Miniaturization and Automation of a Human In Vitro Blood–Brain Barrier Model for the High-Throughput Screening of Compounds in the Early Stage of Drug Discovery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human BBB Models Setting Up
2.1.1. Miniaturization
2.1.2. Automation
Cell Seeding
Immunochemistry and BBB Permeability Assays
Microscopy
2.2. BBB Phenotype Validation of Miniaturized and Automated Human BBB In Vitro Model
2.2.1. Immunocytochemical Characterization
2.2.2. BBB Integrity Assay
2.2.3. RNA Extraction and Gene Expression Analysis
2.2.4. Efflux Pumps Functionality
2.3. BBB Model Applications
2.3.1. Drugs Rate Delivery to the Brain
2.3.2. Compounds BBB Impact
2.3.3. Cell Uptake and Endocytic Route Inhibitors
2.4. Statistical Analysis
3. Results
3.1. Miniaturization and Automation of a Human In Vitro BBB Model
3.1.1. Cell Culture, Plates and Volumes
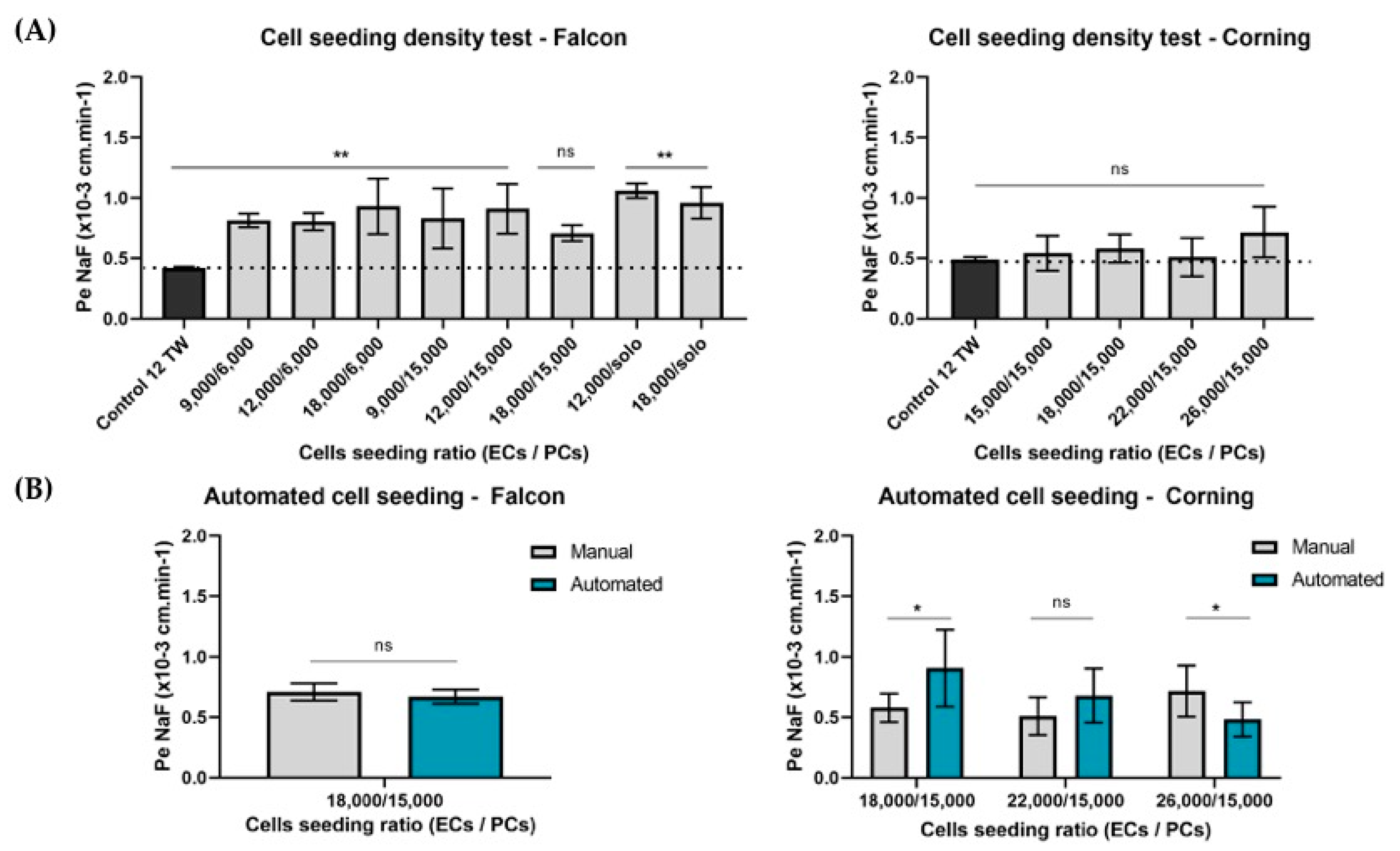
3.1.2. Adaptation of the Cell Densities to the Miniaturization
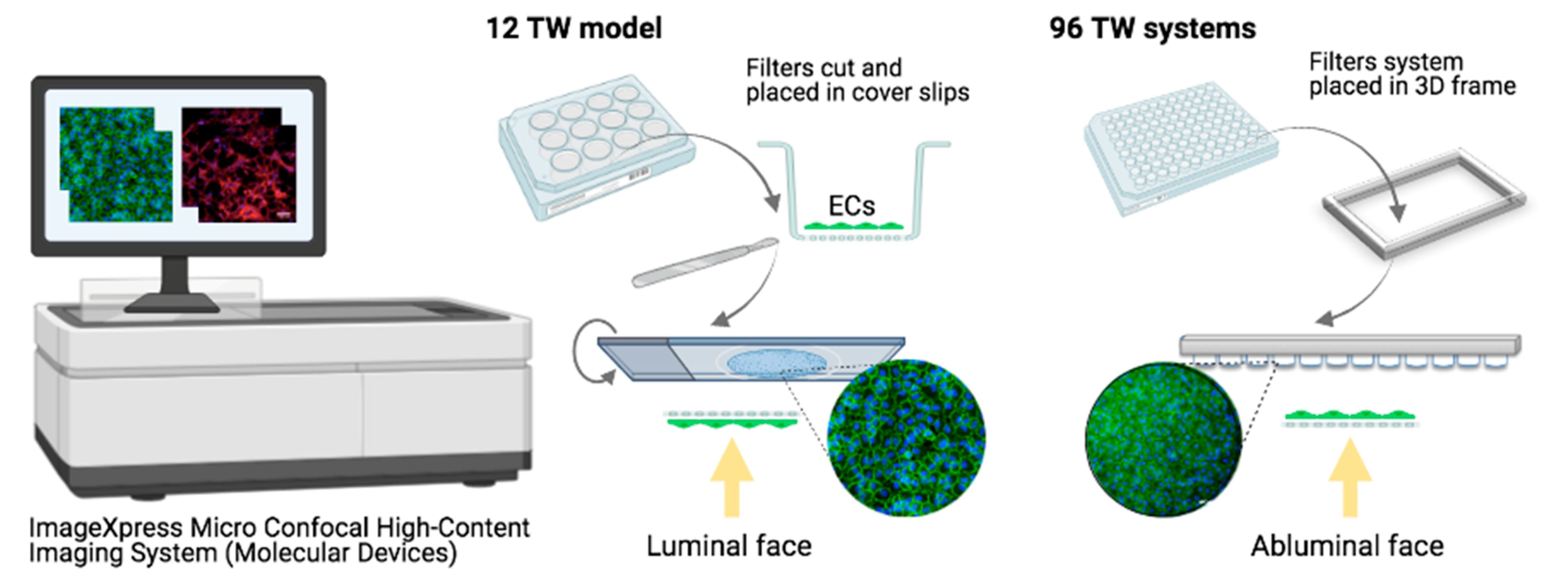
3.1.3. Automation
Cell Seeding Densities
Cell Visualization
Experimental Assays
3.2. BBB Phenotype Maintenance of Miniaturized and Automated Human BBB In Vitro Model Replicate
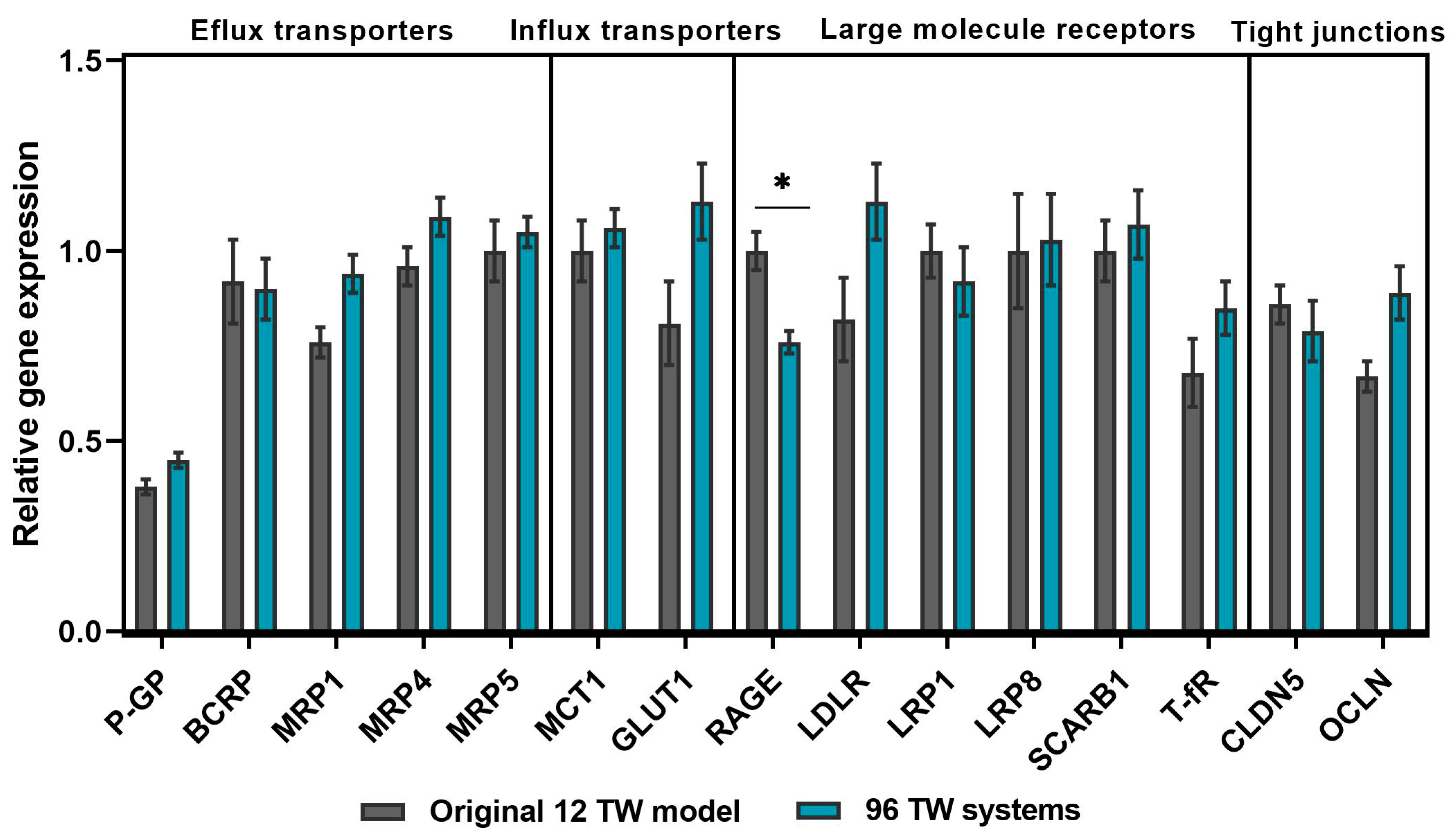
3.2.1. BBB Gene Profile Maintenance
3.2.2. Low Paracellular Permeability of a Tightly Packed BLECs Network
3.2.3. Efflux Pumps Functionality
3.3. Some Applications for Which the Miniaturized and Automated Human BBB In Vitro Model Have Been Successful
3.3.1. Brain Exposure to the Drug Correlations with Human In Vivo Data
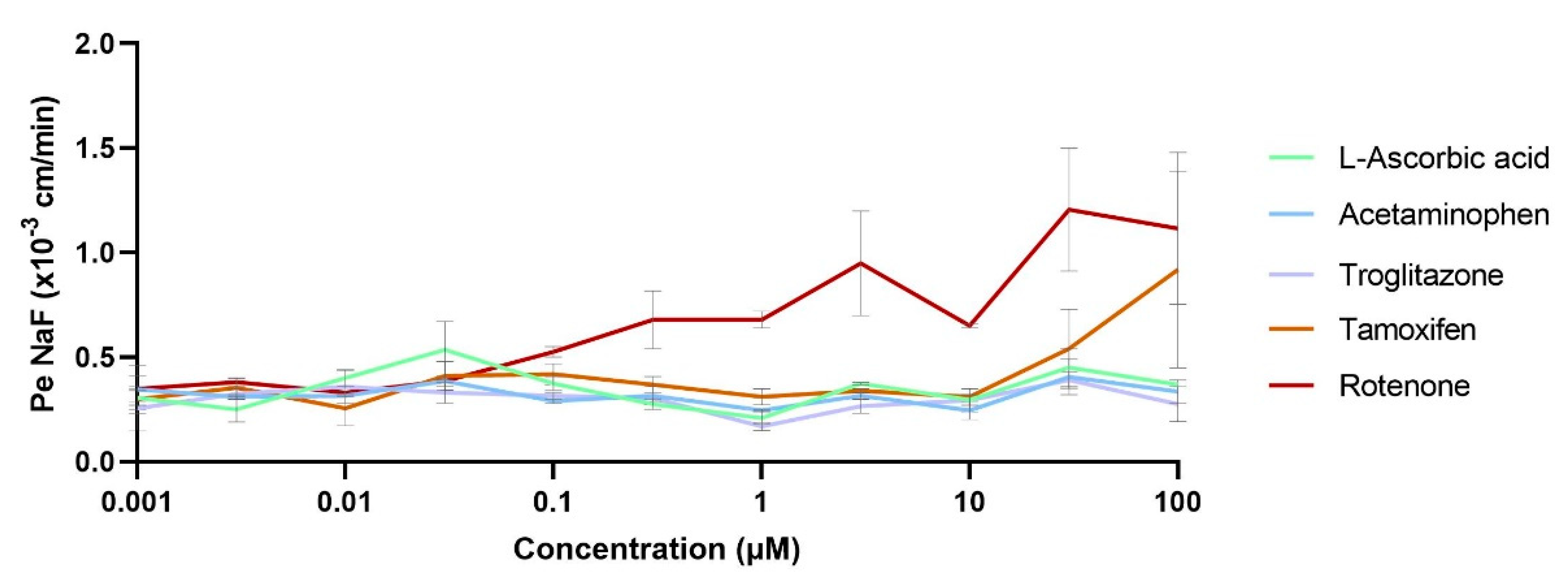
3.3.2. Compound Impact Studies over the BBB
3.3.3. Molecular Internalization and Endocytic Routes Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Statements
References
- World Health Organization. The Top 10 Causes of Death. 22 February 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 15 April 2021).
- World Health Organization. Neurological Disorders Report. 2016. Available online: https://www.who.int/mental_health/neurology/neurological_disorders_report_web.pdf (accessed on 17 March 2021).
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Cecchelli, R.; Berezowski, V.; Lundquist, S.; Culot, M.; Renftel, M.; Dehouck, M.P.; Fenart, L. Modelling of the blood-brain barrier in drug discovery and development. Nat. Rev. Drug Discov. 2007, 6, 650–661. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Reichel, A. The role of blood-brain barrier studies in the pharmaceutical industry. Curr. Drug Metab. 2006, 7, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Kola, I. The state of innovation in drug development. Clin. Pharmacol. Ther. 2008, 83, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Kaitin, K.I.; DiMasi, J.A. Pharmaceutical innovation in the 21st century: New drug approvals in the first decade, 2000–2009. Clin. Pharmacol. Ther. 2011, 89, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, J.V.; Hoekstra, D.; Zuhorn, I.S. Smuggling Drugs into the Brain: An Overview of Ligands Targeting Transcytosis for Drug Delivery across the Blood-Brain Barrier. Pharmaceutics 2014, 6, 557–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J. Control. Release Off. J. Control. Release Soc. 2016, 235, 34–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lombardo, S.M.; Schneider, M.; Türeli, A.E.; Günday Türeli, N. Key for crossing the BBB with nanoparticles: The rational design. Beilstein J. Nanotechnol. 2020, 11, 866–883. [Google Scholar] [CrossRef]
- Gosselet, F.; Loiola, R.A.; Roig, A.; Rosell, A.; Culot, M. Central nervous system delivery of molecules across the blood-brain barrier. Neurochem. Int. 2021, 144, 104952. [Google Scholar] [CrossRef]
- Praça, C.; Rai, A.; Santos, T.; Cristovão, A.C.; Pinho, S.L.; Cecchelli, R.; Dehouck, M.P.; Bernardino, L.; Ferreira, L.S. A nanoformulation for the preferential accumulation in adult neurogenic niches. J. Control. Release Off. J. Control. Release Soc. 2018, 284, 57–72. [Google Scholar] [CrossRef]
- Henrich-Noack, P.; Nikitovic, D.; Neagu, M.; Docea, A.O.; Engin, A.B.; Gelperina, S.; Shtilman, M.; Mitsias, P.; Tzanakakis, G.; Gozes, I.; et al. The blood-brain barrier and beyond: Nano-based neuropharmacology and the role of extracellular matrix. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 359–379. [Google Scholar] [CrossRef]
- Mangas-Sanjuan, V.; González-Álvarez, I.; González-Álvarez, M.; Casabó, V.G.; Bermejo, M. Innovative in vitro method to predict rate and extent of drug delivery to the brain across the blood-brain barrier. Mol. Pharm. 2013, 10, 3822–3831. [Google Scholar] [CrossRef]
- Vandenhaute, E.; Sevin, E.; Hallier-Vanuxeem, D.; Dehouck, M.-P.; Cecchelli, R. Case study: Adapting in vitro blood–brain barrier models for use in early-stage drug discovery. Drug Discov. Today 2012, 17, 285–290. [Google Scholar] [CrossRef]
- Helms, H.C.; Abbott, N.J.; Burek, M.; Cecchelli, R.; Couraud, P.O.; Deli, M.A.; Förster, C.; Galla, H.J.; Romero, I.A.; Shusta, E.V.; et al. In vitro models of the blood-brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2016, 36, 862–890. [Google Scholar] [CrossRef]
- Wolff, A.; Antfolk, M.; Brodin, B.; Tenje, M. In Vitro Blood-Brain Barrier Models-An Overview of Established Models and New Microfluidic Approaches. J. Pharm. Sci. 2015, 104, 2727–2746. [Google Scholar] [CrossRef]
- Bagchi, S.; Chhibber, T.; Lahooti, B.; Verma, A.; Borse, V.; Jayant, R.D. In-vitro blood-brain barrier models for drug screening and permeation studies: An overview. Drug Des. Dev. Ther. 2019, 13, 3591–3605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cecchelli, R.; Aday, S.; Sevin, E.; Almeida, C.; Culot, M.; Dehouck, L.; Coisne, C.; Engelhardt, B.; Dehouck, M.P.; Ferreira, L. A stable and reproducible human blood-brain barrier model derived from hematopoietic stem cells. PLoS ONE 2014, 9, e99733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes regulate the blood-brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heymans, M.; Figueiredo, R.; Dehouck, L.; Francisco, D.; Sano, Y.; Shimizu, F.; Kanda, T.; Bruggmann, R.; Engelhardt, B.; Winter, P.; et al. Contribution of brain pericytes in blood-brain barrier formation and maintenance: A transcriptomic study of co-cultured human endothelial cells derived from hematopoietic stem cells. Fluids Barriers CNS 2020, 17, 48. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, D.C.; Tellechea, A.; Moura, L.; Fidalgo-Carvalho, I.; Duarte, J.; Carvalho, E.; Ferreira, L. Improved survival, vascular differentiation and wound healing potential of stem cells co-cultured with endothelial cells. PLoS ONE 2011, 6, e16114. [Google Scholar] [CrossRef] [Green Version]
- Vandenhaute, E.; Dehouck, L.; Boucau, M.C.; Sevin, E.; Uzbekov, R.; Tardivel, M.; Gosselet, F.; Fenart, L.; Cecchelli, R.; Dehouck, M.P. Modelling the neurovascular unit and the blood-brain barrier with the unique function of pericytes. Curr. Neurovascular Res. 2011, 8, 258–269. [Google Scholar] [CrossRef]
- Fridén, M.; Winiwarter, S.; Jerndal, G.; Bengtsson, O.; Wan, H.; Bredberg, U.; Hammarlund-Udenaes, M.; Antonsson, M. Structure-brain exposure relationships in rat and human using a novel data set of unbound drug concentrations in brain interstitial and cerebrospinal fluids. J. Med. Chem. 2009, 52, 6233–6243. [Google Scholar] [CrossRef]
- Imaging of Cellular Fluorescence on Corning’s Transwell® Permeable Supports. 2019. Available online: https://www.corning.com/catalog/cls/documents/application-notes/CLS-AN-521-A4.pdf (accessed on 14 May 2021).
- Schinkel, A.H.; Jonker, J.W. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: An overview. Adv. Drug Deliv. Rev. 2003, 55, 3–29. [Google Scholar] [CrossRef]
- Weksler, B.B.; Subileau, E.A.; Perrière, N.; Charneau, P.; Holloway, K.; Leveque, M.; Tricoire-Leignel, H.; Nicotra, A.; Bourdoulous, S.; Turowski, P.; et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2005, 19, 1872–1874. [Google Scholar] [CrossRef]
- Santa-Maria, A.R.; Heymans, M.; Walter, F.R.; Culot, M.; Gosselet, F.; Deli, M.A.; Neuhaus, W. Transport Studies Using Blood-Brain Barrier In Vitro Models: A Critical Review and Guidelines. Handb. Exp. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Perrière, N.; Yousif, S.; Cazaubon, S.; Chaverot, N.; Bourasset, F.; Cisternino, S.; Declèves, X.; Hori, S.; Terasaki, T.; Deli, M.; et al. A functional in vitro model of rat blood-brain barrier for molecular analysis of efflux transporters. Brain Res. 2007, 1150, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Heymans, M.; Sevin, E.; Gosselet, F.; Lundquist, S.; Culot, M. Mimicking brain tissue binding in an in vitro model of the blood-brain barrier illustrates differences between in vitro and in vivo methods for assessing the rate of brain penetration. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. 2018, 127, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Culot, M.; Fabulas-da Costa, A.; Sevin, E.; Szorath, E.; Martinsson, S.; Renftel, M.; Hongmei, Y.; Cecchelli, R.; Lundquist, S. A simple method for assessing free brain/free plasma ratios using an in vitro model of the blood brain barrier. PLoS ONE 2013, 8, e80634. [Google Scholar] [CrossRef] [PubMed]
- Loryan, I.; Fridén, M.; Hammarlund-Udenaes, M. The brain slice method for studying drug distribution in the CNS. Fluids Barriers CNS 2013, 10, 6. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Hammarlund-Udenaes, M.; Fridén, M. Understanding the Influence of Nanocarrier-Mediated Brain Delivery on Therapeutic Performance Through Pharmacokinetic-Pharmacodynamic Modeling. J. Pharm. Sci. 2019, 108, 3425–3433. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.L.; Huang, Y.H.; Shen, Y.C.; Huang, H.C.; Liu, P.H. Ascorbic acid prevents blood-brain barrier disruption and sensory deficit caused by sustained compression of primary somatosensory cortex. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2010, 30, 1121–1136. [Google Scholar] [CrossRef] [Green Version]
- Koehn, L.M.; Huang, Y.; Habgood, M.D.; Kysenius, K.; Crouch, P.J.; Dziegielewska, K.M.; Saunders, N.R. Effects of paracetamol (acetaminophen) on gene expression and permeability properties of the rat placenta and fetal brain. F1000Research 2020, 9, 573. [Google Scholar] [CrossRef]
- Low, Y.L.; Jin, L.; Morris, E.R.; Pan, Y.; Nicolazzo, J.A. Pioglitazone Increases Blood-Brain Barrier Expression of Fatty Acid-Binding Protein 5 and Docosahexaenoic Acid Trafficking into the Brain. Mol. Pharm. 2020, 17, 873–884. [Google Scholar] [CrossRef]
- Ravenstijn, P.G.; Merlini, M.; Hameetman, M.; Murray, T.K.; Ward, M.A.; Lewis, H.; Ball, G.; Mottart, C.; de Ville de Goyet, C.; Lemarchand, T.; et al. The exploration of rotenone as a toxin for inducing Parkinson’s disease in rats, for application in BBB transport and PK-PD experiments. J. Pharmacol. Toxicol. Methods 2008, 57, 114–130. [Google Scholar] [CrossRef]
- Walter, F.R.; Veszelka, S.; Pásztói, M.; Péterfi, Z.A.; Tóth, A.; Rákhely, G.; Cervenak, L.; Ábrahám, C.S.; Deli, M.A. Tesmilifene modifies brain endothelial functions and opens the blood-brain/blood-glioma barrier. J. Neurochem. 2015, 134, 1040–1054. [Google Scholar] [CrossRef] [Green Version]
- Hallier-Vanuxeem, D.; Prieto, P.; Culot, M.; Diallo, H.; Landry, C.; Tähti, H.; Cecchelli, R. New strategy for alerting central nervous system toxicity: Integration of blood-brain barrier toxicity and permeability in neurotoxicity assessment. Toxicol. In Vitro 2009, 23, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Gidwani, M.; Singh, A.V. Nanoparticle enabled drug delivery across the blood brain barrier: In vivo and in vitro models, opportunities and challenges. Curr. Pharm. Biotechnol. 2014, 14, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Nag, S. Morphology and properties of brain endothelial cells. Methods Mol. Biol. 2011, 686, 3–47. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.D.; Zhuang, Y.; Ben, J.J.; Qian, L.L.; Huang, H.P.; Bai, H.; Sha, J.H.; He, Z.G.; Chen, Q. Caveolae-dependent endocytosis is required for class A macrophage scavenger receptor-mediated apoptosis in macrophages. J. Biol. Chem. 2011, 286, 8231–8239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okura, T.; Hattori, A.; Takano, Y.; Sato, T.; Hammarlund-Udenaes, M.; Terasaki, T.; Deguchi, Y. Involvement of the pyrilamine transporter, a putative organic cation transporter, in blood-brain barrier transport of oxycodone. Drug Metab. Dispos. Biol. Fate Chem. 2008, 36, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, J.V.; Kalicharan, D.; Couraud, P.O.; Romero, I.A.; Weksler, B.; Hoekstra, D.; Zuhorn, I.S. Surface characteristics of nanoparticles determine their intracellular fate in and processing by human blood-brain barrier endothelial cells in vitro. Mol. Ther. J. Am. Soc. Gene Ther. 2011, 19, 318–325. [Google Scholar] [CrossRef]
- Morad, G.; Carman, C.V.; Hagedorn, E.J.; Perlin, J.R.; Zon, L.I.; Mustafaoglu, N.; Park, T.E.; Ingber, D.E.; Daisy, C.C.; Moses, M.A. Tumor-Derived Extracellular Vesicles Breach the Intact Blood-Brain Barrier via Transcytosis. ACS Nano 2019, 13, 13853–13865. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Covarrubias, L.; Slosky, L.M.; Thompson, B.J.; Davis, T.P.; Ronaldson, P.T. Transporters at CNS barrier sites: Obstacles or opportunities for drug delivery? Curr. Pharm. Des. 2014, 20, 1422–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banks, W.A. From blood-brain barrier to blood-brain interface: New opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 2016, 15, 275–292. [Google Scholar] [CrossRef]
- Deligne, C.; Hachani, J.; Duban-Deweer, S.; Meignan, S.; Leblond, P.; Carcaboso, A.M.; Sano, Y.; Shimizu, F.; Kanda, T.; Gosselet, F.; et al. Development of a human in vitro blood-brain tumor barrier model of diffuse intrinsic pontine glioma to better understand the chemoresistance. Fluids Barriers CNS 2020, 17, 37. [Google Scholar] [CrossRef]
- Mossu, A.; Rosito, M.; Khire, T.; Li Chung, H.; Nishihara, H.; Gruber, I.; Luke, E.; Dehouck, L.; Sallusto, F.; Gosselet, F.; et al. A silicon nanomembrane platform for the visualization of immune cell trafficking across the human blood-brain barrier under flow. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2019, 39, 395–410. [Google Scholar] [CrossRef] [Green Version]




| Target | Gene | AN * | Primer F/R | Primer Sequence |
|---|---|---|---|---|
| P-GP | ABCB1 | NM_001348945 | F | CAGACAGCAGCTGACAGTCCAAGAACAGGACT |
| R | GCCTGGCAGCTGGAAGACAAATACACAAAATT | |||
| BCRP | ABCG2 | NM_001348989 | F | TGGCTGTCATGGCTTGAGTA |
| R | GCCACGTGATTCTTCCACAA | |||
| MRP1 | ABCC1 | NM_004996 | F | GTCCTTAAACAAGGAGGACACG |
| R | TCCTTGGAGGAGTACACAACCT | |||
| MRP4 | ABCC4 | NM_005845 | F | ACCTTGCAAGAGCAGTGTATCA |
| R | TGTCTGCTAACTTCCGCATCTA | |||
| MRP5 | ABCC5 | NR_135125 | F | CCCAGTCCTGGGTATAGAAGTG |
| R | CGAGTTCTCCTGAACTTGGAAT | |||
| MCT1 | SLC16A1 | NM_001166496 | F | AAGGTATATTCCATGCCACCAC |
| R | GCTGATAGGACCTCCACCATAC | |||
| GLUT1 | SLC2A1 | NM_006516 | F | CTTCTCCAACTGGACCTCAAAT |
| R | AGGAGCACAGTGAAGATGATGA | |||
| RAGE | AGER | NM_001206929 | F | GAGTCCGTGTCTACCAGATTCC |
| R | ATCCAAGTGCCAGCTAAGAGTC | |||
| LDLR | LDLR | NM_000527 | F | TTCATGGCTTCATGTACTGGAC |
| R | TTTTCAGTCACCAGCGAGTAGA | |||
| LRP1 | LRP1 | NM_002332 | F | AATGAGTGTCTCAGCCGCAA |
| R | AACGGTTCCTCGTCAGTCAC | |||
| LRP8 | LRP8 | NM_033300 | F | TGTTTTGCATAATCCAGCAATC |
| R | GGTCAACTGCATTTACCCTCTC | |||
| SCARB1 | SCARB1 | NR_160416 | F | ATCCCCTTCTATCTCTCCGTCT |
| R | GTCGTTGTTGTTGAAGGTGATG | |||
| T-FR | T-fR | NM_001313965 | F | ACTTCTTCCGTGCTACTTCCA |
| R | CCACTCTCATGACACGATCATT | |||
| CLDN5 | CLDN5 | NM_003277 | F | GAGGCGTGCTCTACCTGTTTT |
| R | CACAGACGGGTCGTAAAACTC | |||
| OCLN | OCLN | NM_002538 | F | GAGGCTATGGAACTTCCCTTTT |
| R | TAGCTACCAAAGCCACTTCCTC | |||
| RPLP0 | RPLP0 | NM_053275 | F | CAGCTGATCAAGACTGGAGACA |
| R | CACTTCAGGGTTGTAGATGCTG |
| BBB In Vitro System | Original 12 TW Model | 96 TW Systems | |
|---|---|---|---|
| Brand | Corning | Falcon | Corning |
| Cell filter growth area | 1.13 cm2 | 0.0804 cm2 | 0.143 cm2 |
| Manual cell seeding ratio number (PCs well//ECs filter) | ECs 80,000//PCs 50,000 | ECs 18,000//PCs 15,000 | ECs > 15,000//PCs 15,000 |
| Automated cell seeding ratio (PCs well//ECs filter) | - | ECs 18,000//PCs 15,000 | ECs 22,000//PCs 15,000 |
| Filters adaptation for cell visualization by Confocal Microscopy | Cut and placed in cover slip | 96 TW ready for automation | 96 TW ready for automation |
| Bottom plate adapted for cell visualization | Yes | No | Yes |
| Permeability/staining assays | Manually | Automated | Automated |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moya, E.L.J.; Vandenhaute, E.; Rizzi, E.; Boucau, M.-C.; Hachani, J.; Maubon, N.; Gosselet, F.; Dehouck, M.-P. Miniaturization and Automation of a Human In Vitro Blood–Brain Barrier Model for the High-Throughput Screening of Compounds in the Early Stage of Drug Discovery. Pharmaceutics 2021, 13, 892. https://doi.org/10.3390/pharmaceutics13060892
Moya ELJ, Vandenhaute E, Rizzi E, Boucau M-C, Hachani J, Maubon N, Gosselet F, Dehouck M-P. Miniaturization and Automation of a Human In Vitro Blood–Brain Barrier Model for the High-Throughput Screening of Compounds in the Early Stage of Drug Discovery. Pharmaceutics. 2021; 13(6):892. https://doi.org/10.3390/pharmaceutics13060892
Chicago/Turabian StyleMoya, Elisa L. J., Elodie Vandenhaute, Eleonora Rizzi, Marie-Christine Boucau, Johan Hachani, Nathalie Maubon, Fabien Gosselet, and Marie-Pierre Dehouck. 2021. "Miniaturization and Automation of a Human In Vitro Blood–Brain Barrier Model for the High-Throughput Screening of Compounds in the Early Stage of Drug Discovery" Pharmaceutics 13, no. 6: 892. https://doi.org/10.3390/pharmaceutics13060892
APA StyleMoya, E. L. J., Vandenhaute, E., Rizzi, E., Boucau, M.-C., Hachani, J., Maubon, N., Gosselet, F., & Dehouck, M.-P. (2021). Miniaturization and Automation of a Human In Vitro Blood–Brain Barrier Model for the High-Throughput Screening of Compounds in the Early Stage of Drug Discovery. Pharmaceutics, 13(6), 892. https://doi.org/10.3390/pharmaceutics13060892






