Probucol Pharmacological and Bio-Nanotechnological Effects on Surgically Transplanted Graft Due to Powerful Anti-Inflammatory, Anti-Fibrotic and Potential Bile Acid Modulatory Actions
Abstract
:1. Introduction
2. Materials and Methods
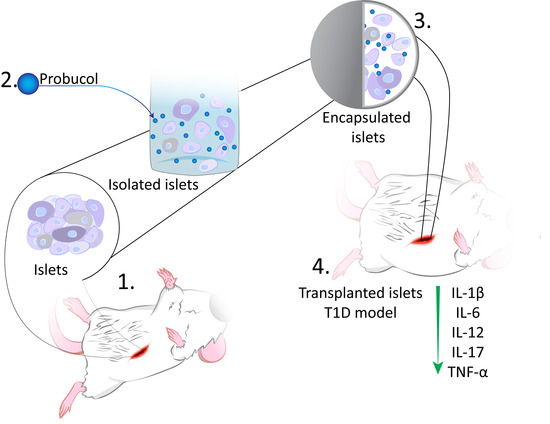
2.1. Formulation and Islet-Microcapsule Formation
2.2. Processes of Islet Extraction and Transplantation (Balb/c Mice)
2.3. Imaging Analyses
2.4. Diabetes Induction and Blood Glucose and Insulin Measurements
2.5. Study Design and Quantification of Cytokines and the Bile Acid Profile
3. Results and Discussion
3.1. Imaging, Topography, and Surface Analysis Measurements
3.2. Blood Glucose and Inflammatory Cytokines Measurements
3.3. Bile Acid Measurements
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Negrulj, R.; Mooranian, A.; Al-Salami, H. Potentials and Limitations of Bile Acids in Type 2 Diabetes Mellitus: Applications of Microencapsulation as a Novel Oral Delivery System. J. Endocrinol. Diabetes Mellit. 2013, 1, 49–59. [Google Scholar]
- Haghighatpanah, M.; Nejad, A.S.M.; Haghighatpanah, M.; Thunga, G.; Mallayasamy, S. Factors that Correlate with Poor Glycemic Control in Type 2 Diabetes Mellitus Patients with Complications. Osong Public Health Res. Perspect 2018, 9, 167–174. [Google Scholar] [CrossRef]
- Llacua, A.; de Haan, B.J.; Smink, S.A.; de Vos, P. Extracellular matrix components supporting human islet function in alginate-based immunoprotective microcapsules for treatment of diabetes. J. Biomed. Mater. Res. A 2016, 104, 1788–1796. [Google Scholar] [CrossRef]
- de Vos, P.; van Hoogmoed, C.G.; van Zanten, J.; Netter, S.; Strubbe, J.H.; Busscher, H.J. Long-term biocompatibility, chemistry, and function of microencapsulated pancreatic islets. Biomaterials 2003, 24, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Mooranian, A.; Negrulj, R.; Takechi, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. Influence of Biotechnological Processes, Speed of Formulation Flow and Cellular Concurrent Stream-Integration on Insulin Production from β-cells as a Result of Co-Encapsulation with a Highly Lipophilic Bile Acid. Cell. Mol. Bioeng. 2018, 11, 65–75. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Takechi, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. Electrokinetic potential-stabilization by bile acid-microencapsulating formulation of pancreatic β-cells cultured in high ratio poly-L-ornithine-gel hydrogel colloidal dispersion: Applications in cell-biomaterials, tissue engineering and biotechnological applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1156–1162. [Google Scholar] [CrossRef] [Green Version]
- Mooranian, A.; Tackechi, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. Innovative Microcapsules for Pancreatic β-Cells Harvested from Mature Double-Transgenic Mice: Cell Imaging, Viability, Induced Glucose-Stimulated Insulin Measurements and Proinflammatory Cytokines Analysis. Pharm. Res. 2017, 34, 1217–1223. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Takechi, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. New Biotechnological Microencapsulating Methodology Utilizing Individualized Gradient-Screened Jet Laminar Flow Techniques for Pancreatic β-Cell Delivery: Bile Acids Support Cell Energy-Generating Mechanisms. Mol. Pharm. 2017, 14, 2711–2718. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Takechi, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. Alginate-combined cholic acid increased insulin secretion of microencapsulated mouse cloned pancreatic β cells. Ther. Deliv. 2017, 8, 833–842. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. The effects of Ionic Gelation-Vibrational Jet Flow technique in fabrication of microcapsules incorporating β-cell: Applications in Type-1 Diabetes. Curr. Diabetes Rev. 2017, 13, 91–96. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. The incorporation of water-soluble gel matrix into bile acid-based microcapsules for the delivery of viable β-cells of the pancreas, in diabetes treatment: Biocompatibility and functionality studies. Drug Deliv. Transl. Res. 2016, 6, 17–23. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. Viability and topographical analysis of microencapsulated β-cells exposed to a biotransformed tertiary bile acid: An ex vivo study. Int. J. Nano Biomater. 2016, 6, 74–82. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. Alginate-deoxycholic Acid Interaction and Its Impact on Pancreatic Β-Cells and Insulin Secretion and Potential Treatment of Type 1 Diabetes. J. Pharm. Innov. 2016, 11, 156–161. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Arfuso, F.; Al-Salami, H. Characterization of a novel bile acid-based delivery platform for microencapsulated pancreatic beta-cells. Artif. Cells Nanomed. Biotechnol. 2016, 44, 194–200. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Al-Salami, H.; Morahan, G.; Jamieson, E. Designing anti-diabetic beta-cells microcapsules using polystyrenic sulfonate, polyallylamine, and a tertiary bile acid: Morphology, bioenergetics, and cytokine analysis. Biotechnol. Prog. 2016, 32, 501–509. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. Flow vibration-doubled concentric system coupled with low ratio amine to produce bile acid-macrocapsules of β-cells. Ther. Deliv. 2016, 7, 171–178. [Google Scholar] [CrossRef]
- de Vos, P.; Faas, M.M.; Strand, B.; Calafiore, R. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials 2006, 27, 5603–5617. [Google Scholar] [CrossRef] [PubMed]
- De Vos, P.; Hamel, A.F.; Tatarkiewicz, K. Considerations for successful transplantation of encapsulated pancreatic islets. Diabetologia 2002, 45, 159–173. [Google Scholar] [CrossRef] [Green Version]
- Mooranian, A.; Raj Wagle, S.; Kovacevic, B.; Takechi, R.; Mamo, J.; Lam, V.; Watts, G.F.; Mikov, M.; Golocorbin-Kon, S.; Stojanovic, G.; et al. Bile acid bio-nanoencapsulation improved drug targeted-delivery and pharmacological effects via cellular flux: 6-months diabetes preclinical study. Sci. Rep. 2020, 10, 106. [Google Scholar] [CrossRef]
- Mooranian, A.; Takechi, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. The effect of molecular weights of microencapsulating polymers on viability of mouse-cloned pancreatic beta-cells: Biomaterials, osmotic forces and potential applications in diabetes treatment. Pharm. Dev. Technol. 2018, 23, 145–150. [Google Scholar] [CrossRef]
- Zucoloto, A.Z.; Manchope, M.F.; Borghi, S.M.; Dos Santos, T.S.; Fattori, V.; Badaro-Garcia, S.; Camilios-Neto, D.; Casagrande, R.; Verri, W.A., Jr. Probucol Ameliorates Complete Freund’s Adjuvant-Induced Hyperalgesia by Targeting Peripheral and Spinal Cord Inflammation. Inflammation 2019, 42, 1474–1490. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Arfuso, F.; Al-Salami, H. Multicompartmental, multilayered probucol microcapsules for diabetes mellitus: Formulation characterization and effects on production of insulin and inflammation in a pancreatic beta-cell line. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1642–1653. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Liu, C.; Chen, S.; Zhao, H.; Zhou, K.; Wang, W.; Yuan, Y.; Li, Z.; Guo, Y.; Shen, Z.; et al. Activation of the Nrf2/ARE signaling pathway by probucol contributes to inhibiting inflammation and neuronal apoptosis after spinal cord injury. Oncotarget 2017, 8, 52078–52093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, R.; Egashira, K.; Machida, Y.; Hayashidani, S.; Takeya, M.; Utsumi, H.; Tsutsui, H.; Takeshita, A. Probucol attenuates left ventricular dysfunction and remodeling in tachycardia-induced heart failure: Roles of oxidative stress and inflammation. Circulation 2002, 106, 362–367. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Chen, W.; An, F.; Tian, H.; Zhang, J.; Peng, J.; Zhang, Y.; Guo, Y. Probucol attenuates inflammation and increases stability of vulnerable atherosclerotic plaques in rabbits. Tohoku. J. Exp. Med. 2011, 225, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Takechi, R.; Pallebage-Gamarallage, M.M.; Lam, V.; Giles, C.; Mamo, J.C. Long-term probucol therapy continues to suppress markers of neurovascular inflammation in a dietary induced model of cerebral capillary dysfunction. Lipids Health Dis. 2014, 13, 91. [Google Scholar] [CrossRef] [Green Version]
- Mooranian, A.; Zamani, N.; Takechi, R.; Luna, G.; Mikov, M.; Golocorbin-Kon, S.; Kovacevic, B.; Arfuso, F.; Al-Salami, H. Modulatory nano/micro effects of diabetes development on pharmacology of primary and secondary bile acids concentrations. Curr. Diabetes Rev. 2020. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. Biological assessments of encapsulated pancreatic β-cells: Their potential transplantation in diabetes. Cell. Mol. Bioeng. 2016, 9, 530–537. [Google Scholar] [CrossRef]
- Negrulj, R.; Mooranian, A.; Chen-Tan, N.; Al-Sallami, H.S.; Mikov, M.; Golocorbin-Kon, S.; Fakhoury, M.; Watts, G.F.; Arfuso, F.; Al-Salami, H. Swelling, mechanical strength, and release properties of probucol microcapsules with and without a bile acid, and their potential oral delivery in diabetes. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Negrulj, R.; Al-Sallami, H.S.; Fang, Z.; Mikov, M.; Golocorbin-Kon, S.; Fakhoury, M.; Watts, G.F.; Matthews, V.; Arfuso, F.; et al. Probucol release from novel multicompartmental microcapsules for the oral targeted delivery in type 2 diabetes. AAPS PharmSciTech. 2015, 16, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Mathavan, S.; Chen-Tan, N.; Arfuso, F.; Al-Salami, H. The role of the bile acid chenodeoxycholic acid in the targeted oral delivery of the anti-diabetic drug gliclazide, and its applications in type 1 diabetes. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Negrulj, R.; Mathavan, S.; Martinez, J.; Sciarretta, J.; Chen-Tan, N.; Mukkur, T.K.; Mikov, M.; Lalic-Popovic, M.; Stojancevic, M.; et al. An advanced microencapsulated system: A platform for optimized oral delivery of antidiabetic drug-bile acid formulations. Pharm. Dev. Technol. 2015, 20, 702–709. [Google Scholar] [CrossRef]
- Mathavan, S.; Chen-Tan, N.; Arfuso, F.; Al-Salami, H. A comprehensive study of novel microcapsules incorporating gliclazide and a permeation enhancing bile acid: Hypoglycemic effect in an animal model of Type-1 diabetes. Drug Deliv. 2016, 23, 2869–2880. [Google Scholar] [CrossRef]
- Mathavan, S.; Ionescu, C.M.; Kovacevic, B.; Mikov, M.; Golocorbin-Kon, S.; Mooranian, A.; Dass, C.R.; Al-Salami, H. Formulation buoyancy of nanoencapsulated gliclazide using primary, conjugated and deconjugated bile acids. Ther. Deliv. 2019. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Zamani, N.; Takechi, R.; Al-Sallami, H.; Mikov, M.; Golocorbin-Kon, S.; Kovacevic, B.; Arfuso, F.; Al-Salami, H. Probucol-poly(meth)acrylate-bile acid nanoparticles increase IL-10, and primary bile acids in prediabetic mice. Ther. Deliv 2019. [Google Scholar] [CrossRef] [PubMed]
- Wagle, S.R.; Kovacevic, B.; Walker, D.; Ionescu, C.M.; Shah, U.; Stojanovic, G.; Kojic, S.; Mooranian, A.; Al-Salami, H. Alginate-based drug oral targeting using bio-micro/nano encapsulation technologies. Expert Opin. Drug Deliv. 2020, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wagle, S.R.; Walker, D.; Kovacevic, B.; Gedawy, A.; Mikov, M.; Golocorbin-Kon, S.; Mooranian, A.; Al-Salami, H. Micro-Nano formulation of bile-gut delivery: Rheological, stability and cell survival, basal and maximum respiration studies. Sci. Rep. 2020, 10, 7715. [Google Scholar] [CrossRef] [PubMed]
- Al-Salami, H.; Butt, G.; Tucker, I.; Golocorbin-Kon, S.; Mikov, M. Probiotics decreased the bioavailability of the bile acid analog, monoketocholic acid, when coadministered with gliclazide, in healthy but not diabetic rats. Eur. J. Drug. Metab. Pharm. 2012, 37, 99–108. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Arfuso, F.; Al-Salami, H. The effect of a tertiary bile acid, taurocholic acid, on the morphology and physical characteristics of microencapsulated probucol: Potential applications in diabetes: A characterization study. Drug Deliv. Transl. Res. 2015, 5, 511–522. [Google Scholar] [CrossRef]
- Mooranian, A.; Zamani, N.; Luna, G.; Al-Sallami, H.; Mikov, M.; Golocorbin-Kon, S.; Stojanovic, G.; Arfuso, F.; Kovacevic, B.; Al-Salami, H. Bile acid-polymer-probucol microparticles: Protective effect on pancreatic beta-cells and decrease in type 1 diabetes development in a murine model. Pharm. Dev. Technol. 2019, 24, 1272–1277. [Google Scholar] [CrossRef]
- Shimizu, H.; Uehara, Y.; Shimomura, Y.; Tanaka, Y.; Kobayashi, I. Probucol attenuated hyperglycemia in multiple low-dose streptozotocin-induced diabetic mice. Life Sci. 1991, 49, 1331–1338. [Google Scholar] [CrossRef]
- Hu, G.; Zhao, Y.; Tang, Y.; Wang, Y.; Shen, J.; Liu, L.; Li, H.; Liu, Y.; Cui, X.; Yu, Y.; et al. Development of a novel model of hypertriglyceridemic acute pancreatitis in hamsters: Protective effects of probucol. Pancreas 2012, 41, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Moretti, A.; Li, Q.; Chmielowski, R.; Joseph, L.B.; Moghe, P.V.; Uhrich, K.E. Nanotherapeutics Containing Lithocholic Acid-Based Amphiphilic Scorpion-Like Macromolecules Reduce In Vitro Inflammation in Macrophages: Implications for Atherosclerosis. Nanomater. 2018, 8, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, J.B.J.; Lajczak, N.K.; Kelly, O.B.; O’Dwyer, A.M.; Giddam, A.K.; Ni Gabhann, J.; Franco, P.; Tambuwala, M.M.; Jefferies, C.A.; Keely, S.; et al. Ursodeoxycholic acid and lithocholic acid exert anti-inflammatory actions in the colon. Am. J. Physiol. Gastrointest Liver Physiol. 2017, 312, G550–G558. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mooranian, A.; Ionescu, C.M.; Wagle, S.R.; Kovacevic, B.; Walker, D.; Jones, M.; Chester, J.; Foster, T.; Johnston, E.; Mikov, M.; et al. Probucol Pharmacological and Bio-Nanotechnological Effects on Surgically Transplanted Graft Due to Powerful Anti-Inflammatory, Anti-Fibrotic and Potential Bile Acid Modulatory Actions. Pharmaceutics 2021, 13, 1304. https://doi.org/10.3390/pharmaceutics13081304
Mooranian A, Ionescu CM, Wagle SR, Kovacevic B, Walker D, Jones M, Chester J, Foster T, Johnston E, Mikov M, et al. Probucol Pharmacological and Bio-Nanotechnological Effects on Surgically Transplanted Graft Due to Powerful Anti-Inflammatory, Anti-Fibrotic and Potential Bile Acid Modulatory Actions. Pharmaceutics. 2021; 13(8):1304. https://doi.org/10.3390/pharmaceutics13081304
Chicago/Turabian StyleMooranian, Armin, Corina Mihaela Ionescu, Susbin Raj Wagle, Bozica Kovacevic, Daniel Walker, Melissa Jones, Jacqueline Chester, Thomas Foster, Edan Johnston, Momir Mikov, and et al. 2021. "Probucol Pharmacological and Bio-Nanotechnological Effects on Surgically Transplanted Graft Due to Powerful Anti-Inflammatory, Anti-Fibrotic and Potential Bile Acid Modulatory Actions" Pharmaceutics 13, no. 8: 1304. https://doi.org/10.3390/pharmaceutics13081304
APA StyleMooranian, A., Ionescu, C. M., Wagle, S. R., Kovacevic, B., Walker, D., Jones, M., Chester, J., Foster, T., Johnston, E., Mikov, M., Atlas, M. D., & Al-Salami, H. (2021). Probucol Pharmacological and Bio-Nanotechnological Effects on Surgically Transplanted Graft Due to Powerful Anti-Inflammatory, Anti-Fibrotic and Potential Bile Acid Modulatory Actions. Pharmaceutics, 13(8), 1304. https://doi.org/10.3390/pharmaceutics13081304