Maturation and Protection Effect of Retinal Tissue-Derived Bioink for 3D Cell Printing Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of RdECM Bioink
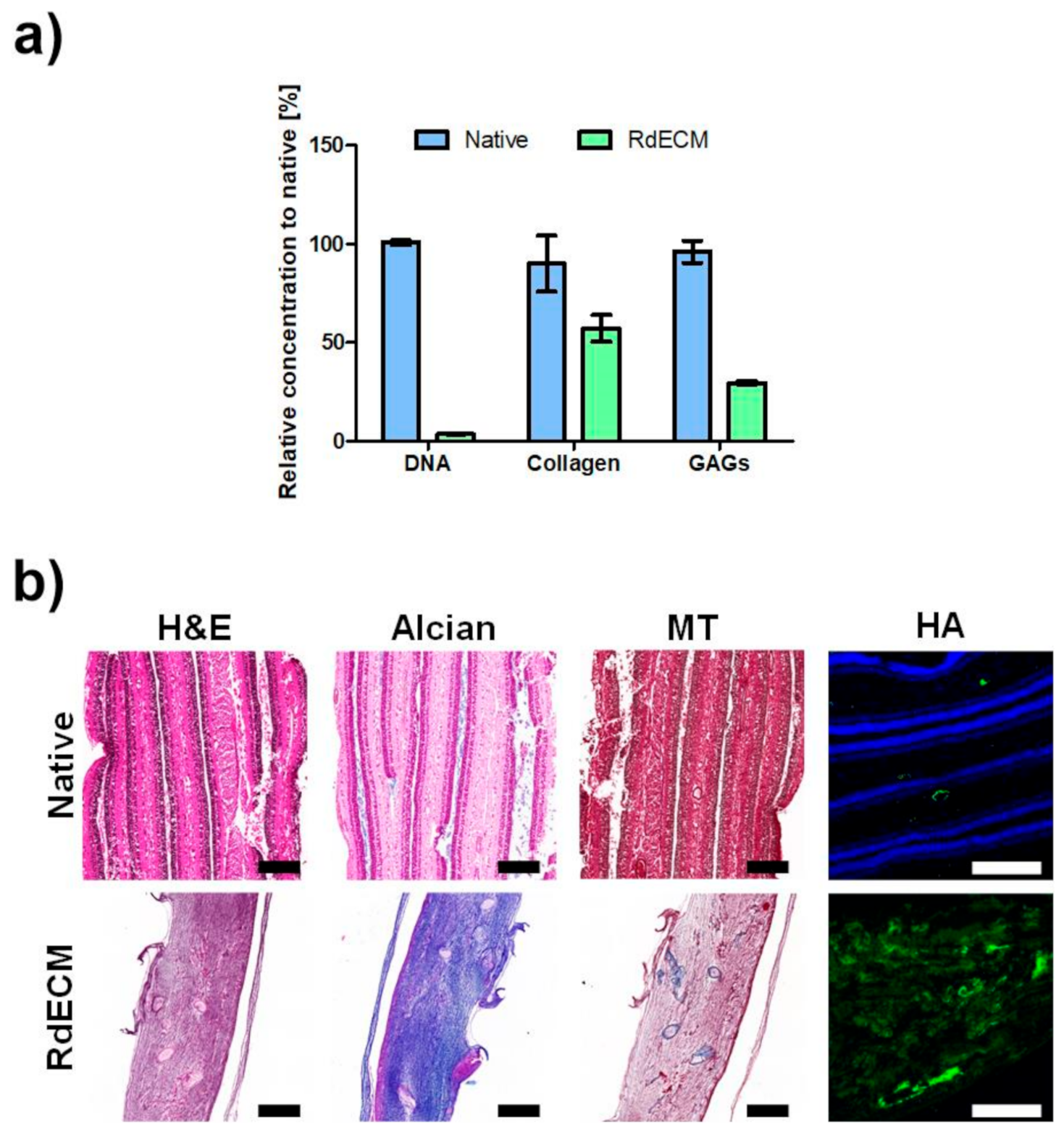
2.2. Biochemical Characterization
2.3. Immunohistochemistry
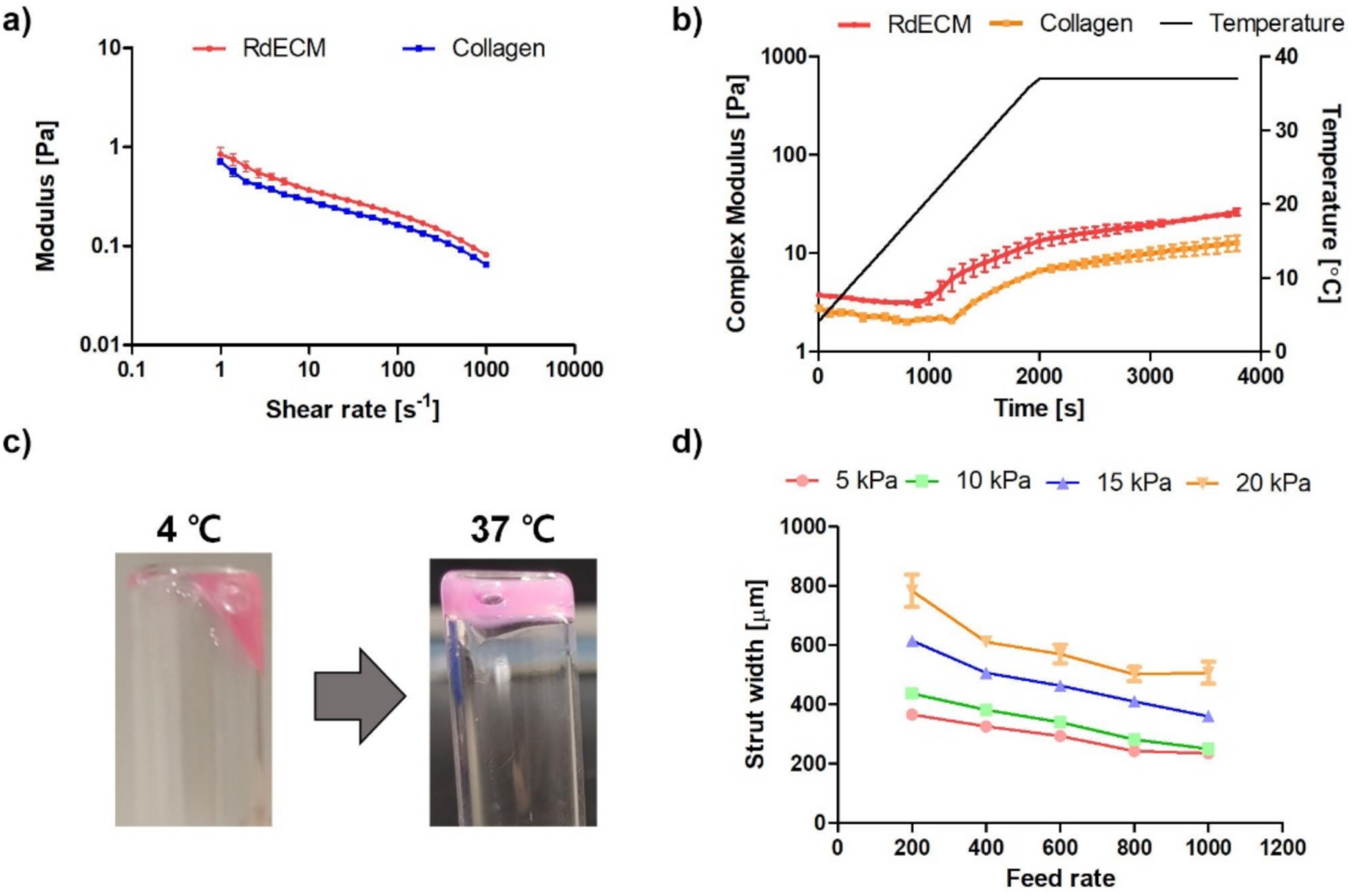
2.4. Rheological Characterization
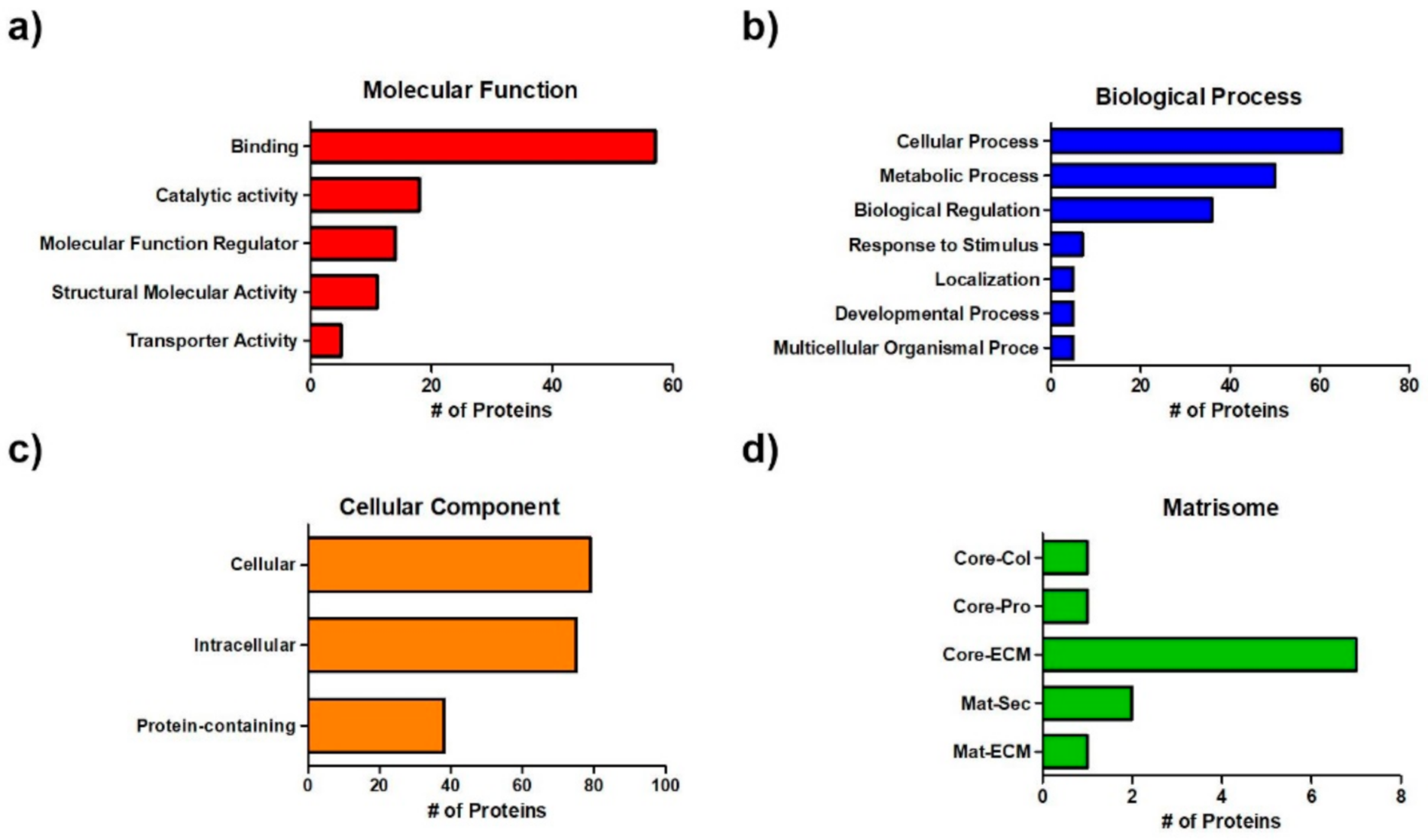
2.5. Proteomic Analysis
2.6. Cell Culture
2.7. Printability Test
2.8. Encapsulation of the Cell and Printing
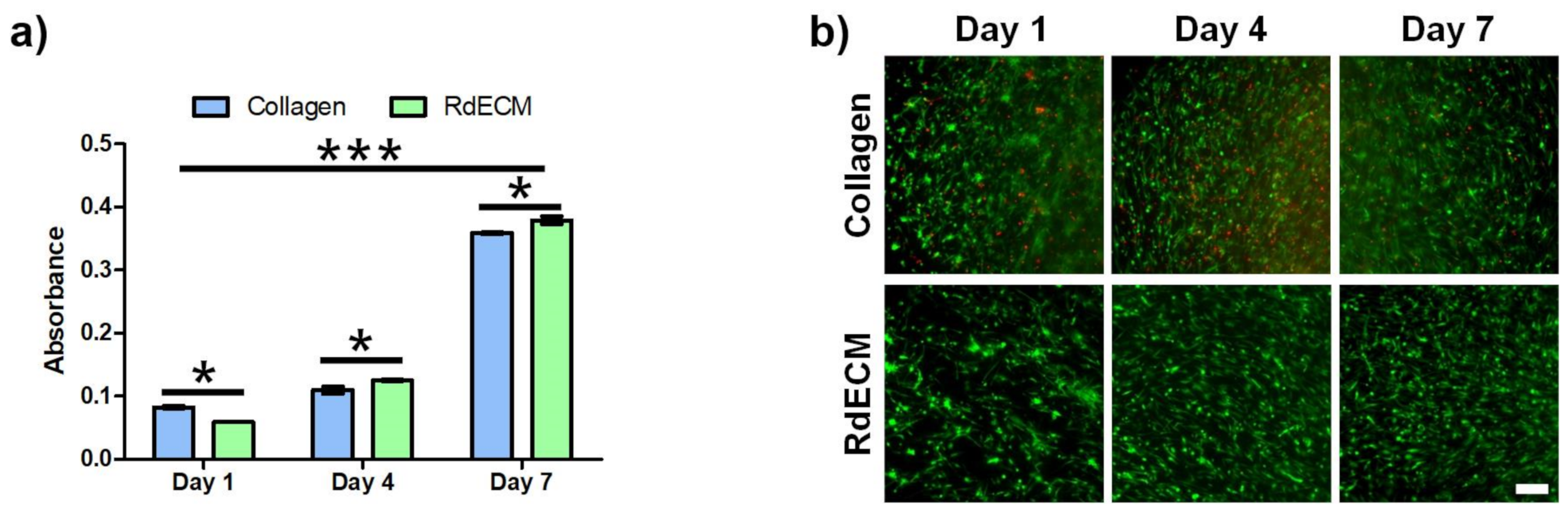
2.9. Proliferation and Live/Dead Assay
2.10. Immunostaining
2.11. Ethics Statement
2.12. Animal Care
2.13. Animal Experiments
2.14. Statistical Analysis
3. Results
3.1. Biochemical Characterization
3.2. Proteomic Analysis
3.3. Rheological Characterization
3.4. Printability Test
3.5. Proliferation and Viability of the Printed Structure
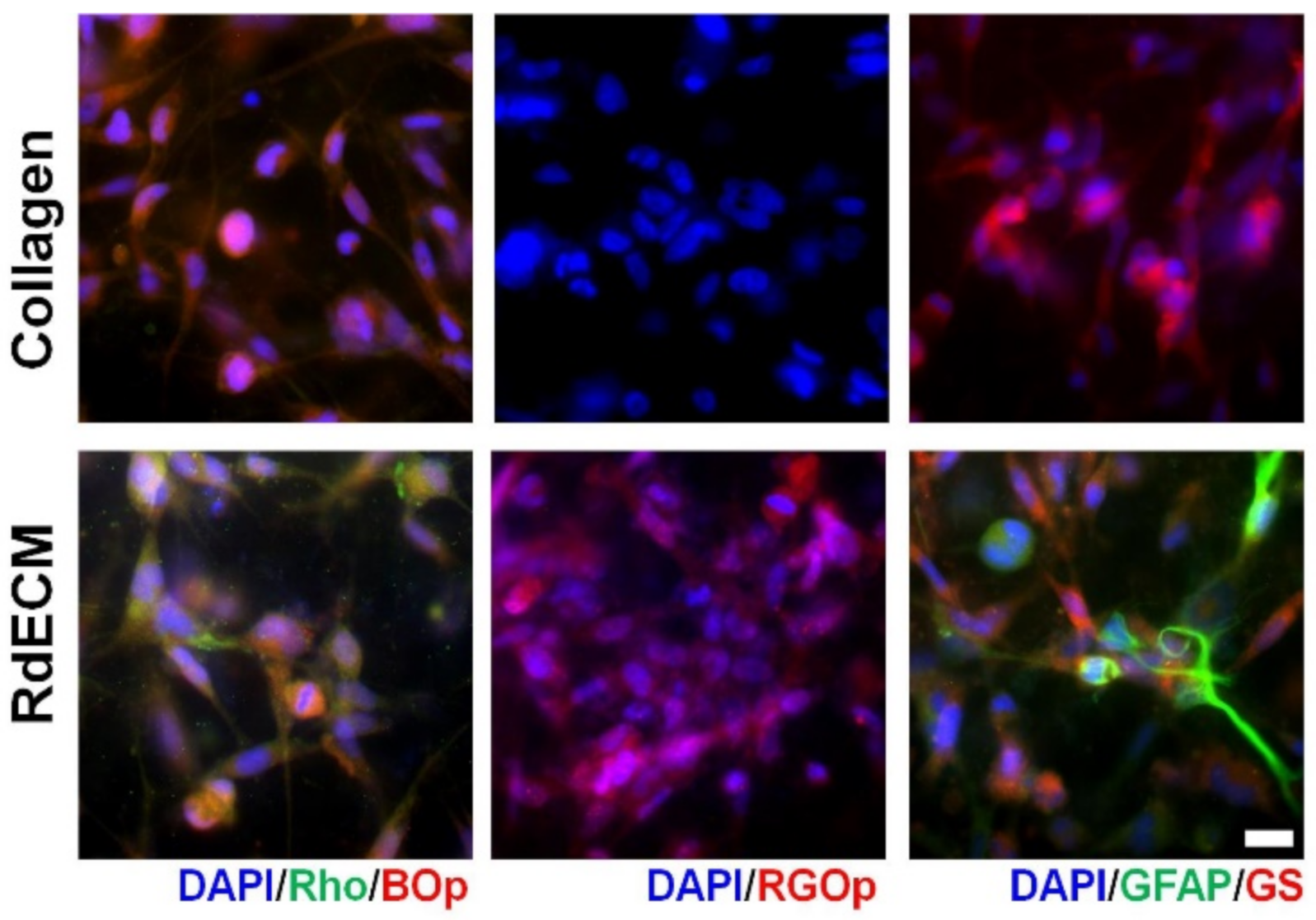
3.6. Effect of RdECM in Muller Cell Differentiation
3.7. Protective Effect of RdECM in Laser-CNV and NMU-RD model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harrison, F.; Crow, M. 4. Functional Vision and Creating Visual Interest. In Living and Learning with Blind Children; University of Toronto Press: Toronto, ON, Canada, 2019; pp. 107–119. [Google Scholar]
- Koretz, J.F.; Handelman, G.H. How the Human Eye Focuses. Sci. Am. 1988, 259, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Buch, H.; Vinding, T.; La Cour, M.; Appleyard, M.; Jensen, G.B.; Nielsen, N.V. Prevalence and causes of visual impairment and blindness among 9980 Scandinavian adults: The Copenhagen City Eye Study. Ophthalmology 2004, 111, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Strong, S.; Liew, G.; Michaelides, M. Retinitis pigmentosa-associated cystoid macular oedema: Pathogenesis and avenues of intervention. Br. J. Ophthalmol. 2017, 101, 31–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, T.Y.; Sabanayagam, C. Strategies to Tackle the Global Burden of Diabetic Retinopathy: From Epidemiology to Artificial Intelligence. Ophthalmologica 2020, 243, 9–20. [Google Scholar] [CrossRef]
- Higuchi, A.; Kumar, S.S.; Benelli, G.; Alarfaj, A.A.; Munusamy, M.A.; Umezawa, A.; Murugan, K. Stem Cell Therapies for Reversing Vision Loss. Trends Biotechnol. 2017, 35, 1102–1117. [Google Scholar] [CrossRef]
- Gu, X.; Yu, X.; Zhao, C.; Duan, P.; Zhao, T.; Liu, Y.; Li, S.; Yang, Z.; Li, Y.; Qian, C.; et al. Efficacy and Safety of Autologous Bone Marrow Mesenchymal Stem Cell Transplantation in Patients with Diabetic Retinopathy. Cell. Physiol. Biochem. 2018, 49, 40–52. [Google Scholar] [CrossRef]
- Luo, J.; Baranov, P.; Patel, S.; Ouyang, H.; Quach, J.; Wu, F.; Qiu, A.; Luo, H.; Hicks, C.; Zeng, J.; et al. Human Retinal Progenitor Cell Transplantation Preserves Vision. J. Biol. Chem. 2014, 289, 6362–6371. [Google Scholar] [CrossRef] [Green Version]
- Seiler, M.J.; Aramant, R.B. Cell replacement and visual restoration by retinal sheet transplants. Prog. Retinal Eye Res. 2012, 31, 661–687. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chen, S.J.; Li, S.Y.; Qu, L.H.; Meng, X.H.; Wang, Y.; Xu, H.W.; Liang, Z.Q.; Yin, Z.Q. Long-term safety of human retinal progenitor cell transplantation in retinitis pigmentosa patients. Stem Cell Res. Ther. 2017, 8, 209. [Google Scholar] [CrossRef] [Green Version]
- Marchena, M.; Villarejo-Zori, B.; Zaldivar-Diez, J.; Palomo, V.; Gil, C.; Sánchez, C.H.; Martínez, A.; De La Rosa, E.J. Small molecules targeting glycogen synthase kinase 3 as potential drug candidates for the treatment of retinitis pigmentosa. J. Enzyme Inhib. Med. Chem. 2017, 32, 522–526. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Sun, X. Complement system and age-related macular degeneration: Drugs and challenges. Drug Des. Dev. Ther. 2019, 13, 2413–2425. [Google Scholar] [CrossRef]
- US-FDA. Guidance for Industry. Nonsterile Semisolid Dosage Forms, Scale-Up and Postapproval Changes: Chemistry, Manufacturing, and Controls; In Vitro Release Testing and In Vivo Bioequivalence Documentation; US-FDA: Silver Spring, MD, USA, 1997.
- Wang, Y.; Yin, Z.; Gao, L.; Sun, D.; Hu, X.; Xue, L.; Dai, J.; Zeng, Y.; Chen, S.; Pan, B.; et al. Curcumin Delays Retinal Degeneration by Regulating Microglia Activation in the Retina of rd1 Mice. Cell. Physiol. Biochem. 2017, 44, 479–493. [Google Scholar] [CrossRef]
- Maminishkis, A.; Chen, S.; Jalickee, S.; Banzon, T.; Shi, G.; Wang, F.E.; Ehalt, T.; Hammer, J.A.; Miller, S.S. Confluent Monolayers of Cultured Human Fetal Retinal Pigment Epithelium Exhibit Morphology and Physiology of Native Tissue. Investig. Opthalmol. Vis. Sci. 2006, 47, 3612–3624. [Google Scholar] [CrossRef] [Green Version]
- Völkner, M.; Zschätzsch, M.; Rostovskaya, M.; Overall, R.; Busskamp, V.; Anastassiadis, K.; Karl, M.O. Retinal Organoids from Pluripotent Stem Cells Efficiently Recapitulate Retinogenesis. Stem Cell Rep. 2016, 6, 525–538. [Google Scholar] [CrossRef] [Green Version]
- Reichman, S.; Slembrouck, A.; Gagliardi, G.; Chaffiol, A.; Terray, A.; Nanteau, C.; Potey, A.; Belle, M.; Rabesandratana, O.; Duebel, J.; et al. Generation of Storable Retinal Organoids and Retinal Pigmented Epithelium from Adherent Human iPS Cells in Xeno-Free and Feeder-Free Conditions. Stem Cells 2017, 35, 1176–1188. [Google Scholar] [CrossRef] [Green Version]
- Achberger, K.; Probst, C.; Haderspeck, J.; Bolz, S.; Rogal, J.; Chuchuy, J.; Nikolova, M.; Cora, V.; Antkowiak, L.; Haq, W.; et al. Merging organoid and organ-on-a-chip technology to generate complex multi-layer tissue models in a human retina-on-a-chip platform. eLife 2019, 8, e46188. [Google Scholar] [CrossRef]
- Al-Ubaidi, M.R.; Naash, M.I.; Conley, S.M. A perspective on the role of the extracellular matrix in progressive retinal degenerative disorders. Investig. Ophthalmol. Vis. Sci. 2013, 54, 8119–8124. [Google Scholar] [CrossRef] [Green Version]
- Baranov, P.Y.; Tucker, B.A.; Young, M.J. Low-Oxygen Culture Conditions Extend the Multipotent Properties of Human Retinal Progenitor Cells. Tissue Eng. 2014, 20, 1465–1475. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kong, J.S.; Han, W.; Kim, B.S.; Cho, D.-W. 3D Cell Printing of Tissue/Organ-Mimicking Constructs for Therapeutic and Drug Testing Applications. Int. J. Mol. Sci. 2020, 21, 7757. [Google Scholar] [CrossRef]
- Jang, J.; Park, J.Y.; Gao, G.; Cho, D.-W. Biomaterials-based 3D cell printing for next-generation therapeutics and diagnostics. Biomaterials 2018, 156, 88–106. [Google Scholar] [CrossRef]
- Gao, G.; Kim, B.S.; Jang, J.; Cho, D.-W. Recent Strategies in Extrusion-Based Three-Dimensional Cell Printing toward Organ Biofabrication. ACS Biomater. Sci. Eng. 2019, 5, 1150–1169. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Jang, J.; Ha, D.-H.; Kim, S.W.; Rhie, J.-W.; Shim, J.-H.; Kim, D.-H.; Cho, D.-W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Park, J.Y.; Kong, J.S.; Lee, H.; Won, J.Y.; Cho, D.W. Development of 3D Printed Bruch’s Membrane-Mimetic Substance for the Maturation of Retinal Pigment Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 1095. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Das, S.; Jang, J.; Cho, D.-W. Decellularized Extracellular Matrix-based Bioinks for Engineering Tissue- and Organ-specific Microenvironments. Chem. Rev. 2020, 120, 10608–10661. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.; Amaral, J.; Becerra, S.P.; Fariss, R.N. A Novel Imaging Technique for Experimental Choroidal Neovascularization. Investig. Opthalmol. Vis. Sci. 2006, 47, 5163–5170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, F.-L.; Lin, C.-H.; Ho, J.-D.; Yen, J.-L.; Chang, H.-M.; Chiou, G.C.Y.; Cheng, Y.-W.; Hsiao, G. The natural retinoprotectant chrysophanol attenuated photoreceptor cell apoptosis in an N-methyl-N-nitrosourea-induced mouse model of retinal degenaration. Sci. Rep. 2017, 7, 41086. [Google Scholar] [CrossRef] [Green Version]
- D’Amato, R.; Wesolowski, E.; Smith, L. Microscopic Visualization of the Retina by Angiography with High-Molecular-Weight Fluorescein-Labeled Dextrans in the Mouse. Microvasc. Res. 1993, 46, 135–142. [Google Scholar] [CrossRef]
- Ying, Y.; Ueta, T.; Jiang, S.; Lin, H.; Wang, Y.; Vavvas, D.; Wen, R.; Chen, Y.-G.; Luo, Z. Metformin inhibits ALK1-mediated angiogenesis via activation of AMPK. Oncotarget 2017, 8, 32794–32806. [Google Scholar] [CrossRef]
- Roggia, M.F.; Imai, H.; Shiraya, T.; Noda, Y.; Ueta, T. Protective Role of Glutathione Peroxidase 4 in Laser-Induced Choroidal Neovascularization in Mice. PLoS ONE 2014, 9, e98864. [Google Scholar] [CrossRef]
- Tomita, M.; Lavik, E.; Klassen, H.; Zahir, T.; Langer, R.; Young, M.J. Biodegradable Polymer Composite Grafts Promote the Survival and Differentiation of Retinal Progenitor Cells. Stem Cells 2005, 23, 1579–1588. [Google Scholar] [CrossRef]
- Park, J.; Baranov, P.; Aydin, A.; Abdelgawad, H.; Singh, D.; Niu, W.; Kurisawa, M.; Spector, M.; Young, M.J. In Situ Cross-linking Hydrogel as a Vehicle for Retinal Progenitor Cell Transplantation. Cell Transplant. 2019, 28, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Tucker, B.A.; Zhang, X.; Checa-Casalengua, P.; Herrero-Vanrell, R.; Young, M.J. Robust cell integration from co-transplantation of biodegradable MMP2-PLGA microspheres with retinal progenitor cells. Biomaterials 2011, 32, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Ko, C.W.; Baranov, P.Y.; Regatieri, C.V.; Redenti, S.; Tucker, B.A.; Mighty, J.; Tao, S.L.; Young, M.J. Enhanced differentiation and delivery of mouse retinal progenitor cells using a micropatterned biodegradable thin-film polycaprolactone scaffold. Tissue Eng. 2015, 21, 1247–1260. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Wang, S.; Xia, T.; Tainsh, L.; Ghiassi-Nejad, M.; Xu, T.; Peng, S.; Adelman, R.A.; Rizzolo, L.J. A biodegradable scaffold enhances differentiation of embryonic stem cells into a thick sheet of retinal cells. Biomaterials 2018, 154, 158–168. [Google Scholar] [CrossRef]
- Redenti, S.; Tao, S.; Yang, J.; Gu, P.; Klassen, H.; Saigal, S.; Desai, T.; Young, M.J. Retinal tissue engineering using mouse retinal progenitor cells and a novel biodegradable, thin-film poly(e-caprolactone) nanowire scaffold. J. Ocul. Biol. Dis. Inform. 2008, 1, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Redenti, S.; Neeley, W.L.; Rompani, S.; Saigal, S.; Yang, J.; Klassen, H.; Langer, R.; Young, M.J. Engineering retinal progenitor cell and scrollable poly(glycerol-sebacate) composites for expansion and subretinal transplantation. Biomaterials 2009, 30, 3405–3414. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Han, W.; Kim, H.; Ha, D.-H.; Jang, J.; Kim, B.S.; Cho, D.-W. Development of Liver Decellularized Extracellular Matrix Bioink for Three-Dimensional Cell Printing-Based Liver Tissue Engineering. Biomacromolecules 2017, 18, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jang, J.; Park, J.; Lee, K.-P.; Lee, S.; Lee, D.-M.; Kim, K.H.; Kim, H.K.; Cho, D.-W. Shear-induced alignment of collagen fibrils using 3D cell printing for corneal stroma tissue engineering. Biofabrication 2019, 11, 035017. [Google Scholar] [CrossRef]
- Han, W.; Singh, N.K.; Kim, J.J.; Kim, H.; Kim, B.S.; Park, J.Y.; Jang, J.; Cho, D.-W. Directed differential behaviors of multipotent adult stem cells from decellularized tissue/organ extracellular matrix bioinks. Biomaterials 2019, 224, 119496. [Google Scholar] [CrossRef]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biol. 2016, 49, 10–24. [Google Scholar] [CrossRef]
- Krasny, L.; Paul, A.; Wai, P.; Howard, B.A.; Natrajan, R.C.; Huang, P.H. Comparative proteomic assessment of matrisome enrichment methodologies. Biochem. J. 2016, 473, 3979–3995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naba, A.; Clauser, K.; Hoersch, S.; Liu, H.; Carr, S.A.; Hynes, R.O. The Matrisome: In Silico Definition and In Vivo Characterization by Proteomics of Normal and Tumor Extracellular Matrices. Mol. Cell. Proteom. 2012, 11, 014647. [Google Scholar] [CrossRef] [Green Version]
- Reinhard, J.; Roll, L.; Faissner, A. Tenascins in Retinal and Optic Nerve Neurodegeneration. Front. Integr. Neurosci. 2017, 11, 30. [Google Scholar] [CrossRef]
- Serjanov, D.; Bachay, G.; Hunter, D.D.; Brunken, W.J. Laminin β2 Chain Regulates Retinal Progenitor Cell Mitotic Spindle Orientation via Dystroglycan. J. Neurosci. 2018, 38, 5996–6010. [Google Scholar] [CrossRef] [Green Version]
- Miri, A.K.; Mirzaee, I.; Hassan, S.; Oskui, S.M.; Nieto, D.; Khademhosseini, A.; Zhang, Y.S. Effective bioprinting resolution in tissue model fabrication. Lab Chip 2019, 19, 2019–2037. [Google Scholar] [CrossRef] [PubMed]
- Morgan, F.L.C.; Moroni, L.; Baker, M.B. Dynamic Bioinks to Advance Bioprinting. Adv. Healthc. Mater. 2020, 9, 1901798. [Google Scholar] [CrossRef] [PubMed]
- Dorgau, B.; Felemban, M.; Sharpe, A.; Bauer, R.; Hallam, D.; Steel, D.H.; Lindsay, S.; Mellough, C.; Lako, M. Laminin γ3 plays an important role in retinal lamination, photoreceptor organisation and ganglion cell differentiation. Cell Death Dis. 2018, 9, 615. [Google Scholar] [CrossRef] [PubMed]
- Hollborn, M.; Ulbricht, E.; Rillich, K.; Dukic-Stefanovic, S.; Wurm, A.; Wagner, L.; Reichenbach, A.; Wiedemann, P.; Limb, G.A.; Bringmann, A.; et al. The human Müller cell line MIO-M1 expresses opsins. Mol. Vis. 2011, 17, 2738–2750. [Google Scholar] [PubMed]
- Kolb, H. Glial Cells of the Retina; National Library of Medicine: Bethesda, MD, USA, 2007.
- Bhatia, B.; Jayaram, H.; Singhal, S.; Jones, M.F.; Limb, G.A. Differences between the neurogenic and proliferative abilities of Müller glia with stem cell characteristics and the ciliary epithelium from the adult human eye. Exp. Eye Res. 2011, 93, 852–861. [Google Scholar] [CrossRef] [Green Version]
- Giannelli, S.G.; Demontis, G.C.; Pertile, G.; Rama, P.; Broccoli, V. Adult Human Müller Glia Cells Are a Highly Efficient Source of Rod Photoreceptors. Stem Cells 2011, 29, 344–356. [Google Scholar] [CrossRef]
- Lawrence, J.M.; Singhal, S.; Bhatia, B.; Keegan, D.J.; Reh, T.A.; Luthert, P.J.; Khaw, P.T.; Limb, G.A. MIO-M1 Cells and Similar Müller Glial Cell Lines Derived from Adult Human Retina Exhibit Neural Stem Cell Characteristics. Stem Cells 2007, 25, 2033–2043. [Google Scholar] [CrossRef]
- Lambert, V.; Lecomte, J.; Hansen, S.; Blacher, S.; Gonzalez, M.-L.A.; Struman, I.; Sounni, N.E.; Rozet, E.; De Tullio, P.; Foidart, J.M.; et al. Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Nat. Protoc. 2013, 8, 2197–2211. [Google Scholar] [CrossRef]
- Rastoin, O.; Dufies, M. Experimental Models in Neovascular Age Related Macular Degeneration. Int. J. Mol. Sci. 2020, 21, 4627. [Google Scholar] [CrossRef]
- Tsubura, A.; Yoshizawa, K.; Kuwata, M.; Uehara, N. Animal models for retinitis pigmentosa induced by MNU; disease progression, mechanisms and therapeutic trials. Histol. Histopathol. 2010, 25, 933–944. [Google Scholar]
- Yan, W.; Long, P.; Wei, D.; Yan, W.; Zheng, X.; Chen, G.; Wang, J.; Zhang, Z.; Chen, T.; Chen, M. Protection of retinal function and morphology in MNU-induced retinitis pigmentosa rats by ALDH2: An in-vivo study. BMC Ophthalmol. 2020, 20, 55. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, R.; Zarembinski, T.I.; Doty, N.; Jiang, C.; Regatieri, C.; Zhang, X.; Young, M.J. The application of hyaluronic acid hydrogels to retinal progenitor cell transplantation. Tissue Eng. 2013, 19, 135–142. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Kong, J.S.; Kim, H.; Han, W.; Won, J.Y.; Cho, D.-W. Maturation and Protection Effect of Retinal Tissue-Derived Bioink for 3D Cell Printing Technology. Pharmaceutics 2021, 13, 934. https://doi.org/10.3390/pharmaceutics13070934
Kim J, Kong JS, Kim H, Han W, Won JY, Cho D-W. Maturation and Protection Effect of Retinal Tissue-Derived Bioink for 3D Cell Printing Technology. Pharmaceutics. 2021; 13(7):934. https://doi.org/10.3390/pharmaceutics13070934
Chicago/Turabian StyleKim, Jongmin, Jeong Sik Kong, Hyeonji Kim, Wonil Han, Jae Yon Won, and Dong-Woo Cho. 2021. "Maturation and Protection Effect of Retinal Tissue-Derived Bioink for 3D Cell Printing Technology" Pharmaceutics 13, no. 7: 934. https://doi.org/10.3390/pharmaceutics13070934
APA StyleKim, J., Kong, J. S., Kim, H., Han, W., Won, J. Y., & Cho, D.-W. (2021). Maturation and Protection Effect of Retinal Tissue-Derived Bioink for 3D Cell Printing Technology. Pharmaceutics, 13(7), 934. https://doi.org/10.3390/pharmaceutics13070934








