Nanovectorization of Prostate Cancer Treatment Strategies: A New Approach to Improved Outcomes
Abstract
1. Introduction
1.1. Prostate Cancer and CRPC Emergence
1.2. Classical Androgen Receptor Pathway in CRPC Progression
1.3. Non-Androgen Receptor Pathways in CRPC Progression
| Treatment | Strenghts | Limitations | Ref |
|---|---|---|---|
| SURGERY | Effective for localized tumours, Combine with pre/postoperative chemo/radiotherapies | Ineffective for metastatic PC Recurrence rate is high | [18] |
| RADIATION THERAPY | Effective with organ specific tumor. Prevents post-operative reoccurrence | Synonymous with high rate of collateral lethality | [19] |
| (HORMONAL THERAPY)ADT | Effective for advanced cancers | High rate of recurrence and emergence of CRPC | [20] |
| CHEMOTHERAPY | Effective in combination with ADT | Synonymous with with high rate of collateral lethality | [21] |
| GENOTHERAPY | Inhibits specific genes that drive Prostate Cancer. More effective in combination with chemotherapy | Ineffective as a monotherapy | [22] |
1.4. Current Prostate Cancer Treatment Strategies and Their Limiting Factors
1.5. Nanoparticles in Prostate Cancer Therapies: The Awaiting Possibilities
1.6. Classification of Therapeutic Nanoparticles in Prostate Cancer
2. EPR Effect and Active Targeting in CRPC Therapy
3. Some Nanoparticles and Their Clinical Adaptations to CRPC Treatment
3.1. Liposomal Nanoparticles
3.1.1. Liposomal Nanoparticle in Therapeutic Gene Delivery
3.1.2. Liposomal Drug Loading and Release
3.2. Micellar Nanoparticles
3.2.1. Polymeric Micelles in Targeted Delivery
3.2.2. Polymeric Micelle Drug Release
3.2.3. Micelles in Chemogene Co-Delivery for CRPC Therapy
3.3. Dendrimer Nanoparticles
3.3.1. Mechanism of Action of Dendrimers
3.3.2. Clinical Significance of Dendrimer
4. Immunologic Response and Nanovectorization-Based Drug Delivery
5. Nanotheranostic Approach for CRPC Therapy
6. Studies on Nanovectorization of Chemical Drugs for CRPC Treatment
7. Studies on Nanovectorization of Therapeutic Oligonucleotides for CRPC Treatment
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Toren, P.J.; Gleave, M.E. Novel non-AR therapeutic targets in castrate resistant prostate cancer. Transl. Androl. Urol. 2013, 2, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J. Updates in advanced prostate cancer 2018. Prostate Cancer Prostatic Dis. 2018, 21, 449. [Google Scholar] [CrossRef] [PubMed]
- Blankfield, R.P. Androgen deprivation therapy for prostate cancer and cardiovascular death. JAMA 2012, 307, 1252–1253. [Google Scholar] [CrossRef]
- Lee, C.-H.; Kantoff, P. Treatment of Metastatic Prostate Cancer in 2018. JAMA Oncol. 2019, 5, 263–264. [Google Scholar] [CrossRef]
- Karantanos, T.; Corn, P.G.; Thompson, T.C. Prostate cancer progression after androgen deprivation therapy: Mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013, 32, 5501–5511. [Google Scholar] [CrossRef] [PubMed]
- Wadosky, K.M.; Koochekpour, S. Molecular mechanisms underlying resistance to androgen deprivation therapy in prostate cancer. Oncotarget 2016, 7, 64447–64470. [Google Scholar] [CrossRef] [PubMed]
- Nadiminty, N.; Gao, A.C. Mechanisms of persistent activation of the androgen receptor in CRPC: Recent advances and future perspectives. World J. Urol. 2012, 30, 287–295. [Google Scholar] [CrossRef]
- Yuan, X.; Balk, S.P. Mechanisms mediating androgen receptor reactivation after castration. Urol. Oncol. Semin. Orig. Investig. 2009, 27, 36–41. [Google Scholar] [CrossRef]
- Dhavale, M.; Abdelaal, M.K.; Alam, A.B.M.N.; Blazin, T.; Mohammed, L.M.; Prajapati, D.; Ballestas, N.P.; Mostafa, J.A. Androgen Receptor Signaling and the Emergence of Lethal Neuroendocrine Prostate Cancer With the Treatment-Induced Suppression of the Androgen Receptor: A Literature Review. Cureus 2021, 13. [Google Scholar] [CrossRef]
- Katsogiannou, M.; Ziouziou, H.; Karaki, S.; Andrieu, C.; de Villeneuve, M.; Rocchi, P. The hallmarks of castration-resistant prostate cancers. Cancer Treat. Rev. 2015, 41, 588–597. [Google Scholar] [CrossRef]
- Rocchi, P.; Beraldi, E.; Ettinger, S.; Fazli, L.; Vessella, R.L.; Nelson, C.; Gleave, M. Increased Hsp27 after androgen ablation facilitates androgen-independent progression in prostate cancer via signal transducers and activators of transcription 3-mediated suppression of apoptosis. Cancer Res. 2005, 65, 11083–11093. [Google Scholar] [CrossRef]
- Baylot, V.; Katsogiannou, M.; Andrieu, C.; Taieb, D.; Acunzo, J.; Giusiano, S.; Fazli, L.; Gleave, M.; Garrido, C.; Rocchi, P. Targeting TCTP as a new therapeutic strategy in castration-resistant prostate cancer. Mol. Ther. J. Am. Soc. Gene Ther. 2012, 20, 2244–2256. [Google Scholar] [CrossRef]
- Colombel, M.; Symmans, F.; Gil, S.; O’Toole, K.M.; Chopin, D.; Benson, M.; Olsson, C.A.; Korsmeyer, S.; Buttyan, R. Detection of the apoptosis-suppressing oncoprotein bc1-2 in hormone-refractory human prostate cancers. Am. J. Pathol. 1993, 143, 390–400. [Google Scholar]
- Hollenhorst, P.C.; Paul, L.; Ferris, M.W.; Graves, B.J. The ETS gene ETV4 is required for anchorage-independent growth and a cell proliferation gene expression program in PC3 prostate cells. Genes Cancer 2011, 1, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Freije, D.; Nusskern, D.R.; Okami, K.; Cairns, P.; Sidransky, D.; Isaacs, W.B.; Bova, G.S. Interfocal heterogeneity of PTEN/MMAC1 gene alterations in multiple metastatic prostate cancer tissues. Cancer Res. 1998, 58, 204–209. [Google Scholar] [PubMed]
- Sette, C. Alternative Splicing Programs in Prostate Cancer. Int. J. Cell Biol. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.P.; Dickinson, J.L.; Holloway, A.F. Epigenetic regulation of prostate cancer. Clin. Epigenet. 2011, 2, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.; Dornbier, R.; Kirshenbaum, E.; Eguia, E.; Sweigert, P.; Baker, M.; Farooq, A.; McVary, K.T.; Gonzalez, C.M.; Gupta, G.; et al. Utilization of Surgery for Post-prostatectomy Incontinence. J. Urol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Herrera, F.G.; Berthold, D.R. Radiation Therapy after Radical Prostatectomy: Implications for Clinicians. Front. Oncol. 2016, 6. [Google Scholar] [CrossRef]
- Brawer, M.K. Hormonal Therapy for Prostate Cancer. Rev. Urol. 2006, 8, S35–S47. [Google Scholar]
- Nader, R.; El Amm, J.; Aragon-Ching, J.B. Role of chemotherapy in prostate cancer. Asian J. Androl. 2018, 20, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-C.; Wang, M.; Chou, M.-C.; Chao, C.-N.; Fang, C.-Y.; Chen, P.-L.; Chang, D.; Shen, C.-H. Gene therapy for castration-resistant prostate cancer cells using JC polyomavirus-like particles packaged with a PSA promoter driven-suicide gene. Cancer Gene Ther. 2019, 26, 208–215. [Google Scholar] [CrossRef]
- Galsky, M.D.; Vogelzang, N.J. Docetaxel-based combination therapy for castration-resistant prostate cancer. Ann. Oncol. 2010, 21, 2135–2144. [Google Scholar] [CrossRef]
- Sriraman, S.K.; Aryasomayajula, B.; Torchilin, V.P. Barriers to drug delivery in solid tumors. Tissue Barriers 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Li, L.; Li, L.; Lin, X.; Wang, F.; Li, Q.; Huang, Y. Overcoming chemotherapy resistance via simultaneous drug-efflux circumvention and mitochondrial targeting. Acta Pharm. Sin. B 2019, 9, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Gillet, J.-P.; Gottesman, M.M. Mechanisms of multidrug resistance in cancer. Methods Mol. Biol. 2010, 596, 47–76. [Google Scholar] [CrossRef]
- Narvekar, M.; Xue, H.Y.; Eoh, J.Y.; Wong, H.L. Nanocarrier for poorly water-soluble anticancer drugs–barriers of translation and solutions. AAPS PharmSciTech 2014, 15, 822–833. [Google Scholar] [CrossRef]
- Hatefi, A.; Amsden, B. Camptothecin Delivery Methods. Pharm. Res. 2002, 19, 1389–1399. [Google Scholar] [CrossRef]
- Ma, P.; Mumper, R.J. Paclitaxel Nano-Delivery Systems: A Comprehensive Review. J. Nanomed. Nanotechnol. 2013, 4, 1000164. [Google Scholar] [CrossRef]
- Price, N.; Jain, V.K.; Sartor, O. Docetaxel Improves Survival in Metastatic Androgen-Independent Prostate Cancer. Clin. Prostate Cancer 2004, 3, 18–20. [Google Scholar] [CrossRef]
- Shelley, M.; Mason, M.D. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar]
- Andreopoulou, E.; Sparano, J.A. Chemotherapy in Patients with Anthracycline- and Taxane-Pretreated Metastatic Breast Cancer: An Overview. Curr. Breast Cancer Rep. 2013, 5, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Han, S.; Duan, C.; Chen, K.; You, Z.; Jia, J.; Lin, S.; Liang, L.; Liu, A.; Long, H.; et al. Role of Taxane and Anthracycline Combination Regimens in the Management of Advanced Breast Cancer. Medicine 2015, 94. [Google Scholar] [CrossRef] [PubMed]
- Elm’hadi, C.; Tanz, R.; Khmamouche, M.R.; Toreis, M.; Mahfoud, T.; Slimani, K.A.; Errihani, H.; Ichou, M. Toxicities of docetaxel: Original drug versus generics—a comparative study about 81 cases. Springerplus 2016, 5. [Google Scholar] [CrossRef]
- Panday, V.R.N.; Huizing, M.T.; Ten Bokkel Huinink, W.W.; Vermorken, J.B.; Beijnen, J.H. Hypersensitivity Reactions to the Taxanes Paclitaxel and Docetaxel. Clin. Drug Investig. 1997, 14, 418–427. [Google Scholar] [CrossRef]
- Min, B.-D.; Kang, H.-W.; Kim, W.-T.; Kim, Y.-J.; Yun, S.J.; Lee, S.C.; Kim, W.-J. Docetaxel-Induced Fatal Interstitial Pneumonitis in a Patient with Castration-Resistant Prostate Cancer. Korean J. Urol. 2012, 53, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Data Sheet 2005. Available online: https://web.archive.org/web/20051228000129/http://medsafe.govt.nz/Profs/datasheet/t/taxotereinf.htm (accessed on 2 January 2020).
- Webster, T.J. Nanomedicine: What’s in a definition? Int. J. Nanomed. 2006, 1, 115–116. [Google Scholar] [CrossRef]
- Sakamoto, J.H.; van de Ven, A.L.; Godin, B.; Blanco, E.; Serda, R.E.; Grattoni, A.; Ziemys, A.; Bouamrani, A.; Hu, T.; Ranganathan, S.I.; et al. Enabling individualized therapy through nanotechnology. Pharmacol. Res. 2010, 62, 57–89. [Google Scholar] [CrossRef]
- Miao, L.; Guo, S.; Zhang, J.; Kim, W.Y.; Huang, L. Nanoparticles with Precise Ratiometric Co-Loading and Co-Delivery of Gemcitabine Monophosphate and Cisplatin for Treatment of Bladder Cancer. Adv. Funct. Mater. 2014, 24, 6601–6611. [Google Scholar] [CrossRef]
- Mou, Q.; Ma, Y.; Ding, F.; Gao, X.; Yan, D.; Zhu, X.; Zhang, C. Two-in-One Chemogene Assembled from Drug-Integrated Antisense Oligonucleotides To Reverse Chemoresistance. J. Am. Chem. Soc. 2019, 141, 6955–6966. [Google Scholar] [CrossRef]
- Liu, S.; Guo, Y.; Huang, R.; Li, J.; Huang, S.; Kuang, Y.; Han, L.; Jiang, C. Gene and doxorubicin co-delivery system for targeting therapy of glioma. Biomaterials 2012, 33, 4907–4916. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.; Chen, C.; Bentobji, M.; Cheillan, F.A.; Piana, J.T.; Qu, F.; Rocchi, P.; Peng, L. Targeted delivery of Dicer-substrate siRNAs using a dual targeting peptide decorated dendrimer delivery system. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1627–1636. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Li, S. Polymeric Micelles: Nanocarriers for Cancer-Targeted Drug Delivery. AAPS PharmSciTech 2014, 15, 862–871. [Google Scholar] [CrossRef]
- Raj, K.K.; Anil, K.S.; Rajesh, K.K. Novel Approaches for Drug Delivery; IGI Global: Hershey, PA, USA, 2016; 540p. [Google Scholar]
- Xiang, Y.; Bernards, N.; Hoang, B.; Zheng, J.; Matsuura, N. Perfluorocarbon nanodroplets can reoxygenate hypoxic tumors in vivo without carbogen breathing. Nanotheranostics 2019, 3, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Asem, H.; Malmström, E. Polymeric Nanoparticles Explored for Drug-Delivery Applications. In Gels and Other Soft Amorphous Solids American Chemical Society; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2018; Volume 1296, pp. 315–331. [Google Scholar] [CrossRef]
- Kröger, A.P.P.; Hamelmann, N.M.; Juan, A.; Lindhoud, S.; Paulusse, J.M.J. Biocompatible Single-Chain Polymer Nanoparticles for Drug Delivery—A Dual Approach. ACS Appl. Mater. Interfaces 2018, 10, 30946–30951. [Google Scholar] [CrossRef] [PubMed]
- Inorganic Nanoparticles for Targeted Drug Delivery—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/B9781845695095500082 (accessed on 20 April 2019).
- Guo, Q.; Shen, X.; Li, Y.; Xu, S. Carbon nanotubes-based drug delivery to cancer and brain. Curr. Med. Sci. 2017, 37, 635–641. [Google Scholar] [CrossRef]
- Villemin, E.; Ong, Y.C.; Thomas, C.M.; Gasser, G. Polymer encapsulation of ruthenium complexes for biological and medicinal applications. Nat. Rev. Chem. 2019, 3, 261. [Google Scholar] [CrossRef]
- Parveen, S.; Sahoo, S.K. Polymeric nanoparticles for cancer therapy. J. Drug Target. 2008, 16, 108–123. [Google Scholar] [CrossRef]
- Zamboni, W.C. Liposomal, nanoparticle, and conjugated formulations of anticancer agents. Clin. Cancer Res. An Off. J. Am. Assoc. Cancer Res. 2005, 11, 8230–8234. [Google Scholar] [CrossRef] [PubMed]
- Malam, Y.; Loizidou, M.; Seifalian, A.M. Liposomes and nanoparticles: Nanosized vehicles for drug delivery in cancer. Trends Pharmacol. Sci. 2009, 30, 592–599. [Google Scholar] [CrossRef]
- Uthaman, S.; Huh, K.M.; Park, I.-K. Tumor microenvironment-responsive nanoparticles for cancer theragnostic applications. Biomater. Res. 2018, 22. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release Off. J. Control. Release Soc. 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A.; Alrokayan, S.A.; Kumar, S. Targeted anticancer therapy: Overexpressed receptors and nanotechnology. Clin. Chim. Acta. 2014, 436, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Milla, P.; Dosio, F.; Cattel, L. PEGylation of proteins and liposomes: A powerful and flexible strategy to improve the drug delivery. Curr. Drug Metab. 2012, 13, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Raj, T.A.S.; Smith, A.M.; Moore, A.S. Vincristine sulfate liposomal injection for acute lymphoblastic leukemia. Int. J. Nanomedicine 2013, 8, 4361–4369. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, X.; Guo, R.; Shen, M.; Zhang, M.; Zhu, M.; Shi, X. Targeted delivery of doxorubicin into cancer cells using a folic acid–dendrimer conjugate. Polym. Chem. 2011, 2, 1754–1760. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®–the first FDA-approved nano-drug: Lessons learned. J. Control. Release Off. J. Control. Release Soc. 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Simberg, D. Nanoparticle transport pathways into tumors. J. Nanopart. Res. 2018, 20. [Google Scholar] [CrossRef]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Maeda, H. Macromolecular therapeutics in cancer treatment: The EPR effect and beyond. J. Control. Release 2012, 164, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Couvreur, P. Les nanotechnologies peuvent-elles contribuer à traiter des maladies sévères?: Chaire d’Innovation Technologique Liliane Bettencourt 2009-2010. Leçon prononcée le jeudi 21 janvier 2010. In Les Nanotechnologies Peuvent-Elles Contribuer à Traiter des Maladies Sévères? Leçons inaugurales; Collège de France: Paris, France, 2013; ISBN 978-2-7226-0098-0. [Google Scholar]
- Yu, B.; Tai, H.C.; Xue, W.; Lee, L.J.; Lee, R.J. Receptor-targeted nanocarriers for therapeutic delivery to cancer. Mol. Membr. Biol. 2010, 27, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.; Wang, E.S. Gemtuzumab ozogamicin for the treatment of acute myeloid leukemia. Expert Rev. Clin. Pharmacol. 2018, 11, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Bareford, L.M.; Swaan, P.W. Endocytic mechanisms for targeted drug delivery. Adv. Drug Deliv. Rev. 2007, 59, 748–758. [Google Scholar] [CrossRef]
- Xu, S.; Olenyuk, B.Z.; Okamoto, C.T.; Hamm-Alvarez, S.F. Targeting receptor-mediated endocytotic pathways with nanoparticles: Rationale and advances. Adv. Drug Deliv. Rev. 2013, 65, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Katsogiannou, M.; Peng, L.; Catapano, C.V.; Rocchi, P. Active-targeted nanotherapy strategies for prostate cancer. Curr. Cancer Drug Targets 2011, 11, 954–965. [Google Scholar] [CrossRef]
- Nahta, R. Molecular Mechanisms of Trastuzumab-Based Treatment in HER2-Overexpressing Breast Cancer. ISRN Oncol. 2012, 2012. [Google Scholar] [CrossRef]
- Marelli, U.K.; Rechenmacher, F.; Sobahi, T.R.A.; Mas-Moruno, C.; Kessler, H. Tumor Targeting via Integrin Ligands. Front. Oncol. 2013, 3. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Cao, Z.; Ji, H.; Yang, X.; Iwamoto, H.; Wahlberg, E.; Länne, T.; Sun, B.; Cao, Y. Anti-VEGF– and anti-VEGF receptor–induced vascular alteration in mouse healthy tissues. Proc. Natl. Acad. Sci. USA 2013, 110, 12018–12023. [Google Scholar] [CrossRef]
- Duda, D.G.; Batchelor, T.T.; Willett, C.G.; Jain, R.K. VEGF-targeted cancer therapy strategies: Current progress, hurdles and future prospects. Trends Mol. Med. 2007, 13, 223–230. [Google Scholar] [CrossRef]
- Haberkorn, U.; Eder, M.; Kopka, K.; Babich, J.W.; Eisenhut, M. New Strategies in Prostate Cancer: Prostate-Specific Membrane Antigen (PSMA) Ligands for Diagnosis and Therapy. Clin. Cancer Res. 2016, 22, 9–15. [Google Scholar] [CrossRef]
- Wüstemann, T.; Haberkorn, U.; Babich, J.; Mier, W. Targeting prostate cancer: Prostate-specific membrane antigen based diagnosis and therapy. Med. Res. Rev. 2019, 39, 40–69. [Google Scholar] [CrossRef] [PubMed]
- Felber, M.; Alberto, R. 99mTc radiolabelling of Fe3O4–Au core–shell and Au–Fe3O4 dumbbell-like nanoparticles. Nanoscale 2015, 7, 6653–6660. [Google Scholar] [CrossRef]
- Felber, M.; Bauwens, M.; Mateos, J.M.; Imstepf, S.; Mottaghy, F.M.; Alberto, R. 99mTc Radiolabeling and Biological Evaluation of Nanoparticles Functionalized with a Versatile Coating Ligand. Chem. A Eur. J. 2015, 21, 6090–6099. [Google Scholar] [CrossRef] [PubMed]
- Yari, H.; Nkepang, G.; Awasthi, V. Surface Modification of Liposomes by a Lipopolymer Targeting Prostate Specific Membrane Antigen for Theranostic Delivery in Prostate Cancer. Materials 2019, 12, 756. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Guo, F.; Chen, Y.; Li, Y.; Yu, B.; Li, Y. Nanoliposomal formulation encapsulating celecoxib and genistein inhibiting COX-2 pathway and Glut-1 receptors to prevent prostate cancer cell proliferation. Cancer Lett. 2019, 448, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bessone, F.; Argenziano, M.; Grillo, G.; Ferrara, B.; Pizzimenti, S.; Barrera, G.; Cravotto, G.; Guiot, C.; Stura, I.; Cavalli, R.; et al. Low-dose curcuminoid-loaded in dextran nanobubbles can prevent metastatic spreading in prostate cancer cells. Nanotechnology 2019, 30, 214004. [Google Scholar] [CrossRef]
- Jiao, J.; Zou, Q.; Zou, M.H.; Guo, R.M.; Zhu, S.; Zhang, Y. Aptamer-modified PLGA nanoparticle delivery of triplex forming oligonucleotide for targeted prostate cancer therapy. Neoplasma 2016, 63, 569–575. [Google Scholar] [CrossRef]
- Yeh, C.-Y.; Hsiao, J.-K.; Wang, Y.-P.; Lan, C.-H.; Wu, H.-C. Peptide-conjugated nanoparticles for targeted imaging and therapy of prostate cancer. Biomaterials 2016, 99, 1–15. [Google Scholar] [CrossRef]
- Li, N.; Xie, X.; Hu, Y.; He, H.; Fu, X.; Fang, T.; Li, C. Herceptin-conjugated liposomes co-loaded with doxorubicin and simvastatin in targeted prostate cancer therapy. Am. J. Transl. Res. 2019, 11, 1255–1269. [Google Scholar]
- Taghdisi, S.M.; Danesh, N.M.; Ramezani, M.; Yazdian-Robati, R.; Abnous, K. A Novel AS1411 Aptamer-Based Three-Way Junction Pocket DNA Nanostructure Loaded with Doxorubicin for Targeting Cancer Cells in Vitro and in Vivo. Mol. Pharm. 2018, 15, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Lillard, J.W.; Singh, R. Reversal of drug resistance by planetary ball milled (PBM) nanoparticle loaded with resveratrol and docetaxel in prostate cancer. Cancer Lett. 2018, 427, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, Z.; Xiang, L.; Li, L.; Zhang, H.; Lin, F.; Cao, H. Codelivery of GRP78 siRNA and docetaxel via RGD-PEG-DSPE/DOPA/CaP nanoparticles for the treatment of castration-resistant prostate cancer. Drug Des. Devel. Ther. 2019, 13, 1357–1372. [Google Scholar] [CrossRef]
- Rahme, K.; Guo, J.; Holmes, J.D. Bioconjugated Gold Nanoparticles Enhance siRNA Delivery in Prostate Cancer Cells. In RNA Interference and Cancer Therapy: Methods and Protocols; Dinesh Kumar, L., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; pp. 291–301. ISBN 978-1-4939-9220-1. [Google Scholar]
- Gupta, S.; Gupta, P.K.; Dharanivasan, G.; Verma, R.S. Current prospects and challenges of nanomedicine delivery in prostate cancer therapy. Nanomedicine 2017, 12, 2675–2692. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.A.; Waterhouse, D.N.; Mayer, L.D.; Cullis, P.R.; Madden, T.D.; Bally, M.B. The liposomal formulation of doxorubicin. Methods Enzymol. 2005, 391, 71–97. [Google Scholar] [CrossRef]
- Mukherjee, A. A Review on Liposomes and Polymeric Nanoparticles as Drug Delivery Vehicles to the Brain. 2017. Available online: https://www.elynsgroup.com/journal/article/a-review-on-liposomes-and-polymeric-nanoparticles (accessed on 25 April 2019).
- Panahi, Y.; Farshbaf, M.; Mohammadhosseini, M.; Mirahadi, M.; Khalilov, R.; Saghfi, S.; Akbarzadeh, A. Recent advances on liposomal nanoparticles: Synthesis, characterization and biomedical applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 788–799. [Google Scholar] [CrossRef]
- Boehlke, L.; Winter, J.N. Sphingomyelin/cholesterol liposomal vincristine: A new formulation for an old drug. Expert Opin. Biol. Ther. 2006, 6, 409–415. [Google Scholar] [CrossRef]
- Udhrain, A.; Skubitz, K.M.; Northfelt, D.W. Pegylated liposomal doxorubicin in the treatment of AIDS-related Kaposi’s sarcoma. Int. J. Nanomed. 2007, 2, 345–352. [Google Scholar]
- Pujade-Lauraine, E.; Wagner, U.; Aavall-Lundqvist, E.; Gebski, V.; Heywood, M.; Vasey, P.A.; Volgger, B.; Vergote, I.; Pignata, S.; Ferrero, A.; et al. Pegylated liposomal doxorubicin and carboplatin compared with paclitaxel and carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. J. Clin. Oncol. 2010, 28, 3323–3329. [Google Scholar] [CrossRef]
- Perez, A.T.; Domenech, G.H.; Frankel, C.; Vogel, C.L. Pegylated Liposomal Doxorubicin (Doxil®) for Metastatic Breast Cancer: The Cancer Research Network, Inc., Experience. In Proceedings of the Cancer Investigation; Marcel Dekker Inc., 2002; Volume 20, pp. 22–29. Available online: https://www.tandfonline.com/doi/abs/10.1081/CNV-120014883 (accessed on 20 October 2020).
- O’Brien, M.E.R.; Wigler, N.; Inbar, M.; Rosso, R.; Grischke, E.; Santoro, A.; Catane, R.; Kieback, D.G.; Tomczak, P.; Ackland, S.P.; et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYXTM/Doxil®) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann. Oncol. 2004, 15, 440–449. [Google Scholar] [CrossRef]
- Ghaferi, M.; Asadollahzadeh, M.J.; Akbarzadeh, A.; Shahmabadi, H.E.; Alavi, S.E. Enhanced efficacy of PEGylated liposomal cisplatin: In vitro and in vivo evaluation. Int. J. Mol. Sci. 2020, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Maeda, O.; Kajiyama, H.; Shibata, K.; Nakamura, S.; Kikkawa, F. Pegylated liposomal doxorubicin/oxaliplatin chemotherapy can overcome cisplatin resistance in spectrin αII-overexpressing ovarian carcinoma. Anticancer Res. 2020, 40, 2497–2507. [Google Scholar] [CrossRef] [PubMed]
- Tannock, I.F.; de Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Théodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- Yaqub, F. Mechanism of action of anthracycline drugs. Lancet Oncol. 2013, 14, e296. [Google Scholar] [CrossRef]
- Narayanan, N.K.; Nargi, D.; Randolph, C.; Narayanan, B.A. Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int. J. Cancer 2009, 125, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Thangapazham, R.L.; Puri, A.; Tele, S.; Blumenthal, R.; Maheshwari, R.K. Evaluation of a nanotechnology-based carrier for delivery of curcumin in prostate cancer cells. Int. J. Oncol. 2008, 32, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Jantscheff, P.; Esser, N.; Graeser, R.; Ziroli, V.; Kluth, J.; Unger, C.; Massing, U. Liposomal gemcitabine (GemLip)-efficient drug against hormone-refractory Du145 and PC-3 prostate cancer xenografts. Prostate 2009, 69, 1151–1163. [Google Scholar] [CrossRef]
- Bode, C.; Trojan, L.; Weiss, C.; Kraenzlin, B.; Michaelis, U.; Teifel, M.; Alken, P.; Michel, M.S. Paclitaxel encapsulated in cationic liposomes: A new option for neovascular targeting for the treatment of prostate cancer. Oncol. Rep. 2009, 22, 321–326. [Google Scholar]
- Pinto, A.C.; Moreira, J.N.; Simões, S. Liposomal imatinib–mitoxantrone combination: Formulation development and therapeutic evaluation in an animal model of prostate cancer. Prostate 2011, 71, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Tyagi, P.; Li, S.; Huang, L. Anisamide-targeted stealth liposomes: A potent carrier for targeting doxorubicin to human prostate cancer cells. Int. J. Cancer 2004, 112, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.A.; Harney, E.; Small, E.J. Liposomal doxorubicin for the treatment of hormone-refractory prostate cancer. Clin. Prostate Cancer 2002, 1, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wang, L.; Guo, Y.; Xiong, X.; Zhu, L.; Fang, K. Inhibition of prostate cancer growth using doxorubicin assisted by ultrasound-targeted nanobubble destruction. Int. J. Nanomed. 2016, 11, 3585–3596. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, X.; Wang, Y.; Yang, L.; Hu, J.; Xiao, W.; Fu, A.; Cai, L.; Li, X.; Ye, X.; et al. Improved tumor-targeting drug delivery and therapeutic efficacy by cationic liposome modified with truncated bFGF peptide. J. Control. Release Off. J. Control. Release Soc. 2010, 145, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Deeken, J.F.; Slack, R.; Weiss, G.J.; Ramanathan, R.K.; Pishvaian, M.J.; Hwang, J.; Lewandowski, K.; Subramaniam, D.; He, A.R.; Cotarla, I.; et al. A phase I study of liposomal-encapsulated docetaxel (LE-DT) in patients with advanced solid tumor malignancies. Cancer Chemother. Pharmacol. 2013, 71, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Hagtvet, E.; Evjen, T.J.; Olsen, D.R.; Fossheim, S.L.; Nilssen, E.A. Ultrasound enhanced antitumor activity of liposomal doxorubicin in mice. J. Drug Target. 2011, 19, 701–708. [Google Scholar] [CrossRef]
- Hagtvet, E.; Røe, K.; Olsen, D.R. Liposomal doxorubicin improves radiotherapy response in hypoxic prostate cancer xenografts. Radiat. Oncol. 2011, 6, 135. [Google Scholar] [CrossRef]
- Jantscheff, P.; Ziroli, V.; Esser, N.; Graeser, R.; Kluth, J.; Sukolinskaya, A.; Taylor, L.A.; Unger, C.; Massing, U. Anti-metastatic effects of liposomal gemcitabine in a human orthotopic LNCaP prostate cancer xenograft model. Clin. Exp. Metastasis 2009, 26, 981–992. [Google Scholar] [CrossRef]
- Pakunlu, R.I.; Wang, Y.; Saad, M.; Khandare, J.J.; Starovoytov, V.; Minko, T. In vitro and in vivo intracellular liposomal delivery of antisense oligonucleotides and anticancer drug. J. Control. Release Off. J. Control. Release Soc. 2006, 114, 153–162. [Google Scholar] [CrossRef]
- Şalva, E.; Turan, S.Ö.; Eren, F.; Akbuğa, J. The enhancement of gene silencing efficiency with chitosan-coated liposome formulations of siRNAs targeting HIF-1α and VEGF. Int. J. Pharm. 2015, 478, 147–154. [Google Scholar] [CrossRef]
- Bolotin, E.M.; Cohen, R.; Bar, L.K.; Emanuel, N.; Ninio, S.; Barenholz, Y.; Lasic, D.D. Ammonium Sulfate Gradients for Efficient and Stable Remote Loading of Amphipathic Weak Bases into Liposomes and Ligandoliposomes. J. Liposome Res. 1994, 4, 455–479. [Google Scholar] [CrossRef]
- Boman, N.L.; Masin, D.; Mayer, L.D.; Cullis, P.R.; Bally, M.B. Liposomal vincristine which exhibits increased drug retention and increased circulation longevity cures mice bearing P388 tumors. Cancer Res. 1994, 54, 2830–2833. [Google Scholar] [PubMed]
- Structural Biochemistry/Lipids/Micelles—Wikibooks, Open Books for an Open World. Available online: https://en.wikibooks.org/wiki/Structural_Biochemistry/Lipids/Micelles (accessed on 22 April 2019).
- Blanco, E.; Kessinger, C.W.; Sumer, B.D.; Gao, J. Multifunctional micellar nanomedicine for cancer therapy. Exp. Biol. Med. 2009, 234, 123–131. [Google Scholar] [CrossRef]
- Nicolas, J.; Couvreur, P. Polymer nanoparticles for the delivery of anticancer drug. Med. Sci. M/S 2017, 33, 11–17. [Google Scholar] [CrossRef][Green Version]
- PEGylation in Anti-Cancer Therapy: An Overview—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S1818087615000860 (accessed on 22 April 2019).
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Thermosensitive and Biodegradable Polymeric Micelles for Paclitaxel Delivery. Available online: https://www.ncbi.nlm.nih.gov/pubmed/15763618 (accessed on 17 April 2019).
- Nasongkla, N.; Bey, E.; Ren, J.; Ai, H.; Khemtong, C.; Guthi, J.S.; Chin, S.-F.; Sherry, A.D.; Boothman, D.A.; Gao, J. Multifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery systems. Nano Lett. 2006, 6, 2427–2430. [Google Scholar] [CrossRef]
- Wiernik, P.H.; Schwartz, E.L.; Strauman, J.J.; Dutcher, J.P.; Lipton, R.B.; Paietta, E. Phase I clinical and pharmacokinetic study of taxol. Cancer Res. 1987, 47, 2486–2493. [Google Scholar]
- Kim, T.-Y.; Kim, D.-W.; Chung, J.-Y.; Shin, S.G.; Kim, S.-C.; Heo, D.S.; Kim, N.K.; Bang, Y.-J. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin. Cancer Res. An Off. J. Am. Assoc. Cancer Res. 2004, 10, 3708–3716. [Google Scholar] [CrossRef]
- Alavi, M.; Hamidi, M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab. Pers. Ther. 2019, 34. [Google Scholar] [CrossRef]
- Duncan, R.; Richardson, S.C.W. Endocytosis and Intracellular Trafficking as Gateways for Nanomedicine Delivery: Opportunities and Challenges. Mol. Pharm. 2012, 9, 2380–2402. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, C.; Das, M.; Jagat, R.K.; Sanjeeb, K.S. Receptor Mediated Tumor Targeting: An Emerging Approach for Cancer Therapy Current Drug Delivery. 2010. Available online: http://www.eurekaselect.com/72923/article (accessed on 25 April 2019).
- Neumann, S.E.; Chamberlayne, C.F.; Zare, R.N. Electrically controlled drug release using pH-sensitive polymer films. Nanoscale 2018, 10, 10087–10093. [Google Scholar] [CrossRef]
- Yang, J.; Lu, W.; Xiao, J.; Zong, Q.; Xu, H.; Yin, Y.; Hong, H.; Xu, W. A positron emission tomography image-guidable unimolecular micelle nanoplatform for cancer theranostic applications. Acta Biomater. 2018, 79, 306–316. [Google Scholar] [CrossRef]
- Gulzar, A.; Gai, S.; Yang, P.; Li, C.; Ansari, M.B.; Lin, J. Stimuli responsive drug delivery application of polymer and silica in biomedicine. J. Mater. Chem. B 2015, 3, 8599–8622. [Google Scholar] [CrossRef]
- de la Fuente, A.; Kramer, S.; Mohr, N.; Pektor, S.; Klasen, B.; Bausbacher, N.; Miederer, M.; Zentel, R.; Rösch, F. 68Ga[Ga]-, 111In[In]-oxine: A novel strategy of in situ radiolabeling of HPMA-based micelles. Am. J. Nucl. Med. Mol. Imaging 2019, 9, 67–83. [Google Scholar]
- Multifunctional Nanoparticles for Combining Ultrasonic Tumor Imaging and Targeted Chemotherapy News-Medical.net. 2007. Available online: https://www.news-medical.net/news/2007/08/22/28987.aspx (accessed on 25 April 2019).
- Le, B.; Powers, G.L.; Tam, Y.T.; Schumacher, N.; Malinowski, R.L.; Steinke, L.; Kwon, G.; Marker, P.C. Multi-drug loaded micelles delivering chemotherapy and targeted therapies directed against HSP90 and the PI3K/AKT/mTOR pathway in prostate cancer. PLoS ONE 2017, 12, e0174658. [Google Scholar] [CrossRef] [PubMed]
- Baylot, V.; Karaki, S.; Rocchi, P. TCTP Has a Crucial Role in the Different Stages of Prostate Cancer Malignant Progression. In TCTP/tpt1-Remodeling Signaling from Stem Cell to Disease; Telerman, A., Amson, R., Eds.; Results and Problems in Cell Differentiation; Springer International Publishing: Cham, Switzerland, 2017; pp. 255–261. ISBN 978-3-319-67591-6. [Google Scholar]
- Karaki, S.; Benizri, S.; Mejías, R.; Baylot, V.; Branger, N.; Nguyen, T.; Vialet, B.; Oumzil, K.; Barthélémy, P.; Rocchi, P. Lipid-oligonucleotide conjugates improve cellular uptake and efficiency of TCTP-antisense in castration-resistant prostate cancer. J. Control. Release Off. J. Control. Release Soc. 2017, 258, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef]
- Esfand, R.; Tomalia, D.A. Poly(amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar] [CrossRef]
- Mendes, L.P.; Pan, J.; Torchilin, V.P. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Li, K.; Li, J.; Wen, S.; Chen, Q.; Shen, M.; Zheng, L.; Zhang, G.; Shi, X. Dendrimer-Assisted Formation of Fe3O4/Au Nanocomposite Particles for Targeted Dual Mode CT/MR Imaging of Tumors. Small 2015, 11, 4584–4593. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Fu, F.; Zhang, M.; Shen, M.; Zhu, M.; Shi, X. Multifunctional dendrimers modified with alpha-tocopheryl succinate for targeted cancer therapy. Medchemcomm 2014, 5, 879–885. [Google Scholar] [CrossRef]
- Wiwattanapatapee, R.; Carreño-Gómez, B.; Malik, N.; Duncan, R. Anionic PAMAM dendrimers rapidly cross adult rat intestine in vitro: A potential oral delivery system? Pharm. Res. 2000, 17, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Fu, F.; Xiong, Z.; Shen, M.; Shi, X. Dendrimer-entrapped gold nanoparticles modified with RGD peptide and alpha-tocopheryl succinate enable targeted theranostics of cancer cells. Colloids Surf. B. Biointerfaces 2015, 133, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Madaan, K.; Kumar, S.; Poonia, N.; Lather, V.; Pandita, D. Dendrimers in drug delivery and targeting: Drug-dendrimer interactions and toxicity issues. J. Pharm. Bioallied Sci. 2014, 6, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiang, W.; Nam, J.; Moon, J.J.; Kim, B.Y.S. Immunomodulating Nanomedicine for Cancer Therapy. Nano Lett. 2018, 18, 6655–6659. [Google Scholar] [CrossRef] [PubMed]
- Shetty, Y.; Prabhu, P.; Prabhakar, B. Emerging vistas in theranostic medicine. Int. J. Pharm. 2018. [Google Scholar] [CrossRef]
- Minchin, R.F.; Martin, D.J. Minireview: Nanoparticles for Molecular Imaging—An Overview. Endocrinology 2010, 151, 474–481. [Google Scholar] [CrossRef]
- de Barros, A.; Tsourkas, A.; Saboury, B.; Cardoso, V.; Alavi, A. Emerging role of radiolabeled nanoparticles as an effective diagnostic technique. EJNMMI Res. 2012, 2, 39. [Google Scholar] [CrossRef]
- Liu, Y.; Welch, M.J. Nanoparticles Labeled with Positron Emitting Nuclides: Advantages, Methods, and Applications. Bioconjug. Chem. 2012, 23, 671–682. [Google Scholar] [CrossRef]
- Xing, Y.; Zhao, J.; Conti, P.S.; Chen, K. Radiolabeled Nanoparticles for Multimodality Tumor Imaging. Theranostics 2014, 4, 290–306. [Google Scholar] [CrossRef]
- Kiessling, F.; Mertens, M.E.; Grimm, J.; Lammers, T. Nanoparticles for Imaging: Top or Flop? Radiology 2014, 273, 10–28. [Google Scholar] [CrossRef]
- Stockhofe, K.; Postema, J.; Schieferstein, H.; Ross, T. Radiolabeling of Nanoparticles and Polymers for PET Imaging. Pharmaceuticals 2014, 7, 392–418. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Zhao, J.; Shi, X.; Conti, P.S.; Chen, K. Recent Development of Radiolabeled Nanoparticles for PET Imaging. Austin J. Nanomed. Nanotechnol. 2014, 2, 1016. [Google Scholar]
- Hong, H.; Zhang, Y.; Sun, J.; Cai, W. Molecular imaging and therapy of cancer with radiolabeled nanoparticles. Nano Today 2009, 4, 399–413. [Google Scholar] [CrossRef]
- Phillips, W.T.; Goins, B.A.; Bao, A. Radioactive liposomes. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 69–83. [Google Scholar] [CrossRef]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-L.; Hu, S.-H. Functional Nanoparticles for Tumor Penetration of Therapeutics. Pharmaceutics 2018, 10, 193. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Ojha, T.; Kiessling, F.; Lammers, T.; Shi, Y. Enhancing Tumor Penetration of Nanomedicines. Biomacromolecules 2017, 18, 1449–1459. [Google Scholar] [CrossRef]
- Cheng, J.; Teply, B.A.; Sherifi, I.; Sung, J.; Luther, G.; Gu, F.X.; Levy-Nissenbaum, E.; Radovic-Moreno, A.F.; Langer, R.; Farokhzad, O.C. Formulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug delivery. Biomaterials 2007, 28, 869–876. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Cheng, J.; Teply, B.A.; Sherifi, I.; Jon, S.; Kantoff, P.W.; Richie, J.P.; Langer, R. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc. Natl. Acad. Sci. USA 2006, 103, 6315–6320. [Google Scholar] [CrossRef]
- Starpharma|Starpharma Commences Dendrimer-Docetaxel Clinical Trial Starpharma. Available online: https://starpharma.com/news/188 (accessed on 26 April 2019).
- Tewari, A. Prostate Cancer: A Comprehensive Perspective; Springer Science & Business Media: Berlin, Germany, 2013; 1089p. [Google Scholar]
- Patel, A.G.; Kaufmann, S.H. How does doxorubicin work? Elife 2012, 1. [Google Scholar] [CrossRef]
- Newling, D.W.W. The use of adriamycin and its derivatives in the treatment of prostatic cancer. Cancer Chemother. Pharmacol. 1992, 30, S90–S94. [Google Scholar] [CrossRef]
- Matsumura, Y.; Hamaguchi, T.; Ura, T.; Muro, K.; Yamada, Y.; Shimada, Y.; Shirao, K.; Okusaka, T.; Ueno, H.; Ikeda, M.; et al. Phase I clinical trial and pharmacokinetic evaluation of NK911, a micelle-encapsulated doxorubicin. Br. J. Cancer 2004, 91, 1775–1781. [Google Scholar] [CrossRef]
- Ki, M.-H.; Kim, J.-E.; Lee, Y.-N.; Noh, S.M.; An, S.-W.; Cho, H.-J.; Kim, D.-D. Chitosan-based hybrid nanocomplex for siRNA delivery and its application for cancer therapy. Pharm. Res. 2014, 31, 3323–3334. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Liu, X.; Bolcato-Bellemin, A.-L.; Wang, Y.; Liu, C.; Erbacher, P.; Qu, F.; Rocchi, P.; Behr, J.-P.; Peng, L. An amphiphilic dendrimer for effective delivery of small interfering RNA and gene silencing in vitro and in vivo. Angew. Chem. Int. Ed. Engl. 2012, 51, 8478–8484. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-X.; Rocchi, P.; Qu, F.-Q.; Zheng, S.-Q.; Liang, Z.-C.; Gleave, M.; Iovanna, J.; Peng, L. PAMAM dendrimers mediate siRNA delivery to target Hsp27 and produce potent antiproliferative effects on prostate cancer cells. ChemMedChem 2009, 4, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Barthelemy, P.; Oumzil, K.; Rocchi, P.; Acunzo, J. Hydrophobically Modified Antisense Oligonucleotides Comprising a Ketal Group. US20160108398A1, 21 April 2016. [Google Scholar]
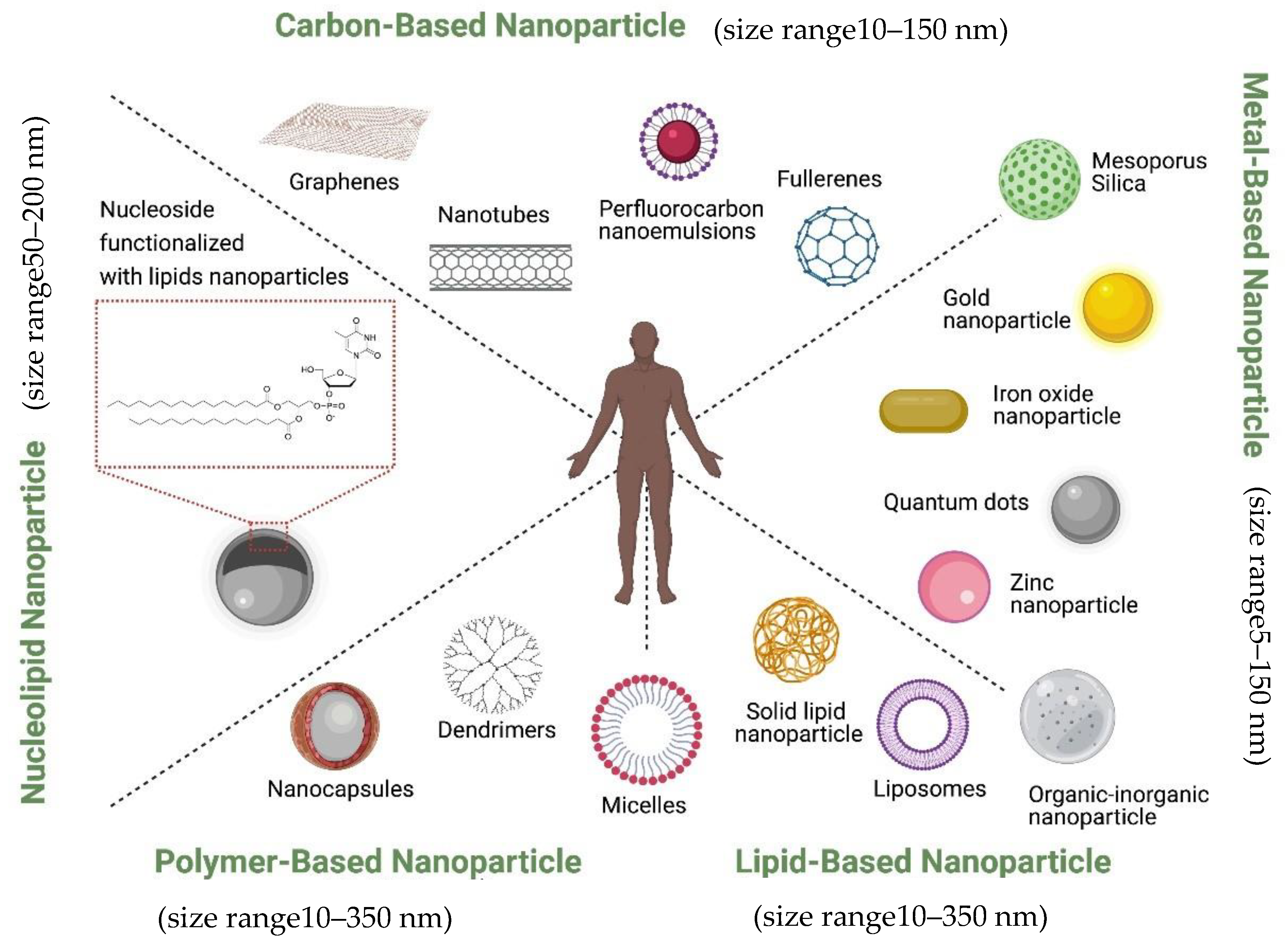
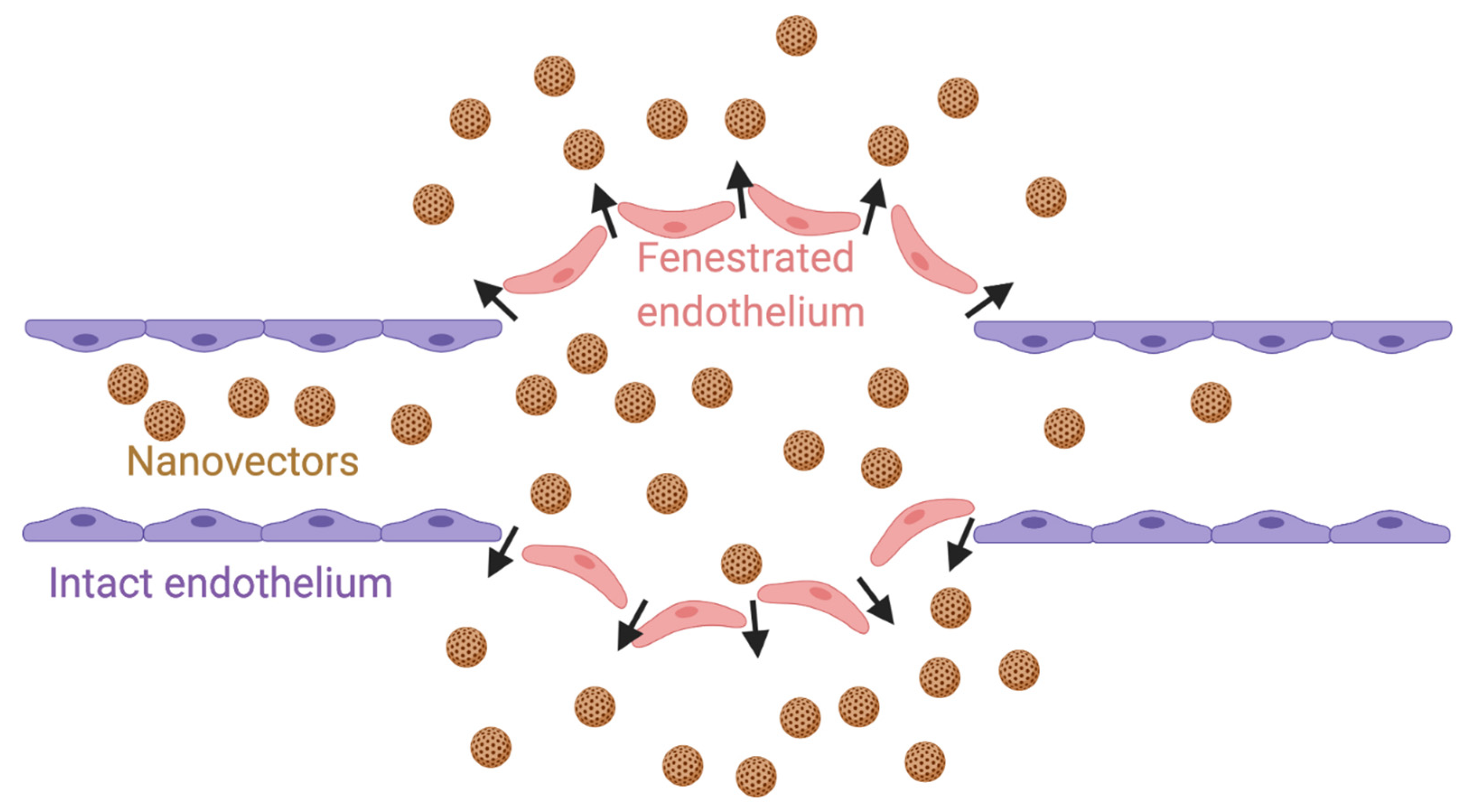


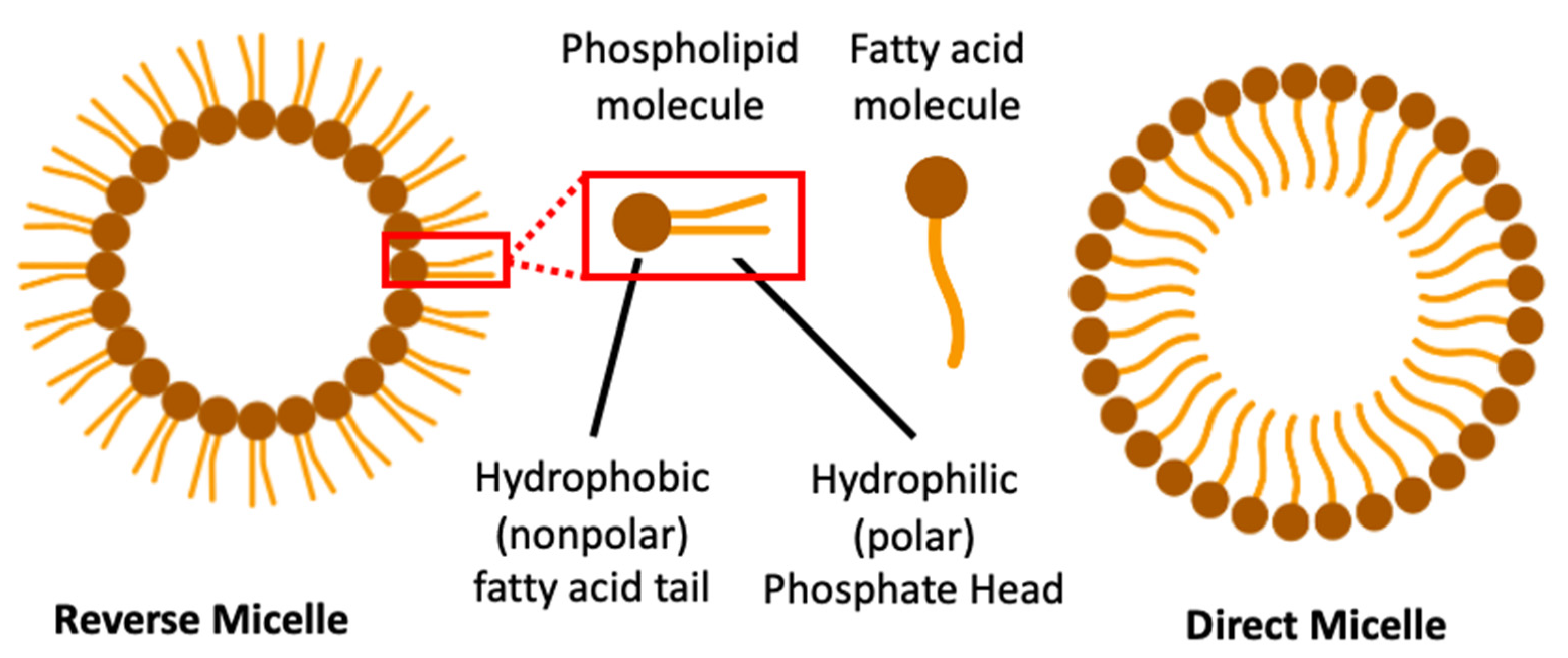

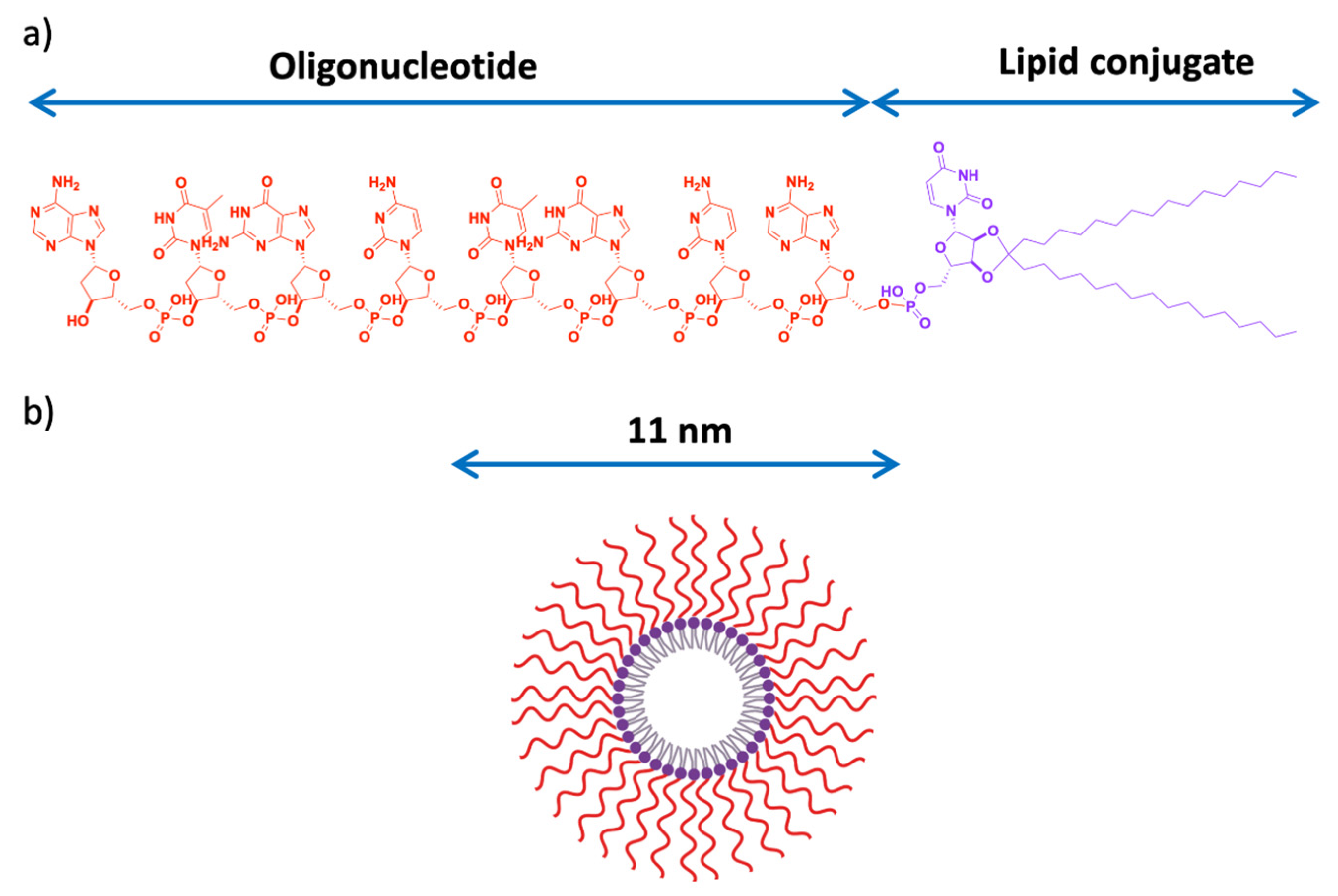
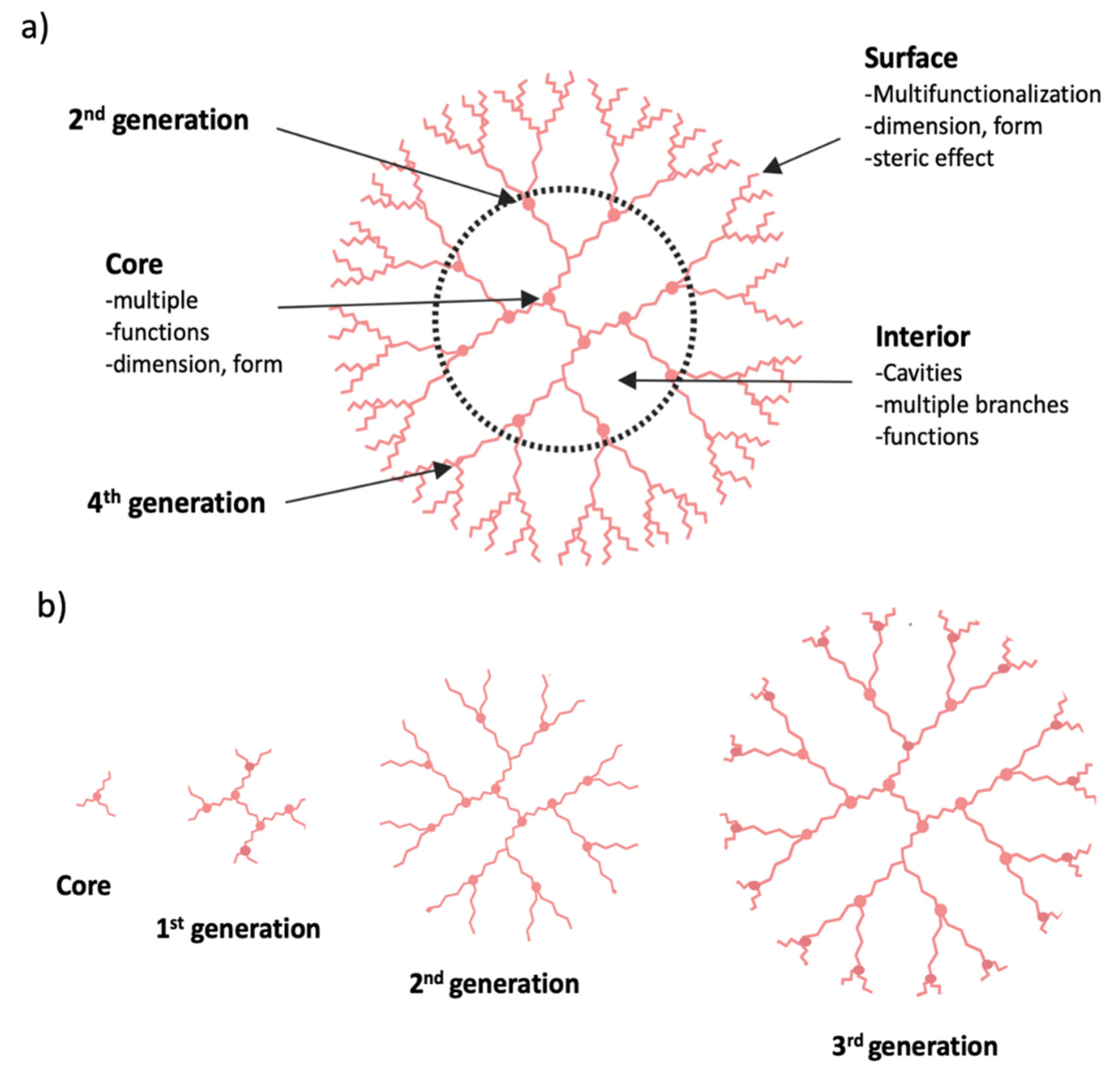


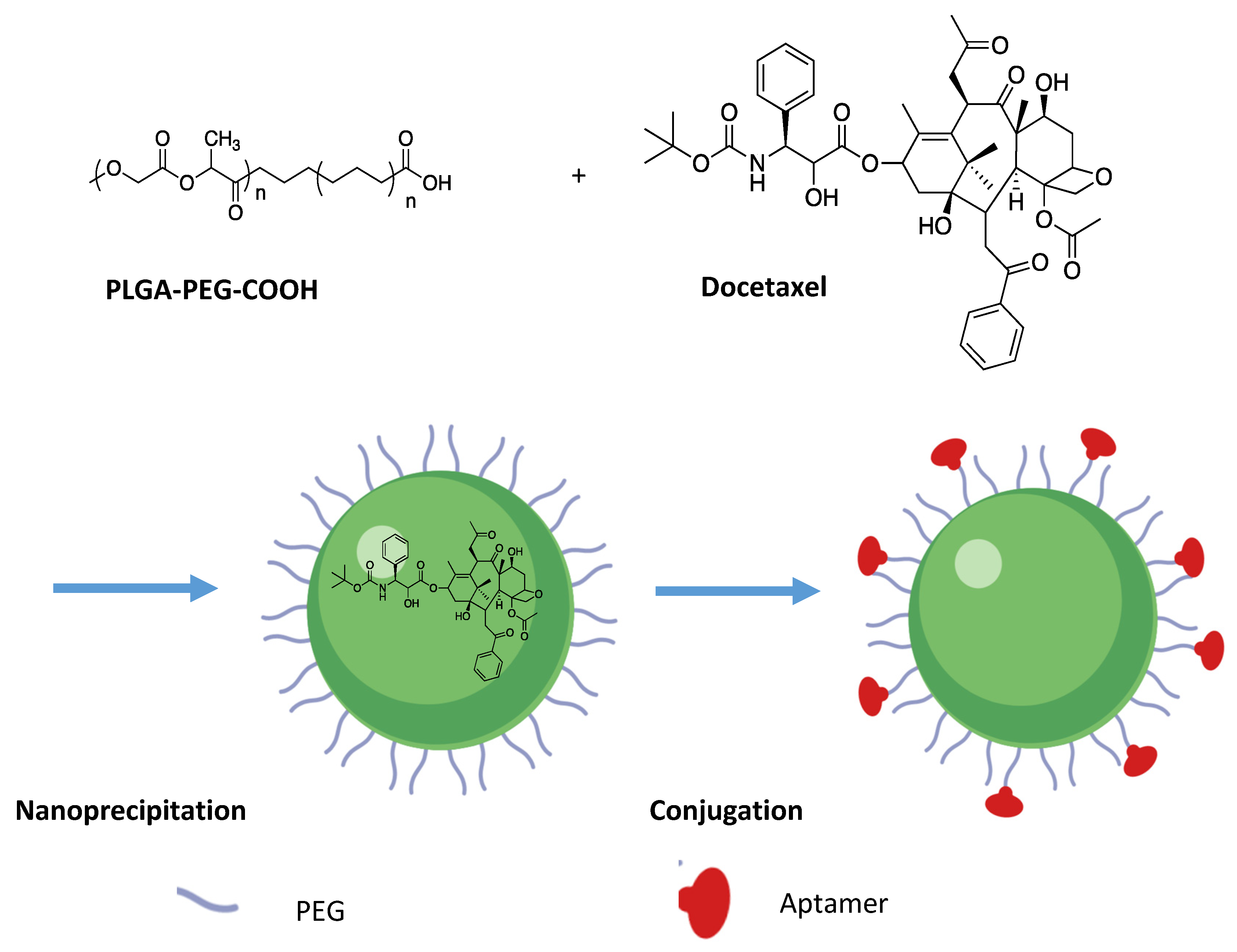
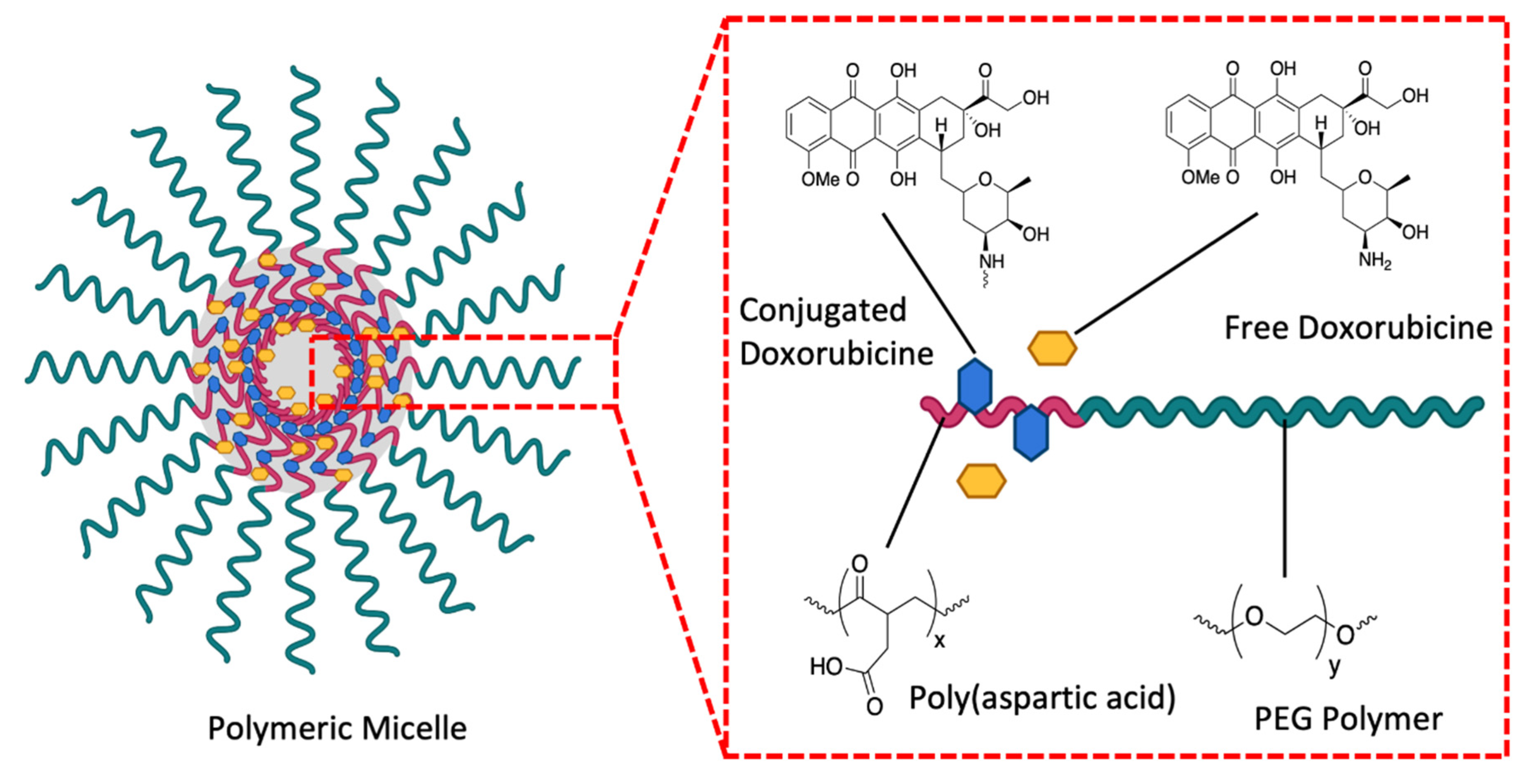
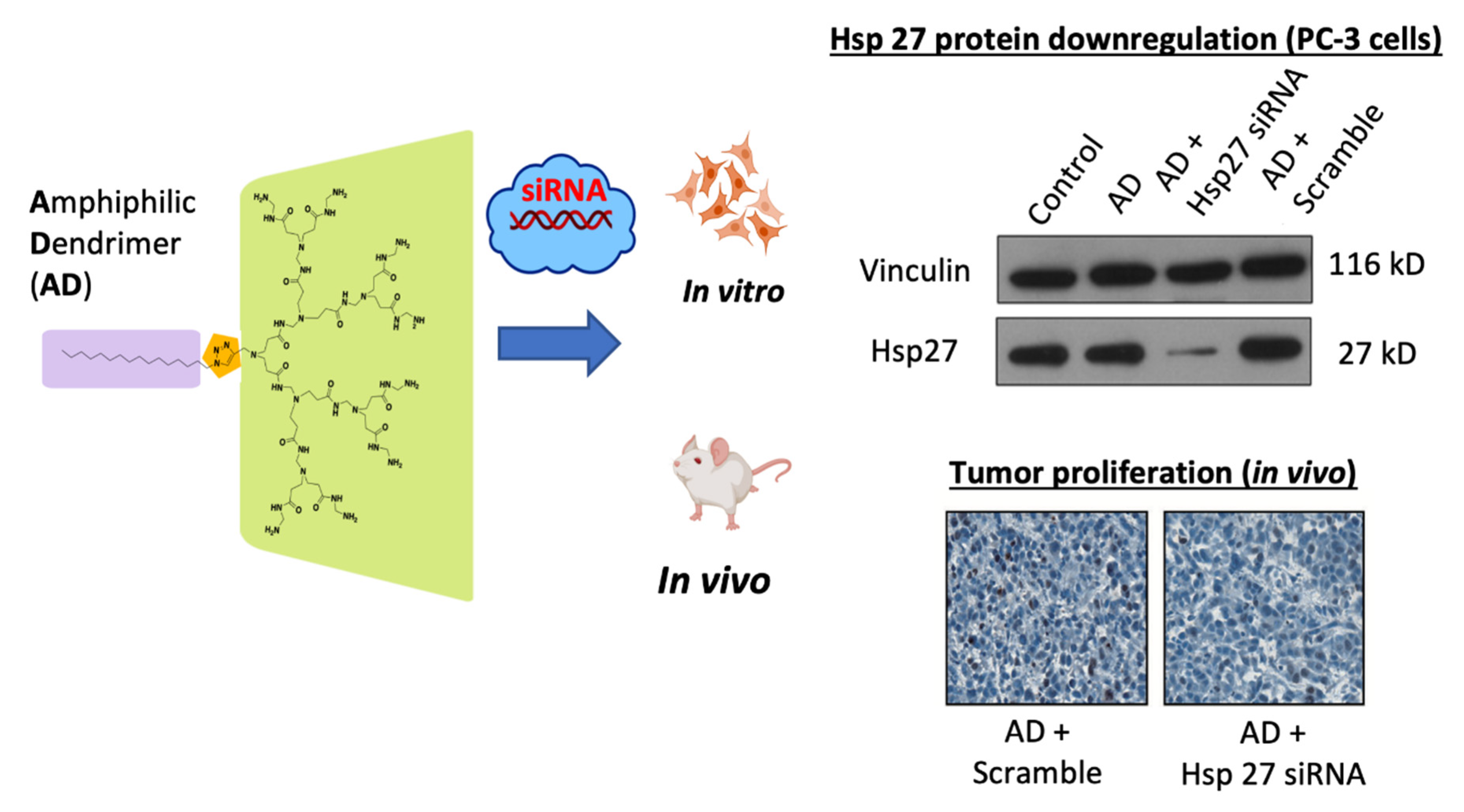
| Stress Response Pathways | Signals Transduction Targets | Cellular Proliferative Targets | Tumor Microenvironment | |
|---|---|---|---|---|
| Targets | Clusterin, HSP90, Bcl-2, HSP27 | PI3K, Akt, mTOR, eIF4E, IGF-IR | Microtubules, PARP1, SERCA Pump | Neurotransmitters, somatostatin, endoglins, VEGF/FGFR, α-integrin |
| Approved therapy | - | - | Docetaxel, cabaxitacel | Denosumab, Radium-223 |
| Experimental therapy | OGX-011, OGX-427 | BEZ235, BKM120, AZD6363, MK2206, AZD8186, Linsitinib, Lapatinib | Tesetaxel, Patupilone, Ixabepilone, G-202 | Sibrotuzumab, TRC-105, EMD525797, BMTP-11, Dovitinib, Beracizumab, pazopanib, phenelzine, pasireotide |
| Generation | Particle | Targeting | Loading | Ref |
|---|---|---|---|---|
| First | Liposome 1 | EPR | Celecoxib/Genistein | [83] |
| Polymeric 2/Nanobuble | EPR | Curcumin | [84] | |
| Liposome | EPR | PEG (avoid RES 3 uptake) | [59] | |
| Second | Liposome 4 | Apatamer | TFO 5 | [85] |
| Liposome 6 | Peptide | Doxorubicine/Vinorebline | [86] | |
| Liposome 7 | Antibody 8 | Doxorubicine | [87] | |
| Third | DNA nanostructure | Apatamer | Doxorubicine | [88] |
| PMB nanoparticle 9 | Small Molecule 10 | Reservatrol/Docetaxel | [89] | |
| Liposome | RGD | siRNA 11/Docetaxel | [90] | |
| Gold Nanoparticle | Small Molecule 10 | siRNA | [91] |
| Drug | Nanoformulation | Phase | Trial Status | Clinicaltrial.Gov Identifier |
|---|---|---|---|---|
| Curcumin | Nanomicellar gel | Second phase | ongoing | NCT02724618 |
| Paclitaxel Lapatinib | Albumin NP | First | Completed | NCT00313599 |
| siRNA for inhibition of M2 subunit of Ribonucleotide reductase (R2) | Cyclodexrin containing polymer stabilized by PEG | First | Terminated in 2013 | NCT00689065 |
| 2-Hydroxyl Flutamide (2-HOF) | Calcium sulphate gel | second | completed | NCT02341404 |
| M-VM3 (TLR5-receptor and its agonist protein 502s) | Adenoviral | First | Ongoing | NCT02654938 |
| IL-12 | Adenoviral | First | completed | NCT00406939 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omabe, K.; Paris, C.; Lannes, F.; Taïeb, D.; Rocchi, P. Nanovectorization of Prostate Cancer Treatment Strategies: A New Approach to Improved Outcomes. Pharmaceutics 2021, 13, 591. https://doi.org/10.3390/pharmaceutics13050591
Omabe K, Paris C, Lannes F, Taïeb D, Rocchi P. Nanovectorization of Prostate Cancer Treatment Strategies: A New Approach to Improved Outcomes. Pharmaceutics. 2021; 13(5):591. https://doi.org/10.3390/pharmaceutics13050591
Chicago/Turabian StyleOmabe, Kenneth, Clément Paris, François Lannes, David Taïeb, and Palma Rocchi. 2021. "Nanovectorization of Prostate Cancer Treatment Strategies: A New Approach to Improved Outcomes" Pharmaceutics 13, no. 5: 591. https://doi.org/10.3390/pharmaceutics13050591
APA StyleOmabe, K., Paris, C., Lannes, F., Taïeb, D., & Rocchi, P. (2021). Nanovectorization of Prostate Cancer Treatment Strategies: A New Approach to Improved Outcomes. Pharmaceutics, 13(5), 591. https://doi.org/10.3390/pharmaceutics13050591







