.8 a | 87.72 ± 11.94 b | 75.86 ± 9.23 b |
| Alanine aminotransferase (ALT) (U/L) | 18.38 ± 2.05 bc | 137.69 ± 7.06 a | 23.77 ± 2 b | 12.2 ± 2.88 c |
| AST/ALT ratio | 4.95 | 0.88 | 3.7 | 6.21 |
| Liver Tissue | Healthy Control | Sick Control | NE-MCFA | cNE-MCFA |
|---|---|---|---|---|
| 0% | 15% | 10% | 5% | |
| Adipose tissue | No alterations | Mild chronic inflammation | Mild chronic inflammation | Mild chronic inflammation/Brown adipose tissue |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agame-Lagunes, B.; Grube-Pagola, P.; García-Varela, R.; Alexander-Aguilera, A.; García, H.S. Effect of Curcumin Nanoemulsions Stabilized with MAG and DAG-MCFAs in a Fructose-Induced Hepatic Steatosis Rat Model. Pharmaceutics 2021, 13, 509. https://doi.org/10.3390/pharmaceutics13040509
Agame-Lagunes B, Grube-Pagola P, García-Varela R, Alexander-Aguilera A, García HS. Effect of Curcumin Nanoemulsions Stabilized with MAG and DAG-MCFAs in a Fructose-Induced Hepatic Steatosis Rat Model. Pharmaceutics. 2021; 13(4):509. https://doi.org/10.3390/pharmaceutics13040509
Chicago/Turabian StyleAgame-Lagunes, Beatriz, Peter Grube-Pagola, Rebeca García-Varela, Alfonso Alexander-Aguilera, and Hugo S. García. 2021. "Effect of Curcumin Nanoemulsions Stabilized with MAG and DAG-MCFAs in a Fructose-Induced Hepatic Steatosis Rat Model" Pharmaceutics 13, no. 4: 509. https://doi.org/10.3390/pharmaceutics13040509