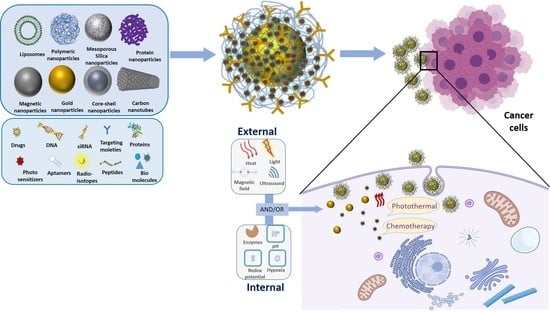
Smart Nanoparticles for Chemo-Based Combinational Therapy
Abstract
:1. Introduction
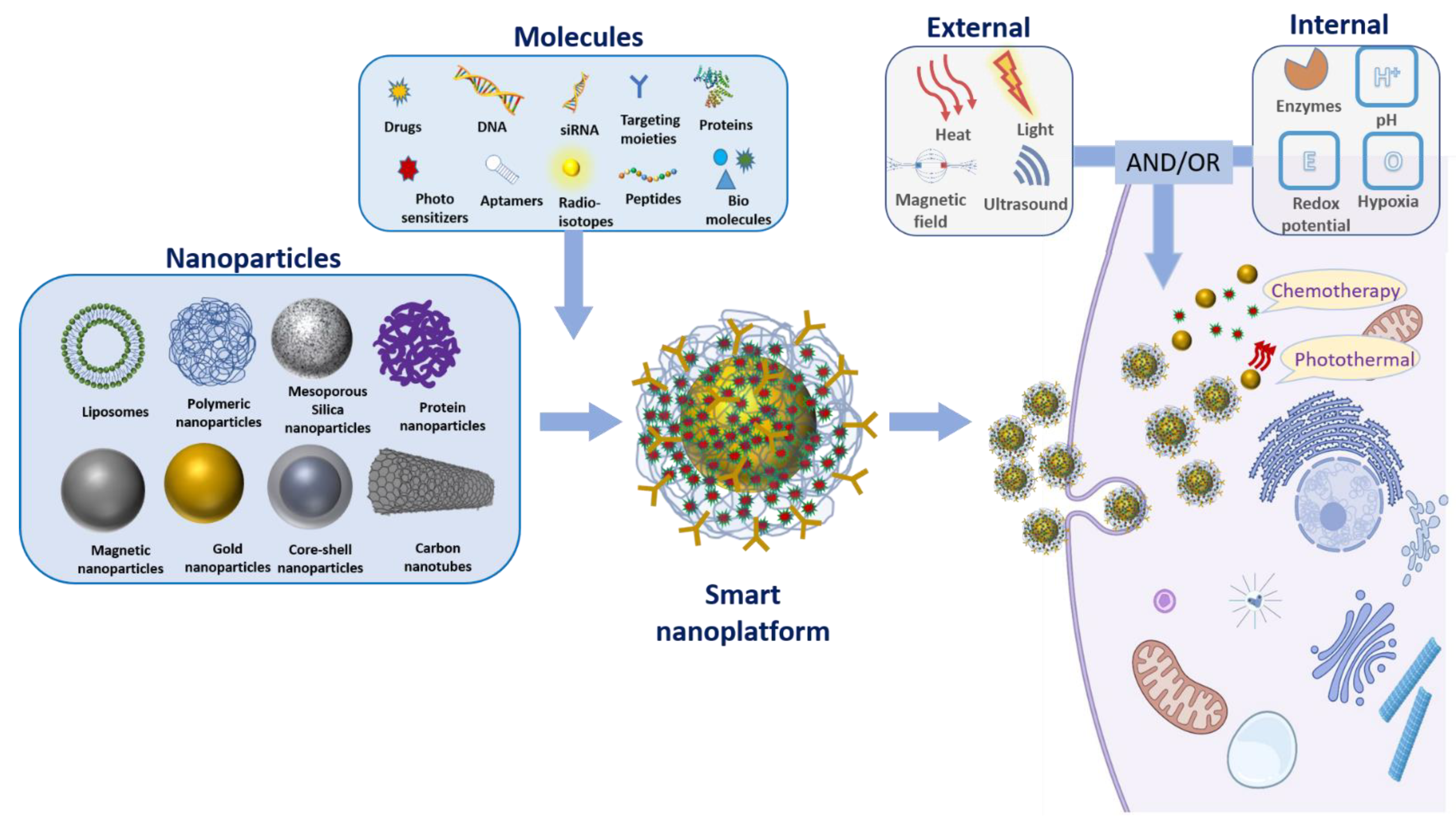
2. Smart Nanoplatforms
2.1. Internal Stimuli
2.1.1. pH
2.1.2. Enzymes
2.1.3. Redox Potential
2.1.4. Hypoxia
2.2. External Stimuli
2.2.1. Light
2.2.2. Thermal
2.2.3. Ultrasound
2.2.4. Magnetic Field
2.3. Multi-Responsive System
3. Smart Nanosystems in Combinational Therapy
3.1. Chemo-Combinational Therapy
3.2. Chemo-Energy Combinational Therapy
3.2.1. Chemo-Photo Combinational Therapy
Chemo-PTT Combination
Chemo-PDT Combination
3.3. Chemo-Ultrasound Combination
3.4. Chemo-Magnetic Combination
3.5. Chemo-Gene Combination
3.6. Chemo-Biomolecules Combination
3.7. Chemo-Immunotherapy Combination
3.8. Chemo-Small Molecules Combination
4. Personalized Medicine Perspective
5. Current Limitations and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K.; et al. Role of tumor microenvironment in tumorigenesis. J. Cancer 2017, 8, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125, 5591. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, A.A.; Aranda, V.; Bardelli, A.; Blanpain, C.; Bock, C.; Borowski, C.; Caldas, C.; Califano, A.; Doherty, M.; Elsner, M.; et al. Toward understanding and exploiting tumor heterogeneity. Nat. Med. 2015, 21, 846–853. [Google Scholar] [CrossRef]
- Hirata, E.; Sahai, E. Tumor microenvironment and differential responses to therapy. Cold Spring Harbor Perspect. Med. 2017, 7, a026781. [Google Scholar] [CrossRef] [Green Version]
- McGranahan, N.; Swanton, C. Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future. Cell 2017, 168, 613–628. [Google Scholar] [CrossRef] [Green Version]
- Shibata, D. Heterogeneity and Tumor History. Science 2012, 336, 304. [Google Scholar] [CrossRef]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and Key Considerations of the Enhanced Permeability and Retention Effect for Nanomedicine Drug Delivery in Oncology. Cancer Res. 2013, 73, 2412. [Google Scholar] [CrossRef] [Green Version]
- Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjug. Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef]
- Golombek, S.K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef]
- Nel, A.; Ruoslahti, E.; Meng, H. New Insights into “Permeability” as in the Enhanced Permeability and Retention Effect of Cancer Nanotherapeutics. ACS Nano 2017, 11, 9567–9569. [Google Scholar] [CrossRef]
- Anarjan, F.S. Active targeting drug delivery nanocarriers: Ligands. Nano-Struct. Nano-Objects 2019, 19, 100370. [Google Scholar]
- Zhong, Y.; Meng, F.; Deng, C.; Zhong, Z. Ligand-Directed Active Tumor-Targeting Polymeric Nanoparticles for Cancer Chemotherapy. Biomacromolecules 2014, 15, 1955–1969. [Google Scholar] [CrossRef]
- Shrestha, B.; Tang, L.; Romero, G. Nanoparticles-mediated combination therapies for cancer treatment. Adv. Ther. 2019, 2, 1900076. [Google Scholar] [CrossRef]
- Lovitt, C.J.; Shelper, T.B.; Avery, V.M. Doxorubicin resistance in breast cancer cells is mediated by extracellular matrix proteins. BMC Cancer 2018, 18, 41. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.; Xu, Z.; Huang, H.; Wang, Y.; Zhao, J.; Guo, X.; Zhou, S. A NIR light triggered disintegratable nanoplatform for enhanced penetration and chemotherapy in deep tumor tissues. Biomaterials 2020, 245, 119840. [Google Scholar] [CrossRef]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef] [Green Version]
- Ayaz, P.; Xu, B.; Zhang, X.; Wang, J.; Yu, D.; Wu, J. A pH and hyaluronidase dual-responsive multilayer-based drug delivery system for resisting bacterial infection. Appl. Surf. Sci. 2020, 527, 146806. [Google Scholar] [CrossRef]
- Liang, S.; Deng, X.; Xu, G.; Xiao, X.; Wang, M.; Guo, X.; Ma, P.A.; Cheng, Z.; Zhang, D.; Lin, J. A Novel Pt–TiO2 Heterostructure with Oxygen-Deficient Layer as Bilaterally Enhanced Sonosensitizer for Synergistic Chemo-Sonodynamic Cancer Therapy. Adv. Funct. Mater. 2020, 30, 1908598. [Google Scholar] [CrossRef]
- Sonawane, S.J.; Kalhapure, R.S.; Govender, T. Hydrazone linkages in pH responsive drug delivery systems. Eur. J. Pharm. Sci. 2017, 99, 45–65. [Google Scholar] [CrossRef]
- San Juan, A.M.T.; Rodgers, T.; Bedolla, C.; Noriega, F.; Romero, G. Layer by layer surface engineering of poly(lactide-co-glycolide) nanoparticles for plasmid DNA delivery. J. Appl. Polym. Sci. 2020, 137, 49377. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Yang, T.; Wu, H. Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int. J. Nanomed. 2018, 13, 2921–2942. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, T.; Muzzio, N.; Watson, C.; Romero, G. Stabilization of Poly (β-Amino Ester) Nanoparticles for the Efficient Intracellular Delivery of PiggyBac Transposon. Bioengneering 2021, 8, 16. [Google Scholar]
- Xiang, B.; Jia, X.-L.; Qi, J.-L.; Yang, L.-P.; Sun, W.-H.; Yan, X.; Yang, S.-K.; Cao, D.-Y.; Du, Q.; Qi, X.-R. Enhancing siRNA-based cancer therapy using a new pH-responsive activatable cell-penetrating peptide-modified liposomal system. Int. J. Nanomed. 2017, 12, 2385–2405. [Google Scholar] [CrossRef] [Green Version]
- Botelho da Silva, S.; Krolicka, M.; van den Broek, L.A.M.; Frissen, A.E.; Boeriu, C.G. Water-soluble chitosan derivatives and pH-responsive hydrogels by selective C-6 oxidation mediated by TEMPO-laccase redox system. Carbohydr. Polym. 2018, 186, 299–309. [Google Scholar] [CrossRef]
- Lee, H.; Park, H.; Noh, G.J.; Lee, E.S. pH-responsive hyaluronate-anchored extracellular vesicles to promote tumor-targeted drug delivery. Carbohydr. Polym. 2018, 202, 323–333. [Google Scholar] [CrossRef]
- Xie, J.; Fan, Z.; Li, Y.; Zhang, Y.; Yu, F.; Su, G.; Xie, L.; Hou, Z. Design of pH-sensitive methotrexate prodrug-targeted curcumin nanoparticles for efficient dual-drug delivery and combination cancer therapy. Int. J. Nanomed. 2018, 13, 1381. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.-L.; Sun, Y.; Ren, L.; Wang, X.; Wang, C.; Li, L.; Yang, Y.-W.; Yu, X.; Yu, J. Supramolecular Nanosystem Based on Pillararene-Capped CuS Nanoparticles for Targeted Chemo-Photothermal Therapy. ACS Appl. Mater. Int. 2018, 10, 29314–29324. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Xu, L.; Liu, Y.; Li, Q.; Zhao, D.; Li, Z.; Zhang, H.; Zhang, H.; Kan, Q.; Wang, Y.; et al. Transformative hyaluronic acid-based active targeting supramolecular nanoplatform improves long circulation and enhances cellular uptake in cancer therapy. Acta Pharm. Sin. B 2019, 9, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wei, R.; Yan, G.-H.; Sun, J.; Li, C.; Wang, H.; Shi, L.; Capobianco, J.A.; Sun, L. Smart Self-Assembled Nanosystem Based on Water-Soluble Pillararene and Rare-Earth-Doped Upconversion Nanoparticles for pH-Responsive Drug Delivery. ACS Appl. Mater. Int. 2018, 10, 4910–4920. [Google Scholar] [CrossRef] [PubMed]
- Gisbert-Garzarán, M.; Berkmann, J.C.; Giasafaki, D.; Lozano, D.; Spyrou, K.; Manzano, M.; Steriotis, T.; Duda, G.N.; Schmidt-Bleek, K.; Charalambopoulou, G.; et al. Engineered pH-Responsive Mesoporous Carbon Nanoparticles for Drug Delivery. ACS Appl. Mater. Int. 2020, 12, 14946–14957. [Google Scholar] [CrossRef] [PubMed]
- Begum, G.; Reddy, T.N.; Kumar, K.P.; Dhevendar, K.; Singh, S.; Amarnath, M.; Misra, S.; Rangari, V.K.; Rana, R.K. In Situ Strategy to Encapsulate Antibiotics in a Bioinspired CaCO3 Structure Enabling pH-Sensitive Drug Release Apt for Therapeutic and Imaging Applications. ACS Appl. Mater. Int. 2016, 8, 22056–22063. [Google Scholar] [CrossRef]
- Suh, M.S.; Shen, J.; Kuhn, L.T.; Burgess, D.J. Layer-by-layer nanoparticle platform for cancer active targeting. Int. J. Pharm. 2017, 517, 58–66. [Google Scholar] [CrossRef]
- Feng, L.; Xie, R.; Wang, C.; Gai, S.; He, F.; Yang, D.; Yang, P.; Lin, J. Magnetic Targeting, Tumor Microenvironment-Responsive Intelligent Nanocatalysts for Enhanced Tumor Ablation. ACS Nano 2018, 12, 11000–11012. [Google Scholar] [CrossRef]
- Yao, Q.; Kou, L.; Tu, Y.; Zhu, L. MMP-Responsive ‘Smart’ Drug Delivery and Tumor Targeting. Trends Pharmacol. Sci. 2018, 39, 766–781. [Google Scholar] [CrossRef]
- Yang, K.; Liu, Y.; Wang, Y.; Ren, Q.; Guo, H.; Matson, J.B.; Chen, X.; Nie, Z. Enzyme-induced in vivo assembly of gold nanoparticles for imaging-guided synergistic chemo-photothermal therapy of tumor. Biomaterials 2019, 223, 119460. [Google Scholar] [CrossRef]
- Wu, L.; Lin, B.; Yang, H.; Chen, J.; Mao, Z.; Wang, W.; Gao, C. Enzyme-responsive multifunctional peptide coating of gold nanorods improves tumor targeting and photothermal therapy efficacy. Acta Biomater. 2019, 86, 363–372. [Google Scholar] [CrossRef]
- Mu, J.; Lin, J.; Huang, P.; Chen, X. Development of endogenous enzyme-responsive nanomaterials for theranostics. Chem. Soc. Rev. 2018, 47, 5554–5573. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Ho, C.J.H.; Aw, J.; Attia, A.B.E.; Mu, J.; Wang, Y.; Wang, X.; Wang, Y.; Liu, X.; Chen, H.; et al. In vivo covalent cross-linking of photon-converted rare-earth nanostructures for tumour localization and theranostics. Nat.Communicat. 2016, 7, 10432. [Google Scholar] [CrossRef] [PubMed]
- Pljesa-Ercegovac, M.; Savic-Radojevic, A.; Matic, M.; Coric, V.; Djukic, T.; Radic, T.; Simic, T. Glutathione Transferases: Potential Targets to Overcome Chemoresistance in Solid Tumors. Int. J. Mol. Sci. 2018, 19, 3785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.; Dai, W.; He, B.; Zhang, H.; Wang, X.; Wang, Y.; Zhang, Q. Current Multistage Drug Delivery Systems Based on the Tumor Microenvironment. Theranostics 2017, 7, 538–558. [Google Scholar] [CrossRef]
- Zhang, C.; Pan, D.; Li, J.; Hu, J.; Bains, A.; Guys, N.; Zhu, H.; Li, X.; Luo, K.; Gong, Q.; et al. Enzyme-responsive peptide dendrimer-gemcitabine conjugate as a controlled-release drug delivery vehicle with enhanced antitumor efficacy. Acta Biomater. 2017, 55, 153–162. [Google Scholar] [CrossRef]
- Choi, K.Y.; Han, H.S.; Lee, E.S.; Shin, J.M.; Almquist, B.D.; Lee, D.S.; Park, J.H. Hyaluronic Acid–Based Activatable Nanomaterials for Stimuli-Responsive Imaging and Therapeutics: Beyond CD44-Mediated Drug Delivery. Adv. Mater. 2019, 31, 1803549. [Google Scholar] [CrossRef]
- Hu, C.; Cun, X.; Ruan, S.; Liu, R.; Xiao, W.; Yang, X.; Yang, Y.; Yang, C.; Gao, H. Enzyme-triggered size shrink and laser-enhanced NO release nanoparticles for deep tumor penetration and combination therapy. Biomaterials 2018, 168, 64–75. [Google Scholar] [CrossRef]
- Shahriari, M.; Zahiri, M.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Enzyme responsive drug delivery systems in cancer treatment. J. Control. Release 2019, 308, 172–189. [Google Scholar] [CrossRef]
- He, Y.; Lei, L.; Cao, J.; Yang, X.; Cai, S.; Tong, F.; Huang, D.; Mei, H.; Luo, K.; Gao, H.; et al. A combinational chemo-immune therapy using an enzyme-sensitive nanoplatform for dual-drug delivery to specific sites by cascade targeting. Sci. Adv. 2021, 7, eaba0776. [Google Scholar] [CrossRef]
- Fukino, T.; Yamagishi, H.; Aida, T. Redox-Responsive Molecular Systems and Materials. Adv. Mater. 2017, 29, 1603888. [Google Scholar] [CrossRef]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharmacol. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Balendiran, G.K.; Dabur, R.; Fraser, D. The role of glutathione in cancer. Cell Biochem. Funct. 2004, 22, 343–352. [Google Scholar] [CrossRef]
- Mo, R.; Gu, Z. Tumor microenvironment and intracellular signal-activated nanomaterials for anticancer drug delivery. Mater. Today 2016, 19, 274–283. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, Z.; Sun, W.; Yang, Y.; Jin, H.; Qiu, L.; Chen, J.; Chen, J. Co-responsive smart cyclodextrin-gated mesoporous silica nanoparticles with ligand-receptor engagement for anti-cancer treatment. Mater. Sci. Eng. C 2019, 103, 109831. [Google Scholar] [CrossRef]
- Raza, A.; Hayat, U.; Rasheed, T.; Bilal, M.; Iqbal, H.M.N. Redox-responsive nano-carriers as tumor-targeted drug delivery systems. Eur.J. Med. Chem. 2018, 157, 705–715. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, Y.; Zou, Y.; Wang, Y.; Niu, D.; He, Q.; Huang, Z.; Zhu, W.; Tian, H.; Shi, J.; et al. Dual Intratumoral Redox/Enzyme-Responsive NO-Releasing Nanomedicine for the Specific, High-Efficacy, and Low-Toxic Cancer Therapy. Adv. Mater. 2018, 30, 1704490. [Google Scholar] [CrossRef]
- Zuo, C.; Dai, X.; Zhao, S.; Liu, X.; Ding, S.; Ma, L.; Liu, M.; Wei, H. Fabrication of Dual-Redox Responsive Supramolecular Copolymers Using a Reducible β-Cyclodextran-Ferrocene Double-Head Unit. ACS Macro Lett. 2016, 5, 873–878. [Google Scholar] [CrossRef]
- Guo, R.; Su, Q.; Zhang, J.; Dong, A.; Lin, C.; Zhang, J. Facile Access to Multisensitive and Self-Healing Hydrogels with Reversible and Dynamic Boronic Ester and Disulfide Linkages. Biomacromolecules 2017, 18, 1356–1364. [Google Scholar] [CrossRef]
- Guo, X.; Cheng, Y.; Zhao, X.; Luo, Y.; Chen, J.; Yuan, W.-E. Advances in redox-responsive drug delivery systems of tumor microenvironment. J. Nanobiotechnol. 2018, 16, 74. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Saw, P.E.; Lin, C.; Nie, Y.; Tao, W.; Farokhzad, O.C.; Zhang, L.; Xu, X. Redox-responsive polyprodrug nanoparticles for targeted siRNA delivery and synergistic liver cancer therapy. Biomaterials 2020, 234, 119760. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, D.; Chen, H.; Lim, W.Q.; Phua, F.S.Z.; An, G.; Yang, P.; Zhao, Y. Reduction-sensitive fluorescence enhanced polymeric prodrug nanoparticles for combinational photothermal-chemotherapy. Biomaterials 2018, 163, 14–24. [Google Scholar] [CrossRef]
- Liu, M.; Khan, A.R.; Ji, J.; Lin, G.; Zhao, X.; Zhai, G. Crosslinked self-assembled nanoparticles for chemo-sonodynamic combination therapy favoring antitumor, antimetastasis management and immune responses. J. Control. Release 2018, 290, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Yuan, Y.; Mao, D.; Wu, W.; Liu, B. Smart activatable and traceable dual-prodrug for image-guided combination photodynamic and chemo-therapy. Biomaterials 2017, 144, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, X.-Y.; Wang, Y.-L.; Li, X.; Yang, X.-H.; Huang, M.; Hu, K.; Li, L.-H.; Wei, Y. Redox-responsive theranostic nanoplatforms based on inorganic nanomaterials. J. Control. Release 2017, 259, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P. The Role of Hypoxia-Induced Factors in Tumor Progression. Oncologist 2004, 9, 10–17. [Google Scholar] [CrossRef]
- Michiels, C.; Tellier, C.; Feron, O. Cycling hypoxia: A key feature of the tumor microenvironment. Biochim. Biophys. Acta (BBA) Rev. Cancer 2016, 1866, 76–86. [Google Scholar] [CrossRef]
- Ahmad, Z.; Lv, S.; Tang, Z.; Shah, A.; Chen, X. Methoxy poly (ethylene glycol)-block-poly (glutamic acid)-graft-6-(2-nitroimidazole) hexyl amine nanoparticles for potential hypoxia-responsive delivery of doxorubicin. J. Biomater. Sci. Polym. Ed. 2016, 27, 40–54. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Meng, X.; Lu, H.; Chang, H.; Dong, H.; Zhang, X. Light-triggered theranostic liposomes for tumor diagnosis and combined photodynamic and hypoxia-activated prodrug therapy. Biomaterials 2018, 185, 301–309. [Google Scholar] [CrossRef]
- Liu, H.; Xie, Y.; Zhang, Y.; Cai, Y.; Li, B.; Mao, H.; Liu, Y.; Lu, J.; Zhang, L.; Yu, R. Development of a hypoxia-triggered and hypoxic radiosensitized liposome as a doxorubicin carrier to promote synergetic chemo-/radio-therapy for glioma. Biomaterials 2017, 121, 130–143. [Google Scholar] [CrossRef]
- Yin, W.; Qiang, M.; Ke, W.; Han, Y.; Mukerabigwi, J.F.; Ge, Z. Hypoxia-responsive block copolymer radiosensitizers as anticancer drug nanocarriers for enhanced chemoradiotherapy of bulky solid tumors. Biomaterials 2018, 181, 360–371. [Google Scholar] [CrossRef]
- Yang, G.; Phua, S.Z.F.; Lim, W.Q.; Zhang, R.; Feng, L.; Liu, G.; Wu, H.; Bindra, A.K.; Jana, D.; Liu, Z.; et al. A Hypoxia-Responsive Albumin-Based Nanosystem for Deep Tumor Penetration and Excellent Therapeutic Efficacy. Adv. Mater. 2019, 31, 1901513. [Google Scholar] [CrossRef]
- Yan, Q.; Guo, X.; Huang, X.; Meng, X.; Liu, F.; Dai, P.; Wang, Z.; Zhao, Y. Gated Mesoporous Silica Nanocarriers for Hypoxia-Responsive Cargo Release. ACS Appl. Mater. Interfaces 2019, 11, 24377–24385. [Google Scholar] [CrossRef]
- Peng, S.; Ouyang, B.; Xin, Y.; Zhao, W.; Shen, S.; Zhan, M.; Lu, L. Hypoxia-degradable and long-circulating zwitterionic phosphorylcholine-based nanogel for enhanced tumor drug delivery. Acta Pharm. Sin. B 2021, 11, 560–571. [Google Scholar] [CrossRef]
- Wei, C.; Liu, Y.; Zhu, X.; Chen, X.; Zhou, Y.; Yuan, G.; Gong, Y.; Liu, J. Iridium/ruthenium nanozyme reactors with cascade catalytic ability for synergistic oxidation therapy and starvation therapy in the treatment of breast cancer. Biomaterials 2020, 238, 119848. [Google Scholar] [CrossRef]
- Cui, D.; Huang, J.; Zhen, X.; Li, J.; Jiang, Y.; Pu, K. A Semiconducting Polymer Nano-prodrug for Hypoxia-Activated Photodynamic Cancer Therapy. Angew. Chem. Int. Ed. 2019, 58, 5920–5924. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, H.; Shen, W.; Liu, W.; Chen, L.; Xiao, C. Hypoxia-Responsive Polypeptide Nanoparticles Loaded with Doxorubicin for Breast Cancer Therapy. ACS Biomater. Sci. Eng. 2020, 6, 2167–2174. [Google Scholar] [CrossRef]
- Huang, C.-C.; Chia, W.-T.; Chung, M.-F.; Lin, K.-J.; Hsiao, C.-W.; Jin, C.; Lim, W.-H.; Chen, C.-C.; Sung, H.-W. An implantable depot that can generate oxygen in situ for overcoming hypoxia-induced resistance to anticancer drugs in chemotherapy. J. Am. Chem. Soc. 2016, 138, 5222–5225. [Google Scholar] [CrossRef]
- Qin, S.; Xu, Y.; Li, H.; Chen, H.; Yuan, Z. Recent advances in in-situ oxygen-generating and oxygen-replenishing strategies for hypoxic-enhanced photodynamic therapy. Biomater. Sci. 2021. [Google Scholar] [CrossRef]
- Chen, Q.; Feng, L.; Liu, J.; Zhu, W.; Dong, Z.; Wu, Y.; Liu, Z. Intelligent Albumin–MnO2 Nanoparticles as pH-/H2O2-Responsive Dissociable Nanocarriers to Modulate Tumor Hypoxia for Effective Combination Therapy. Adv. Mater. 2016, 28, 7129–7136. [Google Scholar] [CrossRef]
- Yang, G.; Xu, L.; Chao, Y.; Xu, J.; Sun, X.; Wu, Y.; Peng, R.; Liu, Z. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat. Commun. 2017, 8, 902. [Google Scholar] [CrossRef] [Green Version]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Xu, X.; Saw, P.E.; Tao, W.; Li, Y.; Ji, X.; Bhasin, S.; Liu, Y.; Ayyash, D.; Rasmussen, J.; Huo, M. ROS-responsive polyprodrug nanoparticles for triggered drug delivery and effective cancer therapy. Adv. Mater. 2017, 29, 1700141. [Google Scholar] [CrossRef]
- Luan, T.; Cheng, L.; Cheng, J.; Zhang, X.; Cao, Y.; Zhang, X.; Cui, H.; Zhao, G. Tailored design of an ROS-responsive drug release platform for enhanced tumor therapy via “sequential induced activation processes”. ACS Appl. Mater. Interfaces 2019, 11, 25654–25663. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Wu, X.; Ding, S.; Lou, X.; Xia, F.; Wang, S.; Hong, Y. Aggregation-Induced Emission Photosensitizers: From Molecular Design to Photodynamic Therapy. J. Med. Chem. 2020, 63, 1996–2012. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Lu, G.; Wei, W.-C.; Huang, Y.-H.; Li, S.; Chen, J.-X.; Cui, X.; Xiao, Y.-F.; Li, X.; Liu, Y.; et al. Stable Organic Photosensitizer Nanoparticles with Absorption Peak beyond 800 Nanometers and High Reactive Oxygen Species Yield for Multimodality Phototheranostics. ACS Nano 2020, 14, 9917–9928. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Z.; Lin, L.; Liu, F.; Wang, Y.; Guo, Z.; Li, Y.; Tian, H.; Chen, X. Porphyrin-based covalent organic framework nanoparticles for photoacoustic imaging-guided photodynamic and photothermal combination cancer therapy. Biomaterials 2019, 223, 119459. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, Y.; Wang, Q.; Liu, T.; Sun, J.; Zhang, R. Remote Light-Responsive Nanocarriers for Controlled Drug Delivery: Advances and Perspectives. Small 2019, 15, 1903060. [Google Scholar] [CrossRef]
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1449. [Google Scholar] [CrossRef]
- Kuo, W.S.; Chang, C.N.; Chang, Y.T.; Yang, M.H.; Chien, Y.H.; Chen, S.J.; Yeh, C.S. Gold nanorods in photodynamic therapy, as hyperthermia agents, and in near-infrared optical imaging. Angew. Chem. Int. Ed. Engl. 2010, 49, 2711–2715. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Yuan, X.; Deng, L.; Yin, Z.; Tian, X.; Bhattacharyya, S.; Liu, H.; Luo, Y.; Luo, L. Graphene oxide activated by 980 nm laser for cascading two-photon photodynamic therapy and photothermal therapy against breast cancer. Appl. Mater. Today 2020, 20, 100665. [Google Scholar] [CrossRef]
- Xu, M.; Yang, G.; Bi, H.; Xu, J.; Feng, L.; Yang, D.; Sun, Q.; Gai, S.; He, F.; Dai, Y.; et al. Combination of CuS and g-C3N4 QDs on upconversion nanoparticles for targeted photothermal and photodynamic cancer therapy. Chem. Eng. J. 2019, 360, 866–878. [Google Scholar] [CrossRef]
- Shao, Y.; Liu, B.; Di, Z.; Zhang, G.; Sun, L.-D.; Li, L.; Yan, C.-H. Engineering of Upconverted Metal–Organic Frameworks for Near-Infrared Light-Triggered Combinational Photodynamic/Chemo-/Immunotherapy against Hypoxic Tumors. J. Am. Chem. Soc. 2020, 142, 3939–3946. [Google Scholar] [CrossRef]
- Ali, M.R.K.; Wu, Y.; Tang, Y.; Xiao, H.; Chen, K.; Han, T.; Fang, N.; Wu, R.; El-Sayed, M.A. Targeting cancer cell integrins using gold nanorods in photothermal therapy inhibits migration through affecting cytoskeletal proteins. Proc. Natl. Acad. Sci. USA 2017, 201703151. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.-P.; Zhou, B.; Lin, Z.; Liang, H.; Shen, X.-C. Recent Advances in Carbon Nanomaterials for Cancer Phototherapy. Chem. Eur. J. 2019, 25, 3993–4004. [Google Scholar] [CrossRef]
- Yang, X.; Li, H.; Qian, C.; Guo, Y.; Li, C.; Gao, F.; Yang, Y.; Wang, K.; Oupicky, D.; Sun, M. Near-infrared light-activated IR780-loaded liposomes for anti-tumor angiogenesis and Photothermal therapy. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2283–2294. [Google Scholar] [CrossRef]
- Yu, L.; Dong, A.; Guo, R.; Yang, M.; Deng, L.; Zhang, J. DOX/ICG Coencapsulated Liposome-Coated Thermosensitive Nanogels for NIR-Triggered Simultaneous Drug Release and Photothermal Effect. ACS Biomater. Sci. Eng. 2018, 4, 2424–2434. [Google Scholar] [CrossRef]
- Shao, J.; Ruan, C.; Xie, H.; Li, Z.; Wang, H.; Chu, P.K.; Yu, X.-F. Black-Phosphorus-Incorporated Hydrogel as a Sprayable and Biodegradable Photothermal Platform for Postsurgical Treatment of Cancer. Adv. Sci. 2018, 5, 1700848. [Google Scholar] [CrossRef]
- Guntnur, R.T.; Muzzio, N.; Morales, M.; Romero, G. Phase transition characterization of poly(oligo(ethylene glycol)methyl ether methacrylate) brushes using the quartz crystal microbalance with dissipation. Soft Matter 2021. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Vibhagool, S.; Zhao, H.; Hoven, V.; Theato, P. Photocaged PNIPAM: A Light Tunable Thermal Responsive Polymer. Macromol. Chem. Phys. 2018, 219, 1800104. [Google Scholar] [CrossRef]
- Zhao, Q.; Ma, C.; Liu, J.; Chen, Z.; Zhao, H.; Li, B.; Yang, X. Synthesis of magnetic covalent organic framework molecularly imprinted polymers at room temperature: A novel imprinted strategy for thermo-sensitive substance. Talanta 2021, 225, 121958. [Google Scholar] [CrossRef] [PubMed]
- Peralta, M.E.; Jadhav, S.A.; Magnacca, G.; Scalarone, D.; Mártire, D.O.; Parolo, M.E.; Carlos, L. Synthesis and in vitro testing of thermoresponsive polymer-grafted core-shell magnetic mesoporous silica nanoparticles for efficient controlled and targeted drug delivery. J. Colloid Interface Sci. 2019, 544, 198–205. [Google Scholar] [CrossRef]
- Amoli-Diva, M.; Sadighi-Bonabi, R.; Pourghazi, K. Switchable on/off drug release from gold nanoparticles-grafted dual light- and temperature-responsive hydrogel for controlled drug delivery. Mater. Sci. Eng. C 2017, 76, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Feng, L.; Liang, C.; Yang, K.; Liu, Z. Ultrasound Triggered Tumor Oxygenation with Oxygen-Shuttle Nanoperfluorocarbon to Overcome Hypoxia-Associated Resistance in Cancer Therapies. Nano Lett. 2016, 16, 6145–6153. [Google Scholar] [CrossRef]
- Beik, J.; Shiran, M.B.; Abed, Z.; Shiri, I.; Ghadimi-Daresajini, A.; Farkhondeh, F.; Ghaznavi, H.; Shakeri-Zadeh, A. Gold nanoparticle-induced sonosensitization enhances the antitumor activity of ultrasound in colon tumor-bearing mice. Med. Phys. 2018, 45, 4306–4314. [Google Scholar] [CrossRef]
- Cheung, V.Y.T. High-intensity focused ultrasound therapy. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 46, 74–83. [Google Scholar] [CrossRef]
- Paris, J.L.; de la Torre, P.; Cabanas, M.V.; Manzano, M.; Grau, M.; Flores, A.I.; Vallet-Regí, M. Vectorization of ultrasound-responsive nanoparticles in placental mesenchymal stem cells for cancer therapy. Nanoscale 2017, 9, 5528–5537. [Google Scholar] [CrossRef] [Green Version]
- Paris, J.L.; Cabañas, M.V.; Manzano, M.; Vallet-Regí, M. Polymer-grafted mesoporous silica nanoparticles as ultrasound-responsive drug carriers. ACS Nano 2015, 9, 11023–11033. [Google Scholar] [CrossRef] [Green Version]
- Yue, W.; Chen, L.; Yu, L.; Zhou, B.; Yin, H.; Ren, W.; Liu, C.; Guo, L.; Zhang, Y.; Sun, L. Checkpoint blockade and nanosonosensitizer-augmented noninvasive sonodynamic therapy combination reduces tumour growth and metastases in mice. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Papa, A.-L.; Korin, N.; Kanapathipillai, M.; Mammoto, A.; Mammoto, T.; Jiang, A.; Mannix, R.; Uzun, O.; Johnson, C.; Bhatta, D.; et al. Ultrasound-sensitive nanoparticle aggregates for targeted drug delivery. Biomaterials 2017, 139, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, X.; Huang, Z.; Huang, P.; Zhang, Y.; Deng, L.; Wang, Z.; Zhou, Z.; Liu, Y.; Kalish, H.; et al. Magneto-Plasmonic Janus Vesicles for Magnetic Field-Enhanced Photoacoustic and Magnetic Resonance Imaging of Tumors. Angew. Chem. Int. Ed. 2016, 55, 15297–15300. [Google Scholar] [CrossRef]
- Tang, X.-L.; Jing, F.; Lin, B.-L.; Cui, S.; Yu, R.-T.; Shen, X.-d.; Wang, T.-W. pH-Responsive Magnetic Mesoporous Silica-Based Nanoplatform for Synergistic Photodynamic Therapy/Chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 15001–15011. [Google Scholar] [CrossRef] [PubMed]
- Rosensweig, R.E. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Di Corato, R.; Béalle, G.; Kolosnjaj-Tabi, J.; Espinosa, A.; Clément, O.; Silva, A.K.A.; Ménager, C.; Wilhelm, C. Combining Magnetic Hyperthermia and Photodynamic Therapy for Tumor Ablation with Photoresponsive Magnetic Liposomes. ACS Nano 2015, 9, 2904–2916. [Google Scholar] [CrossRef]
- Lee, K.; David, A.E.; Zhang, J.; Shin, M.C.; Yang, V.C. Enhanced accumulation of theranostic nanoparticles in brain tumor by external magnetic field mediated in situ clustering of magnetic nanoparticles. J. Ind. Eng. Chem. 2017, 54, 389–397. [Google Scholar] [CrossRef]
- Thirunavukkarasu, G.K.; Cherukula, K.; Lee, H.; Jeong, Y.Y.; Park, I.-K.; Lee, J.Y. Magnetic field-inducible drug-eluting nanoparticles for image-guided thermo-chemotherapy. Biomaterials 2018, 180, 240–252. [Google Scholar] [CrossRef]
- Dey, C.; Baishya, K.; Ghosh, A.; Goswami, M.M.; Ghosh, A.; Mandal, K. Improvement of drug delivery by hyperthermia treatment using magnetic cubic cobalt ferrite nanoparticles. J. Magn. Magn. Mater. 2017, 427, 168–174. [Google Scholar] [CrossRef]
- Chang, D.; Lim, M.; Goos, J.A.C.M.; Qiao, R.; Ng, Y.Y.; Mansfeld, F.M.; Jackson, M.; Davis, T.P.; Kavallaris, M. Biologically Targeted Magnetic Hyperthermia: Potential and Limitations. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Muroski, M.E.; Petit, D.C.M.C.; Mansell, R.; Vemulkar, T.; Morshed, R.A.; Han, Y.; Balyasnikova, I.V.; Horbinski, C.M.; Huang, X.; et al. Rotating magnetic field induced oscillation of magnetic particles for in vivo mechanical destruction of malignant glioma. J. Control. Release 2016, 223, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Betal, S.; Shrestha, B.; Dutta, M.; Cotica, L.F.; Khachatryan, E.; Nash, K.; Tang, L.; Bhalla, A.S.; Guo, R. Magneto-elasto-electroporation (MEEP): In-vitro visualization and numerical characteristics. Sci. Rep. 2016, 6, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Hegazy, M.; Zhou, P.; Wu, G.; Wang, L.; Rahoui, N.; Taloub, N.; Huang, X.; Huang, Y. Construction of polymer coated core–shell magnetic mesoporous silica nanoparticles with triple responsive drug delivery. Polym. Chem. 2017, 8, 5852–5864. [Google Scholar] [CrossRef]
- Falireas, P.G.; Vamvakaki, M. Triple-responsive block copolymer micelles with synergistic pH and temperature response. Macromolecules 2018, 51, 6848–6858. [Google Scholar] [CrossRef]
- Yang, Z.; Cheng, R.; Zhao, C.; Sun, N.; Luo, H.; Chen, Y.; Liu, Z.; Li, X.; Liu, J.; Tian, Z. Thermo-and pH-dual responsive polymeric micelles with upper critical solution temperature behavior for photoacoustic imaging-guided synergistic chemo-photothermal therapy against subcutaneous and metastatic breast tumors. Theranostics 2018, 8, 4097. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Y.; Lian, J.; Shen, Q.; Wang, C.; Ma, B.; Zhang, Y.; Xu, T.; Li, J.; Shao, Y.; et al. Engineering the Surface of Smart Nanocarriers Using a pH-/Thermal-/GSH-Responsive Polymer Zipper for Precise Tumor Targeting Therapy In Vivo. Adv. Mater. 2017, 29, 1702311. [Google Scholar] [CrossRef]
- Amjadi, S.; Hamishehkar, H.; Ghorbani, M. A novel smart PEGylated gelatin nanoparticle for co-delivery of doxorubicin and betanin: A strategy for enhancing the therapeutic efficacy of chemotherapy. Mater. Sci. Eng. C 2019, 97, 833–841. [Google Scholar] [CrossRef]
- Lakkadwala, S.; Singh, J. Co-delivery of doxorubicin and erlotinib through liposomal nanoparticles for glioblastoma tumor regression using an in vitro brain tumor model. Colloids Surf. B Biointerfaces 2019, 173, 27–35. [Google Scholar] [CrossRef]
- Liu, J.; Chi, D.; Pan, S.; Zhao, L.; Wang, X.; Wang, D.; Wang, Y. Effective co-encapsulation of doxorubicin and irinotecan for synergistic therapy using liposomes prepared with triethylammonium sucrose octasulfate as drug trapping agent. Int. J. Pharm. 2019, 557, 264–272. [Google Scholar] [CrossRef]
- Seo, W.; Kapralov, A.A.; Shurin, G.V.; Shurin, M.R.; Kagan, V.E.; Star, A. Payload drug vs. nanocarrier biodegradation by myeloperoxidase- and peroxynitrite-mediated oxidations: Pharmacokinetic implications. Nanoscale 2015, 7, 8689–8694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, L.; Jin, B.; Zhang, S.; Song, C.; Li, Q. Stimuli-free programmable drug release for combination chemo-therapy. Nanoscale 2016, 8, 12553–12559. [Google Scholar] [CrossRef] [PubMed]
- Christowitz, C.; Davis, T.; Isaacs, A.; van Niekerk, G.; Hattingh, S.; Engelbrecht, A.-M. Mechanisms of doxorubicin-induced drug resistance and drug resistant tumour growth in a murine breast tumour model. BMC Cancer 2019, 19, 757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tap, W.D.; Jones, R.L.; Van Tine, B.A.; Chmielowski, B.; Elias, A.D.; Adkins, D.; Agulnik, M.; Cooney, M.M.; Livingston, M.B.; Pennock, G.; et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: An open-label phase 1b and randomised phase 2 trial. Lancet 2016, 388, 488–497. [Google Scholar] [CrossRef] [Green Version]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Sun, R.; Luo, Q.; Li, X.; Huang, X.; Teng, L.; Shen, Z.; Zhu, W. Supramolecular PEGylation of camptothecin for cancer therapy. Mater. Today Nano 2021, 14, 100115. [Google Scholar]
- Ahmadijokani, F.; Tajahmadi, S.; Rezakazemi, M.; Sehat, A.A.; Molavi, H.; Aminabhavi, T.M.; Arjmand, M. Aluminum-based metal-organic frameworks for adsorptive removal of anti-cancer (methotrexate) drug from aqueous solutions. J. Environ. Manag. 2021, 277, 111448. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A review of curcumin and its derivatives as anticancer agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Sun, N.; Cheng, R.; Zhao, C.; Liu, Z.; Li, X.; Liu, J.; Tian, Z. pH multistage responsive micellar system with charge-switch and PEG layer detachment for co-delivery of paclitaxel and curcumin to synergistically eliminate breast cancer stem cells. Biomaterials 2017, 147, 53–67. [Google Scholar] [CrossRef]
- Asghar, K.; Qasim, M.; Dharmapuri, G.; Das, D. Investigation on a smart nanocarrier with a mesoporous magnetic core and thermo-responsive shell for co-delivery of doxorubicin and curcumin: A new approach towards combination therapy of cancer. RSC Adv. 2017, 7, 28802–28818. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, M.; Shafiei-Irannejad, V.; Safa, K.D.; Salehi, R. Multi-branched ionic liquid-chitosan as a smart and biocompatible nano-vehicle for combination chemotherapy with stealth and targeted properties. Carbohydr. Polym. 2018, 196, 299–312. [Google Scholar] [CrossRef]
- Salehi, R.; Rasouli, S.; Hamishehkar, H. Smart thermo/pH responsive magnetic nanogels for the simultaneous delivery of doxorubicin and methotrexate. Int. J. Pharm. 2015, 487, 274–284. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.; Chang, X.; He, F.; Zhuo, R. Efficient co-delivery of doxorubicin and methotrexate by pH-sensitive dual-functional nanomicelles for enhanced synergistic antitumor efficacy. ACS Appl. Bio Mater. 2019, 2, 2271–2279. [Google Scholar] [CrossRef]
- Birault, A.; Giret, S.; Théron, C.; Gallud, A.; Da Silva, A.; Durand, D.; Nguyen, C.; Bettache, N.; Gary-Bobo, M.; Bartlett, J.R. Sequential delivery of synergistic drugs by silica nanocarriers for enhanced tumour treatment. J. Mater. Chem. B 2020, 8, 1472–1480. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, D.; Liu, Y.; Guo, X.; Chen, S.; Zeng, L.; Ma, J.; Zhang, X.; Tian, Z.; Yang, Z. Immunogenic-cell-killing and immunosuppression-inhibiting nanomedicine. Bioact. Mater. 2021, 6, 1513–1527. [Google Scholar] [CrossRef]
- Liu, R.; Xiao, W.; Hu, C.; Xie, R.; Gao, H. Theranostic size-reducible and no donor conjugated gold nanocluster fabricated hyaluronic acid nanoparticle with optimal size for combinational treatment of breast cancer and lung metastasis. J. Control. Release 2018, 278, 127–139. [Google Scholar] [CrossRef]
- Feng, B.; Xu, Z.; Zhou, F.; Yu, H.; Sun, Q.; Wang, D.; Tang, Z.; Yu, H.; Yin, Q.; Zhang, Z.; et al. Near infrared light-actuated gold nanorods with cisplatin–polypeptide wrapping for targeted therapy of triple negative breast cancer. Nanoscale 2015, 7, 14854–14864. [Google Scholar] [CrossRef]
- Zhu, F.; Tan, G.; Zhong, Y.; Jiang, Y.; Cai, L.; Yu, Z.; Liu, S.; Ren, F. Smart nanoplatform for sequential drug release and enhanced chemo-thermal effect of dual drug loaded gold nanorod vesicles for cancer therapy. J. Nanobiotechnol. 2019, 17, 44. [Google Scholar] [CrossRef]
- Xu, W.; Wang, J.; Qian, J.; Hou, G.; Wang, Y.; Ji, L.; Suo, A. NIR/pH dual-responsive polysaccharide-encapsulated gold nanorods for enhanced chemo-photothermal therapy of breast cancer. Mater. Sci. Eng. C 2019, 103, 109854. [Google Scholar] [CrossRef]
- Shen, S.; Wang, S.; Zheng, R.; Zhu, X.; Jiang, X.; Fu, D.; Yang, W. Magnetic nanoparticle clusters for photothermal therapy with near-infrared irradiation. Biomaterials 2015, 39, 67–74. [Google Scholar] [CrossRef]
- Qin, Z.; Wang, Y.; Randrianalisoa, J.; Raeesi, V.; Chan, W.C.W.; Lipiński, W.; Bischof, J.C. Quantitative Comparison of Photothermal Heat Generation between Gold Nanospheres and Nanorods. Sci. Rep. 2016, 6, 29836. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Dai, D.; Lou, X.; Ma, L.; Wang, B.; Yang, Y.-W. Supramolecular nanomaterials based on hollow mesoporous drug carriers and macrocycle-capped CuS nanogates for synergistic chemo-photothermal therapy. Theranostics 2020, 10, 615–629. [Google Scholar] [CrossRef]
- Bao, B.; Su, P.; Song, K.; Cui, Y.; Zhai, X.; Xu, Y.; Liu, J.; Wang, L. A Smart “Sense-and-Treat” Nanoplatform Based on Semiconducting Polymer Nanoparticles for Precise Photothermal-Photodynamic Combined Therapy. Biomacromolecules 2021. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, M.; Wang, Z.; Yang, Y.; Liu, J.; Hu, Q.; Li, L.; Huang, W. Immune remodeling triggered by photothermal therapy with semiconducting polymer nanoparticles in combination with chemotherapy to inhibit metastatic cancers. J. Mater. Chem. B 2021, 9, 2613–2622. [Google Scholar] [CrossRef]
- Lyu, Y.; Zeng, J.; Jiang, Y.; Zhen, X.; Wang, T.; Qiu, S.; Lou, X.; Gao, M.; Pu, K. Enhancing Both Biodegradability and Efficacy of Semiconducting Polymer Nanoparticles for Photoacoustic Imaging and Photothermal Therapy. ACS Nano 2018, 12, 1801–1810. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Pu, K. Multimodal Biophotonics of Semiconducting Polymer Nanoparticles. Acc. Chem. Res. 2018, 51, 1840–1849. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Upputuri, P.K.; Xie, C.; Zeng, Z.; Sharma, A.; Zhen, X.; Li, J.; Huang, J.; Pramanik, M.; Pu, K. Metabolizable Semiconducting Polymer Nanoparticles for Second Near-Infrared Photoacoustic Imaging. Adv. Mater. 2019, 31, 1808166. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zhang, F.; Tang, L.; Ma, J.; Ling, D.; Chen, X.; Sun, X. Redox-responsive dual chemophotothermal therapeutic nanomedicine for imaging-guided combinational therapy. J. Mater. Chem. B 2018, 6, 5362–5367. [Google Scholar] [CrossRef]
- Song, C.W. Effect of Local Hyperthermia on Blood Flow and Microenvironment: A Review. Cancer Res. 1984, 44, 4721s. [Google Scholar]
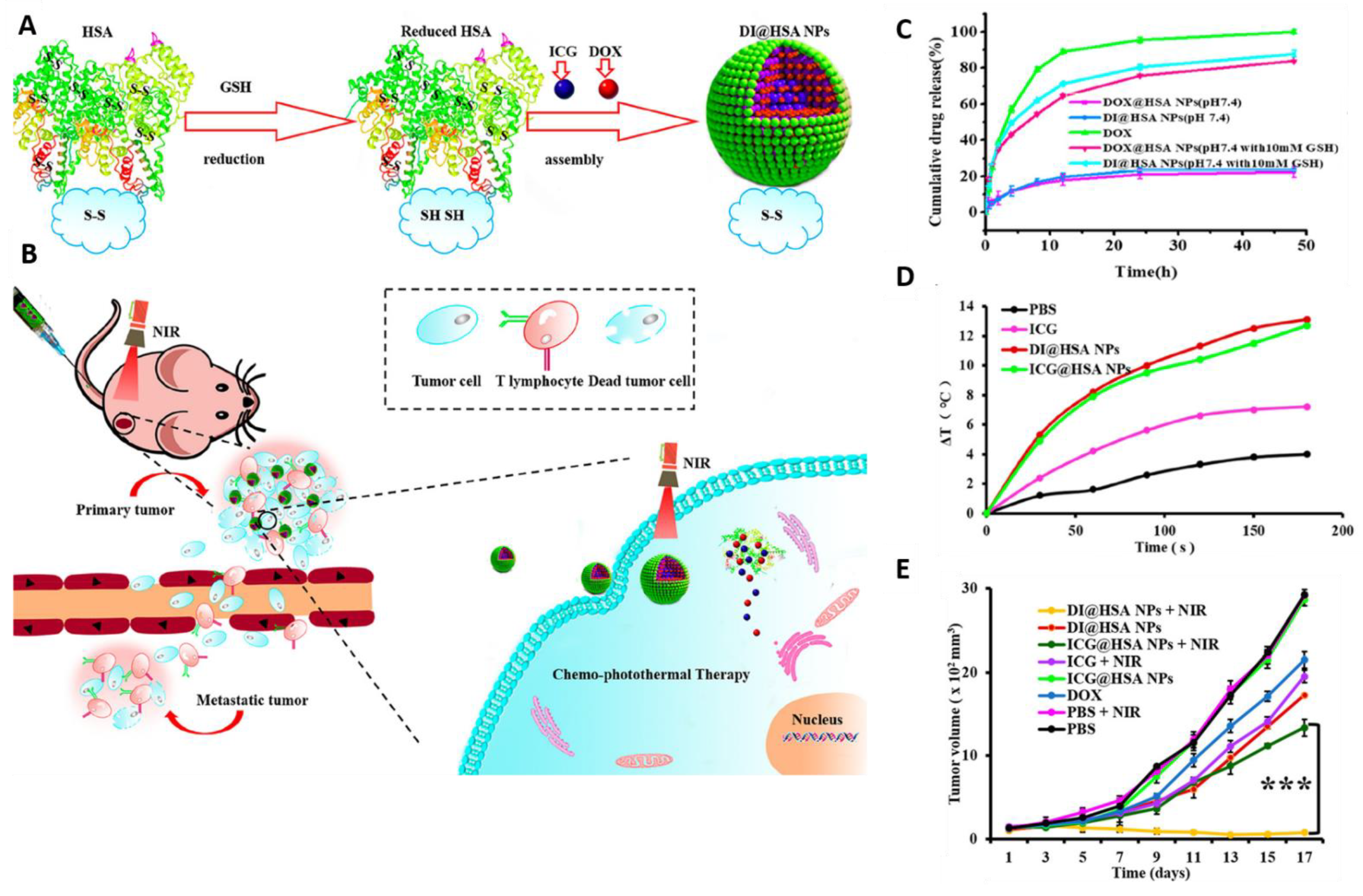
- Ma, H.; Yang, X.; Ke, J.; Wang, C.; Peng, L.; Hu, F.; Yuan, H. Smart Assembled Human Serum Albumin Nanocarrier Enhanced Breast Cancer Treatment and Antitumor Immunity by Chemo-photothermal Therapy. ACS Biomater. Sci. Eng. 2020, 6, 3217–3229. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Yang, D.; Sun, C.; Sun, Q.; He, F.; Gai, S.; Zhong, C.; Li, C.; Yang, P. Fe3O4@MIL-100(Fe)-UCNPs heterojunction photosensitizer: Rational design and application in near infrared light mediated hypoxic tumor therapy. Chem. Eng. J. 2018, 354, 1141–1152. [Google Scholar] [CrossRef]
- Xing, L.; Gong, J.-H.; Wang, Y.; Zhu, Y.; Huang, Z.-J.; Zhao, J.; Li, F.; Wang, J.-H.; Wen, H.; Jiang, H.-L. Hypoxia alleviation-triggered enhanced photodynamic therapy in combination with IDO inhibitor for preferable cancer therapy. Biomaterials 2019, 206, 170–182. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Liu, X.; Wu, X.; Ye, N.; Yang, X.; Li, Z. Boosting Nanomedicine Efficacy with Hyperbaric Oxygen Therapy. In Bio-Nanomedicine for Cancer Therapy; Fontana, F., Santos, H.A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 77–95. [Google Scholar]
- Chang, K.; Liu, Z.; Fang, X.; Chen, H.; Men, X.; Yuan, Y.; Sun, K.; Zhang, X.; Yuan, Z.; Wu, C. Enhanced Phototherapy by Nanoparticle-Enzyme via Generation and Photolysis of Hydrogen Peroxide. Nano Lett. 2017, 17, 4323–4329. [Google Scholar] [CrossRef]
- Chen, H.; Tian, J.; He, W.; Guo, Z. H2O2-Activatable and O2-Evolving Nanoparticles for Highly Efficient and Selective Photodynamic Therapy against Hypoxic Tumor Cells. J. Am. Chem. Soc. 2015, 137, 1539–1547. [Google Scholar] [CrossRef]
- Sheng, D.; Liu, T.; Deng, L.; Zhang, L.; Li, X.; Xu, J.; Hao, L.; Li, P.; Ran, H.; Chen, H.; et al. Perfluorooctyl bromide & indocyanine green co-loaded nanoliposomes for enhanced multimodal imaging-guided phototherapy. Biomaterials 2018, 165, 1–13. [Google Scholar] [CrossRef]
- Qin, X.; Wang, Z.; Guo, C.; Jin, Y. Multi-responsive drug delivery nanoplatform for tumor-targeted synergistic photothermal/dynamic therapy and chemotherapy. New J. Chem. 2020, 44, 3593–3603. [Google Scholar] [CrossRef]
- Son, S.; Kim, J.H.; Wang, X.; Zhang, C.; Yoon, S.A.; Shin, J.; Sharma, A.; Lee, M.H.; Cheng, L.; Wu, J.; et al. Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem. Soc. Rev. 2020, 49, 3244–3261. [Google Scholar] [CrossRef]
- Irajirad, R.; Ahmadi, A.; Najafabad, B.K.; Abed, Z.; Sheervalilou, R.; Khoei, S.; Shiran, M.B.; Ghaznavi, H.; Shakeri-Zadeh, A. Combined thermo-chemotherapy of cancer using 1 MHz ultrasound waves and a cisplatin-loaded sonosensitizing nanoplatform: An in vivo study. Cancer Chemother. Pharmacol. 2019, 84, 1315–1321. [Google Scholar] [CrossRef]
- Teo, P.Y.; Cheng, W.; Hedrick, J.L.; Yang, Y.Y. Co-delivery of drugs and plasmid DNA for cancer therapy. Adv. Drug Deliv. Rev. 2016, 98, 41–63. [Google Scholar] [CrossRef]
- Xia, Y.; Xu, T.; Wang, C.; Li, Y.; Lin, Z.; Zhao, M.; Zhu, B. Novel functionalized nanoparticles for tumor-targeting co-delivery of doxorubicin and siRNA to enhance cancer therapy. Int. J. Nanomed. 2018, 13, 143. [Google Scholar] [CrossRef] [Green Version]
- Sigg, S.J.; Postupalenko, V.; Duskey, J.T.; Palivan, C.G.; Meier, W. Stimuli-responsive codelivery of oligonucleotides and drugs by self-assembled peptide nanoparticles. Biomacromolecules 2016, 17, 935–945. [Google Scholar] [CrossRef]
- Taghdisi, S.M.; Danesh, N.M.; Nameghi, M.A.; Bahreyni, A.; Ramezani, M.; Alibolandi, M.; Emrani, A.S.; Abnous, K. Co-delivery of doxorubicin and α-PCNA aptamer using AS1411-modified pH-responsive nanoparticles for cancer synergistic therapy. J. Drug Deliv. Sci. Technol. 2020, 58, 101816. [Google Scholar] [CrossRef]
- Zhang, B.-C.; Luo, B.-Y.; Zou, J.-J.; Wu, P.-Y.; Jiang, J.-L.; Le, J.-Q.; Zhao, R.-R.; Chen, L.; Shao, J.-W. Co-delivery of Sorafenib and CRISPR/Cas9 Based on Targeted Core–Shell Hollow Mesoporous Organosilica Nanoparticles for Synergistic HCC Therapy. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef]
- Genchi, G.G.; Marino, A.; Grillone, A.; Pezzini, I.; Ciofani, G. Remote control of cellular functions: The role of smart nanomaterials in the medicine of the future. Adv. Healthc. Mater. 2017, 6, 1700002. [Google Scholar] [CrossRef]
- Yao, Y.; Su, Z.; Liang, Y.; Zhang, N. pH-Sensitive carboxymethyl chitosan-modified cationic liposomes for sorafenib and siRNA co-delivery. Int. J. Nanomed. 2015, 10, 6185. [Google Scholar]
- Swami, R.; Kumar, Y.; Chaudhari, D.; Katiyar, S.S.; Kuche, K.; Katare, P.B.; Banerjee, S.K.; Jain, S. pH sensitive liposomes assisted specific and improved breast cancer therapy using co-delivery of SIRT1 shRNA and Docetaxel. Mater. Sci. Eng. C 2021, 120, 111664. [Google Scholar] [CrossRef] [PubMed]
- Younis, M.A.; Khalil, I.A.; Abd Elwakil, M.M.; Harashima, H. A multifunctional lipid-based nanodevice for the highly specific codelivery of sorafenib and midkine siRNA to hepatic cancer cells. Mol. Pharm. 2019, 16, 4031–4044. [Google Scholar] [CrossRef] [PubMed]
- Alex, M.A.; Nehate, C.; Veeranarayanan, S.; Kumar, D.S.; Kulshreshtha, R.; Koul, V. Self assembled dual responsive micelles stabilized with protein for co-delivery of drug and siRNA in cancer therapy. Biomaterials 2017, 133, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Shao, K.; Liu, Y.; Kuang, Y.; Li, J.; An, S.; Guo, Y.; Ma, H.; Jiang, C. Tumor-targeting and microenvironment-responsive smart nanoparticles for combination therapy of antiangiogenesis and apoptosis. ACS Nano 2013, 7, 2860–2871. [Google Scholar] [CrossRef]
- Castillo, R.R.; Lozano, D.; González, B.; Manzano, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery: An update. Expert Opin. Drug Deliv. 2019, 16, 415–439. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.J.; Luan, X.; Feng, H.Y.; Dong, X.; Yang, S.C.; Chen, Z.J.; Cai, Q.Y.; Lu, Q.; Zhang, Y.; Sun, P. Integrated combination treatment using a “smart” chemotherapy and microRNA delivery system improves outcomes in an orthotopic colorectal cancer model. Adv. Funct. Mater. 2018, 28, 1801118. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Tang, C.; Yin, C. Dual-targeting and pH/redox-responsive multi-layered nanocomplexes for smart co-delivery of doxorubicin and siRNA. Biomaterials 2015, 60, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-T.; Liu, Z.-K.; Zhu, Q.-L.; Rong, X.-H.; Liang, C.-L.; Wang, J.; Ma, D.; Sun, J.; Wang, G.-H. Redox-responsive nanocarriers for drug and gene co-delivery based on chitosan derivatives modified mesoporous silica nanoparticles. Colloids Surf. B Biointerfaces 2017, 155, 41–50. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Tang, C.; Yin, C. Co-delivery of doxorubicin and survivin shRNA-expressing plasmid via microenvironment-responsive dendritic mesoporous silica nanoparticles for synergistic cancer therapy. Pharm. Res. 2017, 34, 2829–2841. [Google Scholar] [CrossRef]
- Zhang, B.-C.; Wu, P.-Y.; Zou, J.-J.; Jiang, J.-L.; Zhao, R.-R.; Luo, B.-Y.; Liao, Y.-Q.; Shao, J.-W. Efficient CRISPR/Cas9 gene-chemo synergistic cancer therapy via a stimuli-responsive chitosan-based nanocomplex elicits anti-tumorigenic pathway effect. Chem. Eng. J. 2020, 393, 124688. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: Mechanism, combinations, and clinical outcome. Front. Pharmacol. 2017, 8, 561. [Google Scholar] [CrossRef]
- Emens, L.A.; Ascierto, P.A.; Darcy, P.K.; Demaria, S.; Eggermont, A.M.; Redmond, W.L.; Seliger, B.; Marincola, F.M. Cancer immunotherapy: Opportunities and challenges in the rapidly evolving clinical landscape. Eur. J. Cancer 2017, 81, 116–129. [Google Scholar] [CrossRef]
- Emens, L.A.; Middleton, G. The interplay of immunotherapy and chemotherapy: Harnessing potential synergies. Cancer Immunol. Res. 2015, 3, 436–443. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Huang, Z.; Zheng, L.; Fan, Y. The efficacy and safety of anti-PD-1/PD-L 1 antibodies combined with chemotherapy or CTLA 4 antibody as a first-line treatment for advanced lung cancer. Int. J. Cancer 2018, 142, 2344–2354. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zheng, P. Preserving the CTLA-4 checkpoint for safer and more effective cancer immunotherapy. Trends Pharmacol. Sci. 2020, 41, 4–12. [Google Scholar] [CrossRef]
- Ruan, H.; Bu, L.; Hu, Q.; Cheng, H.; Lu, W.; Gu, Z. Strategies of combination drug delivery for immune checkpoint blockades. Adv. Healthc. Mater. 2019, 8, 1801099. [Google Scholar] [CrossRef]
- Weinmann, H. Cancer immunotherapy: Selected targets and small-molecule modulators. ChemMedChem 2016, 11, 450–466. [Google Scholar] [CrossRef]
- Chen, Y.; Ramjiawan, R.R.; Reiberger, T.; Ng, M.R.; Hato, T.; Huang, Y.; Ochiai, H.; Kitahara, S.; Unan, E.C.; Reddy, T.P. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology 2015, 61, 1591–1602. [Google Scholar] [CrossRef]
- Feng, B.; Zhou, F.; Hou, B.; Wang, D.; Wang, T.; Fu, Y.; Ma, Y.; Yu, H.; Li, Y. Binary cooperative prodrug nanoparticles improve immunotherapy by synergistically modulating immune tumor microenvironment. Adv. Mat. 2018, 30, 1803001. [Google Scholar] [CrossRef]
- Ruan, H.; Hu, Q.; Wen, D.; Chen, Q.; Chen, G.; Lu, Y.; Wang, J.; Cheng, H.; Lu, W.; Gu, Z. A dual-bioresponsive drug-delivery depot for combination of epigenetic modulation and immune checkpoint blockade. Adv. Mater. 2019, 31, 1806957. [Google Scholar] [CrossRef]
- Qi, J.; Jin, F.; Xu, X.; Du, Y. Combination Cancer Immunotherapy of Nanoparticle-Based Immunogenic Cell Death Inducers and Immune Checkpoint Inhibitors. Int. J. Nanomed. 2021, 16, 1435. [Google Scholar] [CrossRef]
- Feng, B.; Hou, B.; Xu, Z.; Saeed, M.; Yu, H.; Li, Y. Self-amplified drug delivery with light-inducible nanocargoes to enhance cancer immunotherapy. Adv. Mater. 2019, 31, 1902960. [Google Scholar] [CrossRef]
- Yazdian-Robati, R.; Arab, A.; Ramezani, M.; Rafatpanah, H.; Bahreyni, A.; Nabavinia, M.S.; Abnous, K.; Taghdisi, S.M. Smart aptamer-modified calcium carbonate nanoparticles for controlled release and targeted delivery of epirubicin and melittin into cancer cells in vitro and in vivo. Drug Dev. Ind. Pharm. 2019, 45, 603–610. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Cong, C.; Li, L.; Luo, L.; He, Y.; Hao, Z.; Gao, D. Sequential intra-intercellular delivery of nanomedicine for deep drug-resistant solid tumor penetration. ACS Appl. Mater. Interfaces 2020, 12, 8978–8988. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Chen, Y.; Ma, J.; Lin, J.; Zhang, Y.; Fan, Z.; Su, G.; Xie, L.; Zhu, X. Integration of phospholipid-hyaluronic acid-methotrexate nanocarrier assembly and amphiphilic drug–drug conjugate for synergistic targeted delivery and combinational tumor therapy. Biomater. Sci. 2018, 6, 1818–1833. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.R.; Jayakrishnan, A.; Kumar, T.S. Combinational delivery of anticancer drugs for osteosarcoma treatment using electrosprayed core shell nanocarriers. J. Mater. Sci. Mater. Med. 2020, 31, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Di Zhang, Z.Y.; Liu, H.; Wang, X.; Hua, J.; Ling, Y.; Wei, L.; Xia, Y.; Sun, S.; Xiao, L. Cell membrane coated smart two-dimensional supraparticle for in vivo homotypic cancer targeting and enhanced combinational theranostics. Nanotheranostics 2021, 5, 275. [Google Scholar] [CrossRef]
- Ren, L.; Liu, X.; Wang, Q.; Zhang, L.; Deng, G.; Zhou, F.; Lu, J. Facile fabrication of a magnetically smart PTX-loaded Cys–Fe3O4/CuS@ BSA nano-drug for imaging-guided chemo-photothermal therapy. Dalton Trans. 2017, 46, 2204–2213. [Google Scholar] [CrossRef]
- Sun, S.; Sun, S.; Sun, Y.; Wang, P.; Zhang, J.; Du, W.; Wang, S.; Liang, X. Bubble-manipulated local drug release from a smart thermosensitive cerasome for dual-mode imaging guided tumor chemo-photothermal therapy. Theranostics 2019, 9, 8138. [Google Scholar] [CrossRef]
- Pan, Q.; Tian, J.; Zhu, H.; Hong, L.; Mao, Z.; Oliveira, J.M.; Reis, R.L.; Li, X. Tumor-targeting polycaprolactone nanoparticles with codelivery of paclitaxel and IR780 for combinational therapy of drug-resistant ovarian cancer. ACS Biomater. Sci. Eng. 2020, 6, 2175–2185. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, Y.-X.; Feng, X.-N.; Cheng, M.; Tang, A.-N.; Kong, D.-M. pH-controlled intracellular in situ reversible assembly of a photothermal agent for smart chemo-photothermal synergetic therapy and ATP imaging. ACS Appl. Mater. Interfaces 2019, 11, 39624–39632. [Google Scholar] [CrossRef]
- Xiang, H.; Chen, H.; Tham, H.P.; Phua, S.Z.F.; Liu, J.-G.; Zhao, Y. Cyclometalated iridium (III)-complex-based micelles for glutathione-responsive targeted chemotherapy and photodynamic therapy. ACS Appl. Mater. Interfaces 2017, 9, 27553–27562. [Google Scholar] [CrossRef]
- Zhuang, H.; Zhao, M.; Ding, S.; Liu, L.; Yuan, W.; Jiang, L.; Han, X.; Jiang, L.; Yi, T. Multifunctional Smart Yolk–Shell Nanostructure with Mesoporous MnO2 Shell for Enhanced Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 38906–38917. [Google Scholar] [CrossRef]
- Shrestha, B.; Wang, L.; Zhang, H.; Hung, C.Y.; Tang, L. Gold Nanoparticles Mediated Drug-Gene Combinational Therapy for Breast Cancer Treatment. Int. J. Nanomed. 2020, 15, 8109. [Google Scholar] [CrossRef]
- Liu, M.; Peng, Y.; Nie, Y.; Liu, P.; Hu, S.; Ding, J.; Zhou, W. Co-delivery of doxorubicin and DNAzyme using ZnO@ polydopamine core-shell nanocomposites for chemo/gene/photothermal therapy. Acta Biomater. 2020, 110, 242–253. [Google Scholar] [CrossRef]
- Li, C.; Yang, X.-Q.; Zhang, M.-Z.; Song, Y.-Y.; Cheng, K.; An, J.; Zhang, X.-S.; Xuan, Y.; Liu, B.; Zhao, Y.-D. In vivo imaging-guided nanoplatform for tumor targeting delivery and combined chemo-, gene-and photothermal therapy. Theranostics 2018, 8, 5662. [Google Scholar] [CrossRef]
- Yue, R.; Chen, M.; Ma, N. Dual MicroRNA-Triggered Drug Release System for Combined Chemotherapy and Gene Therapy with Logic Operation. ACS Appl. Mater. Interfaces 2020, 12, 32493–32502. [Google Scholar] [CrossRef]
- Yang, J.; Ma, S.; Xu, R.; Wei, Y.; Zhang, J.; Zuo, T.; Wang, Z.; Deng, H.; Yang, N.; Shen, Q. Smart biomimetic metal organic frameworks based on ROS-ferroptosis-glycolysis regulation for enhanced tumor chemo-immunotherapy. J. Control. Release 2021, 334, 21–33. [Google Scholar] [CrossRef]
- Duong, H.T.T.; Thambi, T.; Yin, Y.; Lee, J.E.; Seo, Y.K.; Jeong, J.H.; Lee, D.S. Smart pH-Responsive Nanocube-Controlled Delivery of DNA Vaccine and Chemotherapeutic Drugs for Chemoimmunotherapy. ACS Appl. Mater. Interfaces 2019, 11, 13058–13068. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Lin, J.; Wu, H.; Yao, Y.; Zhang, J.; Yang, C. T cell membrane cloaking tumor microenvironment-responsive nanoparticles with a smart “membrane escape mechanism” for enhanced immune-chemotherapy of melanoma. Biomater. Sci. 2021, 9, 3453–3464. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Su, L.; Dai, J.; Li, Z.-W.; Bai, S.; Li, Q.; Chen, X.; Song, J.; Yang, H. Biologically responsive plasmonic assemblies for second near-infrared window photoacoustic imaging-guided concurrent chemo-immunotherapy. ACS Nano 2020, 14, 3991–4006. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, Z.; Dong, Z.; Gong, Y.; Hao, Y.; Tian, L.; Yang, X.; Liu, Z.; Feng, L. CaCO3-Assisted Preparation of pH-Responsive Immune-Modulating Nanoparticles for Augmented Chemo-Immunotherapy. Nano-Micro Lett. 2021, 13, 1–18. [Google Scholar] [CrossRef]
- Ashley, E.A. Towards precision medicine. Nat. Rev. Genet. 2016, 17, 507. [Google Scholar] [CrossRef] [PubMed]
- Schork, N.J. Personalized medicine: Time for one-person trials. Nature 2015, 520, 609. [Google Scholar] [CrossRef]
- Jameson, J.L.; Longo, D.L. Precision medicine—Personalized, problematic, and promising. Obstet. Gynecol. Surv. 2015, 70, 612–614. [Google Scholar] [CrossRef]
- Novelli, G. Personalized genomic medicine. Intern. Emerg. Med. 2010, 5, 81–90. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Rösslein, M. Personalized medicine: The enabling role of nanotechnology. Nanomedicine 2016, 11, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Coccia, M. Converging genetics, genomics and nanotechnologies for groundbreaking pathways in biomedicine and nanomedicine. Int. J. Health Technol. Manag. 2012, 13, 184–197. [Google Scholar] [CrossRef]
- Gao, D.; Guo, X.; Zhang, X.; Chen, S.; Wang, Y.; Chen, T.; Huang, G.; Gao, Y.; Tian, Z.; Yang, Z. Multifunctional phototheranostic nanomedicine for cancer imaging and treatment. Mater. Today Bio 2020, 5, 100035. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Snyder, M.P. Integrative omics for health and disease. Nat. Rev. Genet. 2018, 19, 299. [Google Scholar] [CrossRef]
- Jain, K.K. Innovative diagnostic technologies and their significance for personalized medicine. Mol. Diagn. Ther. 2010, 14, 141–147. [Google Scholar] [CrossRef]


| Types of Combinational Therapy | Nanoparticles Used | Therapeutic Agent | Stimulus | Active Targeting Moieties | Study | Cancer Types/ Cell Lines Used | References |
|---|---|---|---|---|---|---|---|
| Chemo Combinational therapy | Phospholipid-hyaluronic acid based nanoparticles | MTX and HCPT | pH and esterase | Folate and CD44 | In vivo | Breast cancer/ MCF-7 cells | [198] |
| Core: Mesoporous magnetic nanoparticles Shell: Thermo-responsive polymer | DOX and Curcumin | Temperature | None | In vitro | Cervical cancer/ Hela cells | [137] | |
| Core: Chitosan coated HA and DOX Shell: Poly-ε-caprolactone with MTX | DOX and PTX | pH | None | In vitro | Ostrosarcoma/ OMG-63 | [199] | |
| Chemo-Energy Combinational Therapy | Core: MnO2 coated Gold nanorods as a core Shell: Cancer cell membrane | DOX, gold nanorods | GSH, H2O2, Light | Cancer cell membrane | In vivo | Breast cancer/ 4TI | [200] |
| Cystein functionalized iron oxide core and CuS attached BSA shell nanoparticles | PTX and CuS | Light | Magnet | In vivo | Cervical cancer/ HeLa cells | [201] | |
| Cerasome-forming lipid nanoparticles | DOX and DiR | Temperature, Light | None | In vivo | Breast cancer/ 4TI | [202] | |
| poly-ε-caprolactone nanoparticles | PTX and IR780 | Light | LHRH peptide | In vivo | Ovarian cancer/ST30 cells | [203] | |
| ATP-aptamer, rC-DNA, and rG-DNA modified gold nanoparticles | DOX and gold nanoparticles | pH and ATP | None | In vivo | Cervical cancer/ HeLa cells | [204] | |
| Cyclometalated Ir (III) complex micelles | CPT and Ir (III) compound | GSH | Folic acid | In vitro | Cervical cancer/ HeLa cells | [205] | |
| Core: Upconversion/downconversion nanoparticles Shell: Mesoporous MnO2 | DOX and MB | H2O2 and GSH | None | In vivo | Cervical cancer/ HeLa cells | [206] | |
| Chondroitin sulfate-chlorin e6- lipoic acid nanocarrier | DTX and Chlorin e6 | GSH and ultrasound | Chondrotin sulfate | In vivo | Melanoma/ B16F10 | [62] | |
| Chemo-gene Combinational Therapy | PEI coated gold nanospheres | DOX and PLK1 siRNA | pH | None | In vitro | Breast cancer/ SKBR-3 | [207] |
| Core: Zinc oxide Shell: Polydopamine | DOX, DNAzyme, and polydopamine | pH, GSH, and Light | None | In vivo | Lung cancer/ A549 | [208] | |
| PEI weaved mesoporous silica nanoparticles | DOX and miRNA-145 | GSH | WL8 peptide | In vivo | Colorectal cancer/ SW480 | [179] | |
| Ag2S QD coated mesoporous silica nanoparticles | DOX and survivin antisense oligonucleotide | Biotin | Folic acid and desthiobiotin | In vivo | Cervical cancer/ HeLa cells | [209] | |
| DNA functionalized gold nanoparticles | DOX and BCl-2 siRNA | miRNA-21 and miRNA-10b | miRNA-21 and miRNA-10b | In vitro | Breast cancer/ MDA-MB-231 | [210] | |
| Chitosan based nanoparticles | PTX and single guidedVEGFR2/Cas9 plasmid | pH | Lactobionic acid | In vivo | Hepato carcinoma/ H22 | [183] | |
| Chemo-Immuno Combinational Therapy | HA coated Triphenylphosphonium nanoparticles | DOX, Ionidamine, and anti-PD-L1( seperately) | Hyaluronidase and GSH | HA | In vivo | Breast cancer/ 4TI | [49] |
| Metal organic frameworks | DOX and glucose oxidase | GSH | Cancer cell membrane | In vivo | Breast cancer/ 4TI | [211] | |
| Polymeric nanocubes | DOX and plasmid ovalbumin | pH | None | In vivo | Melanoma/ B16/OVA | [212] | |
| T-cell membrane covered HA grafted copolymer nanoparticles | Curcumin and T-cell membrane (acts as PD-L1 antibody) | pH and GSH | HA | In vivo | Melanoma/ B16-F10 | [213] | |
| PEG and poly(SN38-co-4-vinylpyridine) grafted nano gapped gold nanoparticles | SN38 and BLZ-945 | pH and GSH | None | In vivo | Breast cancer/ MCF-7 | [214] | |
| Calcium carbonate containing PLGA-PEG nanoparticles | DOX and alkylated NLG919 | pH | None | In vivo | Breast cancer/ 4TI | [215] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrestha, B.; Wang, L.; Brey, E.M.; Uribe, G.R.; Tang, L. Smart Nanoparticles for Chemo-Based Combinational Therapy. Pharmaceutics 2021, 13, 853. https://doi.org/10.3390/pharmaceutics13060853
Shrestha B, Wang L, Brey EM, Uribe GR, Tang L. Smart Nanoparticles for Chemo-Based Combinational Therapy. Pharmaceutics. 2021; 13(6):853. https://doi.org/10.3390/pharmaceutics13060853
Chicago/Turabian StyleShrestha, Binita, Lijun Wang, Eric M. Brey, Gabriela Romero Uribe, and Liang Tang. 2021. "Smart Nanoparticles for Chemo-Based Combinational Therapy" Pharmaceutics 13, no. 6: 853. https://doi.org/10.3390/pharmaceutics13060853
APA StyleShrestha, B., Wang, L., Brey, E. M., Uribe, G. R., & Tang, L. (2021). Smart Nanoparticles for Chemo-Based Combinational Therapy. Pharmaceutics, 13(6), 853. https://doi.org/10.3390/pharmaceutics13060853