Nanotechnology as a Versatile Tool for 19F-MRI Agent’s Formulation: A Glimpse into the Use of Perfluorinated and Fluorinated Compounds in Nanoparticles
Abstract
:1. Introduction
2. Types of Molecular Imaging
3. Principles of NMR and MRI
3.1. Gadolinium Based Contrast Agents (GBCAs)
3.2. Fluorine as a Contrast Agent
3.3. Similarity between Fluorine and Hydrogen
4. Perfluorocarbons (PFCs) as Contrast Agents for 19F MRI
4.1. Physicochemical and Biological Properties of Perfluorocarbon (PFC) Molecules
4.2. PFC Molecules in a Nanoparticle Formulation
4.3. Types of PFC Molecules
- Perfluorooctyl bromide (PFOB/perflubron) is one of the most used PFC materials in biomedicine [96,97]. It is a tasteless and odourless liquid and is extensively adapted for artificial oxygen carriers [73]. It is a dense liquid with a low diffusion coefficient inside the blood, has a longer blood circulation time, and excreted out faster than most other PFCs. It has a linear structure, low surface tension, high specific gravity, finite lipophilicity due to a covalent bond to bromine which enhances its clearance rates from the body [98]. PFCs with additional chemical elements, such as a bromine atom in PFOB, tend to have a short biologic half-life value [99]. They are scarcely absorbed in the gastrointestinal tract, wherefore could be ingested in large doses for bowel imaging [62]. Albeit PFOB displays multiple 19F peaks (eight peaks, one for each CFn moiety) that compromises its sensitivity, it is possible to minimize undesired resonance peaks by including pre-saturation RF pulses with MRI pulse sequences before readout [98,100].
- Perfluoropolyether (PFPE) is the simplest linear polymer that is an excellent 19F MRI probe as they provide a single sharp resonance for hassle-free identification, maximizing the SNR and eradicating any chemical shift artifact of the PFC [101]. This class of molecule is known for its remarkable thermal stability and high molecular mobility that improves 19F MRI sensitivity [102]. Linear PFPE possesses end groups susceptible to chemical modification by synthetic strategies [103,104] to bolster additional scope in multimodal imaging. This polymer has short T1 (600 ms) and adequately long T2 (160 ms), the desired trait for an imaging agent. They own a linear structure and high content of MR equivalent 19F nuclei per molecule, with >40 chemically equivalent fluorine [88] that should theoretically give them single resonance. The carbon-oxygen bonds stipulate an increased bond rotation that aids them to be better biodegradable [59].
- Using macrocyclic perfluoropolyethers (PFPEs), e.g., the 12, 15, or 18 crown ethers with their high number of equivalent fluorine atoms (16, 20, and 24, respectively) assure an outstanding NMR performance, notably of chemical shift artifacts, SNR, single sharp resonance peak that enable for unambiguous identification, etc. Macrocyclic PFCs such as the perfluoro-15-crown-5 ether (PFCE) assure a substantial improvement in MRI sensitivity with 20 chemically equivalent fluorines (NMR resonance at around ~−92.5 ppm) [98] and is one of the most explored PFC [105,106,107,108,109,110,111,112].
- PERFECTA (suPERFluorinatEdContrasT Agent) has 36 chemically equivalent fluorine atoms per molecule, which gives them a single major resonance in FNMR [113]. Unlike other PFCs, they have a polar hydrocarbon core. They are found to have reliable cellular compatibility from the preliminary in vivo F-MRI experiments [113,114].
4.4. The Sine Qua Non of Fluorinating Agents in 19F MRI for Clinical Translation—Chemical, Physical and Biological Traits
- Significant biological stability and possessing desired chemical traits [91]. The probe must be chemically inert to such an extent that it can endure all of the omnifarious chemicals in the biological milieu until it performs its mission and will be degraded. Most organofluoride compounds easily match this precondition given the strong C–F bond.
- An ideal tracer should possess a restrained T1 relaxation time (reduce acquisition times and increase the number of scans per unit time) and an adequately long T2 relaxation time (to avoid signal intensity loss) [116]. A constant relaxation is anticipated in the complex biological environment. T2/T1 ratio close to unity is desirable for a better SNR. One of the drawbacks of PFC is their long T1. When a PFC has intrinsically long T1 relaxation (PFOB and PFCE have T1 relaxations > 1 s), it will severely limit the rate of data acquisition and its sensitivity [98]. Invariably, in the literature, T2 is an easily manipulable parameter, and this is inspected by modulating the length of fluorinated chains in the probe.
- High number of magnetically equivalent 19F-content: 19FNMR spectrum, a characterization technique used in the initial analysis, for an ideal CA should be simple, preferably with a single, sharp, narrow resonance and intense peak to maximize sensitivity and prevent chemical shift imaging artifacts. The integral of an NMR signal is quantitative [117,118], directly proportional to the imaging agent concentration. The probe should also have a high fluorine content to give a single dominant signal and a good sensitivity, customarily accomplished employing PFCs. One of the undesired attributes of PFCs is that some of them lack proper symmetry, so they have a split signal in the NMR due to the disparate chemical environment of the fluorine in the molecule. This issue is surmounted by methodically applying 19F MRI probes with high symmetry like PFCE/PFPE or polymeric species like dendrimers.
- Prominent SNR enhancement: 19F MRI often suffers from low SNR [119]. The commonly performed strategies to enhance the SNR are to use a CA, modulate the magnetic field strength [120], to improve pulse sequences [121] or hyperpolarization techniques such as dynamic nuclear polarization, chemically induced dynamic nuclear polarization, spin-exchange optical pumping, and parahydrogen-induced polarization that can achieve the same goal [122]. In the case of 19F MRI, using the fluorinated component CA with a considerable amount of fluorine in the probe is also one of the commonly used approaches. Howbeit, high concentrations of CAs might potentially result in toxicity issues.
- Nominal/no in vitro and in vivo toxicity: neither should it modify any biological functions nor degrade to give by-products detrimental for other tissues/organs and hence should possess low immunogenicity.
- Easy and scalable synthesis and formulation of CA: a reproducible synthesis that can sustain the purity of the formulation with as simple as a single-step reaction and adeptness of scaling up.
- Water solubility would be an advantageous feature that would help in the easy application of fluorine. The approach to effectuate water-soluble fluorinated moiety is by chemically modifying the system with hydrophilic compounds or employing hydrophilic hyperfluorinated organofluorine molecules [123]. One requirement for such a probe is possessing a suitable conjugation site. One of the most explored PFC in this regard is PFPE.
- A long shelf life is favoured for a probe (at least six months).
- Finally, it is always preferred to have an easy clearance from the living system to be approved for clinical application.
4.5. Biomedical Applications of PFC Molecules
5. Examples of Nanosystems Used for 19F MRI Studies
5.1. Organic NPs
5.1.1. Polymeric NPs
5.1.2. Hyperbranched
5.1.3. Dendrimers
5.1.4. Nanohydrogel
5.1.5. Lipids
5.1.6. Micelle
5.2. Inorganic NPs
5.2.1. Metal NPs
5.2.2. Silica NPs
5.2.3. Carbon Based
5.3. Mixed/Hybrid NPs
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 19F MRI/FMRI | Fluorine-19 Magnetic Resonance Imaging |
| 19F NMR | Fluorine-19 Nuclear Magnetic Resonance |
| 1H MRI/HMRI | Proton Magnetic Resonance Imaging |
| AFM | Atomic Force Microscopy |
| ATRP | Atom Transfer Radical Polymerization |
| BALB/c | Albino, laboratory-bred strain of the house mouse |
| BODIPy | Boron-dipyrromethene |
| C NMR | Carbon-13 Nuclear Magnetic Resonance |
| CA | Contrast Agent |
| CD | Circular Dichroism Spectroscopy |
| CLSM | Confocal Laser Scanning Microscopy |
| CM | Confocal Microscopy |
| CrM | Correlation Microscopy |
| C-SEM | Scanning Cryo-Electron microscopy |
| CT | Computed Tomography |
| C-TEM | Cryo-Transmission Electron Microscopy |
| CuAAC | Copper-Catalyzed Azide-Alkyne Cycloaddition |
| CyA | Cytotoxicity Assays. The usually used assays are
|
| DC | Dendritic cellsCells |
| DLS | Dynamic Light Scattering |
| DOSY | Diffusion Ordered 2D-NMR Spectroscopy |
| EA | Elemental Analyzer |
| EDX | Energy-Dispersive X-Ray Spectroscopy |
| EGDMA | Ethylene Glycol Dimethylacrylate |
| ELS | Electrophoretic Light Scattering |
| EMA | European Medicines Agency |
| EPR | Electron Paramagnetic Resonance Spectrum |
| FA | Fluorescence Anisotropy |
| FC | Flow Cytometry |
| FDA | The Food and Drug Administration |
| FDK | Fluorinated β-diketones |
| Fe3O4 | Iron Oxide/Magnetite |
| FI | Fluorescence Imaging |
| FITC | Fluorescein Isothiocyanate |
| FLAME | Fluorine Accumulated Silica NP for MRI Contrast Enhancement |
| FM | Fluorescence Microscopy |
| FTIR | Fourier Transform Infrared Spectroscopy |
| GBCAs | Gadolinium Based Contrast Agents |
| Gd(III)/Gd3+ | Gadolinium-III |
| GPC | Gel Permeation Chromatography |
| HAADF-STEM | High-Angle Annular Dark-Field Scanning Transmission Electron Microscopy |
| HBIPF | Hyperbranched Iodopolymer Containing 19F |
| HepG2 Cells | Hepatocellular carcinoma cells |
| HNMR | Proton Nuclear Magnetic Resonance |
| HNT | Halloysite Nanotube |
| HPLC | High-Performance Liquid Chromatography |
| HRTEM | High Resolution Transmission Electron Microscopy |
| ICG | Indocyanine Green |
| ICP-AES | Inductively Coupled Plasma Atomic Emission Spectrometry |
| ICP-MS | Inductively Coupled Plasma Mass Spectrometry |
| KB cells | Human Nasopharyngeal Epidermal Carcinoma |
| LDV | Laser Doppler Velocimetry |
| LSPR | Localized Surface Plasmon Resonance |
| MALDI-TOF-MS | Matrix-Assisted Laser Desorption Ionization-Time-Of-Flight Mass Spectrometry |
| MD | Atomistic Molecular Dynamic Simulations |
| mPEG | Monodisperse Poly (ethylene-glycol) |
| MR | Magnetic Resonance |
| MRI | Magnetic Resonance Imaging |
| MRS | Magnetic Resonance Spectroscopy |
| NIR | Near Infrared |
| NIRS | Near Infrared Spectroscopy and Imaging |
| NMR | Nuclear Magnetic Resonance |
| NP | Nanoparticle |
| NTA | Nanoparticle Tracking Analysis |
| OEGA | Oligo(Ethylene Glycol) Methyl Ether Acrylate |
| PAGE | Polyacrylamide Gel Electrophoresis |
| PAI | Photoacoustic Imaging |
| PEG | Poly (ethylene-glycol) |
| PEGMA | Poly-(ethylene glycol) methyl ether methacrylate |
| PET | Positron Emission Tomography |
| PFC | Perfluorocarbon |
| PFCE | Perfluoro-15-crown-5 ether |
| PFDCO | Perfluorodichlorooctane |
| PFOB | Perfluorooctyl Bromide |
| PFP | Perfluoropropane |
| PFPE | Perfluoropolyether |
| PFTB | Perfluoro-tert-butanol |
| PLGA | Poly (lactic-co-glycolic acid) |
| PRE | Paramagnetic Relaxation Enhancement |
| PTT | Photothermal Therapy |
| QCM | Quartz Crystal Microbalance |
| RAFT | Reversible Addition−Fragmentation Chain-Transfer Polymerization |
| RF | Radiofrequency |
| ROMBP | Ring-Opening Multibranching Polymerization |
| ROS | Reactive Oxygen Species |
| SANS | Small Angle Neutron Scattering |
| SEC | Size Exclusion Chromatography |
| SEM | Scanning Electron Microscope |
| SLS | Static Light Scattering |
| SNR | Signal/Contrast-to-Noise Ratio |
| SPECT | Single-Photon Emission Computed Tomography |
| STEM | Scanning Transmission Electron Microscope |
| TAM | Tumour-associated macrophages |
| TEM | Transmission Electron Microscope |
| TFEA | 2,2,2-trifluoroethyl Acrylate |
| TGA | Thermogravimetric Analysis |
| TM | Turbidometry |
| TPFBME | 1,1,1-tris(perfluorotert- butoxymethyl)ethane |
| UC | Upconversion |
| US | Ultrasound |
| UV–Vis | UV/Vis Absorption Spectra |
| XPS | X-Ray Photoelectron Spectroscopy |
| XRD | X-Ray Diffraction |
| ZP | Zeta Potential |
References
- McMahon, M.T.; Chan, K.W. Developing MR probes for molecular imaging. Adv. Cancer Res. 2014, 124, 297–327. [Google Scholar] [PubMed]
- Chen, Z.Y.; Wang, Y.X.; Lin, Y.; Zhang, J.S.; Yang, F.; Zhou, Q.L.; Liao, Y.Y. Advance of molecular imaging technology and targeted imaging agent in imaging and therapy. Biomed. Res. Int. 2014, 2014, 819324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, M.L.; Gambhir, S.S. A molecular imaging primer: Modalities, imaging agents, and applications. Physiol. Rev. 2012, 92, 897–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debbage, P.; Jaschke, W. Molecular imaging with nanoparticles: Giant roles for dwarf actors. Histochem. Cell Biol. 2008, 130, 845–875. [Google Scholar] [CrossRef] [Green Version]
- Schulz, R.B.; Semmler, W. Fundamentals of Optical Imaging. In Molecular Imaging I. Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2008; Volume 185, pp. 3–22. [Google Scholar]
- Hong, G.; Antaris, A.L.; Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017, 1, 0010. [Google Scholar] [CrossRef]
- Kwon, Y.D.; Byun, Y.; Kim, H.K. 18F-labelled BODIPY dye as a dual imaging agent: Radiofluorination and applications in PET and optical imaging. Nucl. Med. Biol. 2021, 93, 22–36. [Google Scholar] [CrossRef]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef]
- Steinberg, I.; Huland, D.M.; Vermesh, O.; Frostig, H.E.; Tummers, W.S.; Gambhir, S.S. Photoacoustic clinical imaging. Photoacoustics 2019, 14, 77–98. [Google Scholar] [CrossRef]
- Wells, P.N. Ultrasound imaging. Phys. Med. Biol. 2006, 51, R83–R98. [Google Scholar] [CrossRef]
- Alcazar, J.L.; Aubá, M.; Olartecoechea, B. Three-dimensional ultrasound in gynecological clinical practice. Rep. Med. Imaging 2012, 2012, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kramme, R.; Hoffmann, K.; Pozos, R.S.; Buzug, T.M. Springer Handbook of Medical Technology; Springer: Berlin/Heidelberg, Germany, 2011; Volume 413, p. 1497. [Google Scholar]
- Beik, J.; Jafariyan, M.; Montazerabadi, A.; Ghadimi-Daresajini, A.; Tarighi, P.; Mahmoudabadi, A.; Ghaznavi, H.; Shakeri-Zadeh, A. The benefits of folic acid-modified gold nanoparticles in CT-based molecular imaging: Radiation dose reduction and image contrast enhancement. Artif. Cells Nanomed. Biotechnol. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rahmim, A.; Zaidi, H. PET versus SPECT: Strengths, limitations and challenges. Nucl. Med. Commun. 2008, 29, 193–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Lu, Z.R. Molecular imaging of the tumor microenvironment. Adv. Drug Deliv. Rev. 2017, 113, 24–48. [Google Scholar] [CrossRef] [PubMed]
- Molteni, R. X-ray. In Micro-Computed Tomography (Micro-CT) in Medicine and Engineering; Springer: Cham, Switzerland, 2020; pp. 7–25. [Google Scholar] [CrossRef]
- Cho, M.H.; Shin, S.H.; Park, S.H.; Kadayakkara, D.K.; Kim, D.; Choi, Y. Targeted, Stimuli-Responsive, and Theranostic 19F Magnetic Resonance Imaging Probes. Bioconjug. Chem. 2019, 30, 2502–2518. [Google Scholar] [CrossRef]
- Kissane, J.; Neutze, J.A.; Singh, H. Radiology Fundamentals, Introduction to Imaging & Technology, 6th ed.; Springer: Cham, Switzerland, 2020; 431p. [Google Scholar]
- Initiative to Reduce Unnecessary Radiation Exposure from Medical Imaging; Center for Devices and Radiological Health, U.S. Food and Drug Administration: Silver Spring, MD, USA, 2010.
- Balducci, A.; Helfer, B.M.; Ahrens, E.T.; O’Hanlon, C.F., 3rd; Wesa, A.L. Visualizing arthritic inflammation and therapeutic response by fluorine-19 magnetic resonance imaging (19F MRI). J. Inflamm. 2012, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, H.; Yao, D.; Li, J.; Yang, S.; Zhang, C.; Chen, W.; Wang, D. (18)F-labeled magnetic nanoparticles for monitoring anti-angiogenic therapeutic effects in breast cancer xenografts. J. Nanobiotechnol. 2019, 17, 105. [Google Scholar] [CrossRef] [Green Version]
- Belderbos, S.; Gonzalez-Gomez, M.A.; Cleeren, F.; Wouters, J.; Pineiro, Y.; Deroose, C.M.; Coosemans, A.; Gsell, W.; Bormans, G.; Rivas, J.; et al. Simultaneous in vivo PET/MRI using fluorine-18 labeled Fe3O4@Al(OH)3 nanoparticles: Comparison of nanoparticle and nanoparticle-labeled stem cell distribution. EJNMMI Res. 2020, 10, 73. [Google Scholar] [CrossRef]
- Li, S.; Jiang, W.; Yuan, Y.; Sui, M.; Yang, Y.; Huang, L.; Jiang, L.; Liu, M.; Chen, S.; Zhou, X. Delicately Designed Cancer Cell Membrane-Camouflaged Nanoparticles for Targeted 19F MR/PA/FL Imaging-Guided Photothermal Therapy. ACS Appl. Mater. Interfaces 2020, 12, 57290–57301. [Google Scholar] [CrossRef]
- Knight, J.C.; Edwards, P.G.; Paisey, S.J. Fluorinated contrast agents for magnetic resonance imaging; a review of recent developments. RSC Adv. 2011, 1. [Google Scholar] [CrossRef]
- Katti, G.; Ara, S.A.; Shireen, A. Magnetic Resonance Imaging (MRI)—A Review. Int. J. Dent. Clin. 2011, 3, 65–70. [Google Scholar]
- Neil, J.; Ackerman, J.J.H. Magnetic Resonance (MR); Overview. In Encyclopedia of the Neurological Sciences; Elsevier: Amsterdam, The Netherlands, 2014; Volume 6, pp. 971–972. [Google Scholar] [CrossRef]
- Hao, D.; Ai, T.; Goerner, F.; Hu, X.; Runge, V.M.; Tweedle, M. MRI contrast agents: Basic chemistry and safety. J. Magn. Reson. Imaging 2012, 36, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Wahsner, J.; Gale, E.M.; Rodriguez-Rodriguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.D.; Paudel, R.; Liu, J.; Ma, C.; Zhang, Z.S.; Zhou, S.K. MRI contrast agents: Classification and application (Review). Int. J. Mol. Med. 2016, 38, 1319–1326. [Google Scholar] [CrossRef] [Green Version]
- Index to Drug-Specific Information. Available online: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/index-drug-specific-information (accessed on 2 January 2022).
- Lohrke, J.; Frenzel, T.; Endrikat, J.; Alves, F.C.; Grist, T.M.; Law, M.; Lee, J.M.; Leiner, T.; Li, K.C.; Nikolaou, K.; et al. 25 Years of Contrast-Enhanced MRI: Developments, Current Challenges and Future Perspectives. Adv. Ther. 2016, 33, 1–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idee, J.M.; Port, M.; Raynal, I.; Schaefer, M.; Le Greneur, S.; Corot, C. Clinical and biological consequences of transmetallation induced by contrast agents for magnetic resonance imaging: A review. Fundam. Clin. Pharmacol. 2006, 20, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Stockman, J.A. Oral Prednisolone for Preschool Children with Acute Virus-Induced Wheezing. Yearb. Pediatr. 2010, 2010, 515–518. [Google Scholar] [CrossRef]
- De León-Rodríguez, L.M.; Martins, A.F.; Pinho, M.C.; Rofsky, N.M.; Sherry, A.D. Basic MR relaxation mechanisms and contrast agent design. J. Magn. Reson. Imaging 2015, 42, 545–565. [Google Scholar] [CrossRef] [Green Version]
- Hequet, E.; Henoumont, C.; Djouana Kenfack, V.; Lemaur, V.; Lazzaroni, R.; Boutry, S.; Vander Elst, L.; Muller, R.N.; Laurent, S. Design, Characterization and Molecular Modeling of New Fluorinated Paramagnetic Contrast Agents for Dual 1H/19F MRI. Magnetochemistry 2020, 6, 8. [Google Scholar] [CrossRef]
- Grobner, T. Gadolinium--a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol. Dial. Transplant. 2006, 21, 1104–1108. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.B. Fluorinated dendrimers as imaging agents for 19F MRI. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 646–661. [Google Scholar] [CrossRef]
- FDA Gadolinium Retention after Gadolinium Based Contrast Magnetic Resonance Imaging in Patients with Normal Renal Function; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2017; pp. 127–131.
- Do, C.; DeAguero, J.; Brearley, A.; Trejo, X.; Howard, T.; Escobar, G.P.; Wagner, B. Gadolinium-Based Contrast Agent Use, Their Safety, and Practice Evolution. Kidney360 2020, 1, 561–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dekkers, I.A.; Roos, R.; van der Molen, A.J. Gadolinium retention after administration of contrast agents based on linear chelators and the recommendations of the European Medicines Agency. Eur. Radiol. 2018, 28, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA Drug Safety Communication: FDA. In Warns that Gadolinium-Based Contrast Agents (GBCAs) are Retained in the Body; Requires New Class Warnings; FDA: Silver Spring, MR, USA, 2017. [Google Scholar]
- Holland, G.N.; Bottomley, P.A.; Hinshaw, W.S. 19F magnetic resonance imaging. J. Magn. Reson. Imaging 1977, 28, 133–136. [Google Scholar] [CrossRef]
- Weise, G.; Basse-Luesebrink, T.C.; Wessig, C.; Jakob, P.M.; Stoll, G. In vivo imaging of inflammation in the peripheral nervous system by 19F MRI. Exp. Neurol. 2011, 229, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Tirotta, I.; Dichiarante, V.; Pigliacelli, C.; Cavallo, G.; Terraneo, G.; Bombelli, F.B.; Metrangolo, P.; Resnati, G. 19F magnetic resonance imaging (MRI): From design of materials to clinical applications. Chem. Rev. 2015, 115, 1106–1129. [Google Scholar] [CrossRef]
- Otake, Y.; Soutome, Y.; Hirata, K.; Ochi, H.; Bito, Y. Double-tuned radiofrequency coil for 19F and 1H imaging. Magn. Reson. Med. Sci. 2014, 13, 199–205. [Google Scholar] [CrossRef]
- Keupp, J.; Rahmer, J.; Grasslin, I.; Mazurkewitz, P.C.; Schaeffter, T.; Lanza, G.M.; Wickline, S.A.; Caruthers, S.D. Simultaneous dual-nuclei imaging for motion corrected detection and quantification of 19F imaging agents. Magn. Reson. Med. 2011, 66, 1116–1122. [Google Scholar] [CrossRef] [Green Version]
- Wolters, M.; Mohades, S.G.; Hackeng, T.M.; Post, M.J.; Kooi, M.E.; Backes, W.H. Clinical Perspectives of Hybrid Proton-Fluorine Magnetic Resonance Imaging and Spectroscopy. Investig. Radiol. 2013, 48, 341–350. [Google Scholar] [CrossRef]
- Bouvain, P.; Temme, S.; Flogel, U. Hot spot 19 F magnetic resonance imaging of inflammation. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1639. [Google Scholar] [CrossRef]
- Liu, W.; Frank, J.A. Detection and quantification of magnetically labeled cells by cellular MRI. Eur. J. Radiol. 2009, 70, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Grapentin, C.; Barnert, S.; Schubert, R. Monitoring the Stability of Perfluorocarbon Nanoemulsions by Cryo-TEM Image Analysis and Dynamic Light Scattering. PLoS ONE 2015, 10, e0130674. [Google Scholar] [CrossRef] [PubMed]
- Vatsadze, S.Z.; Eremina, O.E.; Veselova, I.A.; Kalmykov, S.N.; Nenajdenko, V.G. 18F-Labelled catecholamine type radiopharmaceuticals in the diagnosis of neurodegenerative diseases and neuroendocrine tumours: Approaches to synthesis and development prospects. Russ. Chem. Rev. 2018, 87, 350–373. [Google Scholar] [CrossRef]
- Harris, R.K.; Becker, E.D.; de Menezes, C.; Sonia, M.; Goodfellow, R.; Granger, P. NMR nomenclature. Nuclear spin properties and conventions for chemical shifts (IUPAC Recommendations 2001). Pure Appl. Chem. 2001, 73, 1795–1818. [Google Scholar] [CrossRef]
- Schmieder, A.H.; Caruthers, S.D.; Keupp, J.; Wickline, S.A.; Lanza, G.M. Recent Advances in 19Fluorine Magnetic Resonance Imaging with Perfluorocarbon Emulsions. Engineering 2015, 1, 475–489. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Cabello, J.; Barnett, B.P.; Bottomley, P.A.; Bulte, J.W. Fluorine 19F MRS and MRI in biomedicine. NMR Biomed. 2011, 24, 114–129. [Google Scholar] [CrossRef]
- Riess, J.G. Understanding the fundamentals of perfluorocarbons and perfluorocarbon emulsions relevant to in vivo oxygen delivery. Artif. Cells Blood Substit. Immobil. Biotechnol. 2005, 33, 47–63. [Google Scholar] [CrossRef]
- Chen, J.; Lanza, G.M.; Wickline, S.A. Quantitative magnetic resonance fluorine imaging: Today and tomorrow. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 431–440. [Google Scholar] [CrossRef] [Green Version]
- Berger, R.; Resnati, G.; Metrangolo, P.; Weber, E.; Hulliger, J. Organic fluorine compounds: A great opportunity for enhanced materials properties. Chem. Soc. Rev. 2011, 40, 3496–3508. [Google Scholar] [CrossRef]
- Shah, P.; Westwell, A.D. The role of fluorine in medicinal chemistry. J. Enzyme Inhib. Med. Chem. 2007, 22, 527–540. [Google Scholar] [CrossRef] [Green Version]
- Barres, A.R.; Molugu, S.K.; Stewart, P.L.; Mecozzi, S. Droplet Core Intermolecular Interactions and Block Copolymer Composition Heavily Influe.ence Oil-In-Water Nanoemulsion Stability. Langmuir 2019, 35, 12765–12772. [Google Scholar] [CrossRef]
- Krafft, M.P.; Riess, J.G. Highly fluorinated amphiphiles and colloida systems, and their applications in the biomedical field. A contribution. Biochimie 1998, 80, 489–514. [Google Scholar] [CrossRef]
- Gladysz, J.A.; Curran, D.P.; Horvath, I.T. Handbook of Fluorous Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weiheim, Germany, 2004. [Google Scholar] [CrossRef]
- Krafft, M.P.; Riess, J.G. Perfluorocarbons: Life sciences and biomedical usesDedicated to the memory of Professor Guy Ourisson, a true RENAISSANCE man. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 1185–1198. [Google Scholar] [CrossRef]
- Jagers, J.; Wrobeln, A.; Ferenz, K.B. Perfluorocarbon-based oxygen carriers: From physics to physiology. Pflug. Arch. 2021, 473, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, V.H.; Rossky, P.J. Molecular origins of fluorocarbon hydrophobicity. Proc. Natl. Acad. Sci. USA 2010, 107, 13603–13607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janjic, J.M.; Ahrens, E.T. Fluorine-containing nanoemulsions for MRI cell tracking. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 492–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.; Gong, X.; Li, A.; Lin, H.; Peng, C.; Zhang, X.; Chen, X.; Gao, J. Cascaded Multiresponsive Self-Assembled 19F MRI Nanoprobes with Redox-Triggered Activation and NIR-Induced Amplification. Nano Lett. 2020, 20, 363–371. [Google Scholar] [CrossRef]
- Bo, S.; Song, C.; Li, Y.; Yu, W.; Chen, S.; Zhou, X.; Yang, Z.; Zheng, X.; Jiang, Z.X. Design and Synthesis of Fluorinated Amphiphile as 19F MRI/Fluorescence Dual-Imaging Agent by Tuning the Self-Assembly. J. Org. Chem. 2015, 80, 6360–6366. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Pal, S.L.; Jana, U.; Manna, P.K.; Mohanta, G.P.; Manavalan, R. Nanoparticle: An overview of preparation and characterization. J. Appl. Pharm. Sci. 2011, 1, 228–234. [Google Scholar]
- Nel, A.E.; Madler, L.; Velegol, D.; Xia, T.; Hoek, E.M.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Gref, R.; Lück, M.; Quellec, P.; Marchand, M.; Dellacherie, E.; Harnisch, S.; Blunk, T.; Müller, R.H. ‘Stealth’ Corona-Core Nanoparticles Surface Modified by Polyethylene Glycol (PEG): Influences of the Corona (PEG Chain Length and Surface Density) and of the Core Com-position on Phagocytic Uptake and Plasma Protein Adsorption. Colloids Surf. B Biointerfaces 2000, 18, 301–313. [Google Scholar] [CrossRef]
- Han, X.; Xu, K.; Taratula, O.; Farsad, K. Applications of nanoparticles in biomedical imaging. Nanoscale 2019, 11, 799–819. [Google Scholar] [CrossRef] [PubMed]
- Spahn, D.R. Blood substitutes. Artificial oxygen carriers: Perfluorocarbon emulsions. Crit. Care 1999, 3, R93–R97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneda, M.M.; Caruthers, S.; Lanza, G.M.; Wickline, S.A. Perfluorocarbon nanoemulsions for quantitative molecular imaging and targeted therapeutics. Ann. Biomed. Eng. 2009, 37, 1922–1933. [Google Scholar] [CrossRef] [PubMed]
- Southworth, R.; Kaneda, M.; Chen, J.; Zhang, L.; Zhang, H.; Yang, X.; Razavi, R.; Lanza, G.; Wickline, S.A. Renal vascular inflammation induced by Western diet in ApoE-null mice quantified by 19F NMR of VCAM-1 targeted nanobeacons. Nanomedicine 2009, 5, 359–367. [Google Scholar] [CrossRef] [Green Version]
- Bailey, M.M.; Kline, S.R.; Anderson, M.D.; Staymates, J.L.; Berkland, C. Chemically modifiable fluorinated copolymer nanoparticles for 19F-MRI contrast enhancement. J. Appl. Polym. Sci. 2012, 126, 1218–1227. [Google Scholar] [CrossRef]
- Achilefu, S.; Raghavachari, R.; Janjic, J.M.; Berlec, A.; Bagia, C.; Liu, L.S.; Jeric, I.; Gach, M.; Janjic, B.M.; Strukelj, B. NIR and MR imaging supported hydrogel based delivery system for anti-TNF alpha probiotic therapy of IBD. In Proceedings of the Reporters, Markers, Dyes, Nanoparticles, and Molecular Probes for Biomedical Applications VIII, San Francisco, CA, USA, 13–18 February 2016. [Google Scholar]
- Bonnet, C.S.; Toth, E. Smart Contrast Agents for Magnetic Resonance Imaging. Chimia 2016, 70, 102–108. [Google Scholar] [CrossRef]
- Gambino, G.; Gambino, T.; Angelovski, G. Combination of bioresponsive chelates and perfluorinated lipid nanoparticles enables in vivo MRI probe quantification. Chem. Commun. 2020, 56, 9433–9436. [Google Scholar] [CrossRef]
- Zhu, X.; Tang, X.; Lin, H.; Shi, S.; Xiong, H.; Zhou, Q.; Li, A.; Wang, Q.; Chen, X.; Gao, J. A Fluorinated Ionic Liquid-Based Activatable 19F MRI Platform Detects Biological Targets. Chem 2020, 6, 1134–1148. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Castro, O.; Nesbitt, A.E.; Lyles, D. Effect of a perfluorocarbon emulsion (Fluosol-DA) on reticuloendothelial system clearance function. Am. J. Hematol. 1984, 16, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Li, A.; Chen, K.; Peng, X.; Zhang, J.; Jiang, M.; Chen, S.; Zheng, X.; Zhou, X.; Jiang, Z.X. Perfluoro-tert-butanol: A cornerstone for high performance fluorine-19 magnetic resonance imaging. Chem. Commun. 2021, 57, 7743–7757. [Google Scholar] [CrossRef] [PubMed]
- Mayer, D.; Ferenz, K.B. Perfluorocarbons for the treatment of decompression illness: How to bridge the gap between theory and practice. Eur. J. Appl. Physiol. 2019, 119, 2421–2433. [Google Scholar] [CrossRef] [Green Version]
- Kosenkov, A.V.; Gulyaev, M.V.; Anisimov, N.V.; Lobyshev, V.I.; Pirogov, Y.A. Investigation of the distribution of heavy nuclei in laboratory animals using multinuclear magnetic resonance imaging. Phys. Wave Phenom. 2015, 23, 311–315. [Google Scholar] [CrossRef]
- Bouvain, P.; Flocke, V.; Kramer, W.; Schubert, R.; Schrader, J.; Flogel, U.; Temme, S. Dissociation of 19F and fluorescence signal upon cellular uptake of dual-contrast perfluorocarbon nanoemulsions. Magn. Reson. Mater. Phys. Biol. Med. 2019, 32, 133–145. [Google Scholar] [CrossRef]
- Banik, B.L.; Fattahi, P.; Brown, J.L. Polymeric nanoparticles: The future of nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 271–299. [Google Scholar] [CrossRef]
- O’Hanlon, C.E.; Amede, K.G.; O’Hear, M.R.; Janjic, J.M. NIR-labeled perfluoropolyether nanoemulsions for drug delivery and imaging. J. Fluor. Chem 2012, 137, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Liu, F.; Liu, S.; Xu, X.; Liu, Z.; Sun, X. Perfluorocarbons-Based 19F Magnetic Resonance Imaging in Biomedicine. Int. J. Nanomed. 2020, 15, 7377–7395. [Google Scholar] [CrossRef]
- Jacoby, C.; Temme, S.; Mayenfels, F.; Benoit, N.; Krafft, M.P.; Schubert, R.; Schrader, J.; Flögel, U. Probing different perfluorocarbons forin vivoinflammation imaging by19F MRI: Image reconstruction, biological half-lives and sensitivity. NMR Biomed. 2014, 27, 261–271. [Google Scholar] [CrossRef]
- Stoll, G.; Basse-Lusebrink, T.; Weise, G.; Jakob, P. Visualization of inflammation using 19 F-magnetic resonance imaging and perfluorocarbons. Wiley Interdiscip Rev. Nanomed. Nanobiotechnol. 2012, 4, 438–447. [Google Scholar] [CrossRef]
- Mason, R.P.; Rodbumrung, W.; Antich, P.P. Hexafluorobenzene: A sensitive 19F NMR indicator of tumor oxygenation. NMR Biomed. 1996, 9, 125–134. [Google Scholar] [CrossRef]
- Sotak, C.H.; Hees, P.S.; Huang, H.N.; Hung, M.H.; Krespan, C.G.; Raynolds, S. A new perfluorocarbon for use in fluorine-19 magnetic resonance imaging and spectroscopy. Magn. Reson. Med. 1993, 29, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Janjic, J.M.; Srinivas, M.; Kadayakkara, D.K.K.; Ahrens, E.T. Self-delivering nanoemulsions for dual fluorine-19 MRI and fluorescence detection. J. Am. Chem. Soc. 2008, 130, 2832–2841. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.X.; Liu, X.; Jeong, E.K.; Yu, Y.B. Symmetry-guided design and fluorous synthesis of a stable and rapidly excreted imaging tracer for 19F MRI. Angew. Chem. Int Ed. Engl. 2009, 48, 4755–4758. [Google Scholar] [CrossRef] [Green Version]
- Goette, M.J.; Keupp, J.; Rahmer, J.; Lanza, G.M.; Wickline, S.A.; Caruthers, S.D. Balanced UTE-SSFP for 19F MR imaging of complex spectra. Magn. Reson. Med. 2015, 74, 537–543. [Google Scholar] [CrossRef] [Green Version]
- Nienhaus, F.; Colley, D.; Jahn, A.; Pfeiler, S.; Flocke, V.; Temme, S.; Kelm, M.; Gerdes, N.; Flogel, U.; Bonner, F. Phagocytosis of a PFOB-Nanoemulsion for 19F Magnetic Resonance Imaging: First Results in Monocytes of Patients with Stable Coronary Artery Disease and ST-Elevation Myocardial Infarction. Molecules 2019, 24, 2058. [Google Scholar] [CrossRef] [Green Version]
- Ahrens, E.T.; Zhong, J. In vivo MRI cell tracking using perfluorocarbon probes and fluorine-19 detection. NMR Biomed. 2013, 26, 860–871. [Google Scholar] [CrossRef] [Green Version]
- Kramer, W.; Schubert, R.; Massing, U. Small-scale preparation of perfluorocarbon-nanoemulsions utilizing dual centrifugation. Int. J. Pharm. 2019, 572, 118753. [Google Scholar] [CrossRef]
- Parak, W.J.; Osinski, M.; Yamamoto, K.I.; Diou, O.; Fattal, E.; Payen, T.; Bridal, S.L.; Valette, J.; Tsapis, N. Nanocapsules of perfluorooctyl bromide for theranostics: From formulation to targeting. In Proceedings of the Colloidal Nanoparticles for Biomedical Applications IX, San Francisco, CA, USA, 1–6 February 2014. [Google Scholar]
- Kadayakkara, D.K.; Damodaran, K.; Hitchens, T.K.; Bulte, J.W.M.; Ahrens, E.T. 19F spin–lattice relaxation of perfluoropolyethers: Dependence on temperature and magnetic field strength (7.0–14.1T). J. Magn. Reson. 2014, 242, 18–22. [Google Scholar] [CrossRef] [Green Version]
- Gerhardt, G.E.; Lagow, R.J. Synthesis of the perfluoropoly(ethylene glycol) ethers by direct fluorination. J. Org. Chem. 1978, 43, 4505–4509. [Google Scholar] [CrossRef]
- Sansotera, M.; Talaeemashhadi, S.; Gambarotti, C.; Pirola, C.; Longhi, M.; Ortenzi, M.A.; Navarrini, W.; Bianchi, C.L. Comparison of Branched and Linear Perfluoropolyether Chains Functionalization on Hydrophobic, Morphological and Conductive Properties of Multi-Walled Carbon Nanotubes. Nanomaterials 2018, 8, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonneaud, C.; Howell, J.; Bongiovanni, R.; Joly-Duhamel, C.; Friesen, C.M. Diversity of Synthetic Approaches to Functionalized Perfluoropolyalkylether Polymers. Macromolecules 2021, 54, 521–550. [Google Scholar] [CrossRef]
- Lemaire, L.; Bastiat, G.; Franconi, F.; Lautram, N.; Duong Thi Dan, T.; Garcion, E.; Saulnier, P.; Benoit, J.P. Perfluorocarbon-loaded lipid nanocapsules as oxygen sensors for tumor tissue pO(2) assessment. Eur. J. Pharm. Biopharm. 2013, 84, 479–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constantinides, C.; McNeill, E.; Carnicer, R.; Al Haj Zen, A.; Sainz-Urruela, R.; Shaw, A.; Patel, J.; Swider, E.; Alonaizan, R.; Potamiti, L.; et al. Improved cellular uptake of perfluorocarbon nanoparticles for in vivo murine cardiac 19F MRS/MRI and temporal tracking of progenitor cells. Nanomedicine 2019, 18, 391–401. [Google Scholar] [CrossRef]
- Flogel, U.; Schluter, A.; Jacoby, C.; Temme, S.; Banga, J.P.; Eckstein, A.; Schrader, J.; Berchner-Pfannschmidt, U. Multimodal assessment of orbital immune cell infiltration and tissue remodeling during development of graves disease by 1 H19 F MRI. Magn. Reson. Med. 2018, 80, 711–718. [Google Scholar] [CrossRef]
- Prinz, C.; Delgado, P.R.; Eigentler, T.W.; Starke, L.; Niendorf, T.; Waiczies, S. Toward 19F magnetic resonance thermometry: Spin-lattice and spin-spin-relaxation times and temperature dependence of fluorinated drugs at 9.4 T. Magn. Reson. Mater. Phys. Biol. Med. 2019, 32, 51–61. [Google Scholar] [CrossRef]
- Weise, G.; Basse-Lusebrink, T.C.; Kleinschnitz, C.; Kampf, T.; Jakob, P.M.; Stoll, G. In vivo imaging of stepwise vessel occlusion in cerebral photothrombosis of mice by 19F MRI. PLoS ONE 2011, 6, e28143. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.H.; Park, E.J.; Min, C.; Choi, S.I.; Jeon, S.; Kim, Y.H.; Kim, D. Tracking Perfluorocarbon Nanoemulsion Delivery by 19F MRI for Precise High Intensity Focused Ultrasound Tumor Ablation. Theranostics 2017, 7, 562–572. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Yan, Y.; Liu, F.; Wu, L.; Shao, M.; Wang, K.; Sun, X.; Li, Y.; Beinpuo, E.S.W.; Shen, B. Folate receptor-targeted 19 F MR molecular imaging and proliferation evaluation of lung cancer. J. Magn. Reson. Imaging 2018, 48, 1617–1625. [Google Scholar] [CrossRef]
- Saini, S.; Korf, H.; Liang, S.; Verbeke, R.; Manshian, B.; Raemdonck, K.; Lentacker, I.; Gysemans, C.; De Smedt, S.C.; Himmelreich, U. Challenges for labeling and longitudinal tracking of adoptively transferred autoreactive T lymphocytes in an experimental type-1 diabetes model. Magn. Reson. Mater. Phys. Biol. Med. 2019, 32, 295–305. [Google Scholar] [CrossRef]
- Tirotta, I.; Mastropietro, A.; Cordiglieri, C.; Gazzera, L.; Baggi, F.; Baselli, G.; Bruzzone, M.G.; Zucca, I.; Cavallo, G.; Terraneo, G.; et al. A superfluorinated molecular probe for highly sensitive in vivo19F-MRI. J. Am. Chem. Soc. 2014, 136, 8524–8527. [Google Scholar] [CrossRef] [PubMed]
- Chirizzi, C.; De Battista, D.; Tirotta, I.; Metrangolo, P.; Comi, G.; Bombelli, F.B.; Chaabane, L. Multispectral MRI with Dual Fluorinated Probes to Track Mononuclear Cell Activity in Mice. Radiology 2019, 291, 351–357. [Google Scholar] [CrossRef] [PubMed]
- 19F MRI Contrast Agents. Available online: Clinicaltrials.gov (accessed on 21 September 2021).
- Arango, J.M.; Padro, D.; Blanco, J.; Lopez-Fernandez, S.; Castellnou, P.; Villa-Valverde, P.; Ruiz-Cabello, J.; Martin, A.; Carril, M. Fluorine Labeling of Nanoparticles and In Vivo 19F Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2021, 13, 12941–12949. [Google Scholar] [CrossRef] [PubMed]
- Weise, G.; Stoll, G. Magnetic resonance imaging of blood brain/nerve barrier dysfunction and leukocyte infiltration: Closely related or discordant? Front. Neuurol. 2012, 3, 178. [Google Scholar] [CrossRef] [Green Version]
- Bharti, S.K.; Roy, R. Quantitative 1H NMR spectroscopy. TrAC Trends Anal. Chem. 2012, 35, 5–26. [Google Scholar] [CrossRef]
- Liang, S.; Dresselaers, T.; Louchami, K.; Zhu, C.; Liu, Y.; Himmelreich, U. Comparison of different compressed sensing algorithms for low SNR 19 F MRI applications-Imaging of transplanted pancreatic islets and cells labeled with perfluorocarbons. NMR Biomed. 2017, 30. [Google Scholar] [CrossRef]
- Waiczies, S.; Rosenberg, J.T.; Kuehne, A.; Starke, L.; Delgado, P.R.; Millward, J.M.; Prinz, C.; Dos Santos Periquito, J.; Pohlmann, A.; Waiczies, H.; et al. Fluorine-19 MRI at 21.1 T: Enhanced spin-lattice relaxation of perfluoro-15-crown-5-ether and sensitivity as demonstrated in ex vivo murine neuroinflammation. Magn. Reson. Mater. Phys. Biol. Med. 2019, 32, 37–49. [Google Scholar] [CrossRef] [Green Version]
- van Heeswijk, R.B.; Colotti, R.; Darcot, E.; Delacoste, J.; Pellegrin, M.; Piccini, D.; Hernando, D. Chemical shift encoding (CSE) for sensitive fluorine-19 MRI of perfluorocarbons with complex spectra. Magn. Reson. Med. 2018, 79, 2724–2730. [Google Scholar] [CrossRef]
- Plaumann, M.; Bommerich, U.; Trantzschel, T.; Lego, D.; Dillenberger, S.; Sauer, G.; Bargon, J.; Buntkowsky, G.; Bernarding, J. Parahydrogen-induced polarization transfer to 19F in perfluorocarbons for 19F NMR spectroscopy and MRI. Chemistry 2013, 19, 6334–6339. [Google Scholar] [CrossRef]
- Tanifum, E.A.; Devkota, L.; Ngwwa, C.; Badachhape, A.A.; Ghaghada, K.B.; Romero, J.; Pautler, R.G.; Annapragada, A.V. A Hyperfluorinated Hydrophilic Molecule for Aqueous 19F MRI Contrast Media. Contrast Media Mol. Imaging 2018, 2018, 1693513. [Google Scholar] [CrossRef]
- Ferenz, K.B.; Steinbicker, A.U. Artificial Oxygen Carriers-Past, Present, and Future-a Review of the Most Innovative and Clinically Relevant Concepts. J. Pharmacol. Exp. Ther. 2019, 369, 300–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stenzel, M.; Mentzel, H.J. Ultrasound elastography and contrast-enhanced ultrasound in infants, children and adolescents. Eur. J. Radiol. 2014, 83, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Paefgen, V.; Doleschel, D.; Kiessling, F. Evolution of contrast agents for ultrasound imaging and ultrasound-mediated drug delivery. Front. Pharmacol. 2015, 6, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podell, S.; Burrascano, C.; Gaal, M.; Golec, B.; Maniquis, J.; Mehlhaff, P. Physical and biochemical stability of Optison, an injectable ultrasound contrast agent. Biotechnol. Appl. Biochem. 1999, 30, 213–223. [Google Scholar] [PubMed]
- Saucedo, A.M.; De La Cerda, J.; Suami, H.; Serda, R.E. Multimodal imaging of the tumor microenvironment and biological responses to immune therapy. Biomed. Microdevices 2018, 20, 105. [Google Scholar] [CrossRef]
- Fox, M.S.; Gaudet, J.M.; Foster, P.J. Fluorine-19 MRI Contrast Agents for Cell Tracking and Lung Imaging. Magn. Reson. Insights 2015, 8, 53–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Hanlon, C.F.; Fedczyna, T.; Eaker, S.; Shingleton, W.D.; Helfer, B.M. Integrating a 19F MRI Tracer Agent into the Clinical Scale Manufacturing of a T-Cell Immunotherapy. Contrast Media Mol. Imaging 2017, 2017, 9548478. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, S.; Padelli, F.; Rinaldi, E.; Gioeni, D.; Aquino, D.; Brizzola, S.; Acocella, F.; Spaggiari, L.; Baggi, F.; Bellomi, M.; et al. 7-T MRI tracking of mesenchymal stromal cells after lung injection in a rat model. Eur. Radiol. Exp. 2020, 4, 54. [Google Scholar] [CrossRef]
- Pavlova, O.S.; Gulyaev, M.V.; Anisimov, N.V.; Silachev, D.N.; Gervits, L.L.; Pirogov, Y.A. New Aspects of Biodistribution of Perfluorocarbon Emulsions in Rats: Thymus Imaging. Appl. Magn. Reson. 2020, 51, 1625–1635. [Google Scholar] [CrossRef]
- Latson, G.W. Perftoran (Vidaphor)-Introduction to Western Medicine. Shock 2019, 52, 65–69. [Google Scholar] [CrossRef]
- Maevsky, E.; Ivanitsky, G.; Bogdanova, L.; Axenova, O.; Karmen, N.; Zhiburt, E.; Senina, R.; Pushkin, S.; Maslennikov, I.; Orlov, A.; et al. Clinical results of Perftoran application: Present and future. Artif. Cells Blood Substit. Immobil. Biotechnol. 2005, 33, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Scholz, A.W.; Eberle, B.; Heussel, C.P.; David, M.; Schmittner, M.D.; Quintel, M.; Schreiber, L.M.; Weiler, N. Ventilation-perfusion ratio in perflubron during partial liquid ventilation. Anesth. Analg. 2010, 110, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Chenoune, M.; De Rochefort, L.; Bruneval, P.; Lidouren, F.; Kohlhauer, M.; Seemann, A.; Ghaleh, B.; Korn, M.; Dubuisson, R.-M.; Ben, Y.A.; et al. Evaluation of lung recovery after static administration of three different perfluorocarbons in pigs. BMC Pharmacol. Toxicol. 2014, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohlhauer, M.; Boissady, E.; Lidouren, F.; de Rochefort, L.; Nadeau, M.; Rambaud, J.; Hutin, A.; Dubuisson, R.M.; Guillot, G.; Pey, P.; et al. A new paradigm for lung-conservative total liquid ventilation. EBioMedicine 2020, 52, 102365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weigel, J.K.; Steinmann, D.; Emerich, P.; Stahl, C.A.; v Elverfeldt, D.; Guttmann, J. High-resolution three-dimensional 19F-magnetic resonance imaging of rat lung in situ: Evaluation of airway strain in the perfluorocarbon-filled lung. Physiol. Meas. 2011, 32, 251–262. [Google Scholar] [CrossRef]
- André Dias, S.; Berdeaux, A.; Darrasse, L.; Demanesse, M.; de Rochefort, L.; Filoche, M.; Ghaleh, B.; Hutin, A.; Isabey, D.; Kunc, T.; et al. ABYSS: Therapeutic hypothermia by total liquid ventilation following cardiac arrest and resuscitation. IRBM 2015, 36, 110–117. [Google Scholar] [CrossRef]
- Hertlein, T.; Sturm, V.; Jakob, P.; Ohlsen, K. 19F magnetic resonance imaging of perfluorocarbons for the evaluation of response to antibiotic therapy in a Staphylococcus aureus infection model. PLoS ONE 2013, 8, e64440. [Google Scholar] [CrossRef] [Green Version]
- de Rochambeau, D.; Barłóg, M.; Edwardson, T.G.W.; Fakhoury, J.J.; Stein, R.S.; Bazzi, H.S.; Sleiman, H.F. “DNA–Teflon” sequence-controlled polymers. Polym. Chem. 2016, 7, 4998–5003. [Google Scholar] [CrossRef]
- Temme, S.; Baran, P.; Bouvain, P.; Grapentin, C.; Kramer, W.; Knebel, B.; Al-Hasani, H.; Moll, J.M.; Floss, D.; Schrader, J.; et al. Synthetic Cargo Internalization Receptor System for Nanoparticle Tracking of Individual Cell Populations by Fluorine Magnetic Resonance Imaging. ACS Nano 2018, 12, 11178–11192. [Google Scholar] [CrossRef]
- Boehm-Sturm, P.; Mengler, L.; Wecker, S.; Hoehn, M.; Kallur, T. In vivo tracking of human neural stem cells with 19F magnetic resonance imaging. PLoS ONE 2011, 6, e29040. [Google Scholar] [CrossRef]
- Sehl, O.C.; Makela, A.V.; Hamilton, A.M.; Foster, P.J. Trimodal Cell Tracking In Vivo: Combining Iron- and Fluorine-Based Magnetic Resonance Imaging with Magnetic Particle Imaging to Monitor the Delivery of Mesenchymal Stem Cells and the Ensuing Inflammation. Tomography 2019, 5, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, G.; Jablonska, A.; Rose, L.; Walczak, P.; Janowski, M. Effect of MRI tags: SPIO nanoparticles and 19F nanoemulsion on various populations of mouse mesenchymal stem cells. Acta Neurobiol. Exp. (Wars) 2015, 75, 144–159. [Google Scholar] [PubMed]
- Flogel, U.; Su, S.; Kreideweiss, I.; Ding, Z.; Galbarz, L.; Fu, J.; Jacoby, C.; Witzke, O.; Schrader, J. Noninvasive detection of graft rejection by in vivo 19 F MRI in the early stage. Am. J. Transplant. 2011, 11, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Deuchar, G.A.; Brennan, D.; Griffiths, H.; Macrae, I.M.; Santosh, C. Perfluorocarbons enhance a T2*-based MRI technique for identifying the penumbra in a rat model of acute ischemic stroke. J. Cereb. Blood Flow Metab. 2013, 33, 1422–1428. [Google Scholar] [CrossRef] [Green Version]
- Khurana, A.; Chapelin, F.; Xu, H.; Acevedo, J.R.; Molinolo, A.; Nguyen, Q.; Ahrens, E.T. Visualization of macrophage recruitment in head and neck carcinoma model using fluorine-19 magnetic resonance imaging. Magn. Reson. Med. 2018, 79, 1972–1980. [Google Scholar] [CrossRef]
- Shin, S.H.; Park, S.H.; Kang, S.H.; Kim, S.W.; Kim, M.; Kim, D. Fluorine-19 Magnetic Resonance Imaging and Positron Emission Tomography of Tumor-Associated Macrophages and Tumor Metabolism. Contrast Media Mol. Imaging 2017, 2017, 4896310. [Google Scholar] [CrossRef]
- Makela, A.V.; Gaudet, J.M.; Foster, P.J. Quantifying tumor associated macrophages in breast cancer: A comparison of iron and fluorine-based MRI cell tracking. Sci. Rep. 2017, 7, 42109. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Nicholson, M.L. The role of perfluorocarbon in organ preservation. Transplantation 2010, 89, 1169–1175. [Google Scholar] [CrossRef]
- Moore, J.K.; Chen, J.; Pan, H.; Gaaut, J.P.; Jain, S.; Wickline, S.A. Quantification of vascular damage in acute kidney injury with fluorine magnetic resonance imaging and spectroscopy. Magn. Reson. Med. 2018, 79, 3144–3153. [Google Scholar] [CrossRef]
- Baete, S.H.; Vandecasteele, J.; Colman, L.; De Neve, W.; De Deene, Y. An oxygen-consuming phantom simulating perfused tissue to explore oxygen dynamics and 19F MRI oximetry. Magn. Reson. Mater. Phys. Biol. Med. 2010, 23, 217–226. [Google Scholar] [CrossRef]
- Jacoby, C.; Borg, N.; Heusch, P.; Sauter, M.; Bonner, F.; Kandolf, R.; Klingel, K.; Schrader, J.; Flogel, U. Visualization of immune cell infiltration in experimental viral myocarditis by 19F MRI in vivo. Magn. Reson. Mater. Phys. Biol. Med. 2014, 27, 101–106. [Google Scholar] [CrossRef] [PubMed]
- van Heeswijk, R.B.; De Blois, J.; Kania, G.; Gonzales, C.; Blyszczuk, P.; Stuber, M.; Eriksson, U.; Schwitter, J. Selective in vivo visualization of immune-cell infiltration in a mouse model of autoimmune myocarditis by fluorine-19 cardiac magnetic resonance. Circ. Cardiovasc. Imaging 2013, 6, 277–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darcot, E.; Colotti, R.; Pellegrin, M.; Wilson, A.; Siegert, S.; Bouzourene, K.; Yerly, J.; Mazzolai, L.; Stuber, M.; van Heeswijk, R.B. Towards Quantification of Inflammation in Atherosclerotic Plaque in the Clinic—Characterization and Optimization of Fluorine-19 MRI in Mice at 3 T. Sci. Rep. 2019, 9, 17488. [Google Scholar] [CrossRef] [PubMed]
- Rothe, M.; Jahn, A.; Weiss, K.; Hwang, J.H.; Szendroedi, J.; Kelm, M.; Schrader, J.; Roden, M.; Flogel, U.; Bonner, F. In vivo 19F MR inflammation imaging after myocardial infarction in a large animal model at 3 T. Magn. Reson. Mater. Phys. Biol. Med. 2019, 32, 5–13. [Google Scholar] [CrossRef]
- Ebner, B.; Behm, P.; Jacoby, C.; Burghoff, S.; French, B.A.; Schrader, J.; Flogel, U. Early assessment of pulmonary inflammation by 19F MRI in vivo. Circ. Cardiovasc. Imaging 2010, 3, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Flogel, U.; Ding, Z.; Hardung, H.; Jander, S.; Reichmann, G.; Jacoby, C.; Schubert, R.; Schrader, J. In vivo monitoring of inflammation after cardiac and cerebral ischemia by fluorine magnetic resonance imaging. Circulation 2008, 118, 140–148. [Google Scholar] [CrossRef] [Green Version]
- Cao, B.; Lyu, X.; Wang, C.; Lu, S.; Xing, D.; Hu, X. Rational collaborative ablation of bacterial biofilms ignited by physical cavitation and concurrent deep antibiotic release. Biomaterials 2020, 262, 120341. [Google Scholar] [CrossRef]
- Riess, J.G. Perfluorocarbon-based oxygen delivery. Artif. Cells Blood Substit. Immobil. Biotechnol. 2006, 34, 567–580. [Google Scholar] [CrossRef]
- Castro, C.I.; Briceno, J.C. Perfluorocarbon-based oxygen carriers: Review of products and trials. Artif. Organs 2010, 34, 622–634. [Google Scholar] [CrossRef]
- Lambert, E.; Gorantla, V.S.; Janjic, J.M. Pharmaceutical design and development of perfluorocarbon nanocolloids for oxygen delivery in regenerative medicine. Nanomedicine 2019, 14, 20. [Google Scholar] [CrossRef]
- Goh, F.; Sambanis, A. In vivo noninvasive monitoring of dissolved oxygen concentration within an implanted tissue-engineered pancreatic construct. Tissue Eng. Part C Methods 2011, 17, 887–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temme, S.; Bonner, F.; Schrader, J.; Flogel, U. 19F magnetic resonance imaging of endogenous macrophages in inflammation. Wiley Interdiscip Rev. Nanomed. Nanobiotechnol. 2012, 4, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Palekar, R.U.; Jallouk, A.P.; Lanza, G.M.; Pan, H.; Wickline, S.A. Molecular imaging of atherosclerosis with nanoparticle-based fluorinated MRI contrast agents. Nanomedicine 2015, 10, 1817–1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivas, M.; Heerschap, A.; Ahrens, E.T.; Figdor, C.G.; de Vries, I.J. 19F MRI for quantitative in vivo cell tracking. Trends Biotechnol. 2010, 28, 363–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivas, M.; Boehm-Sturm, P.; Figdor, C.G.; de Vries, I.J.; Hoehn, M. Labeling cells for in vivo tracking using 19F MRI. Biomaterials 2012, 33, 8830–8840. [Google Scholar] [CrossRef]
- Yahyapour, R.; Farhood, B.; Graily, G.; Rezaeyan, A.; Rezapoor, S.; Abdollahi, H.; Cheki, M.; Amini, P.; Fallah, H.; Najafi, M.; et al. Stem Cell Tracing Through MR Molecular Imaging. Tissue Eng. Regen. Med. 2018, 15, 249–261. [Google Scholar] [CrossRef]
- Heerschap, A. In Vivo 19F Magnetic Resonance Spectroscopy. eMagRes 2016, 5, 1283–1290. [Google Scholar] [CrossRef]
- Bober, Z.; Aebisher, D.; Tabarkiewicz, J.; Guz, W.; Tutka, P.; Bartusik-Aebisher, D. Investigation of pharmaceuticals by nuclear magnetic resonance imaging and spectroscopy. Eur. J. Clin. Exp. Med. 2017, 15, 99–108. [Google Scholar] [CrossRef]
- Waiczies, S.; Srinivas, M.; Flogel, U.; Boehm-Sturm, P.; Niendorf, T. Special issue on fluorine-19 magnetic resonance: Technical solutions, research promises and frontier applications. Magn. Reson. Mater. Phys. Biol. Med. 2019, 32, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Lopez, R.; Tsapis, N.; Fattal, E. Liquid perfluorocarbons as contrast agents for ultrasonography and 19F-MRI. Pharm. Res. 2010, 27, 1–16. [Google Scholar] [CrossRef]
- Cosco, D.; Fattal, E.; Fresta, M.; Tsapis, N. Perfluorocarbon-loaded micro and nanosystems for medical imaging: A state of the art. J. Fluor. Chem. 2015, 171, 18–26. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Q.; Tian, J.-H.; Xing, J.-F.; Guo, W.; Liang, X.-J. Perfluorocarbon-based nanomedicine: Emerging strategy for diagnosis and treatment of diseases. MRS Commun. 2018, 8, 303–313. [Google Scholar] [CrossRef]
- Amiri, H.; Srinivas, M.; Veltien, A.; van Uden, M.J.; de Vries, I.J.; Heerschap, A. Cell tracking using 19F magnetic resonance imaging: Technical aspects and challenges towards clinical applications. Eur. Radiol. 2015, 25, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Riess, J.G. The design and development of improved fluorocarbon-based products for use in medicine and biology. Artif. Cells Blood Substit. Immobil. Biotechnol. 1994, 22, 215–234. [Google Scholar] [CrossRef]
- Riess, J.G.; Pierre, K.M. Fluorinated materials for in vivo oxygen transport (blood substitutes), diagnosis and drug delivery. Biomaterials 1998, 19, 1529–1539. [Google Scholar] [CrossRef]
- Bartusik, D.; Aebisher, D. 19F applications in drug development and imaging—A review. Biomed. Pharmacother. 2014, 68, 813–817. [Google Scholar] [CrossRef]
- Peterson, K.L.; Srivastava, K.; Pierre, V.C. Fluorinated Paramagnetic Complexes: Sensitive and Responsive Probes for Magnetic Resonance Spectroscopy and Imaging. Front. Chem. 2018, 6, 160. [Google Scholar] [CrossRef]
- Jirak, D.; Galisova, A.; Kolouchova, K.; Babuka, D.; Hruby, M. Fluorine polymer probes for magnetic resonance imaging: Quo vadis? Magn. Reson. Mater. Phys. Biol. Med. 2019, 32, 173–185. [Google Scholar] [CrossRef] [Green Version]
- Hequet, E.; Henoumont, C.; Muller, R.N.; Laurent, S. Fluorinated MRI contrast agents and their versatile applications in the biomedical field. Future Med. Chem. 2019, 11, 1157–1175. [Google Scholar] [CrossRef] [Green Version]
- Bartusik-Aebisher, D.; Bober, Z.; Aebisher, D. Selected applications of fluorinated MR contrast agents and fluorine-containing drugs in medicine. Acta Pol. Pharm.—Drug Res. 2020, 77, 403–410. [Google Scholar] [CrossRef]
- Moroz, V.V.; Chernysh, A.M.; Kozlova, E.K. Coronavirus SARS-CoV-2: Hypotheses of Impact on the Circulatory System, Prospects for the Use of Perfluorocarbon Emulsion, and Feasibility of Biophysical Research Methods. Gen. Reanimatol. 2020, 16, 4–13. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Perez-Aranda, M.; Martinez, G.; Merinero, M.; Arguelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, A.; Carreiro, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Wallat, J.D.; Czapar, A.E.; Wang, C.; Wen, A.M.; Wek, K.S.; Yu, X.; Steinmetz, N.F.; Pokorski, J.K. Optical and Magnetic Resonance Imaging Using Fluorous Colloidal Nanoparticles. Biomacromolecules 2017, 18, 103–112. [Google Scholar] [CrossRef]
- Bailey, M.M.; Mahoney, C.M.; Dempah, K.E.; Davis, J.M.; Becker, M.L.; Khondee, S.; Munson, E.J.; Berkland, C. Fluorinated copolymer nanoparticles for multimodal imaging applications. Macromol. Rapid Commun. 2010, 31, 87–92. [Google Scholar] [CrossRef]
- Kaberov, L.I.; Verbraeken, B.; Riabtseva, A.; Brus, J.; Radulescu, A.; Talmon, Y.; Stepanek, P.; Hoogenboom, R.; Filippov, S.K. Fluorophilic–Lipophilic–Hydrophilic Poly(2-oxazoline) Block Copolymers as MRI Contrast Agents: From Synthesis to Self-Assembly. Macromolecules 2018, 51, 6047–6056. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Ta, H.T.; Zhang, C.; Whittaker, A.K. Polymerization-Induced Self-Assembly (PISA)—Control over the Morphology of 19F-Containing Polymeric Nano-objects for Cell Uptake and Tracking. Biomacromolecules 2017, 18, 1145–1156. [Google Scholar] [CrossRef]
- Fu, C.; Herbst, S.; Zhang, C.; Whittaker, A.K. Polymeric 19F MRI agents responsive to reactive oxygen species. Polym. Chem. 2017, 8, 4585–4595. [Google Scholar] [CrossRef]
- Huang, P.; Guo, W.; Yang, G.; Song, H.; Wang, Y.; Wang, C.; Kong, D.; Wang, W. Fluorine Meets Amine: Reducing Microenvironment-Induced Amino-Activatable Nanoprobes for 19F-Magnetic Resonance Imaging of Biothiols. ACS Appl. Mater. Interfaces 2018, 10, 18532–18542. [Google Scholar] [CrossRef]
- Szczech, M.; Lopuszynska, N.; Tomal, W.; Jasinski, K.; Weglarz, W.P.; Warszynski, P.; Szczepanowicz, K. Nafion-Based Nanocarriers for Fluorine Magnetic Resonance Imaging. Langmuir 2020, 36, 9534–9539. [Google Scholar] [CrossRef]
- Srinivas, M.; Cruz, L.J.; Bonetto, F.; Heerschap, A.; Figdor, C.G.; de Vries, I.J. Customizable, multi-functional fluorocarbon nanoparticles for quantitative in vivo imaging using 19F MRI and optical imaging. Biomaterials 2010, 31, 7070–7077. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, M.; Morel, P.A.; Ernst, L.A.; Laidlaw, D.H.; Ahrens, E.T. Fluorine-19 MRI for visualization and quantification of cell migration in a diabetes model. Magn. Reson. Med. 2007, 58, 725–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangala, S.; Jurjen, T.; Gerty, S.; Fernando, B.; Luis-Javier, C.; Houshang, A.; Arend, H.; Carl, G.F.; de Vries, J.M. PLGA-encapsulated perfluorocarbon nanoparticles for simultaneous visualization of distinct cell populations by 19F MRI. Nanomedicine 2015, 10, 2339–2348. [Google Scholar] [CrossRef]
- Koshkina, O.; Lajoinie, G.; Bombelli, F.B.; Swider, E.; Cruz, L.J.; White, P.B.; Schweins, R.; Dolen, Y.; van Dinther, E.A.W.; van Riessen, N.K.; et al. Multicore Liquid Perfluorocarbon-Loaded Multimodal Nanoparticles for Stable Ultrasound and 19F MRI Applied to In Vivo Cell Tracking. Adv. Funct. Mater. 2019, 29, 1806485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swider, E.; Daoudi, K.; Staal, A.H.J.; Koshkina, O.; van Riessen, N.K.; van Dinther, E.; de Vries, I.J.M.; de Korte, C.L.; Srinivas, M. Clinically-Applicable Perfluorocarbon-Loaded Nanoparticles For In vivo Photoacoustic, 19F Magnetic Resonance And Fluorescent Imaging. Nanotheranostics 2018, 2, 258–268. [Google Scholar] [CrossRef] [Green Version]
- Constantinides, C.; Maguire, M.; McNeill, E.; Carnicer, R.; Swider, E.; Srinivas, M.; Carr, C.A.; Schneider, J.E. Fast, quantitative, murine cardiac 19F MRI/MRS of PFCE-labeled progenitor stem cells and macrophages at 9.4T. PLoS ONE 2018, 13, e0190558. [Google Scholar] [CrossRef]
- Mastrogiacomo, S.; Dou, W.; Koshkina, O.; Boerman, O.C.; Jansen, J.A.; Heerschap, A.; Srinivas, M.; Walboomers, X.F. Perfluorocarbon/Gold Loading for Noninvasive in Vivo Assessment of Bone Fillers Using 19F Magnetic Resonance Imaging and Computed Tomography. ACS Appl. Mater. Interfaces 2017, 9, 22149–22159. [Google Scholar] [CrossRef]
- Swider, E.; Staal, A.H.J.; Koen van Riessen, N.; Jacobs, L.; White, P.B.; Fokkink, R.; Janssen, G.-J.; van Dinther, E.; Figdor, C.G.; de Vries, I.; et al. Design of triphasic poly(lactic-co-glycolic acid) nanoparticles containing a perfluorocarbon phase for biomedical applications. RSC Adv. 2018, 8, 6460–6470. [Google Scholar] [CrossRef] [Green Version]
- Staal, A.H.J.; Becker, K.; Tagit, O.; Koen van Riessen, N.; Koshkina, O.; Veltien, A.; Bouvain, P.; Cortenbach, K.R.G.; Scheenen, T.; Flogel, U.; et al. In vivo clearance of 19F MRI imaging nanocarriers is strongly influenced by nanoparticle ultrastructure. Biomaterials 2020, 261, 120307. [Google Scholar] [CrossRef]
- Koshkina, O.; White, P.B.; Staal, A.H.J.; Schweins, R.; Swider, E.; Tirotta, I.; Tinnemans, P.; Fokkink, R.; Veltien, A.; van Riessen, N.K.; et al. Nanoparticles for “two color” 19F magnetic resonance imaging: Towards combined imaging of biodistribution and degradation. J. Colloid Interface Sci. 2020, 565, 278–287. [Google Scholar] [CrossRef]
- Krekorian, M.; Van Riessen, K.; Sandker, G.; Swider, E.; Staal, A.; Koshkina, O.; Heskamp, S.; Srinivas, M.; Aarntzen, E. PLGA nanoparticles for combined SPECT/PET and 19F MRI in vivo cell tracking. Nucl. Med. Biol. 2019, 72–73, S42–S43. [Google Scholar] [CrossRef]
- Hoogendijk, E.; Swider, E.; Staal, A.H.J.; White, P.B.; van Riessen, N.K.; Glasser, G.; Lieberwirth, I.; Musyanovych, A.; Serra, C.A.; Srinivas, M.; et al. Continuous-Flow Production of Perfluorocarbon-Loaded Polymeric Nanoparticles: From the Bench to Clinic. ACS Appl Mater. Interfaces 2020, 12, 49335–49345. [Google Scholar] [CrossRef] [PubMed]
- Vu-Quang, H.; Vinding, M.S.; Xia, D.; Nielsen, T.; Ullisch, M.G.; Dong, M.; Nielsen, N.C.; Kjems, J. Chitosan-coated poly(lactic-co-glycolic acid) perfluorooctyl bromide nanoparticles for cell labeling in 19F magnetic resonance imaging. Carbohydr. Polym. 2016, 136, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Vu-Quang, H.; Vinding, M.S.; Nielsen, T.; Ullisch, M.G.; Nielsen, N.C.; Kjems, J. Theranostic tumor targeted nanoparticles combining drug delivery with dual near infrared and 19F magnetic resonance imaging modalities. Nanomedicine 2016, 12, 1873–1884. [Google Scholar] [CrossRef]
- Vu-Quang, H.; Vinding, M.S.; Jakobsen, M.; Song, P.; Dagnaes-Hansen, F.; Nielsen, N.C.; Kjems, J. Imaging Rheumatoid Arthritis in Mice Using Combined Near Infrared and 19F Magnetic Resonance Modalities. Sci. Rep. 2019, 9, 14314. [Google Scholar] [CrossRef]
- Quang, H.V.; Chang, C.C.; Song, P.; Hauge, E.M.; Kjems, J. Caveolae-mediated mesenchymal stem cell labelling by PSS-coated PLGA PFOB nano-contrast agent for MRI. Theranostics 2018, 8, 2657–2671. [Google Scholar] [CrossRef]
- Diou, O.; Tsapis, N.; Giraudeau, C.; Valette, J.; Gueutin, C.; Bourasset, F.; Zanna, S.; Vauthier, C.; Fattal, E. Long-circulating perfluorooctyl bromide nanocapsules for tumor imaging by 19FMRI. Biomaterials 2012, 33, 5593–5602. [Google Scholar] [CrossRef]
- Somaglino, L.; Mousnier, L.; Giron, A.; Urbach, W.; Tsapis, N.; Taulier, N. In vitro evaluation of polymeric nanoparticles with a fluorine core for drug delivery triggered by focused ultrasound. Colloids Surf. B Biointerfaces 2021, 200, 111561. [Google Scholar] [CrossRef]
- Cruz, L.J.; Que, I.; Aswendt, M.; Chan, A.; Hoehn, M.; Löwik, C. Targeted nanoparticles for the non-invasive detection of traumatic brain injury by optical imaging and fluorine magnetic resonance imaging. Nano Res. 2016, 9, 1276–1289. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Zambito, G.; Deng, S.; Haeck, J.; Gaspar, N.; Himmelreich, U.; Censi, R.; Lowik, C.; Di Martino, P.; Mezzanotte, L. Fluorinated PLGA-PEG-Mannose Nanoparticles for Tumor-Associated Macrophage Detection by Optical Imaging and MRI. Front. Med. 2021, 8, 712367. [Google Scholar] [CrossRef] [PubMed]
- Zerrillo, L.; Gupta, K.; Lefeber, F.; Da Silva, C.G.; Galli, F.; Chan, A.; Veltien, A.; Dou, W.; Censi, R.; Di Martino, P.; et al. Novel Fluorinated Poly (Lactic-Co-Glycolic acid) (PLGA) and Polyethylene Glycol (PEG) Nanoparticles for Monitoring and Imaging in Osteoarthritis. Pharmaceutics 2021, 13, 235. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhou, Q.; Zhu, H.; Zhuang, Y.; Bao, J. Enhanced antitumor efficacy in colon cancer using EGF functionalized PLGA nanoparticles loaded with 5-Fluorouracil and perfluorocarbon. BMC Cancer 2020, 20, 354. [Google Scholar] [CrossRef] [PubMed]
- Neri, G.; Mion, G.; Pizzi, A.; Celentano, W.; Chaabane, L.; Chierotti, M.R.; Gobetto, R.; Li, M.; Messa, P.; Campo, F.D.; et al. Fluorinated-PLGA Nanoparticles for Enhanced Drug Encapsulation and 19F-NMR Detection. Chem.—A Eur. J. 2020, 26, 10057–10063. [Google Scholar] [CrossRef] [PubMed]
- Achilefu, S.; Janjic, J.M.; Patel, S.K.; Patrick, M.J.; Pollock, J.A.; DiVito, E.; Cascio, M.; Raghavachari, R. Suppressing inflammation from inside out with novel NIR visible perfluorocarbon nanotheranostics. In Proceedings of the Reporters, Markers, Dyes, Nanoparticles, and Molecular Probes for Biomedical Applications V, San Francisco, CA, USA, 2–7 February 2013. [Google Scholar]
- Patel, S.K.; Zhang, Y.; Pollock, J.A.; Janjic, J.M. Cyclooxgenase-2 inhibiting perfluoropoly (ethylene glycol) ether theranostic nanoemulsions-in vitro study. PLoS ONE 2013, 8, e55802. [Google Scholar] [CrossRef]
- Patel, S.K.; Patrick, M.J.; Pollock, J.A.; Janjic, J.M. Two-color fluorescent (near-infrared and visible) triphasic perfluorocarbon nanoemuslions. J. Biomed. Opt. 2013, 18, 101312. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Moonshi, S.S.; Han, Y.; Puttick, S.; Peng, H.; Magoling, B.J.A.; Reid, J.C.; Bernardi, S.; Searles, D.J.; Král, P.; et al. PFPE-Based Polymeric 19F MRI Agents: A New Class of Contrast Agents with Outstanding Sensitivity. Macromolecules 2017, 50, 5953–5963. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, T.; Wang, W.; Bell, C.A.; Han, Y.; Fu, C.; Peng, H.; Tan, X.; Kral, P.; Gaus, K.; et al. Tuning of the Aggregation Behavior of Fluorinated Polymeric Nanoparticles for Improved Therapeutic Efficacy. ACS Nano 2020, 14, 7425–7434. [Google Scholar] [CrossRef]
- Fu, C.; Zhang, C.; Peng, H.; Han, F.; Baker, C.; Wu, Y.; Ta, H.; Whittaker, A.K. Enhanced Performance of Polymeric 19F MRI Contrast Agents through Incorporation of Highly Water-Soluble Monomer MSEA. Macromolecules 2018, 51, 5875–5882. [Google Scholar] [CrossRef]
- Kirberger, S.E.; Maltseva, S.D.; Manulik, J.C.; Einstein, S.A.; Weegman, B.P.; Garwood, M.; Pomerantz, W.C.K. Synthesis of Intrinsically Disordered Fluorinated Peptides for Modular Design of High-Signal 19 F MRI Agents. Angew. Chem. Int. Ed. Engl. 2017, 56, 6440–6444. [Google Scholar] [CrossRef]
- Moonshi, S.S.; Zhang, C.; Peng, H.; Puttick, S.; Rose, S.; Fisk, N.M.; Bhakoo, K.; Stringer, B.W.; Qiao, G.G.; Gurr, P.A.; et al. A unique 19F MRI agent for the tracking of non phagocytic cells in vivo. Nanoscale 2018, 10, 8226–8239. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Benaglia, M.; Ortenzi, M.; Micotti, E.; Perego, C.; De Simoni, M.G. Poly(ethylene-glycol)-based fluorinated esters: A readily available entry for novel 19F-MRI agents. Tetrahedron Lett. 2011, 52, 6581–6583. [Google Scholar] [CrossRef]
- Biaggi, C.; Benaglia, M.; Ortenzi, M.; Micotti, E.; Perego, C.; De Simoni, M.-G. Easily available, low cost 19F MRI agents: Poly(ethylene-glycol)-functionalized fluorinated ethers. J. Fluor. Chem. 2013, 153, 172–177. [Google Scholar] [CrossRef]
- Wang, J.; Deng, T.; Liu, Y.; Chen, K.; Yang, Z.; Jiang, Z.X. Monodisperse and Polydisperse PEGylation of Peptides and Proteins: A Comparative Study. Biomacromolecules 2020, 21, 3134–3139. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xiao, Y.; Zhang, H.; Li, Y.; Yuan, Y.; Yang, Z.; Chen, S.; Zheng, X.; Zhou, X.; Jiang, Z.X. Peptidic Monodisperse PEG "combs" with Fine-Tunable LCST and Multiple Imaging Modalities. Biomacromolecules 2019, 20, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, S.; Weng, Z.; Gao, C. Hyperbranched polymers: Advances from synthesis to applications. Chem. Soc. Rev. 2015, 44, 4091–4130. [Google Scholar] [CrossRef]
- Gao, C.; Yan, D. Hyperbranched polymers: From synthesis to applications. Prog. Polym. Sci. 2004, 29, 183–275. [Google Scholar] [CrossRef]
- Wang, K.; Peng, H.; Thurecht, K.J.; Puttick, S.; Whittaker, A.K. Multifunctional hyperbranched polymers for CT/19F MRI bimodal molecular imaging. Polym. Chem. 2016, 7, 1059–1069. [Google Scholar] [CrossRef]
- Zhang, C.; Moonshi, S.S.; Wang, W.; Ta, H.T.; Han, Y.; Han, F.Y.; Peng, H.; Kral, P.; Rolfe, B.E.; Gooding, J.J.; et al. High F-Content Perfluoropolyether-Based Nanoparticles for Targeted Detection of Breast Cancer by 19F Magnetic Resonance and Optical Imaging. ACS Nano 2018, 12, 9162–9176. [Google Scholar] [CrossRef]
- Celentano, W.; Neri, G.; Distante, F.; Li, M.; Messa, P.; Chirizzi, C.; Chaabane, L.; De Campo, F.; Metrangolo, P.; Baldelli Bombelli, F.; et al. Design of fluorinated hyperbranched polyether copolymers for 19F MRI nanotheranostics. Polym. Chem. 2020, 11, 3951–3963. [Google Scholar] [CrossRef]
- Hernández-Ainsa, S.; Barberá, J. Fluorinated liquid crystalline dendrimers. J. Fluor. Chem. 2015, 177, 37–45. [Google Scholar] [CrossRef]
- Ray, S.; Li, Z.; Hsu, C.H.; Hwang, L.P.; Lin, Y.C.; Chou, P.T.; Lin, Y.Y. Dendrimer- and copolymer-based nanoparticles for magnetic resonance cancer theranostics. Theranostics 2018, 8, 6322–6349. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.-M.; Laurent, R.; Delavaux-Nicot, B.; Majoral, J.-P. “Janus” dendrimers: Syntheses and properties. New J. Chem. 2012, 36, 217–226. [Google Scholar] [CrossRef]
- Setyawati, M.I.; Tay, C.Y.; Docter, D.; Stauber, R.H.; Leong, D.T. Understanding and exploiting nanoparticles’ intimacy with the blood vessel and blood. Chem. Soc. Rev. 2015, 44, 8174–8199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Sengar, R.S.; Nigam, A.; Abadjian, M.C.; Potter, D.M.; Grotjahn, D.B.; Wiener, E.C. A Fluorinated Dendrimer-Based Nanotechnology Platform New Contrast Agents for High Field Imaging. Investig. Radiol. 2010, 45, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Kölmel, D.K.; Niegerb, M.; Bräse, S. Highly efficient synthesis of polyfluorinated dendrons suitable for click chemistry. RSC Adv. 2015, 5, 36762–36765. [Google Scholar] [CrossRef]
- Yu, W.; Yang, Y.; Bo, S.; Li, Y.; Chen, S.; Yang, Z.; Zheng, X.; Jiang, Z.-X.; Zhou, X. Design and Synthesis of Fluorinated Dendrimers for Sensitive 19F MRI. J. Org. Chem. 2015, 80, 4443–4449. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, Y.; Bo, S.; Li, Y.; Yang, Z.; Zhou, X.; Chen, S.; Jiang, Z.-X. Monitoring Fluorinated Dendrimer-Based Self-Assembled Drug-Delivery Systems with 19F Magnetic Resonance. Eur. J. Org. Chem. 2017, 2017, 4461–4468. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Q.; Wang, W.; Ni, Q.; Wang, Y.; Song, H.; Zhang, C.; Kong, D.; Liang, X.-J.; Huang, P. Superhydrophilic fluorinated polymer and nanogel for high-performance 19F magnetic resonance imaging. Biomaterials 2020, 256, 120184. [Google Scholar] [CrossRef]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef] [Green Version]
- Dong, R.; Pang, Y.; Su, Y.; Zhu, X. Supramolecular hydrogels: Synthesis, properties and their biomedical applications. Biomater. Sci. 2015, 3, 937–954. [Google Scholar] [CrossRef] [PubMed]
- Eckmann, D.M.; Composto, R.J.; Tsourkas, A.; Muzykantov, V.R. Nanogel Carrier Design for Targeted Drug Delivery. J. Mater. Chem. B 2014, 2, 8085–8097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belabassi, Y.; Moreau, J.; Gheran, V.; Henoumont, C.; Robert, A.; Callewaert, M.; Rigaux, G.; Cadiou, C.; Vander Elst, L.; Laurent, S.; et al. Synthesis and Characterization of PEGylated and Fluorinated Chitosans: Application to the Synthesis of Targeted Nanoparticles for Drug Delivery. Biomacromolecules 2017, 18, 2756–2766. [Google Scholar] [CrossRef]
- Kolouchova, K.; Sedlacek, O.; Jirak, D.; Babuka, D.; Blahut, J.; Kotek, J.; Vit, M.; Trousil, J.; Konefal, R.; Janouskova, O.; et al. Self-Assembled Thermoresponsive Polymeric Nanogels for 19F MR Imaging. Biomacromolecules 2018, 19, 3515–3524. [Google Scholar] [CrossRef] [PubMed]
- Munkhbat, O.; Canakci, M.; Zheng, S.; Hu, W.; Osborne, B.; Bogdanov, A.A.; Thayumanavan, S. 19F MRI of Polymer Nanogels Aided by Improved Segmental Mobility of Embedded Fluorine Moieties. Biomacromolecules 2019, 20, 790–800. [Google Scholar] [CrossRef]
- Li, Q.; Feng, Z.; Song, H.; Zhang, J.; Dong, A.; Kong, D.; Wang, W.; Huang, P. 19F magnetic resonance imaging enabled real-time, non-invasive and precise localization and quantification of the degradation rate of hydrogel scaffolds in vivo. Biomater. Sci. 2020, 8, 3301–3309. [Google Scholar] [CrossRef]
- Patrick, M.J.; Janjic, J.M.; Teng, H.; O’Hear, M.R.; Brown, C.W.; Stokum, J.A.; Schmidt, B.F.; Ahrens, E.T.; Waggoner, A.S. Intracellular pH measurements using perfluorocarbon nanoemulsions. J. Am. Chem. Soc. 2013, 135, 18445–18457. [Google Scholar] [CrossRef] [Green Version]
- Herneisey, M.; Salcedo, P.F.; Domenech, T.; Bagia, C.; George, S.S.; Tunney, R.; Velankar, S.; Hitchens, T.K.; Janjic, J.M. Design of Thermoresponsive Polyamine Cross-Linked Perfluoropolyether Hydrogels for Imaging and Delivery Applications. ACS Med. Chem. Lett. 2020, 11, 2032–2040. [Google Scholar] [CrossRef]
- Kisby, T.; Yilmazer, A.; Kostarelos, K. Reasons for success and lessons learnt from nanoscale vaccines against COVID-19. Nat. Nanotechnol. 2021, 16, 843–850. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil(R)--the first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Dewitte, H.; Geers, B.; Liang, S.; Himmelreich, U.; Demeester, J.; De Smedt, S.C.; Lentacker, I. Design and evaluation of theranostic perfluorocarbon particles for simultaneous antigen-loading and 19F-MRI tracking of dendritic cells. J. Control. Release 2013, 169, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Louchami, K.; Holvoet, B.; Verbeke, R.; Deroose, C.M.; Manshian, B.; Soenen, S.J.; Lentacker, I.; Himmelreich, U. Tri-modal In vivo Imaging of Pancreatic Islets Transplanted Subcutaneously in Mice. Mol. Imaging Biol. 2018, 20, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Tanifum, E.A.; Patel, C.; Liaw, M.E.; Pautler, R.G.; Annapragada, A.V. Hydrophilic fluorinated molecules for spectral 19F MRI. Sci. Rep. 2018, 8, 2889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iima, R.; Takegami, S.; Konishi, A.; Tajima, S.; Minematsu, N.; Kitade, T. Thermal Behavior of 19F Nuclear Magnetic Resonance Signal of 19F-Containing Compound in Lipid Nano-Emulsion for Potential Tumor Diagnosis. AAPS PharmSciTech 2018, 19, 2679–2686. [Google Scholar] [CrossRef]
- Wu, L.; Wen, X.; Wang, X.; Wang, C.; Sun, X.; Wang, K.; Zhang, H.; Williams, T.; Stacy, A.J.; Chen, J.; et al. Local Intratracheal Delivery of Perfluorocarbon Nanoparticles to Lung Cancer Demonstrated with Magnetic Resonance Multimodal Imaging. Theranostics 2018, 8, 563–574. [Google Scholar] [CrossRef]
- Hill, L.K.; Frezzo, J.A.; Katyal, P.; Hoang, D.M.; Ben Youss Gironda, Z.; Xu, C.; Xie, X.; Delgado-Fukushima, E.; Wadghiri, Y.Z.; Montclare, J.K. Protein-Engineered Nanoscale Micelles for Dynamic 19F Magnetic Resonance and Therapeutic Drug Delivery. ACS Nano 2019, 13, 2969–2985. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.-Y.; Liu, K.; Gao, H.-J.; Yu, X.-L.; Cao, Y.; Liu, Z.-X. Upconversion luminescent property and EPR study of NaGdF4:Yb3+/Tm3+ synthesized by the hydrothermal method. Front. Mater. Sci. 2015, 9, 241–246. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, H.; Li, J.; Wang, Y.; Wang, X. Fabrication of pH-responsive PLGA(UCNPs/DOX) nanocapsules with upconversion luminescence for drug delivery. Sci. Rep. 2017, 7, 18014. [Google Scholar] [CrossRef] [Green Version]
- Ashur, I.; Allouche-Arnon, H.; Bar-Shir, A. Calcium Fluoride Nanocrystals: Tracers for In Vivo 19 F Magnetic Resonance Imaging. Angew. Chem. Int. Ed. Engl. 2018, 57, 7478–7482. [Google Scholar] [CrossRef]
- Jones, N.E.; Burnett, C.A.; Salamon, S.; Landers, J.; Wende, H.; Lazzarini, L.; Gibbs, P.; Pickles, M.; Johnson, B.R.G.; Evans, D.J.; et al. Fluoride doped gamma-Fe2O3 nanoparticles with increased MRI relaxivity. J. Mater. Chem. B 2018, 6, 3665–3673. [Google Scholar] [CrossRef]
- Singh, P.; Kumari, K.; Vishvakrma, V.K.; Mehrotra, G.K.; Chandra, R.; Kumar, D.; Patel, R.; Shahare, V.V. Metal NPs (Au, Ag, and Cu): Synthesis, Stabilization, and Their Role in Green Chemistry and Drug Delivery. In Green Technologies and Environmental Sustainability; Springer: Berlin/Heidelberg, Germany, 2017; pp. 309–337. [Google Scholar] [CrossRef]
- Boccalon, M.; Franchi, P.; Lucarini, M.; Delgado, J.J.; Sousa, F.; Stellacci, F.; Zucca, I.; Scotti, A.; Spreafico, R.; Pengo, P.; et al. Gold nanoparticles protected by fluorinated ligands for 19F MRI. Chem. Commun. 2013, 49, 8794–8796. [Google Scholar] [CrossRef] [PubMed]
- Sologan, M.; Padelli, F.; Giachetti, I.; Aquino, D.; Boccalon, M.; Adami, G.; Pengo, P.; Pasquato, L. Functionalized Gold Nanoparticles as Contrast Agents for Proton and Dual Proton/Fluorine MRI. Nanomaterials 2019, 9, 879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michelena, O.; Padro, D.; Carrillo-Carrion, C.; Del Pino, P.; Blanco, J.; Arnaiz, B.; Parak, W.J.; Carril, M. Novel fluorinated ligands for gold nanoparticle labelling with applications in 19F-MRI. Chem. Commun. 2017, 53, 2447–2450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croissant, J.G.; Butler, K.S.; Zink, J.I.; Brinker, C.J. Synthetic amorphous silica nanoparticles: Toxicity, biomedical and environmental implications. Nat. Rev. Mater. 2020, 5, 886–909. [Google Scholar] [CrossRef]
- Pochert, A.; Vernikouskaya, I.; Pascher, F.; Rasche, V.; Lindén, M. Cargo-influences on the biodistribution of hollow mesoporous silica nanoparticles as studied by quantitative 19 F-magnetic resonance imaging. J. Colloid Interface Sci. 2017, 488, 1–9. [Google Scholar] [CrossRef]
- Lee, A.L.; Gee, C.T.; Weegman, B.P.; Einstein, S.A.; Juelfs, A.R.; Ring, H.L.; Hurley, K.R.; Egger, S.M.; Swindlehurst, G.; Garwood, M.; et al. Oxygen Sensing with Perfluorocarbon-Loaded Ultraporous Mesostructured Silica Nanoparticles. ACS Nano 2017, 11, 5623–5632. [Google Scholar] [CrossRef]
- Lee, A.L.; Lee, S.H.; Nguyen, H.; Cahill, M.; Kappel, E.; Pomerantz, W.C.K.; Haynes, C.L. Investigation of the Post-Synthetic Confinement of Fluorous Liquids Inside Mesoporous Silica Nanoparticles. Langmuir 2021, 37, 5222–5231. [Google Scholar] [CrossRef]
- Bouchoucha, M.; van Heeswijk, R.B.; Gossuin, Y.; Kleitz, F.; Fortin, M.A. Fluorinated Mesoporous Silica Nanoparticles for Binuclear Probes in 1H and 19F Magnetic Resonance Imaging. Langmuir 2017, 33, 10531–10542. [Google Scholar] [CrossRef]
- Kikuchi, K. 19F MRI Probes with Tunable Switches and Highly Sensitive 19F MRI Nanoprobes. Bull. Chem. Soc. Jpn. 2015, 88, 518–521. [Google Scholar] [CrossRef] [Green Version]
- Helm, L. Relaxivity in paramagnetic systems: Theory and mechanisms. Prog. Nucl. Magn. Reson. Spectrosc. 2006, 49, 45–64. [Google Scholar] [CrossRef]
- Xie, D.; Yu, M.; Kadakia, R.T.; Que, E.L. 19F Magnetic Resonance Activity-Based Sensing Using Paramagnetic Metals. Acc. Chem. Res. 2020, 53, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, H.; Mizukami, S.; Sugihara, F.; Nakanishi, Y.; Yoshioka, Y.; Kikuchi, K. Multifunctional core-shell silica nanoparticles for highly sensitive 19F magnetic resonance imaging. Angew. Chem. Int. Ed. Engl. 2014, 53, 1008–1011. [Google Scholar] [CrossRef] [PubMed]
- .Nakamura, T.; Sugihara, F.; Matsushita, H.; Yoshioka, Y.; Mizukami, S.; Kikuchi, K. Mesoporous silica nanoparticles for 19F magnetic resonance imaging, fluorescence imaging, and drug delivery. Chem. Sci. 2015, 6, 1986–1990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, T.; Matsushita, H.; Sugihara, F.; Yoshioka, Y.; Mizukami, S.; Kikuchi, K. Activatable 19F MRI nanoparticle probes for the detection of reducing environments. Angew. Chem. Int. Ed. Engl. 2015, 54, 1007–1010. [Google Scholar] [CrossRef]
- de Vries, A.; Moonen, R.; Yildirim, M.; Langereis, S.; Lamerichs, R.; Pikkemaat, J.A.; Baroni, S.; Terreno, E.; Nicolay, K.; Strijkers, G.J.; et al. Relaxometric studies of gadolinium-functionalized perfluorocarbon nanoparticles for MR imaging. Contrast Media Mol. Imaging 2014, 9, 83–91. [Google Scholar] [CrossRef]
- Akazawa, K.; Sugihara, F.; Nakamura, T.; Matsushita, H.; Mukai, H.; Akimoto, R.; Minoshima, M.; Mizukami, S.; Kikuchi, K. Perfluorocarbon-Based 19 F MRI Nanoprobes for In Vivo Multicolor Imaging. Angew. Chem. Int. Ed. Engl. 2018, 57, 16742–16747. [Google Scholar] [CrossRef] [Green Version]
- Holban, A.M.; Grumezescu, A.M.; Andronescu, E. Inorganic nanoarchitectonics designed for drug delivery and anti-infective surfaces. In Surface Chemistry of Nanobiomaterials; William Andrew: Norwich, NY, USA, 2016; pp. 301–327. [Google Scholar] [CrossRef]
- He, H.; Pham-Huy, L.A.; Dramou, P.; Xiao, D.; Zuo, P.; Pham-Huy, C. Carbon nanotubes: Applications in pharmacy and medicine. Biomed. Res. Int. 2013, 2013, 578290. [Google Scholar] [CrossRef] [Green Version]
- Rao, C.N.R.; Govindaraj, A. Chapter 1. Carbon Nanotubes. In Nanotubes and Nanowires; RSC Publishing: London, UK, 2011; pp. 1–242. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Zheng, Y.; Zhang, H.; Wang, X.; Bai, L.; Wu, Y.; Ba, X. Development of a halloysite nanotube-based 19F NMR probe as a promising detection tool for H2O2. J. Nanoparticle Res. 2020, 22, 342. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, T.; Ji, Y.; Han, L.; Wu, Y.; Song, H.; Bai, L.; Ba, X. Selective Modification of Halloysite Nanotubes with 1-Pyrenylboronic Acid: A Novel Fluorescence Probe with Highly Selective and Sensitive Response to Hyperoxide. ACS Appl. Mater. Interfaces 2015, 7, 23805–23811. [Google Scholar] [CrossRef]
- Cheng, C.; Gao, Y.; Song, W.; Zhao, Q.; Zhang, H.; Zhang, H. Halloysite nanotube-based H2O2-responsive drug delivery system with a turn on effect on fluorescence for real-time monitoring. Chem. Eng. J. 2020, 380. [Google Scholar] [CrossRef]
- Kislukhin, A.A.; Xu, H.; Adams, S.R.; Narsinh, K.H.; Tsien, R.Y.; Ahrens, E.T. Paramagnetic fluorinated nanoemulsions for sensitive cellular fluorine-19 magnetic resonance imaging. Nat. Mater. 2016, 15, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.; Stares, E.; Adams, S.R.; Lister, D.; Leach, B.; Ahrens, E.T. Paramagnetic Fluorinated Nanoemulsions for in vivo F-19 MRI. Mol. Imaging Biol. 2020, 22, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Song, M.; Tang, J.; Hu, G.; Xu, S.; Guo, Z.; Li, N.; Cui, J.; Zhang, X.; Chen, X.; et al. Ultrahigh 19F Loaded Cu1.75S Nanoprobes for Simultaneous 19F Magnetic Resonance Imaging and Photothermal Therapy. ACS Nano 2016, 10, 1355–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Peng, H.; Thurecht, K.J.; Whittaker, A.K. Fluorinated POSS-Star Polymers for 19F MRI. Macromol. Chem. Phys. 2016, 217, 2262–2274. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Sudeep, P.M.; Park, J.H.; Woellner, C.F.; Maladonado, K.; Galvao, D.S.; Kaipparettu, B.A.; Tiwary, C.S.; Ajayan, P.M. Multifunctional Hybrids Based on 2D Fluorinated Graphene Oxide and Superparamagnetic Iron Oxide Nanoparticles. Part. Part. Syst. Charact. 2017, 34, 1700245. [Google Scholar] [CrossRef]
- Cui, J.; Jiang, R.; Guo, C.; Bai, X.; Xu, S.; Wang, L. Fluorine Grafted Cu7S4-Au Heterodimers for Multimodal Imaging Guided Photothermal Therapy with High Penetration Depth. J. Am. Chem. Soc. 2018, 140, 5890–5894. [Google Scholar] [CrossRef]
- Niu, D.; Wang, X.; Li, Y.; Zheng, Y.; Li, F.; Chen, H.; Gu, J.; Zhao, W.; Shi, J. Facile synthesis of magnetite/perfluorocarbon co-loaded organic/inorganic hy.ybrid vesicles for dual-modality ultrasound/magnetic resonance imaging and imaging-guided high-intensity focused ultrasound ablation. Adv. Mater. 2013, 25, 2686–2692. [Google Scholar] [CrossRef]
- Hu, G.; Tang, J.; Bai, X.; Xu, S.; Wang, L. Superfluorinated copper sulfide nanoprobes for simultaneous 19F magnetic resonance imaging and photothermal ablation. Nano Res. 2016, 9, 1630–1638. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Liu, G.; Deng, Z.; Wu, S.; Li, P.; Xu, Z.; Xu, H.; Chu, P.K. Magnetite-loaded fluorine-containing polymeric micelles for magnetic resonance imaging and drug delivery. Biomaterials 2012, 33, 3013–3024. [Google Scholar] [CrossRef]
- Zhang, C.; Li, L.; Han, F.Y.; Yu, X.; Tan, X.; Fu, C.; Xu, Z.P.; Whittaker, A.K. Integrating Fluorinated Polymer and Manganese-Layered Double Hydroxide Nanoparticles as pH-activated 19 F MRI Agents for Specific and Sensitive Detection of Breast Cancer. Small 2019, 15, e1902309. [Google Scholar] [CrossRef]
- Wang, J.; Hu, X.; Ding, H.; Huang, X.; Xu, M.; Li, Z.; Wang, D.; Yan, X.; Lu, Y.; Xu, Y.; et al. Fluorine and Nitrogen Co-Doped Carbon Dot Complexation with Fe(III) as a T1 Contrast Agent for Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2019, 11, 18203–18212. [Google Scholar] [CrossRef] [PubMed]
- Mali, A.; Kaijzel, E.L.; Lamb, H.J.; Cruz, L.J. 19F-nanoparticles: Platform for in vivo delivery of fluorinated biomaterials for 19F-MRI. J. Control. Release 2021, 338, 870–889. [Google Scholar] [CrossRef] [PubMed]
- Piloni, A.; Simonutti, R.; Stenzel, M.H. The effect of cationic groups on the stability of 19F MRI contrast agents in nanoparticles. J. Polym. Sci. Part. A Polym. Chem. 2019, 57, 1994–2001. [Google Scholar] [CrossRef]
- Pochert, A.; Ziller, S.; Kapetanovic, S.; Neusser, G.; Kranz, C.; Lindén, M. Intermediate pickering emulsion formation as a means for synthesizing hollow mesoporous silica nanoparticles. New J. Chem. 2016, 40, 4217–4222. [Google Scholar] [CrossRef]
- Astafyeva, K.; Somaglino, L.; Desgranges, S.; Berti, R.; Patinote, C.; Langevin, D.; Lazeyras, F.; Salomir, R.; Polidori, A.; Contino-Pepin, C.; et al. Perfluorocarbon nanodroplets stabilized by fluorinated surfactants: Characterization and potentiality as theranostic agents. J. Mater. Chem. B 2015, 3, 2892–2907. [Google Scholar] [CrossRef] [Green Version]

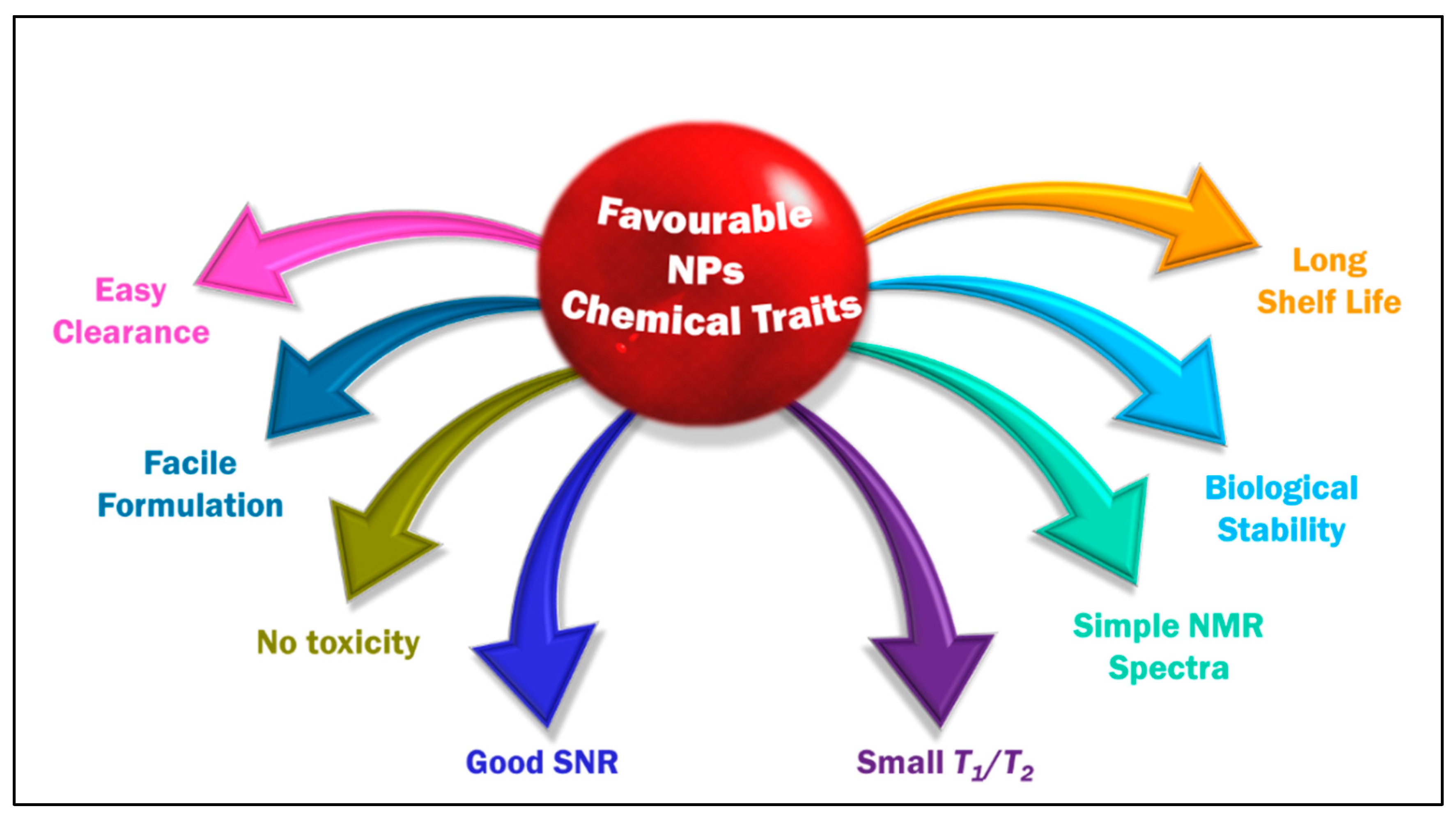





| Technique | Emission Source | Need of Contrast Agents | Spatial Resolution | Acquisition Time | Target |
|---|---|---|---|---|---|
| Optical Imaging | Visible and Near-Infrared Light | ✓ | millimeters (mm) | Seconds (S) to Minutes (Min) | Soft tissues |
| Photoacoustic (PAI) | Laser | ✓ | centimeters (cm) | Soft tissues | |
| Ultrasound (US) | Sound Waves | ✓ | cm | S | Soft tissues |
| Computed Tomography (CT), Positron Emission Tomography (PET) Single-Photon Emission Computed Tomography (SPECT) | Gamma Rays | ✓ | mm | Min | Hard tissues and soft tissues |
| X-ray | X-rays | ✓ | micrometer (µm) | S | Hard tissues and soft tissues |
| Magnetic Resonance Imaging (MRI) | Radiofrequency Waves | ✓ | µm–mm | Seconds (S) to Hours | Deep soft tissue |
| Parameter | 1H | 19F |
|---|---|---|
| Natural abundance (%) | 99.98 | 100 |
| Spin | 1/2 | 1/2 |
| Gyromagnetic ratio (γ) in MHz/T | 42.576 | 40.076 |
| Relative sensitivity | 1.0 | 0.834 |
| Van de Waals’ radius (in Å) | 1.2 (H–C) | 1.35 (F–C) |
| The population ratio (NE/NG) | 0.9999802 | 0.9999814 |
| ∆ϵ/kT at 3T | 1.98 × 10−5 | 1.86 × 10−5 |
| Lattice spacing | 4.97 Å (Hydrocarbon) | 5.9 Å (fluorocarbon) |
| Chemical shifts in ppm (NMR) | 0 to 15 | >350 |
| Aromatic PFCs |  Hexafluorobenzene (HFB) MF = C6F6 Mw = 186.05 B.P = 80.2 °C FNMR signals = S | 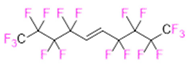 Trans-1,2-bis(perfluoro-N-butyl)ethylene (TBPE) MF = C10H2F18 Mw = 464.09 B.P = 64,3 °C D = 1.675 FNMR signals = M |  2,3,4,5,6-Pentafluorostyrene (PFS) MF = C8H3F5 Mw = 194.10 B.P = 139–140 °C D = 1.406 FNMR signals = 3 major peaks with spitting |
| Saturated Linear PFCs |  Perfluoro-tert-butanol (PFTB) MF = C4HF9O Mw = 236.04 B.P = 45.0 °C FNMR signals = S |  Perfluoropropane (PFP) MF = C3F8 Mw = 188.02 B.P = −36.6 °C FNMR signals = 2 major peaks with spitting |  Perfluorohexane (PFH) MF = C6F14 Mw = 338.04 B.P = 56.6–57.2 °C FNMR signals = 3 major peaks with spitting |
 Perfluorononane (PFN) MF = C9F20 Mw = 488.06 B.P = 125–126 °C D = 1.799 FNMR signals = 3 major peaks with spitting | 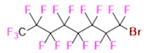 Perfluorooctyl bromide (PFOB) MF = C8BrF17 Mw = 498.96 B.P = 142 °C D = 1.93 FNMR signals = M |  Perfluorooctanoic acid (PFOA) MF = C8HF15O2 Mw = 414.07 B.P = 189.0–192 °C D = 1.792 FNMR signals = M | |
| Saturated Ring System PFCs |  Perfluorodecalin (PFD) MF = C10F18 Mw = 462.08 B.P = 142 °C D = 1.908 FNMR signals = M |  Perfluoro-1,3-dimethylcyclohexane (PFDCH) MF = C8F16 Mw = 400.06 B.P = 101–102 °C D = 1.828 FNMR signals = M |  Perfluoroperhydrophenanthrene (PFPHP) MF = C14F24 Mw = 624.11 B.P = 212–218 °C D = 2.03 FNMR signals = M |
| Perfluoroethers and Polyethers | 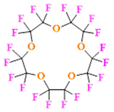 Perfluoro-15-crown-5 ether (PFCE) MF = C10F20O5 Mw = 580.07 B.P = 145 °C D = 1.780 FNMR signals = S | 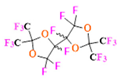 Perfluoro-2,2,2’,2’-tetramethyl-4,4’-bis(1,3-dioxolane) (PTBD) MF = C10F18O4 Mw = 526.08 B.P = ~ 160 °C D = ~1.9 FNMR signals = M | 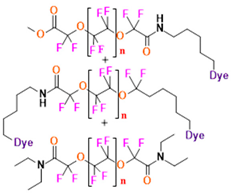 Fluorescent‘blended’ PFPE Amides (FBPA) MF, Mw, B.P = Depends on repeat unit and the dye attached FNMR signals = 1 major peak, 4 minor peaks |
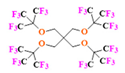 Superfluorinated probe (PERFECTA) MF = C21H8F36O4 Mw = 1008.23 FNMR signals = S | 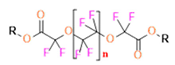 Perfluoropolyether (PFPE) MF, Mw, B.P = Depends on repeat unit and the R-group attached FNMR signals = 1 major peak and 1 minor peak | ||
| Perfluoroamines |  Perfluorotriethylamine(PFTA) MF = C6F15N Mw = 371.05 B.P = 68–69 °C D = 1.736 FNMR signals = 2 major peaks with spitting | 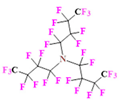 Perfluorotributylamine (FC-43) MF = C12F27N Mw = 671.09 B.P = 178.0 °C D = 1.884 FNMR signals = M | 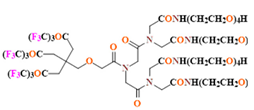 19F Imaging Tracer (19FIT) MF = C63H94F27N7O27 Mw = 1894.41 FNMR signals = S |
| Perflurosilanes |  (Pentafluorophenyl)triethoxysilane (PFPTS) MF = C12H15F5O3Si Mw = 330.32 B.P = 69 °C D = 1.242 FNMR signals = 3 major peaks with spitting |  1H,1H,2H,2H-Perfluorooctyltriethoxysilane (PFOTS) MF = C14H19F13O3Si Mw = 510.36 B.P = 220 °C D = 1.329 FNMR signals = M |  Trichloro(1H,1H,2H,2H-perfluorooctyl)silane (TCPFOS) MF = C8H4Cl3F13Si Mw = 481.54 B.P = 192 °C D = 1.30 FNMR signals = M |
| Type | Name | Preparation Technique | Fluorine Component | Characterisation * | Pros and Cons | Application | Ref |
|---|---|---|---|---|---|---|---|
| POLYMERIC | Fluorous colloidal NPs | Copolymer by ATRP. NPs formation by self-assembly to micelle | Trifluoroethyl methacrylate | DLS (260 nm), TEM, FMRI, FC, CM, UV-Vis, CyA–on macrophage cells, animal studies–female athymic NCR nude mice for breast cancer | Simple preparation of copolymer | Immune cell tracking and systemic disease monitoring | [187] |
| No surfactant | |||||||
| Little off target accumulation | |||||||
| Tumour-homing | |||||||
| Poly(OEGA-co-TFEA)-b-poly(St-co-VBA) | Polymerisation by RAFT and NPs by PISA | 2,2,2-trifluoroethyl acrylate | FMRI and NMR, DLS, TEM, CM | Little or no cytotoxicity–Chinese Hamster Ovarian cells | In vivo cell tracking | [190] | |
| Multiple NPs morphologies by controlling reaction time and polymer chain length in one preparation (spherical, worm, vesicle) | |||||||
| ROS-responsive fluorinated polymers | Polymer by ATRP and NPs by self-assembly | 2,2,2-trifluoroethyl methacrylate | H and F NMR, FMRI, DLS (62, 32 and 18 nm), UV-Vis | Enhanced sensitivity for acidic microenvironment and the presence of ROS | ROS/pH dual-responsive 19F MRI agent | [191] | |
| The concentration of H2O2 studied (~1 M) were higher than biological levels (50–100 μM) | |||||||
| 6-step synthesis that requires purification | |||||||
| “OFF–ON” regulation of NPs to acidic environment | |||||||
| Amino activable nanoprobe- p(mPEGMA)-co-poly(AMA-DNBS-F) (PEDF nanoprobe) | Copolymers by RAFT polymerisation and nanoprobe by nanoprecipitation | Trifluoromethyl-containing segments | H and F NMR, DLS (33 nm), FMRI, TEM, FTIR, CLSM, in vivo imaging in tumours–xenograft tumour models in mice | 2 step preparations for monomers | In vivo bio-thiols imaging | [192] | |
| Highly sensitive to bio-thiols | |||||||
| Water soluble | |||||||
| Fluorinated block copolymers NPs | RAFT for the block polymers and NPs by self-assembly in aqueous solution | 2,2,2-trifluoroethylamide L-arginine methacrylamide | H, F- NMR, DLS (25 to 60 nm), TEM | Fluorinated functionalities in the hydrophilic shell | MRI Imaging | [300] | |
| Increased T2 | |||||||
| 19F MRI-detectable drug delivery system | Layer-by-layer technique deposition of polyelectrolyte shells on nanoemulsion drops | Polyelectrolyte Nafion–fluorinated anionic polymer | DLS (170 nm), LDV, NTA, C-SEM, QCM, FMRI | Sufficient SNR ratio | Passive tumour targeting and drug delivery | [193] | |
| Highly cationic particle (+68 ± 5 mV) | |||||||
| Self-assembled 19F nanoprobes | Self-assembly of amphiphilic redox-responsive 19F-containing polymers and NIR-absorbing ICG molecules | 3,5-Bis(trifluoromethyl) benzoic acid part in the polymer | TEM, DLS (40 nm), UV–Vis, FNMR and MRI, TEM | Water-soluble | Accurate sensing and imaging of tumours | [66] | |
| In vivo and in vitro studies–HepG2 tumour-bearing cells and mice | |||||||
| High SNR ratio | |||||||
| Good biocompatibility | |||||||
| 5 steps for preparation with purification requirement and moderate yield | |||||||
| Novel system which has potential to be extended for imaging other tumour targets | |||||||
| Multi-functional fluorocarbon NPs | Single and double emulsion | PFD, PFH, perfluorooctane, PFOB, PFCE | DLS (200 nm–200 µm), SEM, CM, FC, FI, FMRI, Cell viability–primary human dendritic cells, histology | Customizable NPs, minimal toxicity | In vivo imaging and targeting applications | [194] | |
| Size smaller than 200 nm is not formed by this NP formation | |||||||
| PLGA PFPE | Emulsification (Sonicator)–1:1 molar ratio of autoclaved PFPE and sterile filtered Pluronic | PFPE | DLS (103 nm), FNMR and MRI, FM, cellular viability–diabetogenic mice T cells | Specificity for the labelled cells | Non-invasive monitoring the trafficking of cellular therapeutics | [195] | |
| Reliable estimates of the apparent number of cells from image data | |||||||
| PFCE encapsulated PLGA | Single emulsion | PFCE | DLS, FNMR and MRI, SANS, animal studies–male Wistar rats, mouse, mice, cell studie–primary murine/human dendritic cells | Biocompatible NPs | US and 19F MRI | [197] | |
| Better acquisition time | Murine cardiac 19F MRI/MRS | [199] | |||||
| Obtains complimentary information when in combination with other imaging agents | In vivo PAI, 19F MRI and fluorescent imaging (FI) | [198] | |||||
| NPs loaded with chemotherapeutic drugs could give it a theranostic effect, Resomer RG 502 H, lactide: glycolide molar ratio 48:52 to 52:48 is the mostly used PLGA. The other ratios of lactide: glycolide and also their end group might give interesting results. The encapsulation efficiency of PFC could be studied each time to better understand the sensitivity | FMRI and CT (with gold NPs) | [200] | |||||
| SPECT/PET and 19F MRI | [204] | ||||||
| Chitosan coated PLGA -PFOB NPs | Single emulsion by homogenisation followed by sonication using 1.5% sodium cholate | PFOB | DLS (170 nm), CLSM, FC, FNMR and FMRI, TEM | Background-free signal compared to Gd (III) and super paramagnetic iron oxides NPs | Labelling and tracking therapeutic cells in vivo | [206] | |
| As chitosan coating is just a physical adsorption, the stability of it has to be verified in biological environment | |||||||
| Size of NPs is increased (200–400 nm) after the chitosan coating | |||||||
| PEGylated PLGA NPs (PLGA NP (NIR700 + PFC)-PEG-800 CW | O/W emulsion and solvent evaporation-extraction method | PFCE | DLS (240–250 nm), TEM, FMRI, TEM, FM, histology, cell culture–murine breast carcinoma cell line | Quantitative 3D information from deeper tissues | In vivo imaging | [212] | |
| Rapid qualitative optical monitoring | |||||||
| PLGA–PEG folate-receptor-targeted NPs | Single emulsion-evaporation (1.5% sodium cholate surfactant) | PFOB | DLS (150 nm), FC, CLSM, F MRI, NIRS, CyA-KB cells | Encapsulate imaging agent and drug | Theranostic NP | [207] | |
| Insufficient SNR in vivo for FMRI | |||||||
| The loading capacity of the NPs is low for doxorubicin and ICG (0.04% and 0.127%) | |||||||
| Doxorubicin-conjugated PFPE NPs | Polymers by RAFT polymerization | PFPE | DLS (8.1, 9.3 and 8.3), FNMR, MD | Improved cellular uptake | Improved therapeutic efficacy | [222] | |
| Deep tumour penetration | |||||||
| Studies done using 3D tumour spheroids | |||||||
| F3-PLGA and F9-PLGA | Nanoprecipitation–surfactant free | Fluorinated PLGA (2,2,2-trifluoroethanolamine, nonafluoro-t-butoxyethylamine) | DLS (~54 nm and 58 nm), TEM, F NMR, FM, CyA–immortalized human glomerular endothelial cells and podocytes | No surfactant used | Theranostic NPs | [217] | |
| Encapsulate hydrophobic drugs | |||||||
| The reaction yield of the fluorinated polymer is not understood | |||||||
| HYPERBRANCHED | Multifunctional hyperbranched polymers containing 19F | RAFT polymerization for polymer, NPs by self-assembly in water | 2,2,2-trifluoroethylacrylate | DLS (∼13 nm), GPC, TEM, FNMR and MRI, CT | Direct dissolution in water | Quantitative 19F MRI CA | [232] |
| Biodegradable | |||||||
| 3 step preparation and the final product is not pure (3 mixture products) | |||||||
| FNMR with multiple peaks | |||||||
| T2 shortened | |||||||
| PFPE based hyperbranched NPs conjugated with targeting aptamers | RAFT polymerization–for NPs, click chemistry for aptamers attaching | PFPE | F-DOSY (<10 nm), FM, FC, CrM, MD, FNMR and MRI | Superior MR imaging sensitivity and fluorine content -breast cancer cells | Quantitative 19F MRI CA | [233] | |
| Low-cost fluorescence imaging | |||||||
| Unsuitable for long term studies due to faster clearance from the body | |||||||
| Accumulation of polymer in the liver was observed after 48 h and the 19F signal could be still detected in the liver | |||||||
| Fluorinated hyperbranched polyether copolymers | ROMBP and copolymerization for polymers and self-assembly of the colloids | 2-[(2,2,2-trifluoroethoxy) methyl]oxirane/epifluorohydrin | DLS (160–200 nm), H NMR and F MRI, FM, HPLC, cytotoxicity studies - immortalized human glomerular endothelial cells and immortalized human podocytes | Repair damaged kidney glomerular cells in vitro | New generation 19F MRI nanotheranostics | [234] | |
| Negligible cytotoxicity | |||||||
| Narrow size distribution | |||||||
| Relatively long T1 | |||||||
| Higher amount of F gives less SNR | |||||||
| DENDRIMERS | Fluorinated Gd(III)-DOTA complexes | Convergent synthesis for polymer and self-assembly for NPs | Fluorinated amino acid group | F NMR, DOSY, H and F MRI, KB cells for in vitro cytotoxicity study, animal imaging—Sprague Dawley female rats | Substantial improvement in relaxation rate and SNR ratio | CA for high field imaging | [239] |
| Easily cleared through the kidneys | |||||||
| The fluorine in the surface layer of dendrimers is toxic which can be diminished by burying the fluorine further into the dendrimer interior | |||||||
| Second-generation dendron | Sonogashira coupling, alkyne deprotection and CuAAC | PFTB group attached to the dendron | FNMR | Higher number of equivalent fluorine than commercially available 19F MRI probes | Probes for 19F MRI | [240] | |
| Too unpolar to be water-soluble | |||||||
| Just one characterisation technique used | |||||||
| Pseudo-symmetrical fluorines dendrimers | Polymer prep–bromination and Williamson ether synthesis, NPs by self-assembly | Bis(4-fluorophenyl) trifluoromethyl carbinol group | FNMR and MRI | Large amount of fluorine with a single NMR peak | 19F MRI-guided drug therapy | [241] | |
| Optimize 19F relaxation time | |||||||
| High sensitivity | |||||||
| Reliable quantification | |||||||
| Comparatively low yield (8%) for 11 synthesis steps | |||||||
| Self-assembled fluorinated amphiphiles | Convergent way–Sonogashira coupling and Williamson ether synthesis for polymer, NPs by self-assembly | Fluorinated benzyl group | FNMR, DLS (6.3 nm), TEM | Quantifying drugs, detecting drug microenvironments and weak interactions | 19F NMR/MRI guided drug therapy. | [242] | |
| Several synthetic step for the preparation with most of them requiring separation | |||||||
| NANOHYDROGELS | Chitosan | Ionic gelation using hyaluronic acid and tripolyphosphate | 4,4,4-trifluorobutyric acid | DLS (274 nm), ELS (+30 mV), FNMR (−66 ppm), HNMR, TGA, DOSY, IR | Good biocompatibility toward murine macrophages cell line | Chitosan drug delivery systems for MRI lymphography | [247] |
| Degree of substitution is comparatively low (0.3% and 20%) and varies between different substitutes, and determination is laborious | |||||||
| Diblock polymers | Self-assembly by heating in aqueous solution | Poly[N(2,2 difluoroethyl)acrylamide] | SLS (100 and 67 nm), TEM, C-TEM, FNMR | Good sensitivity | 19F MR imaging–angiogenesis imaging or the labelling of pancreatic islets | [248] | |
| Non-cytotoxic for several cell lines | |||||||
| Long synthesis steps for preparation of polymers | |||||||
| Fluorinated amphiphilic polymers | Self-assembly of polymers–direct dissolution of amphiphilic polymers in PBS buffer | -CF3 groups attached to the chains of polymer | DLS (6- 14 nm), FNMR, FMRI–phantom and animal imaging, CM, CyA-HeLa cells | Enhancement in T2 relaxation times by increasing the segment mobility | Multimodal imaging and therapeutic applications. | [249] | |
| Superhydrophilic 19F MRI CA | Hydrogel matrix attached to zwitterionic, fluorinated and alkynyl molecule by click chemistry | The fluorine atoms on trifluoromethyl groups | HMRS, FTIR, GPC, CD, Rheometer, SEM, FMRI, degradation study–female BALB/c mice, CyA-Dendritic cells, NIH 3T3 cells | Gelation properties of hydrogels unaffected by labelling CA | Real-time FMRI to precisely locate and quantify the degradation rate of hydrogel scaffolds in vivo | [250] | |
| 3D-stereoscopic and 2D-anatomical information | |||||||
| LIPIDS | Antigen-loaded PFC particles | High-frequency mixing of the liquid PFC with a cationic lipid mixture-particles coat with PEG | PFH or PFCE | CM, TEM, F NMR, Cytotoxicity in transplanted pancreatic islets and beta cell-like cells and T-cell proliferation assay | Improving pancreatic islets transplantation technique | Theranostic PFC NPs | [255,256] |
| Good cell viability and no change in cells’ phenotypical properties | |||||||
| High resolution localization of transplanted cells | |||||||
| The use of PFCE is better than PFH because the latter have 3 peaks in FNMR which reduces its sensitivity | |||||||
| Thermally responsive lipid nano-emulsion | Nano-emulsion | Modified α-tocopherol | FNMR, DLS (50 nm), ZP | Proved that T2 changes more than T1 due to variation in temperature for FNMR | Potential tumour diagnosis | [258] | |
| The temperature studied is extreme (37 and 42 °C) compared to real tumour | |||||||
| Multifunctional paramagnetic PFC NP | Microfluidization | PFCE | DLS (132 nm), AFM, UV–vis, FM, cellular toxicity on bronchial epithelium, FC, clinical pathology, FMRI | Enhanced intratumoural penetration | PFC NP delivery from intravenous applications to intratracheal use (for lung cancer) | [259] | |
| NPs stored under very special condition | |||||||
| The lipid surfactant used have a laborious preparation | |||||||
| The studied NPs contain Gd3+ as Gd-lipid chelates | |||||||
| MICELLE | Fluorinated thermoresponsive assembled protein (F-TRAP) | Self-assembly micelle | Fluorinated amino acids within a protein (5,5,5-DL-trifluoroleucines) | DLS (30 nm), FA, SLS, CD, MALDI-TOF-MS, TEM, turbidometry, FNMR, FMRI, Animal studies-mouse xenograft model of human breast cancer | No change in T1 | Thermoresponsive 19F MRI/MRS-traceable theranostic agents | [260] |
| Doxorubicin encapsulation and thermoresponsive release | |||||||
| Zero echo time 19F MRI was used to get the direct imaging of protein as after micelle formation, there is a reduction in T2 | |||||||
| The release of drug is at 45 °C (usually tumour temperature range is 37 °C to 39 °C) | |||||||
| INORGANIC | Gold NPs protected by fluorinated ligands (F- MPC) | Homogeneous phase synthesis | Fluorinated tetraethylene glycol part of the ligand | DLS (10 nm), TEM, HAADF-STEM, FNMR, UV-Vis, ESR, CLSM, cell interaction with HeLa cells | Elimination of the use of surfactants | Nanovector | [266] |
| Size may help to reach small vasculature vessels | |||||||
| Soluble in many organic solvents | |||||||
| The preparation of fluorinated ligands contains 6 steps, most of them requiring purification | |||||||
| Functionalized gold NPs | Homogeneous phase synthesis | Fluorinated tetraethylene glycol part of the ligand | DLS, HNMR, TEM (1.5–2 nm), UV-Vis, FNMR and MRI | Water-soluble | Dual 1H/19F MRI | [267] | |
| Good quality MRI images | |||||||
| Same as ref [266] + Gd(III) is embedded deep in the layer of Au NPs that causes reduction in T1 relaxation times of bulk water proton | |||||||
| Gold NP functionalised with fluorine atoms | Reduction of HAuCl4 in the presence of NaBH4 | PFTB | ICP-MS, F-NMR/MRI, UV-Vis, TEM, Cell viability and apoptosis assays -MDA-MB-231, C33-A and MDA-MB-435S cell lines, MTS CyA | Colloidal stability in water and other solvents | 19F MR imaging | [268] | |
| Single chemical shift | |||||||
| Long storage | |||||||
| High fluorine loading | |||||||
| Long preparation and purification procedure for the fluorine ligands | |||||||
| The position of fluorine in the NPs is not established | |||||||
| Hollow mesoporous silica NPs (HMSN-PFCE) | Modified protocol from [301] | PFCE | DLS, SEM (290 nm), TEM, MRI, NMR, PAGE | Prolonged circulation time | Dual MRI (1H and 19F) | [270] | |
| Helps in understanding the effect of loading agent on the biodistribution of NPs | |||||||
| Better biodistribution of NPs | |||||||
| The study is majorly applicable to systems whose cargo is on the outer surface | |||||||
| Fluorinated mesoporous silica NPs (FMSNs and polyFMSNs) | Repeated impregnation-calcination process | Fluorosilane or polyfluorosiloxane | TGA, TEM (140 nm), DLS, FNMR and MRI, XPS, relaxometric properties | Colloidal stability | Dual MRI (1H and 19F) | [273] | |
| Increase in 19F relaxivities | |||||||
| Meticulous NPs preparation | |||||||
| Contains Gd3+ | |||||||
| The detection of probe might be impeded by the strong reduction of T2 after NPs formation | |||||||
| PEG modified silica NP | Dehydration polymerizing reaction–PFCE including micelle as a platform | PFCE | TEM, DLS (50 nm), 1H/19F MRI | High sensitivity | Tumour imaging | [274] | |
| Water stability | |||||||
| Information on long term stability, encapsulation efficiency of PFCE is deficient | |||||||
| Silica multifunctional core–shell NPs (FLAMEs) | PFCE-phospholipid nanoemulsion by sol-gel process using a novel surfactant, PAP | PFCE | DMS (76 nm), F NMR and MRI, TEM, biocompatibility by MTT assay-colon-26 cells, Passive targeting, and accumulation- mice bearing a tumour | High sensitivity | Detection of gene expression and in vivo tumour imaging | [277] | |
| Modifiability of the surface, biocompatibility | |||||||
| In vivo stability | |||||||
| The FLAME NPs needs to be PEGylated as naked NPs is trapped immediately by the RES | |||||||
| The information on long term stability of NPs is lacking | |||||||
| Mesoporous FLAME (mFLAME) | PFCE emulsion by Sol–gel process | PFCE | DLS (165 nm), TEM, FNMR and MRI, CLSM, FC, MTT CyA-KB cells, FM | Ample cellular uptake and drug release in folate receptor-overexpressing tumour cells | Theranostic cancer treatment | [278] | |
| Drug release abilities at lower pH. (pH 5) | |||||||
| Efficient tumour cell internalization | |||||||
| Gd3+ complexes on FLAME NPs surface (FLAME-SS-Gd3+) | Gd3+ complexes were attached to the FLAME surface by disulfide linkers | PFCE | DLS (53.4 nm), FNMR and MRI, ICP-AES | Smart nanoprobe–based on PRE effect | Novel 19F MRI probes that visualize reducing environments | [279] | |
| In vivo imaging | |||||||
| High SNR ratio | |||||||
| PFC based 19F MRI nanoprobes (PFC@SiO2, FLAME) | PFC emulsion by sol–gel process | PFCE, PFOB, FC-43, PFN, PFDCO, TPFBME | DLS, TEM (40–120 nm), FI-RAW264.7 cells, H MRI and FMRI, hepatic uptake in mouse | T2 values -relatively longer than polymer-based or inorganic 19F MRI nanoprobes | Multicolour MRI probes | [281] | |
| In vivo triple-colour 19F MRI | |||||||
| The shelf-life information is lacking for the NPs | |||||||
| Fluorinated paramagnetic CAs | Multistep synthesis–cycloaddition reaction | Nonafluorinated carboxylic acid | FNMR, relaxivity measurements, MD | Relaxation times depending on the lanthanide ion | 19F MRI | [35] | |
| Low solubility in aqueous media | |||||||
| Hexagonal-phase NaGdF4:Yb3+/Tm3+ NPs | Hydrothermal method | NH4F/NaF | XRD, SEM, EDX, UV, photoluminescence spectra, EPR | Conducive to the UV light | IR tomography and MRI | [261] | |
| Good water solubility | |||||||
| Lanthanide-based upconversion NPs | |||||||
| Inorganic nanocrystals-PEG-coated CaF2 nanocrystals | Solvothermal approach | CaF2 | H and C and F-NMR, DLS (<10 nm), TEM, XRD, EDX, FTIR, TGA, mouse model of inflammation | Maximal 19F density | Imaging tracers for in vivo 19F MRI | [263] | |
| Average out homonuclear dipolar interactions | |||||||
| Direct and real-time in vivo 19F MRI | |||||||
| Chemically surface modifiable | |||||||
| Long T2 | |||||||
| Halloysite nanotubes- benzeneboronic acids (HNTs-6FBB) | One-pot synthesis | 3,5-bis(trifluoromethyl) benzeneboronic acid | FNMR (−60 ppm), XRD, FTIR, XPS, TEM, EA (0.31% F) | Relatively long T2 | Selective response toward H2O2 | [285] | |
| Water dispersibility | |||||||
| Detection of H2O2 is based on a very minute shift in FNMR (0.2 ppm) | |||||||
| Low cell cytotoxicity | |||||||
| MIXED/HYBRID | Fe(III) tris-β-diketonate with PFPE (‘FETRIS’) | Microfluidization –metal-binding β-diketones conjugated to PFPE using pluronic surfactant | PFPE and PFPE derivatives, PFOB | DLS (140 nm to 200 nm), FNMR and FMRI, cell labelling-rodent glioma cell line | Ability to tune T1 by Fe concentration | In vivo detection of cell therapies and inflammatory cells | [288] |
| Low cytotoxicity | |||||||
| Small rates of metal leakage in the presence of EDTA in vitro and after cell labelling | |||||||
| Cu1.75S–19F@OFP–SiO2 | One-pot encapsulation method-PFCE anchored to Cu1.75S NPs and trapped within the silica shell | PFCE | DLS (20.8 nm), TEM, FMRI, PTT | Ultrahigh F signal | Ablation and sensitive multimodal imaging | [290] | |
| Biocompatible | |||||||
| Capable of both in vivo imaging (F-MRI) and photothermal ablation | |||||||
| Presence of excess of metals in a single probe! | |||||||
| The degradation of this complex should be evaluated since without the SiO2 coating it is cytotoxic | |||||||
| Fluorinated POSS-star polymers | Synthesis of star polymers by RAFT polymerization and polymer formation in water | 2,2,2-Trifluoroethyl acrylate in the ligands attached to POSS | DLS (8–10 nm), FNMR and FMRI | High imaging intensity | Theranostic agents for cancer diagnosis and treatment | [291] | |
| No surfactants | |||||||
| The yield for the formation of star polymers is low and extreme conditions for preparation | |||||||
| Hybrid of fluorinated graphene oxide and iron oxide (IFGO) | Graphene oxide-Hummer’s method. Hybrid–co-precipitation | Fluorinated graphene | DLS (8–10 nm), FMRI, XRD, XPS, SEM and HRTEM, FTIR, MTT CyA-benign breast epithelial cell line, Raman, UV-Vis, hysteresis | Additional imaging modality–magnetic targeted drug delivery | Superior CAs for MRI and fluorescent imaging | [292] | |
| Increased magnetic saturation-better contrast | |||||||
| Cu7S4−Au heterodimer Cu7S4−Au@PSI−19F/PEG nanocomposites | Wet-chemical method for Cu7S4-Au nano seeds followed by click chemistry | 2,2,2-trifluoro-N-2-propyn-1-yl-acetamide | DLS, HRTEM (27 nm), XRD, EDX, HAADF-STEM, XPS, STEM, F NMR and MRI, CT, cell viability-4T1 cell lines, PTT–liver of female mice | Deep penetration | Multimodal imaging guided photothermal therapy | [293] | |
| High spatial resolution | |||||||
| Enhances the photothermal efficacy | |||||||
| Long preparation for the nanocomposite | |||||||
| Mn-LDH@PFPE NPs | Composite system by conjugating a PFPE onto the surface of manganese-incorporated layered double hydroxide | PFPE | NMR and MRI, DLS (10 nm), TEM, GPC, CM, MTT assay–MDA-MB- 468 breast cancer cells, histopathologic examination | High specificity to breast cancer cells | Potential “smart” 19F MRI agent for detection of cancer diseases | [297] | |
| Fe3+@F,N-CD (fluorine and nitrogen co-doped carbon dot) | Simple microwave-assisted thermal decomposition method–from glucose and levofloxacin | Levofloxacin | DLS (16 nm), TEM, GPC, FTIR, XPS, FM, ESR, cytotoxic studies–HeLa cells, In vivo experiments -4T1 tumour bearing BALB/c mice, FMRI, CLSM | High T1 relaxivity | T1-weighted MRI CA | [298] | |
| Strong photoluminescence | |||||||
| Low synthetic cost | |||||||
| Low toxicity | |||||||
| Cannot be used for long term imaging in the body as they are excreted in a very short time from the body |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joseph, J.M.; Gigliobianco, M.R.; Firouzabadi, B.M.; Censi, R.; Di Martino, P. Nanotechnology as a Versatile Tool for 19F-MRI Agent’s Formulation: A Glimpse into the Use of Perfluorinated and Fluorinated Compounds in Nanoparticles. Pharmaceutics 2022, 14, 382. https://doi.org/10.3390/pharmaceutics14020382
Joseph JM, Gigliobianco MR, Firouzabadi BM, Censi R, Di Martino P. Nanotechnology as a Versatile Tool for 19F-MRI Agent’s Formulation: A Glimpse into the Use of Perfluorinated and Fluorinated Compounds in Nanoparticles. Pharmaceutics. 2022; 14(2):382. https://doi.org/10.3390/pharmaceutics14020382
Chicago/Turabian StyleJoseph, Joice Maria, Maria Rosa Gigliobianco, Bita Mahdavi Firouzabadi, Roberta Censi, and Piera Di Martino. 2022. "Nanotechnology as a Versatile Tool for 19F-MRI Agent’s Formulation: A Glimpse into the Use of Perfluorinated and Fluorinated Compounds in Nanoparticles" Pharmaceutics 14, no. 2: 382. https://doi.org/10.3390/pharmaceutics14020382
APA StyleJoseph, J. M., Gigliobianco, M. R., Firouzabadi, B. M., Censi, R., & Di Martino, P. (2022). Nanotechnology as a Versatile Tool for 19F-MRI Agent’s Formulation: A Glimpse into the Use of Perfluorinated and Fluorinated Compounds in Nanoparticles. Pharmaceutics, 14(2), 382. https://doi.org/10.3390/pharmaceutics14020382









