Film-Forming Systems for Dermal Drug Delivery
Abstract
:1. Introduction
1.1. Skin
1.2. Skin Anatomy
1.3. Biochemistry of the Stratum Corneum
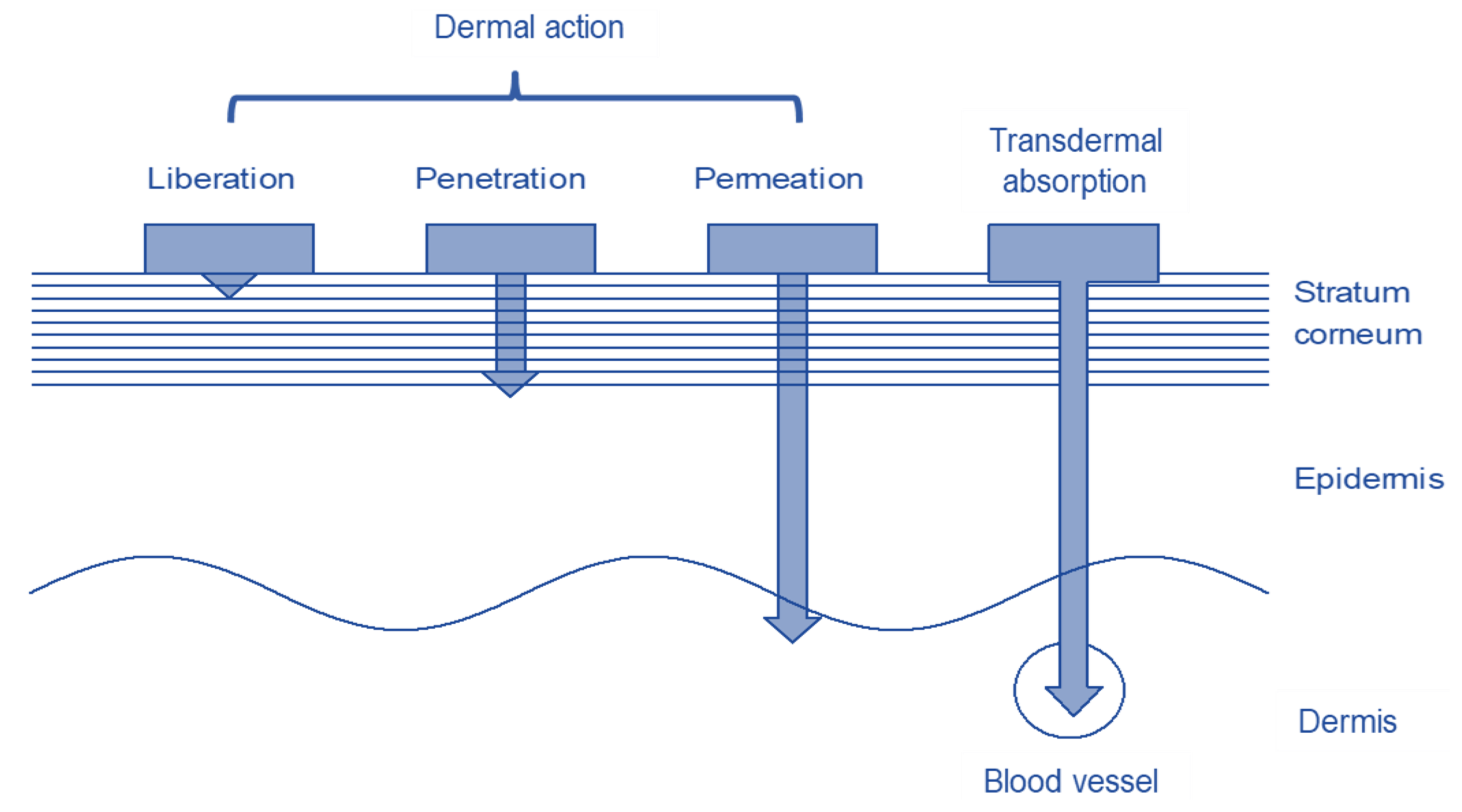
1.4. Transdermal Transport
1.5. Dermal Systems
1.6. Transdermal Systems
2. Film-Forming Systems
2.1. Components of Film-Forming Systems
2.1.1. Drug
2.1.2. Polymer
2.1.3. Solvent
2.1.4. Plasticizer
2.2. Film-Forming Systems on the Market
| Ingrediencies | Axiron® | Lamisil Once® | Hansaplast® Sprühpflaster |
|---|---|---|---|
| Formulation | Solution | Solution | Solution |
| Drug | Testosterone | Terbinafine HCl | - |
| Polymer | Polyvinylpyrrolidone | Poly(acrylamide-co-isooctylacrylat), Hydroxypropylcellulose | Acrylic copolymer, polyurethane polymer |
| Solvent | Ethanol 96%, Isopropyl alcohol | Ethanol | Ethanol, water, dimethylether |
| Plasticizer | Medium chain triglyceride | ||
| other | Octisalate (UV protection) | ||
| Application | Apply once a day to one axilla only | Single application of 2 g solution, drying time 1–2 min | Spray on a thin film of the plaster spray from a distance of 5–10 cm and allow to dry for 1 min. |
| Company | Eli Lilly | GlaxoSmithKlineConsumer Healthcare | Beiersdorf |
3. Development and Investigation of Film-Forming Systems
3.1. Film-Forming Solutions
3.2. Film-Forming-Gels
3.3. Film-Forming Emulsions
4. Conclusions and Further Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nontagna, W.P.; Paul, F. The Sturcture and Function of Skin; Academic Press: Cambridge, MA, USA, 1974. [Google Scholar]
- Honari, G.; Andersen, R.; Maibach, H.L. Sensitive Skin Syndrome, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Hong, J.; Koo, B.; Koo, J. The psychosocial and occupational impact of chronic skin disease. Dermatol. Ther. 2008, 21, 54–59. [Google Scholar] [CrossRef]
- Elias, P.M. Stratum Corneum Defensive Functions: An Integrated View. J. Investig. Dermatol. 2005, 125, 183–200. [Google Scholar] [CrossRef]
- Wilhelm, K.P.; Elsner, P.; Berardesca, E.; Maibach, H.I. Bioengineering of the Skin: Skin Imaging and Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Schlüter, H.; Wepf, R.; Moll, I.; Franke, W.W. Sealing the live part of the skin: The integrated meshwork of desmosomes, tight junctions and curvilinear ridge structures in the cells of the uppermost granular layer of the human epidermis. Eur. J. Cell Biol. 2004, 83, 655–665. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; Honeywell-Nguyen, P.L. Skin structure and mode of action of vesicles. Adv. Drug Deliv. Rev. 2002, 54, S41–S55. [Google Scholar] [CrossRef]
- Lai-Cheong, J.E.M.; John, A. Structure and function of skin, hair and nails. Medicine 2009, 37, 223–226. [Google Scholar] [CrossRef]
- Zhang, J.; Michniak-Kohn, B. Investigation of microemulsion microstructures and their relationship to transdermal permeation of model drugs: Ketoprofen, lidocaine, and caffeine. Int. J. Pharm. 2011, 421, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Scheuplein, R.J.; Blank, I.H. Permeability of the Skin. Physiol. Rev. 1971, 51, 702–747. [Google Scholar] [CrossRef] [PubMed]
- Kerscher, W. Trüeb, Funktionen und Aufbau der Haut; Dermatokosmetik, M.K., Williams, S., Trüeb, R.M., Eds.; Springer: Hamburg, Germany, 2009; pp. 1–26. [Google Scholar]
- Sarunyoo, S. An Overview of skin penetration enhancers: Penetration enhancing activity, skin irritation potential and mechanism of action. Songklanakarin J. Sci. Technol. 2009, 31, 299–321. [Google Scholar]
- Zhai, H.; Maibach, H.I. Effects of Skin Occlusion on Percutaneous Absorption: An Overview. Skin Pharmacol. Physiol. 2001, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Toll, R.; Jacobi, U.; Richter, H.; Lademann, J.; Schaefer, H.; Blume-Peytavi, U. Penetration Profile of Microspheres in Follicular Targeting of Terminal Hair Follicles. J. Investig. Dermatol. 2004, 123, 168–176. [Google Scholar] [CrossRef] [Green Version]
- Bouthillette, M.; Beccati, D.; Akthakul, A.; Ramadurai, N.; Nashat, A.; Langer, R.; Anderson, R.R.; Sakamoto, F.H. A crosslinked polymer skin barrier film for moderate to severe atopic dermatitis: A pilot study in adults. J. Am. Acad. Dermatol. 2020, 82, 895–901. [Google Scholar] [CrossRef]
- Roy, S. Preformulation aspects of transdermal drug delivery systems. In Transdermal and Topical Drug Delivery Systems; Ghosh, T.K., Pfister, W.R., Yum, S.I., Eds.; Interpharm Press Inc.: Hauppauge, NY, USA, 1997; pp. 141–166. [Google Scholar]
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef]
- Yu, B.; Kang, S.-Y.; Akthakul, A.; Ramadurai, N.; Pilkenton, M.; Patel, A.; Nashat, A.; Anderson, D.G.; Sakamoto, F.H.; Gilchrest, B.A.; et al. An elastic second skin. Nat. Mater. 2016, 15, 911–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frederiksen, K.; Guy, R.H.; Petersson, K. The potential of polymeric film-forming systems as sustained delivery platforms for topical drugs. Expert Opin. Drug Deliv. 2016, 13, 349–360. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, F.F.D.; Menezes, L.; Tavares, M.I.B. Film-Forming Systems in Topically Administered Pharmaceutical Formulations. Mater. Sci. Appl. 2020, 11, 576–590. [Google Scholar]
- Kathe, K.; Kathpalia, H. Film forming systems for topical and transdermal drug delivery. Asian J. Pharm. Sci. 2017, 12, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Alberti, I.; Grenier, A.; Kraus, H.; Carrara, D.N. Pharmaceutical development and clinical effectiveness of a novel gel technology for transdermal drug delivery. Expert Opin. Drug Deliv. 2005, 2, 935–950. [Google Scholar] [CrossRef]
- Kalia, Y.; Merino, V.; Guy, R.H. Transdermal Drug Delivery: Clinical Aspects. Dermatol. Clin. 1998, 16, 289–299. [Google Scholar] [CrossRef]
- Guy, R.H.; Hadgraft, J. Transdermal drug delivery: A simplified pharmacokinetic approach. Int. J. Pharm. 1985, 24, 267–274. [Google Scholar] [CrossRef]
- Matousek, J.L.; Campbell, K.L.; Kakoma, I.; Solter, P.F.; Schaeffer, D. Evaluation of the effect of pH on in vitro growth of Malassezia pachydermatis. Can. J. Vet. Res. Rev. Can. Rech. Vétérinaire 2003, 67, 56–59. [Google Scholar]
- Bucher, K.E.; Walz, D. Irritant actions of unphysiological pH values. A controlled procedure to test for topical irritancy. Agents Actions 1979, 9, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.; Kim, H.; Na, S.-J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef] [Green Version]
- Felton, L.A. Mechanisms of polymeric film formation. Int. J. Pharm. 2013, 457, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Verband der Chemischen Industrie. Available online: https://www.vci.de/themen/chemikaliensicherheit/reach/vci-position-echa-vorschlag-beschraenkung-polymere-als-absichtlich-eingesetztes-mikroplastik.jsp (accessed on 22 June 2021).
- Chandak, A.R.; Verma, P.R.P. Development and Evaluation of HPMC Based Matrices for Transdermal Patches of Tramadol. Clin. Res. Regul. Aff. 2008, 25, 13–30. [Google Scholar] [CrossRef]
- Patel, D.P.; Setty, C.M.; Mistry, G.N.; Patel, S.L.; Patel, T.J.; Mistry, P.C.; Rana, A.K.; Patel, P.K.; Mishra, R.S. Development and Evaluation of Ethyl Cellulose-Based Transdermal Films of Furosemide for Improved In Vitro Skin Permeation. AAPS Pharm. Sci. Technol. 2009, 10, 437–442. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.S.; Kim, D.Y.; Seo, H.W.; Jeong, S.H.; Kim, J.H.; Kim, M.S. Preparation of erythromycin-loaded poly(vinylalcohol) film and investigation of its feasibility as a transdermal delivery carrier. Tissue Eng. Regen. Med. 2014, 11, 211–216. [Google Scholar] [CrossRef]
- Ranade, S.; Bajaj, A.; Londhe, V.; Babul, N.; Kao, D. Fabrication of topical metered dose film forming sprays for pain management. Eur. J. Pharm. Sci. 2017, 100, 132–141. [Google Scholar] [CrossRef]
- Padula, C.; Nicoli, S.; Santi, P. Innovative formulations for the delivery of levothyroxine to the skin. Int. J. Pharm. 2009, 37, 12–16. [Google Scholar] [CrossRef]
- Bornare, S.S.; Aher, S.S.; Saudagar, R.B. A Review: Film Forming Gel Novel Drug Delivery System. Int. J. Curr. Pharm. Res. 2018, 10, 25–28. [Google Scholar] [CrossRef] [Green Version]
- Gennari, C.G.; Selmin, F.; Franzè, S.; Musazzi, U.M.; Quaroni, G.M.; Casiraghi, A.; Cilurzo, F. A glimpse in critical attributes to design cutaneous film forming systems based on ammonium methacrylate. J. Drug Deliv. Sci. Technol. 2017, 41, 157–163. [Google Scholar] [CrossRef]
- Güngör, S.; Erdal, M.; Özsoy, Y. Plasticizers in Transdermal Drug Delivery Systems. Recent Adv. Plast. 2012, 1, 91–92. [Google Scholar]
- Ammar, H.; Ghorab, M.; Mahmoud, A.A.; Makram, T.S.; Ghoneim, A.M. Rapid pain relief using transdermal film forming polymeric solution of ketorolac. Pharm. Dev. Technol. 2013, 18, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Felton, L.A. Film Coating of Oral Solid Dosage Forms. In Encyclopedia of Pharmaceutical Technology; Swarbrick, J., Ed.; Informa Healthcare: New York, NY, USA, 2007; pp. 1729–1747. [Google Scholar]
- Amnuaikit, C.; Ikeuchi, I.; Ogawara, K.-I.; Higaki, K.; Kimura, T. Skin permeation of propranolol from polymeric film containing terpene enhancers for transdermal use. Int. J. Pharm. 2005, 289, 167–178. [Google Scholar] [CrossRef]
- Crawford, R.R.; Esmerian, O.K. Effect of Plasticizers on Some Physical Properties of Cellulose Acetate Phthalate Films. J. Pharm. Sci. 1971, 60, 312–314. [Google Scholar] [CrossRef]
- Rao, P.; Diwan, P.V. Permeability studies of cellulose acetate free films for transdermal use: Influence of plasticizers. Pharm. Acta Helvetiae 1997, 72, 47–51. [Google Scholar] [CrossRef]
- Bodmeier, R.; Paeratakul, O. Dry and wet strengths of polymeric films prepared from an aqueous colloidal polymer dispersion, Eudragit RS30D. Int. J. Pharm. 1993, 96, 129–138. [Google Scholar] [CrossRef]
- Polymers BASF. Available online: BASF_PS_Pharma_Product-Overview.pdf (accessed on 16 April 2021).
- Jobmann, S.; Stein, T.; Heinz, S.M.; Hoffmann, R. Versorgung akuter traumatischer Wunden. CME 2012, 9, 53–63. [Google Scholar] [CrossRef]
- Schuren, J.; Becker, A.; Sibbald, R.G. A liquid film-forming acrylate for peri-wound protection: A systematic review and meta-analysis (3M™ Cavilon™ no-sting barrier film). Int. Wound J. 2005, 2, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Lamisil Once. Glaxo Smith Klinenconsumer Healthcare. Available online: https://www.lamisil-info.de/lamisil-mittel-gegen-hautpilz/lamisil-once/#:~:text=Lamisil%20Once%20bildet%20direkt%20nach%20dem%20Auftragen%20einen (accessed on 12 May 2021).
- Liqui-patch. Available online: https://www.aescuvest.de/projekte/liqui-patch/ (accessed on 16 April 2021).
- MedSpray. Available online: https://www.medpharm.com/en/discovery/delivery-technology/ (accessed on 16 April 2021).
- Durapeel. Available online: http://www.crescitatherapeutics.com/technology/durapeel/ (accessed on 16 April 2021).
- Liqui-patch Further Projects. Available online: https://www.miradarx.com/licensing-liqui-patch-in-hrt (accessed on 16 April 2021).
- Axiron. Available online: https://www.drugs.com/dosage/axiron.html (accessed on 16 April 2021).
- Axiron. Available online: https://beipackzetteln.de/axiron-30mg15ml (accessed on 16 April 2021).
- GlaxoSmithKline Consumer Healthcare. Available online: https://www.medicines.org.uk/emc/product/6481/smpc (accessed on 30 July 2017).
- Hansaplast Sprühpflaster. Available online: https://www.hansaplast.de/produkte/wundversorgung/sprueh-pflaster (accessed on 16 April 2021).
- Li, B.S.; Cary, J.H.; Maibach, H.I. Stratum corneum substantivity: Drug development implications. Arch. Dermatol. Res. 2018, 310, 537–549. [Google Scholar] [CrossRef]
- Schroeder, I.Z.; Franke, P.; Schaefer, U.F.; Lehr, C.-M. Development and characterization of film forming polymeric solutions for skin drug delivery. Eur. J. Pharm. Biopharm. 2007, 65, 111–121. [Google Scholar] [CrossRef]
- Edwards, A.; Qi, S.; Liu, F.; Brown, M.; McAuley, W. Rationalising polymer selection for supersaturated film forming systems produced by an aerosol spray for the transdermal delivery of methylphenidate. Eur. J. Pharm. Biopharm. 2017, 114, 164–174. [Google Scholar] [CrossRef]
- Garvie-Cook, H.; Frederiksen, K.; Petersson, K.; Guy, R.H.; Gordeev, S.N. Biophysical elucidation of the mechanism of enhanced drug release and topical delivery from polymeric film-forming systems. J. Control. Release 2015, 212, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Garvie-Cook, H.; Frederiksen, K.; Petersson, K.; Guy, R.H.; Gordeev, S. Characterization of topical film-forming systems using atomic force microscopy and Raman microspectroscopy. Mol. Pharm. 2015, 12, 751–757. [Google Scholar] [CrossRef] [Green Version]
- Misra, A.; Raghuvanshi, R.S.; Ganga, S.; Diwan, M.; Talwar, G.; Singh, O. Formulation of a transdermal system for biphasic delivery of testosterone. J. Control. Release 1996, 39, 1–7. [Google Scholar] [CrossRef]
- Schroeder, I.Z.; Franke, P.; Schaefer, U.F.; Lehr, C.-M. Delivery of ethinylestradiol from film forming polymeric solutions across human epidermis in vitro and in vivo in pigs. J. Control. Release 2007, 118, 196–203. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Abbott, P.V. Local Applications of Antibiotics and Antibiotic-Based Agents in Endodontics. In Endodontic Irrigation: Chemical Disinfection of the Root Canal System; Basrani, B., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 253–266. [Google Scholar]
- Gao, P.; Nie, X.; Zou, M.; Shi, Y.; Cheng, G. Recent advances in materials for extended-release antibiotic delivery system. J. Antibiot. 2011, 64, 625–634. [Google Scholar] [CrossRef] [Green Version]
- PREVAIL-FX ONE STEP: Povidone-Iodine and Isopropyl Alcohol Solution CareFusion 2200 Inc. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=39195cf1-3c59-498e-9403-f1889a39e44e&audience=consumer (accessed on 16 April 2021).
- Mori, N.M.; Patel, P.; Sheth, N.R.; Rathod, L.V.; Ashara, K.C. Fabrication and characterization of film-forming voriconazole transdermal spray for the treatment of fungal infection. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 41–51. [Google Scholar] [CrossRef]
- Marra, F.; Nicoli, S.; Padula, C.; Santi, P. Amikacin reverse iontophoresis: Optimization of in vitro extraction. Int. J. Pharm. 2013, 440, 216–220. [Google Scholar] [CrossRef]
- Nicoli, S.; Santi, P. Transdermal delivery of aminoglycosides: Amikacin transport and iontophoretic non-invasive monitoring. J. Control. Release 2006, 111, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Contardi, M.; Heredia-Guerrero, J.A.; Perotto, G.; Valentini, P.; Pompa, P.P.; Spanò, R.; Goldoni, L.; Bertorelli, R.; Athanassiou, A.; Bayer, I.S. Transparent ciprofloxacin-povidone antibiotic films and nanofiber mats as potential skin and wound care dressings. Eur. J. Pharm. Sci. 2017, 104, 133–144. [Google Scholar] [CrossRef]
- Yang, S.; Yang, Y.; Cui, S.; Feng, Z.; Du, Y.; Song, Z.; Tong, Y.; Yang, L.; Wang, Z.; Zeng, H.; et al. Chitosan-polyvinyl alcohol nanoscale liquid film-forming system facilitates MRSA-infected wound healing by enhancing antibacterial and antibiofilm properties. Int. J. Nanomed. 2018, 13, 4987–5002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Paula, E.; Cereda, C.; Tofoli, G.R.; Franz-Montan, M.; Fraceto, L.F.; de Araújo, D.R. Drug Delivery Systems for Local Anesthetics. Recent Pat. Drug Deliv. Formul. 2010, 4, 23–34. [Google Scholar] [CrossRef]
- Padula, C.; Nicoli, S.; Colombo, P.; Santi, P. Single-layer transdermal film containing lidocaine: Modulation of drug release. Eur. J. Pharm. Biopharm. 2007, 66, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Padula, C.; Colombo, G.; Nicoli, S.; Catellani, P.L.; Massimo, G.; Santi, P. Bioadhesive film for the transdermal delivery of lidocaine: In vitro and in vivo behavior. J. Control. Release 2003, 88, 277–285. [Google Scholar] [CrossRef]
- de Araujo, D.R.; Padula, C.; Cereda, C.M.S.; Tófoli, G.R.; Brito, R.B., Jr.; de Paula, E.; Nicoli, S.; Santi, P. Bioadhesive films containing benzocaine: Correlation between in vitro permeation and in vivo local anesthetic effect. Pharm. Res. 2010, 27, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Femenía-Font, A.; Padula, C.; Marra, F.; Balaguer-Fernández, C.; Merino, V.; Castellano, A.L.; Nicoli, S.; Santi, P. Bioadhesive monolayer film for the in vitro transdermal delivery of sumatriptan. J. Pharm. Sci. 2006, 95, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Nicoli, S.; Penna, E.; Padula, C.; Colombo, P.; Santi, P. New transdermal bioadhesive film containing oxybutynin: In vitro permeation across rabbit ear skin. Int. J. Pharm. 2006, 325, 2–7. [Google Scholar] [CrossRef]
- Frederiksen, K.; Guy, R.H.; Petersson, K. Formulation considerations in the design of topical, polymeric film-forming systems for sustained drug delivery to the skin. Eur. J. Pharm. Biopharm. 2015, 91, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.W.; Kim, K.S.; Seo, Y.G.; Lee, B.-J.; Park, Y.J.; Youn, Y.S.; Kim, J.O.; Yong, C.S.; Jin, S.G.; Choi, H.-G. Novel sodium fusidate-loaded film-forming hydrogel with easy application and excellent wound healing. Int. J. Pharm. 2015, 495, 67–74. [Google Scholar] [CrossRef]
- Guo, R.; Du, X.; Zhang, R.; Deng, L.; Dong, A.; Zhang, J. Bioadhesive film formed from a novel organic-inorganic hybrid gel for transdermal drug delivery system. Eur. J. Pharm. Biopharm. 2011, 79, 574–583. [Google Scholar] [CrossRef]
- Liu, X.; Fu, L.; Dai, W.; Liu, W.; Zhao, J.; Wu, Y.; Teng, L.; Sun, F.; Li, Y. Design of transparent film-forming hydrogels of tolterodine and their effects on stratum corneum. Int. J. Pharm. 2014, 471, 322–331. [Google Scholar] [CrossRef]
- Otto, A.; Du Plessis, J.; Wiechers, J.W. Formulation effects of topical emulsions on transdermal and dermal delivery. Int. J. Cosmet. Sci. 2009, 31, 1–19. [Google Scholar] [CrossRef]
- Daniels, R. Compliance messen und verbessern. Pharm. Ztg. 2005, 150, 20–25. [Google Scholar]
- Lunter, D.J.; Daniels, R. New film forming emulsions containing Eudragit(R) NE and/or RS 30D for sustained dermal delivery of nonivamide. Eur. J. Pharm. Biopharm. 2012, 82, 291–298. [Google Scholar] [CrossRef]
- Lunter, D.; Daniels, R. In vitro skin permeation and penetration of nonivamide from novel film-forming emulsions. Skin Pharm. Physiol. 2013, 26, 139–146. [Google Scholar] [CrossRef]
- Rottke, M.; Lunter, D.J.; Daniels, R. In vitro studies on release and skin permeation of nonivamide from novel oil-in-oil-emulsions. Eur. J. Pharm. Biopharm. 2014, 86, 260–266. [Google Scholar] [CrossRef]
- Heck, R.; Lukić, M.Ž.; Savić, S.D.; Daniels, R.; Lunter, D.J. Ex vivo skin permeation and penetration of nonivamide from and in vivo skin tolerability of film-forming formulations containing porous silica. Eur. J. Pharm. Sci. 2017, 106, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Heck, R.; Hermann, S.; Lunter, D.J.; Daniels, R. Film-forming formulations containing porous silica for the sustained delivery of actives to the skin. Eur. J. Pharm. Biopharm. 2016, 108, 1–8. [Google Scholar] [CrossRef]
- Schmidberger, M.; Nikolic, I.; Pantelic, I.; Lunter, D. Optimization of Rheological Behaviour and Skin Penetration of Thermogelling Emulsions with Enhanced Substantivity for Potential Application in Treatment of Chronic Skin Diseases. Pharmaceutics 2019, 11, 361. [Google Scholar] [CrossRef] [Green Version]
- Schmidberger, M.; Daniels, R.; Lunter, D.J. Method to determine the impact of substantivity on ex vivo skin-permeation. Eur. J. Pharm. Biopharm. 2018, 131, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, S.; Daniels, R.; Lunter, D. Methods for the determination of the substantivity of topical formulations. Pharm. Dev. Technol. 2017, 22, 487–491. [Google Scholar] [CrossRef] [PubMed]



| Polymer | Properties |
|---|---|
| Polymer | Properties |
| Carbopol (polyacrylat) | water-soluble, pH sensitive |
| Chitosan (Poly-D-Glucosamin) | water-soluble at pH < 7 |
| Crosslinked polymer layer XPL | adhesive, elastic |
| Dermacryl 79 (Carboxylates Ac rylpolymer) | water-insoluble |
| Ethylcellulose | non-toxic, not irritating, anti-allergic |
| Eudragit NE (ethylacrylate methylmethacrylate copolymer) | water-insoluble, transparent, elastic, adhesive |
| Eudragit RL-100 (polymethacrylate polymere) | water-insoluble, transparent, elastic, adhesive |
| Eudragit RS-100 (polymethacrylate polymere) | water-insoluble, transparent, elastic, adhesive |
| Eudragit L30D-55 (methacrylate-ethylacrylate-copolymer) | water dispersible at pH 2–3 |
| Hydroxypropyl-beta-cyclodextrin | water-insoluble, increases bioavailability |
| Hydroxypropylmethylcellulose (HPMC) | water-soluble, non-ionic |
| KIucel (Hydroxypropyl cellulose) | water-soluble, non-ionic |
| Macrogol | water-soluble |
| Methyl cellulose | water-soluble |
| Poloxamer (polyethylenepolypropylene glycol) | thermoreversible |
| Plastoid (Butyl methacrylate-methylmethacrylate copolymer) | water-insoluble |
| Polydimethylsiloxane (PDMS) | water-insoluble, non-toxic |
| Polyvinyl alcohol (PVA) | water-soluble, adhesive, non-toxic |
| Polyvinyl pyrrolidine (PVP) | water-soluble, adhesive, increase bioavailability |
| Quaternary polymethacrylat (QPM) | water-insoluble |
| Sepineo P600 (acrylamide/sodium acryloldimethyltaurate) | water-insoluble |
| Silicone | water-soluble, non-occlusive |
| Solvent | Properties |
|---|---|
| Benzyl alcohole | lipophilic, organic solvent |
| Butanol | organic solvent |
| Ethanol | organic solvent, volatile, hydrophil |
| Ethylacetat | organic solvent, lipophilic |
| Isopropanol | organic solvent, volatile, hydrophilic |
| Isopropyl myristat | organic solvent, lipophilic, penetration enhancer |
| Polyethylene glycole | hydrophilic, penetration enhancer |
| Water | hydrophilic |
| Plasticizer | Properties | Polymer | Concentration in % |
|---|---|---|---|
| Dibutylphthalat | Plasticizer | Eudragit E 100, Ethyl cellulose, Polyvinylpyrollidone, Hydroxypropyl methyl cellulose, | 10–40 |
| Glycerol | Plasticizer | Polyvinyl Alcohol, Polyvinylpyrrolidone | 10–30 |
| Polyethylenglycol 300 or 400 | Plasticizer | Hydroxypropyl cellulose, Eudragit E 100, Carbopol, | 5 |
| Polysorbat 80 | Non-ionic solubilizer, plasticizer, emulsifier, co-emulsifier | Cellulose Acetate | 20–50 |
| Propylene glycol | Polymeric solubilizer, plasticizer | Polyvinyl Alcohol, Polyvinylpyrrolidone, Eudragit L100, Hydroxypropyl methyl cellulose, Ethylcellulose, Carboxy methyl cellulose | 5–50 |
| Sorbitol | Plasticizer | Polyvinyl Alcohol, Chitosan | 2–20 |
| Triacetin | Versatile water or oil miscible solvent. plasticizer | Eudragit E 100 | 1.43–5.48 |
| Triethyl citrate | Plasticizer | Hydroxypropyl methyl cellulose, Eudragit RL and NE, Acrylate copolymer, Polyvinylpyrrolidone, Polyvinyl Alcohol | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pünnel, L.C.; Lunter, D.J. Film-Forming Systems for Dermal Drug Delivery. Pharmaceutics 2021, 13, 932. https://doi.org/10.3390/pharmaceutics13070932
Pünnel LC, Lunter DJ. Film-Forming Systems for Dermal Drug Delivery. Pharmaceutics. 2021; 13(7):932. https://doi.org/10.3390/pharmaceutics13070932
Chicago/Turabian StylePünnel, Larissa Carine, and Dominique Jasmin Lunter. 2021. "Film-Forming Systems for Dermal Drug Delivery" Pharmaceutics 13, no. 7: 932. https://doi.org/10.3390/pharmaceutics13070932
APA StylePünnel, L. C., & Lunter, D. J. (2021). Film-Forming Systems for Dermal Drug Delivery. Pharmaceutics, 13(7), 932. https://doi.org/10.3390/pharmaceutics13070932







