Continuous Melt Granulation for Taste-Masking of Ibuprofen
Abstract
:1. Introduction: The Need for Taste-Masked Formulations
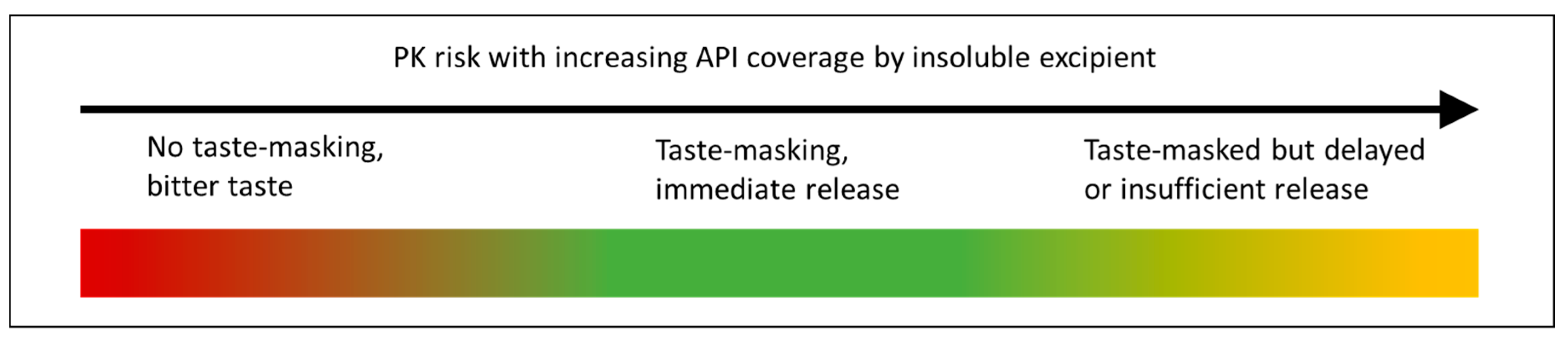
1.1. Taste-Masking Formulation Approaches
- Cover the flavor: Provided the API’s flavor is not too unpleasant and does not cause local irritation, the addition of flavors or sweeteners to the dosage form can be enough to improve the taste of the drug;
- Prevent dissolution by chemical means;
- ○
- Drug solubility is insufficient in saliva;
- ○
- Modification of the molecule to produce a prodrug that is not soluble in saliva;
- ○
- Selection of a salt that is not soluble in saliva;
- ○
- Complexation with excipients that prevent dissolution of the drug in saliva, e.g., cyclodextrins, ion-exchange resins, ionic polymers, often taking advantage of pH-dependent solubility;
- Prevent dissolution by forming a physical barrier to dissolution;
- ○
- Matrix encapsulation where the drug is embedded in a lipid or polymer, typically a small quantity of drug is on the surface so a certain amount of drug dissolution in saliva must be tolerable;
- ○
- Membrane encapsulation where the drug is completely isolated by a layer of lipid or polymer to prevent dissolution in saliva;
- Combinations of these techniques: for example, a membrane around a matrix particle with a flavor and sweetener system in an orally disintegrating tablet (ODT).
1.2. Taste-Masking Assessment
1.3. Continuous Twin-Screw Melt Granulation for Taste-Masking
2. Materials and Methods
2.1. Materials
2.2. Formulation Screening
2.3. Pilot-Scale Melt Granulation
2.4. Granule Characterization
2.5. Physical Form of Ibuprofen in Melt Granules
2.6. Small-Volume Dissolution Method
3. Results
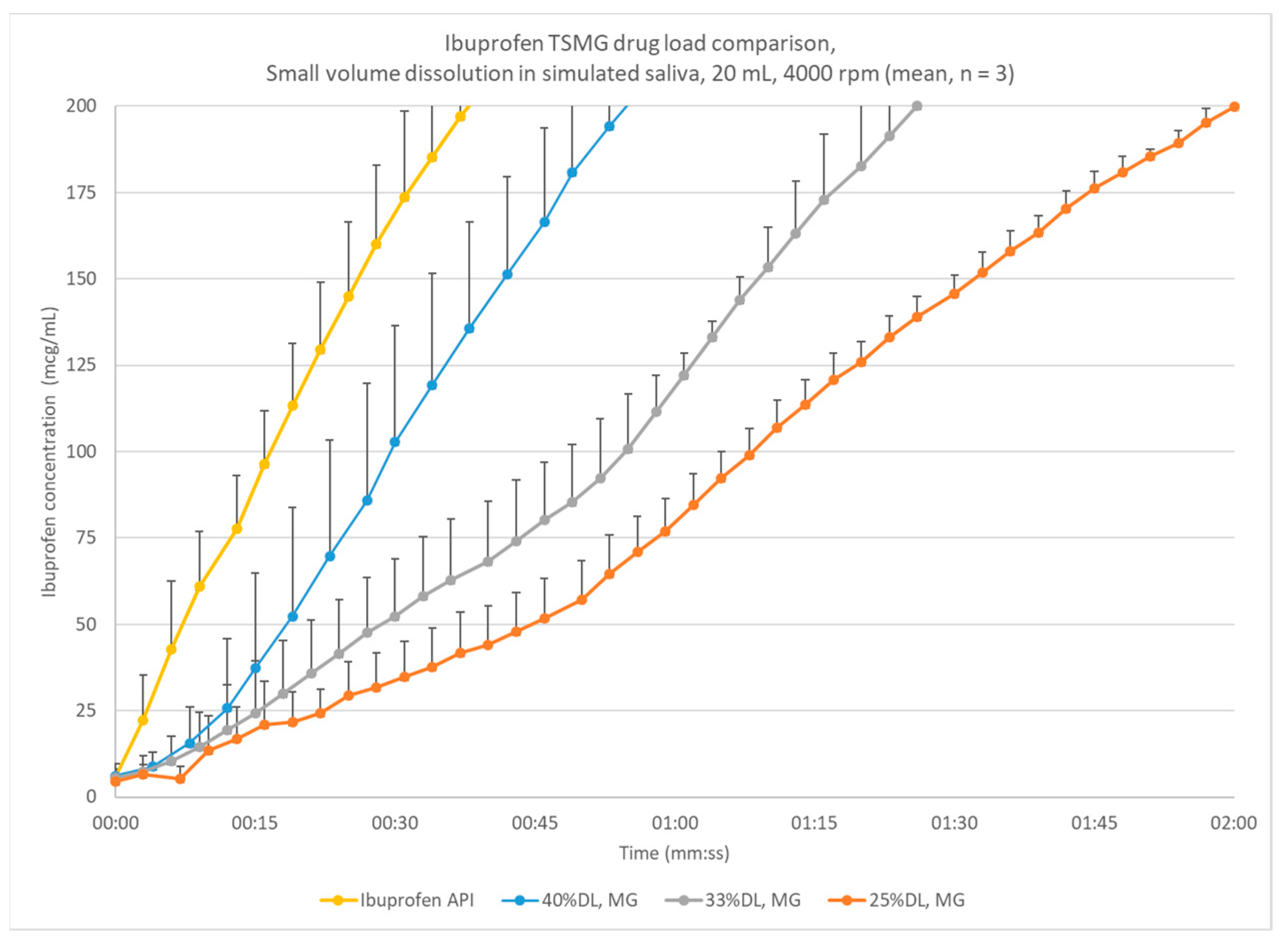
3.1. Protoype Process to Screen Drug Loading
3.2. Pilot-Scale Process with 33% DL and 40% DL Ibuprofen
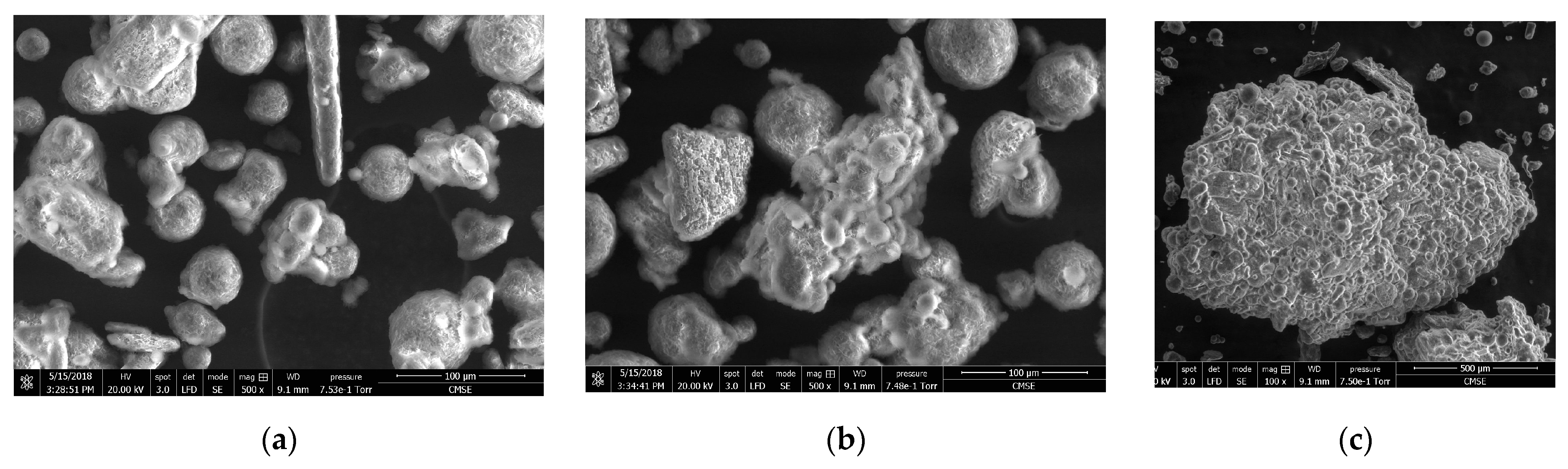
3.3. Granule Particle Size and Morphology
3.4. Physical Characterization
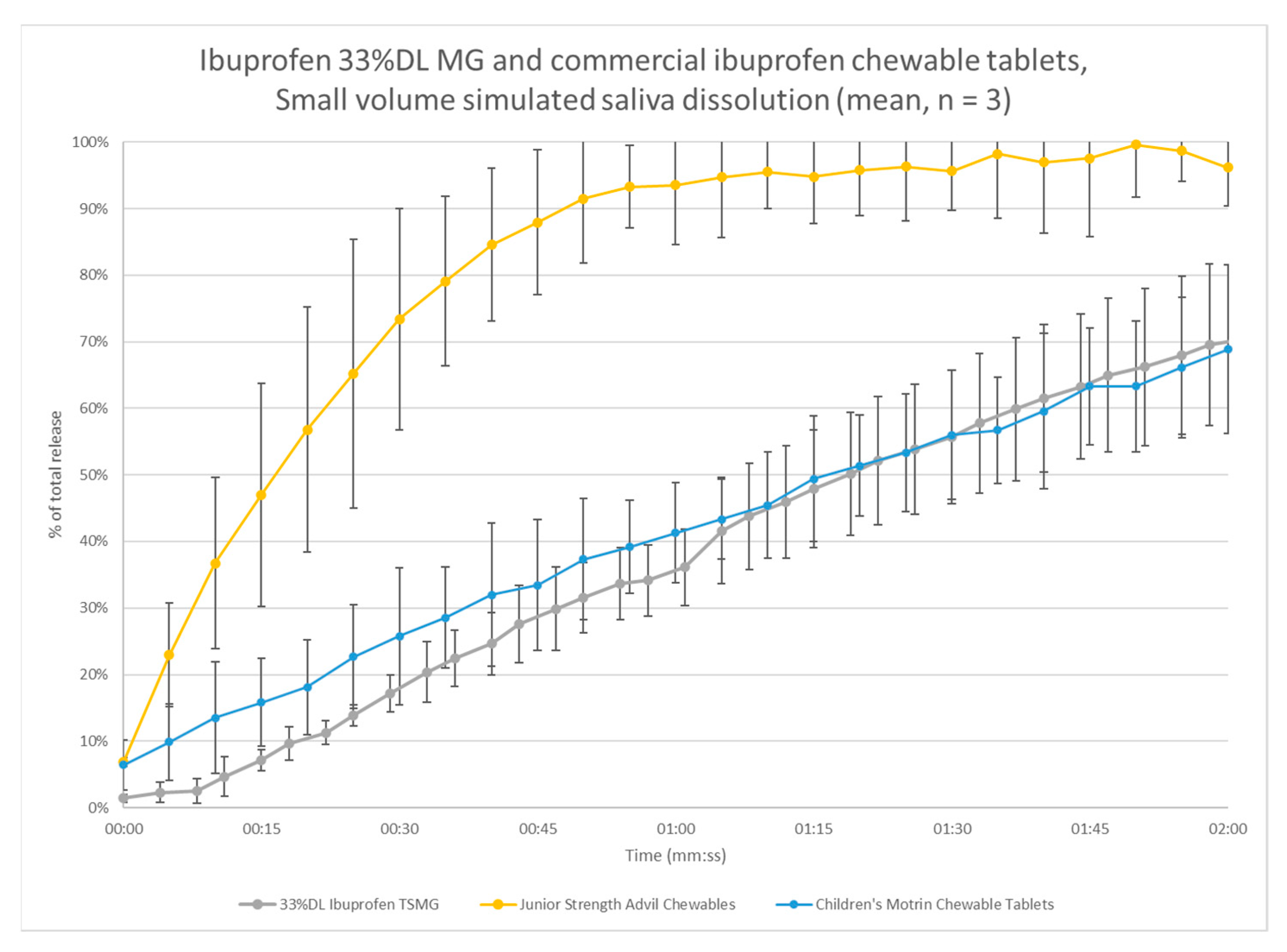
3.5. Small-Volume Dissolution
4. Analysis
4.1. Continuous Melt Granulation Mechanism
4.2. Granulation Process Analysis
4.3. Dissolution Model for Ibuprofen Melt Granulation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haynes, R.B.; McDonald, H.; Garg, A.X.; Montague, P. Interventions for helping patients to follow prescriptions for medications. Cochrane Database Syst. Rev. 2002, 2002, CD000011. [Google Scholar] [CrossRef]
- Mahoney, J.J.; Ansell, B.J.; Fleming, W.K.; Butterworth, S.W. The Unhidden Cost of Noncompliance. J. Manag. Care Pharm. 2008, 14, 1–30. [Google Scholar] [CrossRef]
- Bracken, L.; McDonough, E.; Ashleigh, S.; Wilson, F.; Ohia, U.; Mistry, P.; Jones, H.; Kanji, N.; Liu, F.; Peak, M. O23 Can children swallow tablets? outcome data from a feasibility study to assess the swallowability and acceptability of different sized placebo tablets in children and young people (creating acceptable tablets—Cat). BMJ Open 2019, 104. [Google Scholar] [CrossRef] [Green Version]
- Van Arnum, P. Pediatric formulations: Technical and regulatory considerations. Pharm. Technol. 2009, 2009, 43. Available online: https://www.pharmtech.com/view/pediatric-formulations-technical-and-regulatory-considerations (accessed on 10 June 2021).
- (EMEA) EMA. Reflection Paper: Formulations of Choice for the Pediatric Population; EMEA: London, UK, 2006. [Google Scholar]
- Douroumis, D. Practical approaches of taste masking technologies in oral solid forms. Expert Opin. Drug Deliv. 2007, 4, 417–426. [Google Scholar] [CrossRef]
- Ayenew, Z.; Puri, V.; Kumar, L.; Bansal, A.K. Trends in pharmaceutical taste masking technologies: A patent review. Recent Patents Drug Deliv. Formul. 2009, 3, 26–39. [Google Scholar] [CrossRef]
- Faisal, W.; Farag, F.; Abdellatif, A.A.H.; Abbas, A. Taste Masking Approaches for Medicines. Curr. Drug Deliv. 2018, 15, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Al-Kasmi, B.; Alsirawan, M.B.; Bashimam, M.; El-Zein, H. Mechanical microencapsulation: The best technique in taste masking for the manufacturing scale—Effect of polymer encapsulation on drug targeting. J. Control. Release 2017, 260, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Forster, S.; Dippold, E.; Chiang, T. Twin-Screw Melt Granulation for Oral Solid Pharmaceutical Products. Pharmaceutics 2021, 13, 665. [Google Scholar] [CrossRef]
- Morris, J.B.; Tisi, D.A.; Tan, D.C.T.; Worthington, J.H. Development and Palatability Assessment of Norvir® (Ritonavir) 100 mg Powder for Pediatric Population. Int. J. Mol. Sci. 2019, 20, 1718. [Google Scholar] [CrossRef] [Green Version]
- Pein, M.; Preis, M.; Eckert, C.; Kiene, F.E. Taste-masking assessment of solid oral dosage forms—A critical review. Int. J. Pharm. 2014, 465, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Woertz, K.; Tissen, C.; Kleinebudde, P.; Breitkreutz, J. A comparative study on two electronic tongues for pharmaceutical formu-lation development. J. Pharm. Biomed. Anal. 2011, 55, 272–281. [Google Scholar] [CrossRef]
- Siewert, M.; Dressman, J.; Brown, C.K.; Shah, V.P.; Aiache, J.-M.; Aoyagi, N.; Bashaw, D.; Brown, W.; Burgess, D.; Crison, J.; et al. FIP/AAPS guidelines to dissolution/in vitro release testing of novel/special dosage forms. AAPS PharmSciTech 2003, 4, 43–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagerlöf, F.; Dawes, C. The volume of saliva in the mouth before and after swallowing. J. Dent. Res. 1984, 63, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Gittings, S.; Turnbull, N.; Henry, B.; Roberts, C.; Gershkovich, P. Characterisation of human saliva as a platform for oral dissolution medium development. Eur. J. Pharm. Biopharm. 2015, 91, 16–24. [Google Scholar] [CrossRef]
- Beck, M.H.; Cataldo, M.; Slifer, K.J.; Pulbrook, V.; Guhman, J.K. Teaching Children with Attention Deficit Hyperactivity Disorder (ADHD) and Autistic Disorder (AD) How to Swallow Pills. Clin. Pediatr. 2005, 44, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Hamashita, T.; Matsuzaki, M.; Ono, T.; Ono, M.; Tsunenari, Y.; Aketo, T.; Watano, S. Granulation of Core Particles Suitable for Film Coating by Agitation Fluidized Bed II. A Proposal of a Rapid Dissolution Test for Evaluation of Bitter Taste of Ibuprofen. Chem. Pharm. Bull. 2008, 56, 883–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, D.; Chopra, R.; Bedi, N. Development and evaluation of paracetamol taste masked orally disintegrating tablets using polymer coating technique. Int. J. Pharm. Pharm. Sci. 2012, 4, 129–134. [Google Scholar]
- Albertini, B.; Cavallari, C.; Passerini, N.; Voinovich, D.; González-Rodríguez, M.L.; Magarotto, L.; Rodriguez, L. Characterization and taste-masking evaluation of acetaminophen granules: Comparison between different preparation methods in a high-shear mixer. Eur. J. Pharm. Sci. 2004, 21, 295–303. [Google Scholar] [CrossRef]
- Steffens, K.E.; Wagner, K.G. Improvement of tabletability via twin-screw melt granulation: Focus on binder distribution. Int. J. Pharm. 2019, 570. [Google Scholar] [CrossRef]
- Vasanthavada, M.; Wang, Y.; Haefele, T.; Lakshman, J.P.; Mone, M.; Tong, W.; Joshi, Y.M.; Serajuddin, A.T. Application of Melt Granulation Technology Using Twin-screw Extruder in Development of High-dose Modified-Release Tablet Formulation. J. Pharm. Sci. 2011, 100, 1923–1934. [Google Scholar] [CrossRef]
- Münster, M.; Schoch, C.; Schmidt, C.; Breitkreutz, J. Multiparticulate system combining taste masking and immediate release properties for the aversive compound praziquantel. Eur. J. Pharm. Sci. 2017, 109, 446–454. [Google Scholar] [CrossRef]
- Rosiaux, Y.; Forest, A.; Girard, J.-M.; Deleglise, C.; Sheehan, L.; Marchaud, D. High shear blending with glyceryl distearate provides individually coated drug particles for effective taste masking. J. Drug Deliv. Sci. Technol. 2018, 48, 437–449. [Google Scholar] [CrossRef]
- Lo, J.B.; Appel, L.E.; Herbig, S.M.; McCray, S.B.; Thombre, A.G. Formulation design and pharmaceutical development of a novel con-trolled release form of azithromycin for single-dose therapy. Drug Dev. Ind. Pharm. 2009, 35, 1522–1529. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dumpa, N.; Bandari, S.; Durig, T.; Repka, M.A. Fabrication of Taste-Masked Donut-Shaped Tablets Via Fused Filament Fabrication 3D Printing Paired with Hot-Melt Extrusion Techniques. AAPS PharmSciTech 2020, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gryczke, A.; Schminke, S.; Maniruzzaman, M.; Beck, J.; Douroumis, D. Development and evaluation of orally disintegrating tablets (ODTs) containing Ibuprofen granules prepared by hot melt extrusion. Colloids Surf. B Biointerfaces 2011, 86, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.L.; O’Connor, T.F.; Yang, X.; Cruz, C.N.; Chatterjee, S.; Madurawe, R.D.; Moore, C.M.V.; Yu, L.X.; Woodcock, J. Modernizing Pharmaceutical Manufacturing: From Batch to Continuous Production. J. Pharm. Innov. 2015, 10, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Schaber, S.D.; Gerogiorgis, D.I.; Ramachandran, R.; Evans, J.M.B.; Barton, P.I.; Trout, B.L. Economic Analysis of Integrated Continuous and Batch Pharmaceutical Manufacturing: A Case Study. Ind. Eng. Chem. Res. 2011, 50, 10083–10092. [Google Scholar] [CrossRef] [Green Version]
- Vanhoorne, V.; Vervaet, C. Recent progress in continuous manufacturing of oral solid dosage forms. Int. J. Pharm. 2020, 579. [Google Scholar] [CrossRef]
- Kittikunakorn, N.; Liu, T.; Zhang, F. Twin-screw melt granulation: Current progress and challenges. Int. J. Pharm. 2020, 588. [Google Scholar] [CrossRef]
- Kittikunakorn, N.; Koleng, J.J., III; Listro, T.; Sun, C.C.; Zhang, F. Effects of thermal binders on chemical stabilities and tabletability of gabapentin granules prepared by twin-screw melt granulation. Int. J. Pharm. 2019, 559, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Kittikunakorn, N.; Sun, C.C.; Zhang, F. Effect of screw profile and processing conditions on physical transformation and chemical degradation of gabapentin during twin-screw melt granulation. Eur. J. Pharm. Sci. 2019, 131, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Kittikunakorn, N.; Paul, S.; Koleng, J.J., III; Liu, T.; Cook, R.; Yang, F.; Bi, V.; Durig, T.; Sun, C.C.; Kumar, A.; et al. How Does the Dissimilarity of Screw Geometry Impact Twin-screw Melt Granulation? Eur. J. Pharm. Sci. 2021, 157. [Google Scholar] [CrossRef] [PubMed]
- Steffens, K.E.; Wagner, K.G. Compression behaviour of granules produced via twin-screw melt granulation: Effect of initial par-ticle size on granulation efficiency. Powder Technol. 2020, 374, 430–442. [Google Scholar] [CrossRef]
- Mu, B.; Thompson, M. Examining the mechanics of granulation with a hot melt binder in a twin-screw extruder. Chem. Eng. Sci. 2012, 81, 46–56. [Google Scholar] [CrossRef]
- Vercruysse, J.; Díaz, D.C.; Peeters, E.; Fonteyne, M.; Delaet, U.; Van Assche, I.; De Beer, T.; Remon, J.; Vervaet, C. Continuous twin screw granulation: Influence of process variables on granule and tablet quality. Eur. J. Pharm. Biopharm. 2012, 82, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Vercruysse, J.; Burggraeve, A.; Fonteyne, M.; Cappuyns, P.; Delaet, U.; Van Assche, I.; De Beer, T.; Remon, J.; Vervaet, C. Impact of screw configuration on the particle size distribution of granules produced by twin screw granulation. Int. J. Pharm. 2015, 479, 171–180. [Google Scholar] [CrossRef]
- Thompson, M. Twin screw granulation–review of current progress. Drug Dev. Ind. Pharm. 2015, 41, 1223–1231. [Google Scholar] [CrossRef]
- Thompson, M.; Sun, J. Wet Granulation in a Twin-Screw Extruder: Implications of Screw Design. J. Pharm. Sci. 2010, 99, 2090–2103. [Google Scholar] [CrossRef]
- Torrecillas, C.M.; Gorringe, L.J.; Rajoub, N.; Robertson, J.; Elkes, R.G.; Lamprou, D.A.; Halbert, G.W. The impact of channel fill level on internal forces during continuous twin screw wet granulation. Int. J. Pharm. 2019, 558, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Tu, W.-D.; Ingram, A.; Seville, J. Regime map development for continuous twin screw granulation. Chem. Eng. Sci. 2013, 87, 315–326. [Google Scholar] [CrossRef]
- O’Neil, M.J. (Ed.) The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 14th ed.; Merck: Whitehouse Station, NJ, USA, 2006. [Google Scholar]
- Hughes, L.; Gehris, A. A New Method of Characterizing the Buccal Dissolution of Drugs; Rohm and Haas Research Laboratories-Spring House: Bristol, PA, USA, 2003. [Google Scholar]
- Dhenge, R.M.; Fyles, R.S.; Cartwright, J.J.; Doughty, D.G.; Hounslow, M.J.; Salman, A.D. Twin screw wet granulation: Granule properties. Chem. Eng. J. 2010, 164, 322–329. [Google Scholar] [CrossRef]
- Dinge, A. Eutectic Mixtures of Drugs with Poor Aqueous Solubility—Solid State Characterization and Dissolution Studies. Mater’s Thesis, Temple University, Philadelphia, PA, USA, 2012. [Google Scholar]
- Passerini, N.; Albertini, B.; González-Rodríguez, M.L.; Cavallari, C.; Rodriguez, L. Preparation and characterisation of ibuprofen–poloxamer 188 granules obtained by melt granulation. Eur. J. Pharm. Sci. 2002, 15, 71–78. [Google Scholar] [CrossRef]
- Kimura, S.-i.; Uchida, S.; Kanada, K.; Namiki, N. Effect of granule properties on rough mouth feel and palatability of orally disin-tegrating tablets. Int. J. Pharm. 2015, 484, 156–162. [Google Scholar] [CrossRef]
- Hamdani, J.; Moës, A.J.; Amighi, K. Development and evaluation of prolonged release pellets obtained by the melt pelletization process. Int. J. Pharm. 2002, 245, 167–177. [Google Scholar] [CrossRef]
- Schæfer, T.; Mathiesen, C. Melt pelletization in a high shear mixer. IX. Effects of binder particle size. Int. J. Pharm. 1996, 139, 139–148. [Google Scholar] [CrossRef]
- Iveson, S.M.; Wauters, P.A.; Forrest, S.; Litster, J.D.; Meesters, G.M.; Scarlett, B. Growth regime map for liquid-bound granules: Further development and experimental validation. Powder Technol. 2001, 117, 83–97. [Google Scholar] [CrossRef]
- Brown, C.; DiNunzio, J.; Eglesia, M.; Forster, S.; Lamm, M.; Lowinger, M.; Marsac, P.; McKelvey, C.; Meyer, R.; Schenck, L.; et al. HME for Solid Dispersions: Scale-Up and Late-Stage Development. Amorphous Solid Dispersion; Springer: New York, NY, USA, 2014; pp. 231–260. [Google Scholar]
- Osorio, J.G.; Sayin, R.; Kalbag, A.V.; Litster, J.D.; Martinez-Marcos, L.; Lamprou, D.A.; Halbert, G.W. Scaling of continuous twin screw wet granulation. AIChE J. 2016, 63, 921–932. [Google Scholar] [CrossRef] [Green Version]
- Gorringe, L.; Kee, G.; Saleh, M.; Fa, N.; Elkes, R. Use of the channel fill level in defining a design space for twin screw wet granulation. Int. J. Pharm. 2017, 519, 165–177. [Google Scholar] [CrossRef]
- Lute, S.V.; Dhenge, R.M.; Salman, A.D. Twin Screw Granulation: An Investigation of the Effect of Barrel Fill Level. Pharmaceutics 2018, 10, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier, R.; Moll, K.-P.; Krumme, M.; Kleinebudde, P. Impact of fill-level in twin-screw granulation on critical quality attributes of granules and tablets. Eur. J. Pharm. Biopharm. 2017, 115, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Dhenge, R.; Washino, K.; Cartwright, J.J.; Hounslow, M.J.; Salman, A.D. Twin screw granulation using conveying screws: Effects of viscosity of granulation liquids and flow of powders. Powder Technol. 2013, 238, 77–90. [Google Scholar] [CrossRef]
- Hattori, Y.; Haruna, Y.; Otsuka, M. Dissolution process analysis using model-free Noyes–Whitney integral equation. Colloids Surf. B Biointerfaces 2013, 102, 227–231. [Google Scholar] [CrossRef] [PubMed]














| Zone | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Screw and barrel design | Vent | Feed | Convey | Convey | Convey to Mix | Convey | Convey | Convey | Convey | Convey to Discharge |
| Temperature set point (°C) | 25 | 25 | 25 | 50–60 | 50–60 | 50–60 | 50–60 | 40 | 30 | 30 |
| Temperature (°C) | Screw Speed (rpm) | Throughput (kg/h) | |
|---|---|---|---|
| Low | 50 | 50 | 0.25 |
| Center | 55 | 100 | 0.50 |
| High | 60 | 150 | 0.75 |
| Process Temperature (°C) | Screw Speed (rpm) | Feed Rate (kg/h) | Average (µm) | Standard Deviation (µm) | ||||
|---|---|---|---|---|---|---|---|---|
| ×10 | ×50 | ×90 | ×10 | ×50 | ×90 | |||
| 50 | 50 | 0.25 | 55 | 143 | 1543 | 1.7 | 27 | 198 |
| 50 | 50 | 0.75 | 48 | 91 | 898 | 1.0 | 5 | 441 |
| 50 | 100 | 0.5 | 50 | 100 | 607 | 3.2 | 8 | 458 |
| 50 | 150 | 0.25 | 57 | 194 | 1147 | 2.1 | 68 | 320 |
| 50 | 150 | 0.75 | 53 | 114 | 1155 | 1.2 | 5 | 144 |
| 55 | 50 | 0.25 | 50 | 99 | 1152 | 1.0 | 2 | 317 |
| 55 | 50 | 0.75 | 48 | 93 | 1334 | 1.5 | 5 | 398 |
| 55 | 100 | 0.5 | 50 | 107 | 1571 | 1.0 | 7 | 364 |
| 55 | 100 | 0.5 | 51 | 104 | 1170 | 2.3 | 12 | 549 |
| 55 | 150 | 0.25 | 57 | 183 | 1071 | 2.0 | 22 | 178 |
| 55 | 150 | 0.75 | 52 | 126 | 1476 | 3.0 | 31 | 480 |
| 60 | 50 | 0.25 | 80 | 557 | 1359 | 8.2 | 217 | 502 |
| 60 | 50 | 0.75 | 58 | 159 | 982 | 1.2 | 13 | 143 |
| 60 | 100 | 0.5 | 92 | 486 | 959 | 11.7 | 66 | 89 |
| 60 | 150 | 0.25 | 566 | 1545 | 2331 | 95.2 | 191 | 70 |
| 60 | 150 | 0.75 | 106 | 551 | 1023 | 23.1 | 44 | 56 |
| Process Temperature (°C) | Screw Speed (rpm) | Feed rate (kg/h) | Average (µm) | Standard Deviation (µm) | ||||
|---|---|---|---|---|---|---|---|---|
| ×10 | ×50 | ×90 | ×10 | ×50 | ×90 | |||
| 50 | 50 | 0.25 | 49 | 103 | 968 | 2.1 | 19 | 574 |
| 50 | 50 | 0.75 | 47 | 89 | 1209 | 1.0 | 4 | 612 |
| 50 | 100 | 0.5 | 54 | 165 | 1031 | 2.6 | 52 | 265 |
| 50 | 150 | 0.25 | 59 | 205 | 862 | 3.0 | 62 | 210 |
| 50 | 150 | 0.75 | 50 | 103 | 981 | 0.6 | 5 | 316 |
| 55 | 50 | 0.25 | 51 | 107 | 819 | 1.5 | 6 | 196 |
| 55 | 50 | 0.75 | 53 | 100 | 1306 | 5.3 | 8 | 250 |
| 55 | 100 | 0.5 | 51 | 107 | 1156 | 1.2 | 6 | 240 |
| 55 | 150 | 0.25 | 68 | 272 | 843 | 8.4 | 86 | 137 |
| 55 | 150 | 0.75 | 54 | 120 | 1005 | 1.0 | 4 | 274 |
| 60 | 50 | 0.25 | 88 | 655 | 1410 | 10.0 | 31 | 224 |
| 60 | 50 | 0.75 | 64 | 278 | 1154 | 3.1 | 78 | 354 |
| 60 | 100 | 0.5 | 90 | 730 | 1764 | 12.2 | 177 | 400 |
| 60 | 150 | 0.25 | 187 | 800 | 1319 | 58.4 | 80 | 245 |
| 60 | 150 | 0.75 | 96 | 620 | 1431 | 2.6 | 34 | 431 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forster, S.P.; Lebo, D.B. Continuous Melt Granulation for Taste-Masking of Ibuprofen. Pharmaceutics 2021, 13, 863. https://doi.org/10.3390/pharmaceutics13060863
Forster SP, Lebo DB. Continuous Melt Granulation for Taste-Masking of Ibuprofen. Pharmaceutics. 2021; 13(6):863. https://doi.org/10.3390/pharmaceutics13060863
Chicago/Turabian StyleForster, Seth P., and David B. Lebo. 2021. "Continuous Melt Granulation for Taste-Masking of Ibuprofen" Pharmaceutics 13, no. 6: 863. https://doi.org/10.3390/pharmaceutics13060863






