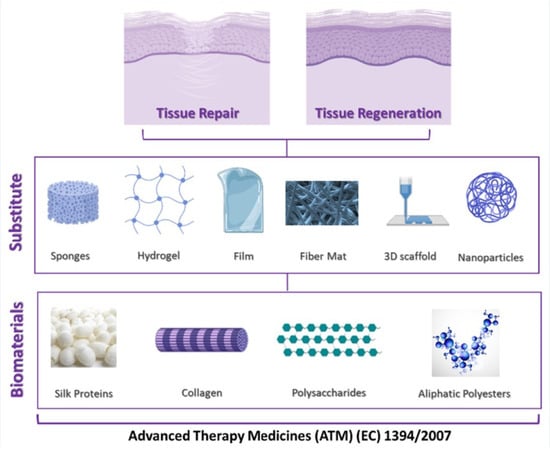
Biomaterials for Soft Tissue Repair and Regeneration: A Focus on Italian Research in the Field
Abstract
1. Introduction
2. Silk Proteins
2.1. Silk Fibroin
2.1.1. Sponges
2.1.2. Hydrogels
2.1.3. Films
2.1.4. Matrices and Fibers
2.1.5. 3D Printed Scaffolds
2.1.6. Nanoparticles
2.2. Silk Sericin
2.2.1. Sponges
2.2.2. Hydrogels
2.2.3. Films
2.2.4. Nanoparticles
3. Collagen
4. Polysaccharides
4.1. Chitosan and Chitosan Derivatives
4.2. Alginic Acid
4.3. Gellan
4.4. Glycosaminoglycans
5. Biodegradable Aliphatic Polyesters
5.1. Synthesis and Properties
5.2. Examples of PLA and Derivatives Scaffolds for Tissue Regeneration
6. Regulatory Aspects
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1L | 1 layer |
| 2D | two-dimensional |
| 2L | 2 layers |
| 3D | three-dimensional |
| 3L | 3 layers |
| AgNPs | Silver nanoparticles |
| AgSD | silver sulfadiazine |
| ALG | alginate |
| ATM | Advanced Therapy Medicines |
| CaALG | calcium alginate |
| CAD | computer Aided Design |
| CATMPs | Combined advanced therapy medicinal products |
| CHS | Chitosan |
| CHSG | chitosan glutamate |
| CL | Collagen |
| CS | chondroitin sulfate |
| c-SLN | coated Solid Lipid Nanoparticles |
| CVR | carvacrol |
| DHT | dehydrothermal treatment |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DoE | Design of Experiment |
| ECM | extracellular matrix |
| EDC | Ethyl-3-(3-dimethylaminopropyl)carbodiimide)-based |
| EMA | European Medicine Agency |
| ETO: | Ethylene oxide |
| FDA | Food and Drug Administration |
| GAG | glycosaminoglycans |
| GFs | growth factors |
| GG | gellan |
| GLY | glycine |
| GNPs | graphene nanoplatelets |
| GP | glycerophosphate |
| HA | hyaluronic acid |
| HAL | halloysite |
| HC | homochiral crystallites |
| H-chain | heavy chain |
| HCHS | chitosan hydrochloride |
| HTNs | halloysite nanotubes |
| HYBD | clay hybrid |
| IBD | Inflammatory Bowel Disease |
| IL | interleukin |
| L-chain | light chain |
| M0 | naive macrophages |
| M1 | pro-inflammatory macrophages |
| M2 | anti-inflammatory macrophages |
| Mats | matrices |
| MBG | mesoporous bioactive glasses |
| MH | Manuka Honey |
| MMPS | metalloproteinases |
| mMSC | murine mesenchymal stem cells |
| MMT | montmorillonite |
| MPC | 5-methyl-pyrrolidinone chitosan |
| MSC | mesenchymal stem cells |
| MW | molecular weight |
| NCs | nanocomposites |
| NPs | nanoparticles |
| OCMCHS | O-carboxymethyl chitosan |
| PCL | poly(ε-caprolactone) |
| PDGF-AB | Platelet-Derived Growth Factor-AB |
| PDLA | poly(d-lactic acid) |
| PDLLA | poly(dl-lactic acid) |
| PEC | pectin |
| PEO | polyethylene oxide |
| PGA | polyglycolide |
| PHC | palygorskite |
| pI | protein isoelectric point |
| PL | platelet lysate |
| PLA | polylactide |
| PLA-PCL | Polylactide-co-polycaprolactone |
| PLGA | Polylactide-co-glycolide |
| PLLA | poly(l-lactic acid) |
| p-MSCs | bone marrow mesenchymal stem cells |
| PRP | platelet rich plasma |
| Pul | pullulan |
| PUR | polyurethane |
| PVA | poly(vinylalcohol) |
| PVP | poly (vinylpyrrolidone) |
| pxch | chemically effective crosslinking |
| pxel | elastically effective crosslinking |
| RC-33 | 1-[3-(1,10-biphen)-4-yl] butylpiperidine |
| RGD | Arg-Gly-Asp |
| ROP | ring-opening polymerization |
| RSF | regenerated silk fibroin |
| SC | stereocomplex crystallites |
| SD | substitution degree |
| SF | silk fibroin |
| SS | sericin |
| TA | tranexamic acid |
| TEP | tissue-engineered products or medicines |
| TMC | Trimethyl chitosan |
| TNF | Tumor Necrosis Factor |
| VCM | vancomycin hydrochloride |
References
- Christman, K.L. Biomaterials for tissue repair. Science 2019, 363, 340–341. [Google Scholar] [CrossRef] [PubMed]
- Vert, M.; Doi, Y.; Hellwich, K.H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schue, F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–408. [Google Scholar] [CrossRef]
- Guimaraes, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 2020, 5, 351–370. [Google Scholar] [CrossRef]
- Mamidi, N.; Romo, I.L.; Gutierrez, H.M.L.; Barrera, E.V.; Elias-Zuniga, A. Development of forcespun fiber-aligned scaffolds from gelatin-zein composites for potential use in tissue engineering and drug release. MRS Commun. 2018, 8, 885–892. [Google Scholar] [CrossRef]
- Mamidi, N.; Delgadillo, R.M.V.; Gonzalez-Ortiz, A. Engineering of carbon nano-onion bioconjugates for biomedical applications. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 120, 111698. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, H.-J.; Vunjak-Novakovic, G.; Kaplan, D.L. Stem cell-based tissue engineering with silk biomaterials. Biomaterials 2006, 27, 6064–6082. [Google Scholar] [CrossRef]
- Yucel, T.; Lovett, M.L.; Keplan, D.L. Silk-based biomaterials for sustained drug delivery. J. Control. Release 2014, 190, 381–397. [Google Scholar] [CrossRef]
- Crivelli, B.; Perteghella, S.; Bari, E.; Sorrenti, M.; Tripodo, G.; Chlapanidas, T.; Torre, M.L. Silk nanoparticles: From inert supports to bioactive natural carriers for drug delivery. Soft Matter 2018, 14, 546–557. [Google Scholar] [CrossRef]
- Chlapanidas, T.; Farago, S.; Lucconi, G.; Perteghella, S.; Galuzzi, M.; Mantelli, M.; Avanzini, M.A.; Tosca, M.C.; Marazzi, M.; Vigo, D.; et al. Sericins exhibit ROS-scavenging, anti-tyrosinase, anti-elastase, and in vitro immunomodulatory activities. Int. J. Biol. Macromol. 2013, 58, 47–56. [Google Scholar] [CrossRef]
- Arango, M.C.; Montoya, Y.; Peresin, M.S.; Bustamante, J.; Alvarez-Lopez, C. Silk sericin as a biomaterial for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2020, 1–15. [Google Scholar] [CrossRef]
- Gupta, D.; Agrawal, A.; Rangi, A. Extraction and characterization of silk sericin. Indian J. Fibre Text. Res. 2014, 39, 364–372. [Google Scholar]
- Kunz, R.I.; Brancalhao, R.M.C.; Ribeiro, L.D.C.; Natali, M.R.M. Silkworm sericin: Properties and biomedical applications. Biomed Res. Int. 2016, 8175701. [Google Scholar] [CrossRef]
- Aramwit, P.; Siritientong, T.; Srichana, T. Potential applications of silk sericin, a natural protein from textile industry by-products. Waste Manag. Res. 2012, 30. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, B. Biodegradation of silk biomaterials. Int. J. Mol. Sci. 2009, 10, 1514–1524. [Google Scholar] [CrossRef]
- Ferreira, B.M.P.; Andersson, N.; Atterling, E.; Engqvist, J.; Hall, S.; Dicko, C. 3D Structure and mechanics of silk sponge scaffolds is governed by larger pore sizes. Front. Mater. 2020, 7, 211. [Google Scholar] [CrossRef]
- Wang, Y.; Rudym, D.D.; Walsh, A.; Abrahamsen, L.; Kim, H.J.; Kim, H.S.; Kirker-Head, C.; Kaplan, D.L. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials 2008, 29, 3415–3428. [Google Scholar] [CrossRef]
- Johari, N.; Moroni, L.; Samadikuchaksaraei, A. Tuning the conformation and mechanical properties of silk fibroin hydrogels. Eur. Polym. J. 2020, 134, 109842. [Google Scholar] [CrossRef]
- Han, K.S.; Song, J.E.; Tripathy, N.; Kim, H.; Moon, B.M.; Park, C.H.; Khang, G. Effect of pore sizes of silk scaffolds for cartilage tissue engineering. Macromol. Res. 2015, 23, 1091–1097. [Google Scholar] [CrossRef]
- Burger, D.; Beaumont, M.; Rosenau, T.; Tamada, Y. Porous silk fibroin/cellulose hydrogels for bone tissue engineering via a novel combined process based on sequential regeneration and porogen leaching. Molecules 2020, 25, 5097. [Google Scholar] [CrossRef]
- Kim, M.H.; Park, W.H. Chemically cross-linked silk fibroin hydrogel with enhanced elastic properties, biodegradability, and biocompatibility. Int. J. Nanomed. 2016, 11, 2967–2978. [Google Scholar] [CrossRef]
- Jiang, C.Y.; Wang, X.Y.; Gunawidjaja, R.; Lin, Y.H.; Gupta, M.K.; Kaplan, D.L.; Naik, R.R.; Tsukruk, V.V. Mechanical properties of robust ultrathin silk fibroin films. Adv. Funct. Mater. 2007, 17, 2229–2237. [Google Scholar] [CrossRef]
- Fan, S.N.; Zhang, Y.P.; Shao, H.L.; Hu, X.C. Electrospun regenerated silk fibroin mats with enhanced mechanical properties. Int. J. Biol. Macromol. 2013, 56, 83–88. [Google Scholar] [CrossRef]
- Amiraliyan, N.; Nouri, M.; Kish, M.H. Structural characterization and mechanical properties of electrospun silk fibroin nanofiber mats. Polym. Sci. Ser. A 2010, 52, 407–412. [Google Scholar] [CrossRef]
- Chen, J.P.; Chen, S.H.; Lai, G.J. Preparation and characterization of biomimetic silk fibroin/chitosan composite nanofibers by electrospinning for osteoblasts culture. Nanoscale Res. Lett. 2012, 7, 1–11. [Google Scholar] [CrossRef]
- Wang, Q.S.; Han, G.C.; Yan, S.Q.; Zhang, Q. 3D Printing of silk fibroin for biomedical applications. Materials 2019, 12, 504. [Google Scholar] [CrossRef]
- Kanokpanont, S.; Damrongsakkul, S.; Ratanavaraporn, J.; Aramwit, P. An innovative bi-layered wound dressing made of silk and gelatin for accelerated wound healing. Int. J. Pharm. 2012, 436, 141–153. [Google Scholar] [CrossRef]
- Jang, M.J.; Um, I.C. Effect of sericin concentration and ethanol content on gelation behavior, rheological properties, and sponge characteristics of silk sericin. Eur. Polym. J. 2017, 93, 761–774. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.S.; Zhang, J.X.; Huang, L.; Liu, J.; Li, Y.K.; Zhang, G.Z.; Kundu, S.C.; Wang, L. Exploring natural silk protein sericin for regenerative medicine: An injectable, photoluminescent, cell-adhesive 3D hydrogel. Sci. Rep. 2014, 4, 7064. [Google Scholar] [CrossRef]
- Nayak, S.; Talukdar, S.; Kundu, S.C. Potential of 2D crosslinked sericin membranes with improved biostability for skin tissue engineering. Cell Tissue Res. 2012, 347, 783–794. [Google Scholar] [CrossRef]
- Park, C.J.; Ryoo, J.; Ki, C.S.; Kim, J.W.; Kim, I.S.; Bae, D.G.; Um, I.C. Effect of molecular weight on the structure and mechanical properties of silk sericin gel, film, and sponge. Int. J. Biol. Macromol. 2018, 119, 821–832. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, H.; Wei, K.; Yang, Y.; Zheng, R.Y.; Kim, I.S.; Zhang, K.Q. A review of structure construction of silk fibroin biomaterials from single structures to multi-level structures. Int. J. Mol. Sci. 2017, 18, 237. [Google Scholar] [CrossRef]
- Inoue, S.; Tanaka, K.; Arisaka, F.; Kimura, S.; Ohtomo, K.; Mizuno, S. Silk fibroin of Bombyx mori is secreted, assembling a high molecular mass elementary unit consisting of H-chain, L-chain, and P25, with a 6:6:1 molar ratio. J. Biol. Chem. 2000, 275, 40517–40528. [Google Scholar] [CrossRef]
- Sun, W.Z.; Gregory, D.A.; Tomeh, M.A.; Zhao, X.B. Silk fibroin as a functional biomaterial for tissue engineering. Int. J. Mol. Sci. 2021, 22, 1499. [Google Scholar] [CrossRef]
- Perteghella, S.; Martella, E.; de Girolamo, L.; Orfei, C.P.; Pierini, M.; Fumagalli, V.; Pintacuda, D.V.; Chlapanidas, T.; Vigano, M.; Farago, S.; et al. Fabrication of innovative silk/alginate microcarriers for mesenchymal stem cell delivery and tissue regeneration. Int. J. Mol. Sci. 2017, 18, 1829. [Google Scholar] [CrossRef] [PubMed]
- Orfei, C.P.; Talo, G.; Vigano, M.; Perteghella, S.; Lugano, G.; Fontana, F.F.; Ragni, E.; Colombini, A.; De Luca, P.; Moretti, M.; et al. Silk/fibroin microcarriers for mesenchymal stem cell delivery: Optimization of cell seeding by the design of experiment. Pharmaceutics 2018, 10, 200. [Google Scholar] [CrossRef]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.S.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef]
- Li, M.Z.; Wu, Z.Y.; Zhang, C.S.; Lu, S.Z.; Yan, H.J.; Huang, D.; Ye, H.L. Study on porous silk fibroin materials. II. Preparation and characteristics of spongy silk fibroin materials. J. Appl. Polym. Sci. 2001, 79, 2192–2199. [Google Scholar] [CrossRef]
- Nazarov, R.; Jin, H.J.; Kaplan, D.L. Porous 3-D scaffolds from regenerated silk fibroin. Biomacromolecules 2004, 5, 718–726. [Google Scholar] [CrossRef]
- Lv, Q.; Feng, Q.L. Preparation of 3-D regenerated fibroin scaffolds with freeze drying method and freeze drying/foaming technique. J. Mater. Sci. Mater. Med. 2006, 17, 1349–1356. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, S.Q.; Li, M.Z. Silk fibroin based porous materials. Materials 2009, 2, 2276–2295. [Google Scholar] [CrossRef]
- Saleem, M.; Rasheed, S.; Chen, Y.G. Silk fibroin/hydroxyapatite scaffold: A highly compatible material for bone regeneration. Sci. Technol. Adv. Mater. 2020, 21, 242–266. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.J.; Park, J.Y.; Li, C.M.; Jin, H.J.; Valluzzi, R.; Kaplan, D.L. Structure and properties of silk hydrogels. Biomacromolecules 2004, 5, 786–792. [Google Scholar] [CrossRef]
- Matsumoto, A.; Chen, J.; Collette, A.L.; Kim, U.J.; Altman, G.H.; Cebe, P.; Kaplan, D.L. Mechanisms of silk fibroin sol-gel transitions. J. Phys. Chem. B 2006, 110, 21630–21638. [Google Scholar] [CrossRef]
- Yucel, T.; Cebe, P.; Kaplan, D.L. Vortex-Induced Injectable Silk Fibroin Hydrogels. Biophys. J. 2009, 97, 2044–2050. [Google Scholar] [CrossRef]
- Wang, X.; Kluge, J.A.; Leisk, G.G.; Kaplan, D.L. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials 2008, 29, 1054–1064. [Google Scholar] [CrossRef]
- Floren, M.; Migliaresi, C.; Motta, A. Processing techniques and applications of silk hydrogels in bioengineering. J. Funct. Biomater. 2016, 7, 26. [Google Scholar] [CrossRef]
- Leisk, G.G.; Lo, T.J.; Yucel, T.; Lu, Q.; Kaplan, D.L. Electrogelation for protein adhesives. Adv. Mater. 2010, 22, 711–715. [Google Scholar] [CrossRef]
- Floren, M.L.; Spilimbergo, S.; Motta, A.; Migliaresi, C. Carbon dioxide induced silk protein gelation for biomedical applications. Biomacromolecules 2012, 13, 2060–2072. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Cho, H.; Gil, E.S.; Mandal, B.B.; Min, B.H.; Kaplan, D.L. Silk-fibrin/hyaluronic acid composite gels for nucleus pulposus tissue regeneration. Tissue Eng. Part A 2011, 17, 2999–3009. [Google Scholar] [CrossRef]
- Chlapanidas, T.; Tosca, M.C.; Farago, S.; Perteghella, S.; Galuzzi, M.; Lucconi, G.; Antonioli, B.; Ciancio, F.; Rapisarda, V.; Vigo, D.; et al. Formulation and characterization of silk fibroin films as a scaffold for derived stem cells in skin tissue engineering. Int. J. Immunopathol. Pharmacol. 2013, 26, 43–49. [Google Scholar] [CrossRef]
- Chlapanidas, T.; Farago, S.; Mingotto, F.; Crovato, F.; Tosca, M.C.; Antonioli, B.; Bucco, M.; Lucconi, G.; Scalise, A.; Vigo, D.; et al. Regenerated silk fibroin scaffold and iinfrapatellar adipose stromal vascular fraction as feeder-layer: A new product for cartilage advanced therapy. Tissue Eng. Part A 2011, 17, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, L.K.; Chen, J.L.; Wang, L.S.; Gui, X.X.; Ran, J.S.; Xu, G.W.; Zhao, H.S.; Zeng, M.F.; Ji, J.F.; et al. Silk fibroin biomaterial shows safe and effective wound healing in animal models and a randomized controlled clinical trial. Adv. Healthc. Mater. 2017, 6, 1700121. [Google Scholar] [CrossRef]
- Chlapanidas, T.; Perteghella, S.; Farago, S.; Boschi, A.; Tripodo, G.; Vigani, B.; Crivelli, B.; Renzi, S.; Dotti, S.; Preda, S.; et al. Platelet lysate and adipose mesenchymal stromal cells on silk fibroin nonwoven mats for wound healing. J. Appl. Polym. Sci. 2016, 133, 42942. [Google Scholar] [CrossRef]
- Alessandrino, A.; Marelli, B.; Arosio, C.; Fare, S.; Tanzi, M.C.; Freddi, G. Electrospun silk fibroin mats for tissue engineering. Eng. Life Sci. 2008, 8, 219–225. [Google Scholar] [CrossRef]
- Perteghella, S.; Vigani, B.; Mastracci, L.; Grillo, F.; Antonioli, B.; Galuzzi, M.; Tosca, M.C.; Crivelli, B.; Preda, S.; Tripodo, G.; et al. Stromal vascular fraction loaded silk fibroin mats effectively support the survival of diabetic mice after pancreatic islet transplantation. Macromol. Biosci. 2017, 17, 1700131. [Google Scholar] [CrossRef] [PubMed]
- Vigani, B.; Mastracci, L.; Grillo, F.; Perteghella, S.; Preda, S.; Crivelli, B.; Antonioli, B.; Galuzzi, M.; Tosca, M.C.; Marazzi, M.; et al. Local biological effects of adipose stromal vascular fraction delivery systems after subcutaneous implantation in a murine model. J. Bioact. Compat. Polym. 2016, 31, 600–612. [Google Scholar] [CrossRef]
- Crivelli, B.; Bari, E.; Perteghella, S.; Catenacci, L.; Sorrenti, M.; Mocchi, M.; Farago, S.; Tripodo, G.; Prina-Mello, A.; Torre, M.L. Silk fibroin nanoparticles for celecoxib and curcumin delivery: ROS-scavenging and anti-inflammatory activities in an in vitro model of osteoarthritis. Eur. J. Pharm. Biopharm. 2019, 137, 37–45. [Google Scholar] [CrossRef]
- Perteghella, S.; Crivelli, B.; Catenacci, L.; Sorrenti, M.; Bruni, G.; Necchi, V.; Vigani, B.; Sorlini, M.; Torre, M.L.; Chlapanidas, T. Stem cell-extracellular vesicles as drug delivery systems: New frontiers for silk/curcumin nanoparticles. Int. J. Pharm. 2017, 520, 86–97. [Google Scholar] [CrossRef]
- Perteghella, S.; Sottani, C.; Cocce, V.; Negri, S.; Cavicchini, L.; Alessandri, G.; Cottica, D.; Torre, M.L.; Grignani, E.; Pessina, A. Paclitaxel-loaded silk fibroin nanoparticles: Method validation by UHPLC-MS/MS to assess an exogenous approach to load cytotoxic drugs. Pharmaceutics 2019, 11, 285. [Google Scholar] [CrossRef]
- Bari, E.; Serra, M.; Paolillo, M.; Bernardi, E.; Tengattini, S.; Piccinini, F.; Lanni, C.; Sorlini, M.; Bisbano, G.; Calleri, E.; et al. Silk fibroin nanoparticle functionalization with Arg-Gly-Asp cyclopentapeptide promotes active targeting for tumor site-specific delivery. Cancers 2021, 13, 1185. [Google Scholar] [CrossRef]
- Orlandi, G.; Farago, S.; Menato, S.; Sorlini, M.; Butti, F.; Mocchi, M.; Donelli, I.; Catenacci, L.; Sorrenti, M.L.; Croce, S.; et al. Eco-sustainable silk sericin from by-product of textile industry can be employed for cosmetic, dermatology and drug delivery. J. Chem. Technol. Biotechnol. 2020, 95, 2549–2560. [Google Scholar] [CrossRef]
- Tengattini, S.; Orlandi, G.; Perteghella, S.; Bari, E.; Amadio, M.; Calleri, E.; Massolini, G.; Torre, M.L.; Temporini, C. Chromatographic profiling of silk sericin for biomedical and cosmetic use by complementary hydrophylic, reversed phase and size exclusion chromatographic methods. J. Pharm. Biomed. Anal. 2020, 186, 113291. [Google Scholar] [CrossRef]
- Chlapanidas, T.; Perteghella, S.; Leoni, F.; Farago, S.; Marazzi, M.; Rossi, D.; Martino, E.; Gaggeri, R.; Collina, S. TNF-alpha blocker effect of naringenin-loaded sericin microparticles that are potentially useful in the treatment of psoriasis. Int. J. Mol. Sci. 2014, 15, 13624–13636. [Google Scholar] [CrossRef]
- Bari, E.; Arciola, C.R.; Vigani, B.; Crivelli, B.; Moro, P.; Marrubini, G.; Sorrenti, M.; Catenacci, L.; Bruni, G.; Chlapanidas, T.; et al. In vitro effectiveness of microspheres based on silk sericin and Chlorella vulgaris or Arthrospira platensis for wound healing applications. Materials 2017, 10, 983. [Google Scholar] [CrossRef]
- Bari, E.; Perteghella, S.; Farago, S.; Torre, M.L. Association of silk sericin and platelet lysate: Premises for the formulation of wound healing active medications. Int. J. Biol. Macromol. 2018, 119, 37–47. [Google Scholar] [CrossRef]
- Bari, E.; Perteghella, S.; Marrubini, G.; Sorrenti, M.; Catenacci, L.; Tripodo, G.; Mastrogiacomo, M.; Mandracchia, D.; Trapani, A.; Farago, S.; et al. In vitro efficacy of silk sericin microparticles and platelet lysate for intervertebral disk regeneration. Int. J. Biol. Macromol. 2018, 118, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.B.; Priya, A.S.; Kundu, S.C. Novel silk sericin/gelatin 3-D scaffolds and 2-D films: Fabrication and characterization for potential tissue engineering applications. Acta Biomater. 2009, 5, 3007–3020. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Chen, H.G.; Li, Y.L.; Fang, A.; Wu, T.F.; Shen, C.Y.; Zhao, Y.Y.; Zhang, G.Z. A transparent sericin-polyacrylamide interpenetrating network hydrogel as visualized dressing material. Polym. Test. 2020, 87, 106517. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Liu, J.; Huang, L.; Wang, Z.; Wang, L. Design and performance of a sericin-alginate interpenetrating network hydrogel for cell and drug delivery. Sci. Rep. 2015, 5, 12374. [Google Scholar] [CrossRef]
- Chen, C.S.; Zeng, F.; Xiao, X.; Wang, Z.; Li, X.L.; Tan, R.W.; Liu, W.Q.; Zhang, Y.S.; She, Z.D.; Li, S.J. Three-dimensionally printed silk-sericin-based hydrogel scaffold: A promising visualized dressing material for real-time monitoring of wounds. Acs Appl. Mater. Interfaces 2018, 10, 33879–33890. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Akbari, M.; Hadisi, Z.; Omidi, M.; Chen, X.B. Development of the PVA/CS nanofibers containing silk protein sericin as a wound dressing: In vitro and in vivo assessment. Int. J. Biol. Macromol. 2020, 149, 513–521. [Google Scholar] [CrossRef]
- Napavichayanun, S.; Yamdech, R.; Aramwit, P. Development of bacterial cellulose incorporating silk sericin, polyhexamethylene biguanide, and glycerin with enhanced physical properties and antibacterial activities for wound dressing application. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 61–67. [Google Scholar] [CrossRef]
- Kwak, H.W.; Lee, H.; Lee, M.E.; Jin, H.J. Facile and green fabrication of silk sericin films reinforced with bamboo-derived cellulose nanofibrils. J. Clean. Prod. 2018, 200, 1034–1042. [Google Scholar] [CrossRef]
- Orlandi, G.; Bari, E.; Catenacci, L.; Sorrenti, M.; Segale, L.; Farago, S.; Sorlini, M.; Arciola, C.R.; Torre, M.L.; Perteghella, S. Polyphenols-Loaded Sericin Self-Assembling Nanoparticles: A Slow-Release for Regeneration by Tissue-Resident Mesenchymal Stem/Stromal Cells. Pharmaceutics 2020, 12, 381. [Google Scholar] [CrossRef]
- Kolacna, L.; Bakesova, J.; Varga, F.; Kostakova, E.; Planka, L.; Necas, A.; Lukas, D.; Amler, E.; Pelouch, V. Biochemical and biophysical aspects of collagen nanostructure in the extracellular matrix. Physiol. Res. 2007, 56, S51–S60. [Google Scholar]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Terzi, A.; Sannino, A.; Madaghiele, M. Mimicking the hierarchical organization of natural collagen: Toward the development of ideal scaffolding material for tissue regeneration. Front. Bioeng. Biotechnol. 2021, 9, 644595. [Google Scholar] [CrossRef]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Campa, L.; Lunetti, P.; Madaghiele, M.; Blasi, F.S.; Corallo, A.; Capobianco, L.; Sannino, A. Marine collagen and its derivatives: Versatile and sustainable bio-resources for healthcare. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 113, 110963. [Google Scholar] [CrossRef]
- Gorgieva, S.; Kokol, V. Collagen- vs. gelatine-based biomaterials and their biocompatibility: Review and perspectives. In Biomaterials Applications for Nanomedicine; Pignatello, R., Ed.; IntechOpen: London, UK, 2011; pp. 17–52. ISBN 978-953-307-661-4. [Google Scholar]
- Gaspar-Pintiliescu, A.; Stanciuc, A.M.; Craciunescu, O. Natural composite dressings based on collagen, gelatin and plant bioactive compounds for wound healing: A review. Int. J. Biol. Macromol. 2019, 138, 854–865. [Google Scholar] [CrossRef]
- Shojaati, G.; Khandaker, I.; Sylakowski, K.; Funderburgh, M.L.; Du, Y.Q.; Funderburgh, J.L. Compressed collagen enhances stem cell therapy for corneal scarring. Stem Cells Transl. Med. 2018, 7, 487–494. [Google Scholar] [CrossRef]
- Scarano, A.; Lorusso, F.; Orsini, T.; Morra, M.; Iviglia, G.; Valbonetti, L. Biomimetic surfaces coated with covalently immobilized collagen type I: An X-Ray photoelectron spectroscopy, atomic force microscopy, micro-CT and histomorphometrical study in rabbits. Int. J. Mol. Sci. 2019, 20, 724. [Google Scholar] [CrossRef]
- Iviglia, G.; Kargozar, S.; Baino, F. Biomaterials, current strategies, and novel nano-technological approaches for periodontal regeneration. J. Funct. Biomater. 2019, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Gigante, A.; Cesari, E.; Busilacchi, A.; Manzotti, S.; Kyriakidou, K.; Greco, F.; Di Primio, R.; Mattioli-Belmonte, M. Collagen I membranes for tendon repair: Effect of collagen fiber orientation on cell behavior. J. Orthop. Res. 2009, 27, 826–832. [Google Scholar] [CrossRef]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.N.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The collagen suprafamily: From biosynthesis to advanced biomaterial development. Adv. Mater. 2019, 31, 1–39. [Google Scholar] [CrossRef]
- Gu, L.S.; Shan, T.T.; Ma, Y.X.; Tay, F.R.; Niu, L.N. Novel biomedical applications of crosslinked collagen. Trends Biotechnol. 2019, 37, 464–491. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, L.; Calo, E.; Bonfrate, V.; Pedone, D.; Gallo, N.; Natali, M.L.; Sannino, A.; Madaghiele, M. Exploring the effects of the crosslink density on the physicochemical properties of collagen-based scaffolds. Polym. Test. 2021, 93, 106966. [Google Scholar] [CrossRef]
- Madaghiele, M.; Calo, E.; Salvatore, L.; Bonfrate, V.; Pedone, D.; Frigione, M.; Sannino, A. Assessment of collagen crosslinking and denaturation for the design of regenerative scaffolds. J. Biomed. Mater. Res. Part A 2016, 104, 186–194. [Google Scholar] [CrossRef]
- Terzi, A.; Storelli, E.; Bettini, S.; Sibillano, T.; Altamura, D.; Salvatore, L.; Madaghiele, M.; Romano, A.; Siliqi, D.; Ladisa, M.; et al. Effects of processing on structural, mechanical and biological properties of collagen-based substrates for regenerative medicine. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Montalbano, G.; Borciani, G.; Cerqueni, G.; Licini, C.; Banche-Niclot, F.; Janner, D.; Sola, S.; Fiorilli, S.; Mattioli-Belmonte, M.; Ciapetti, C.; et al. Collagen hybrid formulations for the 3D printing of nanostructured bone scaffolds: An optimized genipin-crosslinking strategy. Nanomaterials 2020, 10, 1681. [Google Scholar] [CrossRef]
- Zhou, J.A.; Cao, C.B.; Ma, X.L.; Lin, J. Electrospinning of silk fibroin and collagen for vascular tissue engineering. Int. J. Biol. Macromol. 2010, 47, 514–519. [Google Scholar] [CrossRef]
- Montalbano, G.; Toumpaniari, S.; Popov, A.; Duan, P.; Chen, J.; Dalgarno, K.; Scott, W.E.; Ferreira, A.M. Synthesis of bioinspired collagen/alginate/fibrin based hydrogels for soft tissue engineering. Mater. Sci. Eng. C-Mater. Biol. Appl. 2018, 91, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Michalska-Sionkowska, M.; Walczak, M. Preparation and characterization of collagen/hyaluronic acid/chitosan film crosslinked with dialdehyde starch. Int. J. Biol. Macromol. 2020, 149, 290–295. [Google Scholar] [CrossRef]
- Caddeo, S.; Mattioli-Belmonte, M.; Cassino, C.; Barbani, N.; Dicarlo, M.; Gentile, P.; Baino, F.; Sartori, S.; Vitale-Brovarone, C.; Ciardelli, G. Newly-designed collagen/polyurethane bioartificial blend as coating on bioactive glass-ceramics for bone tissue engineering applications. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 96, 218–233. [Google Scholar] [CrossRef]
- Colaco, E.; Brouri, D.; Aissaoui, N.; Cornette, P.; Dupres, V.; Domingos, R.F.; Lambert, J.F.; Maisonhaute, E.; El Kirat, K.; Landoulsi, J. Hierarchical collagen-hydroxyapatite nanostructures designed through layer-by-layer assembly of crystal-decorated fibrils. Biomacromolecules 2019, 20, 4522–4534. [Google Scholar] [CrossRef]
- Yu, L.; Rowe, D.W.; Perera, I.P.; Zhang, J.Y.; Suib, S.L.; Xin, X.N.; Wei, M. Intrafibrillar mineralized collagen-hydroxyapatite-based scaffolds for bone regeneration. Acs Appl. Mater. Interfaces 2020, 12, 18235–18249. [Google Scholar] [CrossRef]
- Debons, N.; Dems, D.; Helary, C.; Le Grill, S.; Picaut, L.; Renaud, F.; Delsuc, N.; Schanne-Klein, M.C.; Coradin, T.; Aime, C. Differentiation of neural-type cells on multi-scale ordered collagen-silica bionanocomposites. Biomater. Sci. 2020, 8, 569–576. [Google Scholar] [CrossRef]
- Miele, D.; Catenacci, L.; Rossi, S.; Sandri, G.; Sorrenti, M.; Terzi, A.; Giannini, C.; Riva, F.; Ferrari, F.; Caramella, C.; et al. Collagen/PCL nanofibers electrospun in green solvent by DOE assisted process. An insight into collagen contribution. Materials 2020, 13, 4698. [Google Scholar] [CrossRef]
- Oh, G.W.; Nguyen, V.T.; Heo, S.Y.; Ko, S.C.; Kim, C.S.; Park, W.S.; Choi, I.W.; Jung, W.K. 3D PCL/fish collagen composite scaffolds incorporating osteogenic abalone protein hydrolysates for bone regeneration application: In vitro and in vivo studies. J. Biomater. Sci. Polym. Ed. 2021, 32, 355–371. [Google Scholar] [CrossRef]
- Dulnik, J.; Denis, P.; Sajkiewicz, P.; Kolbuk, D.; Choinska, E. Biodegradation of bicomponent PCL/gelatin and PCL/collagen nanofibers electrospun from alternative solvent system. Polym. Degrad. Stab. 2016, 130, 10–21. [Google Scholar] [CrossRef]
- Dulnik, J.; Kolbuk, D.; Denis, P.; Sajkiewicz, P. The effect of a solvent on cellular response to PCL/gelatin and PCL/collagen electrospun nanofibres. Eur. Polym. J. 2018, 104, 147–156. [Google Scholar] [CrossRef]
- Gouveia, P.J.; Hodgkinson, T.; Amado, I.; Sadowska, J.M.; Ryan, A.J.; Romanazzo, S.; Carroll, S.; Cryan, S.A.; Kelly, D.J.; O’Brien, F.J. Development of collagen-poly(caprolactone)-based core-shell scaffolds supplemented with proteoglycans and glycosaminoglycans for ligament repair. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 120, 111657. [Google Scholar] [CrossRef]
- Qiao, X.C.; Russell, S.J.; Yang, X.B.; Tronci, G.; Wood, D.J. Compositional and in vitro evaluation of nonwoven type I collagen/poly-dl-lactic acid scaffolds for bone regeneration. J. Funct. Biomater. 2015, 6, 667. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Z.; Lee, K.; Yang, Y.N.; Kawazoe, N.; Chen, G.P. PLGA-collagen-ECM hybrid meshes mimicking stepwise osteogenesis and their influence on the osteogenic differentiation of hMSCs. Biofabrication 2020, 12, 025027. [Google Scholar] [CrossRef] [PubMed]
- Bellini, D.; Cencetti, C.; Sacchetta, A.C.; Battista, A.M.; Martinelli, A.; Mazzucco, L.; D’Abusco, A.S.; Matricardi, P. PLA-grafting of collagen chains leading to a biomaterial with mechanical performances useful in tendon regeneration. J. Mech. Behav. Biomed. Mater. 2016, 64, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, F.; Giavaresi, G.; Bellini, D.; Casagranda, V.; Pressato, D.; Fini, M. Evaluation of a new collagen-based medical device (ElastiCo (R)) for the treatment of acute Achilles tendon injury and prevention of peritendinous adhesions: An in vitro biocompatibility and in vivo investigation. J. Tissue Eng. Regen. Med. 2020, 14, 1113–1125. [Google Scholar] [CrossRef]
- Salvatore, L.; Carofiglio, V.E.; Stufano, P.; Bonfrate, V.; Calo, E.; Scarlino, S.; Nitti, P.; Centrone, D.; Cascione, M.; Leporatti, S.; et al. Potential of electrospun poly(3-hydroxybutyrate)/collagen blends for tissue engineering applications. J. Healthc. Eng. 2018, 2018, 6573947. [Google Scholar] [CrossRef]
- Achilli, M.; Mantovani, D. Tailoring mechanical properties of collagen-based scaffolds for vascular tissue engineering: The effects of pH, temperature and ionic strength on gelation. Polymers 2010, 2, 664–680. [Google Scholar] [CrossRef]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online 2019, 18, 1–74. [Google Scholar] [CrossRef]
- Rodriguez-Vazquez, M.; Vega-Ruiz, B.; Ramos-Zuniga, R.; Saldana-Koppel, D.A.; Quinones-Olvera, L.F. Chitosan and its potential use as a scaffold for tissue engineering in regenerative medicine. Biomed Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef]
- Hoven, V.P.; Tangpasuthadol, V.; Angkitpaiboon, Y.; Vallapa, N.; Kiatkamjornwong, S. Surface-charged chitosan: Preparation and protein adsorption. Carbohydr. Polym. 2007, 68, 44–53. [Google Scholar] [CrossRef]
- Saranya, N.; Moorthi, A.; Saravanan, S.; Devi, M.P.; Selvamurugan, N. Chitosan and its derivatives for gene delivery. Int. J. Biol. Macromol. 2011, 48, 234–238. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Sandri, G.; Rossi, S.; Ferrari, F.; Caramella, C. Chitosan and its salts for mucosal and transmucosal delivery. Expert Opin. Drug Deliv. 2009, 6, 923–939. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, L.; Mao, Z.W.; Gao, C.Y. Chitosan-based biomaterials for tissue repair and regeneration. Chitosan Biomater. II 2011, 244, 81–127. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and applications in biomedical engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Balagangadharan, K.; Dhivya, S.; Selvamurugan, N. Chitosan based nanofibers in bone tissue engineering. Int. J. Biol. Macromol. 2017, 104, 1372–1382. [Google Scholar] [CrossRef]
- Sultankulov, B.; Berillo, D.; Sultankulova, K.; Tokay, T.; Saparov, A. Progress in the development of chitosan-based biomaterials for tissue engineering and regenerative medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef]
- Croisier, F.; Jerome, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.P.; Luo, Y.; Ke, C.H.; Qiu, H.F.; Wang, W.; Zhu, Y.B.; Hou, R.X.; Xu, L.; Wu, S.Z. Chitosan-based functional materials for skin wound repair: Mechanisms and applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, D.Y.; Lu, S.T.; Li, P.W.; Li, S.D. Chitosan-based composite materials for prospective hemostatic applications. Mar. Drugs 2018, 16, 273. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohydr. Polym. 2009, 76, 167–182. [Google Scholar] [CrossRef]
- Rossi, S.; Ferrari, F.; Sandri, G.; Bonferoni, M.C.; Del Fante, C.; Perotti, C.; Caramella, C. Wound healing: Hemoderivatives and biopolymers. In Concise Encyclopedia of Biomedical Polymers and Polymeric Biomaterials; Mishra, M., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2017; pp. 1642–1660. [Google Scholar]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Caramella, C.M.; Ferrari, F. Hyaluronic acid and chitosan-based nanosystems: A new dressing generation for wound care. Expert Opin. Drug Deliv. 2019, 16, 715–740. [Google Scholar] [CrossRef]
- Lin, C.W.; Chen, Y.K.; Lu, M.; Lou, K.L.; Yu, J.S. Photo-crosslinked keratin/chitosan membranes as potential wound dressing materials. Polymers 2018, 10, 987. [Google Scholar] [CrossRef]
- Sharma, S.; Swetha, K.L.; Roy, A. Chitosan-Chondroitin sulfate based polyelectrolyte complex for effective management of chronic wounds. Int. J. Biol. Macromol. 2019, 132, 97–108. [Google Scholar] [CrossRef]
- Rossi, S.; Faccendini, A.; Bonferoni, M.C.; Ferrari, F.; Sandri, G.; Del Fante, C.; Perotti, C.; Caramella, C.M. “Sponge-like” dressings based on biopolymers for the delivery of platelet lysate to skin chronic wounds. Int. J. Pharm. 2013, 440, 207–215. [Google Scholar] [CrossRef]
- Mori, M.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G.; Chlapanidas, T.; Torre, M.L.; Caramella, C. Sponge-like dressings based on the association of chitosan and sericin for the treatment of chronic skin ulcers. I. Design of experiments assisted development. J. Pharm. Sci. 2016, 105, 1180–1187. [Google Scholar] [CrossRef]
- Mori, M.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G.; Riva, F.; Tenci, M.; Del Fante, C.; Nicoletti, G.; Caramella, C. Sponge-like dressings based on the association of chitosan and sericin for the treatment of chronic skin ulcers. II. Loading of the hemoderivative platelet lysate. J. Pharm. Sci. 2016, 105, 1188–1195. [Google Scholar] [CrossRef]
- Tenci, M.; Rossi, S.; Bonferoni, M.C.; Sandri, G.; Boselli, C.; Di Lorenzo, A.; Daglia, M.; Cornaglia, A.I.; Gioglio, L.; Perotti, C.; et al. Particulate systems based on pectin/chitosan association for the delivery of manuka honey components and platelet lysate in chronic skin ulcers. Int. J. Pharm. 2016, 509, 59–70. [Google Scholar] [CrossRef]
- Dellera, E.; Bonferoni, M.C.; Sandri, G.; Rossi, S.; Ferrari, F.; Del Fante, C.; Perotti, C.; Grisoli, P.; Caramella, C. Development of chitosan oleate ionic micelles loaded with silver sulfadiazine to be associated with platelet lysate for application in wound healing. Eur. J. Pharm. Biopharm. 2014, 88, 643–650. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Sandri, G.; Dellera, E.; Rossi, S.; Ferrari, F.; Mori, M.; Caramella, C. Ionic polymeric micelles based on chitosan and fatty acids and intended for wound healing. Comparison of linoleic and oleic acid. Eur. J. Pharm. Biopharm. 2014, 87, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Bonferoni, M.C.; Riva, F.; Invernizzi, A.; Dellera, E.; Sandri, G.; Rossi, S.; Marrubini, G.; Bruni, G.; Vigani, B.; Caramella, C.; et al. Alpha tocopherol loaded chitosan oleate nanoemulsions for wound healing. Evaluation on cell lines and ex vivo human biopsies, and stabilization in spray dried Trojan microparticles. Eur. J. Pharm. Biopharm. 2018, 123, 31–41. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Sandri, G.; Rossi, S.; Usai, D.; Liakos, I.; Garzoni, A.; Fiamma, M.; Zanetti, S.; Athanassiou, A.; Caramella, C.; et al. A novel ionic amphiphilic chitosan derivative as a stabilizer of nanoemulsions: Improvement of antimicrobial activity of Cymbopogon citratus essential oil. Colloids Surf. B-Biointerfaces 2017, 152, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Shen, E.C.; Chou, T.C.; Gau, C.H.; Tu, H.P.; Chen, Y.T.; Fu, E. Releasing growth factors from activated human platelets after chitosan stimulation: A possible bio-material for platelet-rich plasma preparation. Clin. Oral Implant. Res. 2006, 17, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Marciello, M.; Sandri, G.; Ferrari, F.; Bonferoni, M.C.; Papetti, A.; Caramella, C.; Dacarro, C.; Grisoli, P. Wound dressings based on chitosans and hyaluronic acid for the release of chlorhexidine diacetate in skin ulcer therapy. Pharm. Dev. Technol. 2007, 12, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Tenci, M.; Rossi, S.; Bonferoni, M.C.; Sandri, G.; Mentori, I.; Boselli, C.; Cornaglia, A.I.; Daglia, M.; Marchese, A.; Caramella, C.; et al. Application of DoE approach in the development of mini-capsules, based on biopolymers and manuka honey polar fraction, as powder formulation for the treatment of skin ulcers. Int. J. Pharm. 2017, 516, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Tenci, M.; Rossi, S.; Aguzzi, C.; Carazo, E.; Sandri, G.; Bonferoni, M.C.; Grisoli, P.; Viseras, C.; Caramella, C.M.; Ferrari, F. Carvacrol/clay hybrids loaded into in situ gelling films. Int. J. Pharm. 2017, 531, 676–688. [Google Scholar] [CrossRef]
- Rossi, S.; Marciello, M.; Bonferoni, M.C.; Ferrari, F.; Sandri, G.; Dacarro, C.; Grisoli, P.; Caramella, C. Thermally sensitive gels based on chitosan derivatives for the treatment of oral mucositis. Eur. J. Pharm. Biopharm. 2010, 74, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Bonferoni, M.C.; Ferrari, F.; Rossi, S.; Mori, M.; Caramella, C. Opportunities offered by chitosan-based nanotechnology in mucosal/skin drug delivery. Curr. Top. Med. Chem. 2015, 15, 401–412. [Google Scholar] [CrossRef]
- Rossi, S.; Vigani, B.; Puccio, A.; Bonferoni, M.C.; Sandri, G.; Ferrari, F. Chitosan ascorbate nanoparticles for the vaginal delivery of antibiotic drugs in atrophic vaginitis. Mar. Drugs 2017, 15, 319. [Google Scholar] [CrossRef]
- Aguzzi, C.; Sandri, G.; Bonferoni, C.; Cerezo, P.; Rossi, S.; Ferrari, F.; Caramella, C.; Viseras, C. Solid state characterisation of silver sulfadiazine loaded on montmorillonite/chitosan nanocomposite for wound healing. Colloids Surf. B-Biointerfaces 2014, 113, 152–157. [Google Scholar] [CrossRef]
- Sandri, G.; Bonferoni, M.C.; Ferrari, F.; Rossi, S.; Aguzzi, C.; Mori, M.; Grisoli, P.; Cerezo, P.; Tenci, M.; Viseras, C.; et al. Montmorillonite-chitosan-silver sulfadiazine nanocomposites for topical treatment of chronic skin lesions: In vitro biocompatibility, antibacterial efficacy and gap closure cell motility properties. Carbohydr. Polym. 2014, 102, 970–977. [Google Scholar] [CrossRef]
- Sandri, G.; Aguzzi, C.; Rossi, S.; Bonferoni, M.C.; Bruni, G.; Boselli, C.; Cornaglia, A.I.; Riva, F.; Viseras, C.; Caramella, C.; et al. Halloysite and chitosan oligosaccharide nanocomposite for wound healing. Acta Biomater. 2017, 57, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, M.; Manolova, N.; Rashkov, I. Electrospun antibacterial chitosan-based fibers. Macromol. Biosci. 2013, 13, 860–872. [Google Scholar] [CrossRef]
- Hadipour-Goudarzi, E.; Montazer, M.; Latifi, M.; Aghaji, A.A.G. Electrospinning of chitosan/sericin/PVA nanofibers incorporated with in situ synthesis of nano silver. Carbohydr. Polym. 2014, 113, 231–239. [Google Scholar] [CrossRef]
- Sarhan, W.A.; Azzazy, H.M.E. High concentration honey chitosan electrospun nanofibers: Biocompatibility and antibacterial effects. Carbohydr. Polym. 2015, 122, 135–143. [Google Scholar] [CrossRef]
- Adeli, H.; Khorasani, M.T.; Parvazinia, M. Wound dressing based on electrospun PVA/chitosan/starch nanofibrous mats: Fabrication, antibacterial and cytocompatibility evaluation and in vitro healing assay. Int. J. Biol. Macromol. 2019, 122, 238–254. [Google Scholar] [CrossRef]
- Poornima, B.; Korrapati, P.S. Fabrication of chitosan-polycaprolactone composite nanofibrous scaffold for simultaneous delivery of ferulic acid and resveratrol. Carbohydr. Polym. 2017, 157, 1741–1749. [Google Scholar] [CrossRef]
- Kang, Y.O.; Yoon, I.S.; Lee, S.Y.; Kim, D.D.; Lee, S.J.; Park, W.H.; Hudson, S.M. Chitosan-coated poly(vinyl alcohol) nanofibers for wound dressings. J. Biomed. Mater. Res. Part B-Appl. Biomater. 2010, 92B, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Li, W.Z.; Lv, X.X.; Lei, Z.J.; Bian, Y.Q.; Deng, H.B.; Wang, H.J.; Li, J.Q.; Li, X.Y. Biomimetic LBL structured nanofibrous matrices assembled by chitosan/collagen for promoting wound healing. Biomaterials 2015, 53, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Vigani, B.; Rossi, S.; Milanesi, G.; Bonferoni, M.C.; Sandri, G.; Bruni, G.; Ferrari, F. Electrospun alginate fibers: Mixing of two different poly(ethylene oxide) grades to improve fiber functional properties. Nanomaterials 2018, 8, 971. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Miele, D.; Faccendini, A.; Bonferoni, M.C.; Rossi, S.; Grisoli, P.; Taglietti, A.; Ruggeri, M.; Bruni, G.; Vigani, B.; et al. Chitosan/Glycosaminoglycan Scaffolds: The Role of Silver Nanoparticles to Control Microbial Infections in Wound Healing. Polymers 2019, 11, 1207. [Google Scholar] [CrossRef]
- Sandri, G.; Rossi, S.; Bonferoni, M.C.; Miele, D.; Faccendini, A.; Del Favero, E.; Di Cola, E.; Cornaglia, A.I.; Boselli, C.; Luxbacher, T.; et al. Chitosan/glycosaminoglycan scaffolds for skin reparation. Carbohydr. Polym. 2019, 220, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Faccendini, A.; Ruggeri, M.; Miele, D.; Rossi, S.; Bonferoni, M.C.; Aguzzi, C.; Grisoli, P.; Viseras, C.; Vigani, B.; Sandri, G.; et al. Norfloxacin-loaded electrospun scaffolds: Montmorillonite nanocomposite vs. free drug. Pharmaceutics 2020, 12, 325. [Google Scholar] [CrossRef]
- Vigani, B.; Valentino, C.; Sandri, G.; Listro, R.; Fagiani, F.; Collina, S.; Lanni, C.; Bonferoni, M.C.; Caramella, C.M.; Rossi, S.; et al. A composite nanosystem as a potential tool for the local treatment of glioblastoma: Chitosan-coated solid lipid nanoparticles embedded in electrospun nanofibers. Polymers 2021, 13, 1371. [Google Scholar] [CrossRef] [PubMed]
- Grijalvo, S.; Nieto-Diaz, M.; Maza, R.M.; Eritja, R.; Diaz, D.D. Alginate hydrogels as scaffolds and delivery systems to repair the damaged spinal cord. Biotechnol. J. 2019, 14, 1900275. [Google Scholar] [CrossRef] [PubMed]
- Sahana, T.G.; Rekha, P.D. Biopolymers: Applications in wound healing and skin tissue engineering. Mol. Biol. Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef] [PubMed]
- Varela, P.; Sartori, S.; Viebahn, R.; Salber, J.; Ciardelli, G. Macrophage immunomodulation: An indispensable tool to evaluate the performance of wound dressing biomaterials. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019830355. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Rossi, S.; Bonferoni, M.C.; Ferrari, F.; Sandri, G.; Riva, F.; Del Fante, C.; Perotti, C.; Caramella, C. Calcium alginate particles for the combined delivery of platelet lysate and vancomycin hydrochloride in chronic skin ulcers. Int. J. Pharm. 2014, 461, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Milanesi, G.; Bruni, G.; Ferrari, F. Coated electrospun alginate-containing fibers as novel delivery systems for regenerative purposes. Int. J. Nanomed. 2018, 13, 6531–6550. [Google Scholar] [CrossRef]
- Murakami, K.; Aoki, H.; Nakamura, S.; Takikawa, M.; Hanzawa, M.; Kishimoto, S.; Hattori, H.; Tanaka, Y.; Kiyosawa, T.; Sato, Y.; et al. Hydrogel blends of chitin/chitosan, fucoidan and alginate as healing-impaired wound dressings. Biomaterials 2010, 31, 83–90. [Google Scholar] [CrossRef]
- Roh, D.H.; Kang, S.Y.; Kim, J.Y.; Kwon, Y.B.; Kweon, H.Y.; Lee, K.G.; Park, Y.H.; Baek, R.M.; Heo, C.Y.; Choe, J.; et al. Wound healing effect of silk fibroin/alginate-blended sponge in full thickness skin defect of rat. J. Mater. Sci. Mater. Med. 2006, 17, 547–552. [Google Scholar] [CrossRef]
- Xie, H.X.; Chen, X.L.; Shen, X.R.; He, Y.; Chen, W.; Luo, Q.; Ge, W.H.; Yuan, W.H.; Tang, X.; Hou, D.Y.; et al. Preparation of chitosan-collagen-alginate composite dressing and its promoting effects on wound healing. Int. J. Biol. Macromol. 2018, 107, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Chen, S.; Zhang, B.J.; Li, M.; Diao, K.; Zhang, Z.L.; Li, J.; Xu, Y.; Wang, X.H.; Chen, H. In situ injectable nano-composite hydrogel composed of curcumin, N,O-carboxymethyl chitosan and oxidized alginate for wound healing application. Int. J. Pharm. 2012, 437, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Mori, M.; Vigani, B.; Bonferoni, M.C.; Sandri, G.; Riva, F.; Caramella, C.; Ferrari, F. A novel dressing for the combined delivery of platelet lysate and vancomycin hydrochloride to chronic skin ulcers: Hyaluronic acid particles in alginate matrices. Eur. J. Pharm. Sci. 2018, 118, 87–95. [Google Scholar] [CrossRef]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Rui, M.; Collina, S.; Fagiani, F.; Lanni, C.; Ferrari, F. Dual-functioning scaffolds for the treatment of spinal cord injury: Alginate nanofibers loaded with the Sigma 1 Receptor (S1R) Agonist RC-33 in chitosan films. Mar. Drugs 2020, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Budai-Szucs, M.; Ruggeri, M.; Faccendini, A.; Leber, A.; Rossi, S.; Varga, G.; Bonferoni, M.C.; Valyi, P.; Burian, K.; Csanyi, E.; et al. Electrospun scaffolds in periodontal wound healing. Polymers 2021, 13, 307. [Google Scholar] [CrossRef] [PubMed]
- Jahanbakhsh, A.; Nourbakhsh, M.S.; Bonakdar, S.; Shokrgozar, M.A.; Haghighipour, N. Evaluation of alginate modification effect on cell-matrix interaction, mechanotransduction and chondrogenesis of encapsulated MSCs. Cell Tissue Res. 2020, 381, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Shelton, R.M.; Perrie, Y.; Harris, J.J. An initial evaluation of gellan gum as a material for tissue engineering applications. J. Biomater. Appl. 2007, 22, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, T.; Song, J.E.; Khang, G. Biological role of gellan gum in improving scaffold drug delivery, cell adhesion properties for tissue engineering applications. Molecules 2019, 24, 4514. [Google Scholar] [CrossRef]
- Oliveira, J.T.; Martins, L.; Picciochi, R.; Malafaya, I.B.; Sousa, R.A.; Neves, N.M.; Mano, J.F.; Reis, R.L. Gellan gum: A new biomaterial for cartilage tissue engineering applications. J. Biomed. Mater. Res. Part A 2010, 93A, 852–863. [Google Scholar] [CrossRef]
- Oliveira, J.T.; Gardel, L.S.; Rada, T.; Martins, L.; Gomes, M.E.; Reis, R.L. Injectable gellan gum hydrogels with autologous cells for the treatment of rabbit articular cartilage defects. J. Orthop. Res. 2010, 28, 1193–1199. [Google Scholar] [CrossRef]
- Pereira, D.R.; Silva-Correia, J.; Caridade, S.G.; Oliveira, J.T.; Sousa, R.A.; Salgado, A.J.; Oliveira, J.M.; Mano, J.F.; Sousa, N.; Reis, R.L. Development of gellan gum-based microparticles/hydrogel matrices for application in the intervertebral disc regeneration. Tissue Eng. Part C-Methods 2011, 17, 961–972. [Google Scholar] [CrossRef]
- Pereira, D.R.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L.; Pandit, A.; Biggs, M.J. Nanocellulose reinforced gellan-gum hydrogels as potential biological substitutes for annulus fibrosus tissue regeneration. Nanomed.-Nanotechnol. Biol. Med. 2018, 14, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Van Uden, S.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Current strategies for treatment of intervertebral disc degeneration: Substitution and regeneration possibilities. Biomater. Res. 2017, 21, 22. [Google Scholar] [CrossRef] [PubMed]
- Manda, M.G.; da Silva, L.P.; Cerqueira, M.T.; Pereira, D.R.; Oliveira, M.B.; Mano, J.F.; Marques, A.P.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L. Gellan gum-hydroxyapatite composite spongy-like hydrogels for bone tissue engineering. J. Biomed. Mater. Res. Part A 2018, 106, 479–490. [Google Scholar] [CrossRef]
- Bonifacio, M.A.; Cochis, A.; Cometa, S.; Scalzone, A.; Gentile, P.; Procino, G.; Milano, S.; Scalia, A.C.; Rimondini, L.; De Giglio, E. Advances in cartilage repair: The influence of inorganic clays to improve mechanical and healing properties of antibacterial Gellan gum-Manuka honey hydrogels. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 108, 110444. [Google Scholar] [CrossRef]
- Gantar, A.; da Silva, L.P.; Oliveira, J.M.; Marques, A.P.; Correlo, V.M.; Novak, S.; Reis, R.L. Nanoparticulate bioactive-glass-reinforced gellan-gum hydrogels for bone-tissue engineering. Mater. Sci. Eng. C-Mater. Biol. Appl. 2014, 43, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Douglas, T.E.L.; Krawczyk, G.; Pamula, E.; Declercq, H.A.; Schaubroeck, D.; Bucko, M.M.; Balcaen, L.; Van Der Voort, P.; Bliznuk, V.; van den Vreken, N.M.F.; et al. Generation of composites for bone tissue-engineering applications consisting of gellan gum hydrogels mineralized with calcium and magnesium phosphate phases by enzymatic means. J. Tissue Eng. Regen. Med. 2016, 10, 938–954. [Google Scholar] [CrossRef]
- Thangavelu, M.; Kim, D.; Jeong, Y.W.; Lee, W.; Jung, J.J.; Song, J.E.; Reis, R.L.; Khang, G. Enhancing osteochondral tissue regeneration of gellan gum by incorporating Gallus gallus var Domesticus-derived demineralized bone particle. Biomimicked Biomater. Adv. Tissue Eng. Regen. Med. 2020, 1250, 79–93. [Google Scholar] [CrossRef]
- Kim, D.; Thangavelu, M.; Cheolui, S.; Kim, H.S.; Choi, M.J.; Song, J.E.; Khang, G. Effect of different concentration of demineralized bone powder with gellan gum porous scaffold for the application of bone tissue regeneration. Int. J. Biol. Macromol. 2019, 134, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Choi, J.H.; Shin, M.E.; Kim, J.W.; Kim, P.Y.; Kim, N.; Song, J.E.; Khang, G. Evaluation of cartilage regeneration of chondrocyte encapsulated gellan gum-based hyaluronic acid blended hydrogel. Int. J. Biol. Macromol. 2019, 141, 51–59. [Google Scholar] [CrossRef]
- Shin, E.Y.; Park, J.H.; Shin, M.E.; Song, J.E.; Carlomagno, C.; Khang, G. Evaluation of Chondrogenic Differentiation Ability of Bone Marrow Mesenchymal Stem Cells in Silk Fibroin/Gellan Gum Hydrogels Using miR-30. Macromol. Res. 2019, 27, 369–376. [Google Scholar] [CrossRef]
- Baek, J.S.; Carlomagno, C.; Muthukumar, T.; Kim, D.; Park, J.H.; Song, J.E.; Migliaresi, C.; Motta, A.; Reis, R.L.; Khang, G. Evaluation of Cartilage Regeneration in Gellan Gum/agar Blended Hydrogel with Improved Injectability. Macromol. Res. 2019, 27, 558–564. [Google Scholar] [CrossRef]
- Kouhi, M.; Varshosaz, J.; Hashemibeni, B.; Sarmadi, A. Injectable gellan gum/lignocellulose nanofibrils hydrogels enriched with melatonin loaded forsterite nanoparticles for cartilage tissue engineering: Fabrication, characterization and cell culture studies. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 115, 111114. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, D.; Jeong, Y.W.; Choi, M.J.; Lee, G.W.; Thangavelu, M.; Song, J.E.; Khang, G. Engineering retinal pigment epithelial cells regeneration for transplantation in regenerative medicine using PEG/Gellan gum hydrogels. Int. J. Biol. Macromol. 2019, 130, 220–228. [Google Scholar] [CrossRef]
- Silva-Correia, J.; Miranda-Goncalves, V.; Salgado, A.J.; Sousa, N.; Oliveira, J.M.; Reis, R.M.; Reis, R.L. Angiogenic potential of gellan-gum-based hydrogels for application in nucleus pulposus regeneration: In vivo study. Tissue Eng. Part A 2012, 18, 1203–1212. [Google Scholar] [CrossRef]
- Silva-Correia, J.; Gloria, A.; Oliveira, M.B.; Mano, J.F.; Oliveira, J.M.; Ambrosio, L.; Reis, R.L. Rheological and mechanical properties of acellular and cell-laden methacrylated gellan gum hydrogels. J. Biomed. Mater. Res. Part A 2013, 101, 3438–3446. [Google Scholar] [CrossRef] [PubMed]
- Tenci, M.; Rossi, S.; Giannino, V.; Vigani, B.; Sandri, G.; Bonferoni, M.C.; Daglia, M.; Longo, L.M.; Macelloni, C.; Ferrari, F. An In Situ Gelling System for the Local Treatment of Inflammatory Bowel Disease (IBD). The loading of Maqui (Aristotelia chilensis) Berry extract as an antioxidant and anti-inflammatory agent. Pharmaceutics 2019, 11, 611. [Google Scholar] [CrossRef]
- Vigani, B.; Valentino, C.; Cavalloro, V.; Catenacci, L.; Sorrenti, M.; Sandri, G.; Bonferoni, M.C.; Bozzi, C.; Collina, S.; Rossi, S.; et al. Gellan-based composite system as a potential tool for the treatment of nervous tissue injuries: Cross-linked electrospun nanofibers embedded in a RC-33-loaded freeze-dried matrix. Pharmaceutics 2021, 13, 164. [Google Scholar] [CrossRef]
- Sodhi, H.; Panitch, A. Glycosaminoglycans in tissue engineering: A review. Biomolecules 2021, 11, 29. [Google Scholar] [CrossRef]
- Trabucchi, E.; Pallotta, S.; Morini, M.; Corsi, F.; Franceschini, R.; Casiraghi, A.; Pravettoni, A.; Foschi, D.; Minghetti, P. Low molecular weight hyaluronic acid prevents oxygen free radical damage to granulation tissue during wound healing. Int. J. Tissue React.-Exp. Clin. Asp. 2002, 24, 65–71. [Google Scholar]
- Cencetti, C.; Bellini, D.; Longinotti, C.; Martinelli, A.; Matricardi, P. Preparation and characterization of a new gellan gum and sulphated hyaluronic acid hydrogel designed for epidural scar prevention. J. Mater. Sci.-Mater. Med. 2011, 22, 263–271. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Khan, S.; Minhas, M.U.; de Matas, M.; Sikstone, V.; Hussain, Z.; Abbasi, M.; Kousar, M. Biopolymer-based biomaterials for accelerated diabetic wound healing: A critical review. Int. J. Biol. Macromol. 2019, 139, 975–993. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Bonferoni, M.C.; Rossi, S.; Ferrari, F.; Mori, M.; Cervio, M.; Riva, F.; Liakos, I.; Athanassiou, A.; Saporito, F.; et al. Platelet lysate embedded scaffolds for skin regeneration. Expert Opin. Drug Deliv. 2015, 12, 525–545. [Google Scholar] [CrossRef] [PubMed]
- Saporito, F.; Sandri, G.; Rossi, S.; Bonferoni, M.C.; Riva, F.; Malavasi, L.; Caramella, C.; Ferrari, F. Freeze dried chitosan acetate dressings with glycosaminoglycans and traxenamic acid. Carbohydr. Polym. 2018, 184, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Faccendini, A.; Longo, M.; Ruggeri, M.; Rossi, S.; Bonferoni, M.C.; Miele, D.; Prina-Mello, A.; Aguzzi, C.; Viseras, C.; et al. Halloysite- and Montmorillonite-loaded scaffolds as enhancers of chronic wound healing. Pharmaceutics 2020, 12, 179. [Google Scholar] [CrossRef]
- Backes, E.H.; Fernandes, E.M.; Diogo, G.S.; Marques, C.F.; Silva, T.H.; Costa, L.C.; Passador, F.R.; Reis, R.L.; Pessan, L.A. Engineering 3D printed bioactive composite scaffolds based on the combination of aliphatic polyester and calcium phosphates for bone tissue regeneration. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 122, 111928. [Google Scholar] [CrossRef] [PubMed]
- Kesavan, A.; Rajakumar, T.; Karunanidhi, M.; Ravi, A. Synthesis and characterization of random copolymerization of aliphatic biodegradable reunite D-Mannitol. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Svyntkivska, M.; Makowski, T.; Piorkowska, E.; Brzezinski, M.; Herc, A.; Kowalewska, A. Modification of polylactide nonwovens with carbon nanotubes and ladder poly(silsesquioxane). Molecules 2021, 26, 1353. [Google Scholar] [CrossRef] [PubMed]
- Dorati, R.; Colonna, C.; Tomasi, C.; Genta, I.; Modena, T.; Conti, B. Design of 3D hybrid composite scaffolds: Effect of composition on scaffold structure and cell proliferation. Macromol. Symp. 2013, 334, 106–116. [Google Scholar] [CrossRef]
- Lee, B.K.; Yun, Y.; Park, K. PLA micro- and nano-particles. Adv. Drug Deliv. Rev. 2016, 107, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Li, T.T.; Xie, X.X.; Feng, Y.; Chen, Z.Y.; Yang, H.; Wu, C.H.; Deng, S.Q.; Liu, Y.Y. PLGA-based drug delivery systems for remotely triggered cancer therapeutic and diagnostic applications. Front. Bioeng. Biotechnol. 2020, 8, 381. [Google Scholar] [CrossRef] [PubMed]
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and safety of PLA and its copolymers. Adv. Drug Deliv. Rev. 2016, 107, 153–162. [Google Scholar] [CrossRef]
- Shao, J.; Xu, L.L.; Pu, S.Z.; Hou, H.Q. The crystallization behavior of poly(l-lactide)/poly(d-lactide) blends: Effect of stirring time during solution mixing. Polym. Bull. 2021, 78, 147–163. [Google Scholar] [CrossRef]
- Dorati, R.; Colonna, C.; Genta, I.; Modena, T.; Conti, B. Effect of porogen on the physico-chemical properties and degradation performance of PLGA scaffolds. Polym. Degrad. Stab. 2010, 95, 694–701. [Google Scholar] [CrossRef]
- Low, Y.J.; Andriyana, A.; Ang, B.C.; Zainal Abidin, N.I. Bioresorbable and degradable behaviors of PGA: Current state and future prospects. Polym. Eng. Sci. 2020, 60, 2657–2675. [Google Scholar] [CrossRef]
- Boucher, D.S. Solubility parameters and solvent affinities for polycaprolactone: A comparison of methods. J. Appl. Polym. Sci. 2020, 137, 48908. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Poly-epsilon-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef]
- Elsawy, M.A.; Kim, K.H.; Park, J.W.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Vey, E.; Roger, C.; Meehan, L.; Booth, J.; Claybourn, M.; Miller, A.F.; Saiani, A. Degradation mechanism of poly(lactic-co-glycolic) acid block copolymer cast films in phosphate buffer solution. Polym. Degrad. Stab. 2008, 93, 1869–1876. [Google Scholar] [CrossRef]
- Proikakis, C.S.; Mamouzelos, N.J.; Tarantili, P.A.; Andreopoulos, A.G. Swelling and hydrolytic degradation of poly(D,L-lactic acid) in aqueous solutions. Polym. Degrad. Stab. 2006, 91, 614–619. [Google Scholar] [CrossRef]
- Vieira, A.C.; Vieira, J.C.; Ferra, J.M.; Magalhaes, F.D.; Guedes, R.M.; Marques, A.T. Mechanical study of PLA-PCL fibers during in vitro degradation. J. Mech. Behav. Biomed. Mater. 2011, 4, 451–460. [Google Scholar] [CrossRef]
- Schliecker, G.; Schmidt, C.; Fuchs, S.; Kissel, T. Characterization of a homologous series of D,L-lactic acid oligomers; a mechanistic study on the degradation kinetics in vitro. Biomaterials 2003, 24, 3835–3844. [Google Scholar] [CrossRef]
- Siepmann, J.; Gopferich, A. Mathematical modeling of bioerodible, polymeric drug delivery systems. Adv. Drug Deliv. Rev. 2001, 48, 229–247. [Google Scholar] [CrossRef]
- Dorati, R.; Genta, I.; Colonna, C.; Modena, T.; Pavanetto, F.; Perugini, P.; Conti, B. Investigation of the degradation behaviour of poly(ethylene glycol-co-D,L-lactide) copolymer. Polym. Degrad. Stab. 2007, 92, 1660–1668. [Google Scholar] [CrossRef]
- Dorati, R.; De Trizio, A.; Marconi, S.; Ferrara, A.; Auricchio, F.; Genta, I.; Modena, T.; Benazzo, M.; Benazzo, A.; Volpato, G.; et al. Design of a bioabsorbable multilayered patch for esophagus tissue engineering. Macromol. Biosci. 2017, 17, 1600426. [Google Scholar] [CrossRef]
- Dorati, R.; Genta, I.; Montanari, L.; Cilurzo, F.; Buttafava, A.; Faucitano, A.; Conti, B. The effect of gamma-irradiation on PLGA/PEG microspheres containing ovalbumin. J. Control. Release 2005, 107, 78–90. [Google Scholar] [CrossRef]
- Dorati, R.; Colonna, C.; Serra, M.; Genta, I.; Modena, T.; Pavanetto, F.; Perugini, P.; Conti, B. Gamma-irradiation of PEGd,lPLA and PEG-PLGA multiblock copolymers: I. Effect of irradiation doses. AAPS PharmSciTech 2008, 9, 718–725. [Google Scholar] [CrossRef]
- Jain, S.; Yassin, M.A.; Fuoco, T.; Mohamed-Ahmed, S.; Vindenes, H.; Mustafa, K.; Finne-Wistrand, A. Understanding of how the properties of medical grade lactide based copolymer scaffolds influence adipose tissue regeneration: Sterilization and a systematic in vitro assessment. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 124, 112020. [Google Scholar] [CrossRef]
- Dorati, R.; Colonna, C.; Tomasi, C.; Genta, I.; Modena, T.; Faucitano, A.; Buttafava, A.; Conti, B. Gamma-irradiation of PEGd,IPLA and PEG-PLGA multiblock copolymers: II. Effect of oxygen and EPR investigation. AAPS PharmSciTech 2008, 9, 1110–1118. [Google Scholar] [CrossRef][Green Version]
- Dorati, R.; Colonna, C.; Tomasi, C.; Bruni, G.; Genta, I.; Modena, T.; Conti, B. Long-term effect of gamma irradiation on the functional properties and cytocompatibility of multiblock co-polymer films. J. Biomater. Sci.-Polym. Ed. 2012, 23, 2223–2240. [Google Scholar] [CrossRef]
- Dorati, R.; Colonna, C.; Tomasi, C.; Genta, I.; Bruni, G.; Conti, B. Design of 3D scaffolds for tissue engineering testing a tough polylactide-based graft copolymer. Mater. Sci. Eng. C-Mater. Biol. Appl. 2014, 34, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Prabhath, A.; Vernekar, V.N.; Vasu, V.; Badon, M.; Avochinou, J.E.; Asandei, A.D.; Kumbar, S.G.; Weber, E.; Laurencin, C.T. Kinetic degradation and biocompatibility evaluation of polycaprolactone-based biologics delivery matrices for regenerative engineering of the rotator cuff. J. Biomed. Mater. Res. Part A 2021. [Google Scholar] [CrossRef] [PubMed]
- Dorati, R.; Colonna, C.; Genta, I.; Bruni, G.; Visai, L.; Conti, B. Preparation and characterization of an advanced medical device for bone regeneration. AAPS PharmSciTech 2014, 15, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Verardi, S.; Lombardi, T.; Stacchi, C. Clinical and radiographic evaluation of nanohydroxyapatite powder in combination with polylactic acid/polyglycolic acid copolymer as bone replacement graft in the surgical treatment of intrabony periodontal defects: A retrospective case series study. Materials 2020, 13, 269. [Google Scholar] [CrossRef]
- Lopresti, F.; Pavia, F.C.; Ceraulo, M.; Capuana, E.; Brucato, V.; Ghersi, G.; Botta, L.; La Carrubba, V. Physical and biological properties of electrospun poly(d,l-lactide)/nanoclay and poly(d,l-lactide)/nanosilica nanofibrous scaffold for bone tissue engineering. J. Biomed. Mater. Res. Part A 2021, 4. [Google Scholar] [CrossRef]
- De Santis, R.; Russo, T.; Rau, J.V.; Papallo, I.; Martorelli, M.; Gloria, A. Design of 3D additively manufactured hybrid structures for cranioplasty. Materials 2021, 14, 181. [Google Scholar] [CrossRef]
- Dorati, R.; Pisani, S.; Maffeis, G.; Conti, B.; Modena, T.; Chiesa, E.; Bruni, G.; Musazzi, U.M.; Genta, I. Study on hydrophilicity and degradability of chitosan/polylactide-co-polycaprolactone nanofibre blend electrospun membrane. Carbohydr. Polym. 2018, 199, 150–160. [Google Scholar] [CrossRef]
- Pisani, S.; Croce, S.; Chiesa, E.; Dorati, R.; Lenta, E.; Genta, I.; Bruni, G.; Mauramati, S.; Benazzo, A.; Cobianchi, L.; et al. Tissue engineered esophageal patch by mesenchymal stromal cells: Optimization of electrospun patch engineering. Int. J. Mol. Sci. 2020, 21, 1764. [Google Scholar] [CrossRef]
- Pisani, S.; Genta, I.; Dorati, R.; Kavatzikidou, P.; Angelaki, D.; Manousaki, A.; Karali, K.; Ranella, A.; Stratakis, E.; Conti, B. Biocompatible polymeric electrospun matrices: Micro-nanotopography effect on cell behavior. J. Appl. Polym. Sci. 2020, 137, 49223. [Google Scholar] [CrossRef]
- Dorati, R.; Chiesa, E.; Pisani, S.; Genta, I.; Modena, T.; Bruni, G.; Brambilla, C.R.M.; Benazzo, M.; Conti, B. The effect of process parameters on alignment of tubular electrospun nanofibers for tissue regeneration purposes. J. Drug Deliv. Sci. Technol. 2020, 58, 101781. [Google Scholar] [CrossRef]
- Pisani, S.; Dorati, R.; Genta, I.; Benazzo, M.; Conti, B.; Prina-Mello, A. A study focused on macrophages modulation induced by the Polymeric Electrospun Matrices (EL-Ms) for application in tissue regeneration: In vitro proof of concept. Int. J. Pharm. 2021, 603, 120712. [Google Scholar] [CrossRef]
- Chiesa, E.; Dorati, R.; Pisani, S.; Bruni, G.; Rizzi, L.G.; Conti, B.; Modena, T.; Genta, I. Graphene nanoplatelets for the development of reinforced PLA-PCL electrospun fibers as the next-generation of biomedical mats. Polymers 2020, 12, 1390. [Google Scholar] [CrossRef] [PubMed]
- Theryo, G.; Jing, F.; Pitet, L.M.; Hillmyer, M.A. Tough polylactide graft copolymers. Macromolecules 2010, 43, 7394–7397. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Mohanty, A.K. Modification of brittle polylactide by novel hyperbranched polymer-based nanostructures. Biomacromolecules 2007, 8, 2476–2484. [Google Scholar] [CrossRef]


| Biomaterial | Scaffold Type | Biocompatibility | Biodegradability | Sterilizability | Mechanical Properties | Porosity-Pore Size |
|---|---|---|---|---|---|---|
| Silk fibroin | Sponges | Good | Months-Years [14] | Autoclave/Irradiation | 5–100 kPa [15] | 100–1000 µm [16] |
| Hydrogels | Good | Days-Months [14] | Filtration 0.22 µm/Irradiation | 20–90 MPa [17] | 10–350 µm [18,19,20] | |
| Films | Good | Days-Months [14] | Filtration 0.22 µm/Irradiation | 10–100 MPa [21] | Not reported | |
| Mats and fibers | Good | Months-Years [14] | Autoclave/Irradiation | 2–18 MPa [22,23] | 0.5–12 µm [23,24] | |
| 3D Printed scaffold | Good | Not reported | Filtration 0.22 µm/Irradiation | Modulable [25] | Modulable [25] | |
| Silk sericin | Sponges | Good | Hours-days [26] | Filtration 0.22 µm/Irradiation | 0.2–1 kPa [27] | Not reported |
| Hydrogels | Good | Hours-days [28] | Filtration 0.22 µm/Irradiation | 0.6–6 kPa [27] | 20–300 µm [28] | |
| Films | Good | Hours-days [29] | Filtration 0.22 µm/Irradiation | 10–40 MPa [30] | Not reported |
| Scaffold Types | Biocompatibility | Biodegradability | Sterilizability | Mechanical Properties (E Modulus) | Porosity |
|---|---|---|---|---|---|
| Hydrogels [80,81,89,91] Fibers [90,97,99,100,106] Films [83,92] Hybrids [82,93,94,95,96,103,107] Sponges [79] | Good as component of ECM matrix [75,80] Possible concerns of immunogenic effects [79,80] | Hours/days if not crosslinked [97,99] Modulable up to 6–24 months after crosslinking [80,84,85,87,89] | Gamma irradiation or ethylene oxide [80,108] | Generally poor without crosslinking For hydrogels, up to kPa. For fibers and films up to MPa after chemical crosslinking [80,84,85] Modulable by grafting/mixing with polymers [97,99,104] | Tuning by cross linking [85] and by nanofiber engineering [86] |
| Biomaterial | Scaffold Type | Biocompatibility | Biodegradability | Sterilizability | Mechanical Properties (Young’s Modulus) | Porosity-Pore Size |
|---|---|---|---|---|---|---|
| Polylactic acid | Fibers (nano and micro) | Good [204] Approved by FDA and EMA for human use in injectable drug products | Months, depending on its Mw [210,214] | Ionizing radiations (gamma and beta), Ethylene oxide (ETO) [216,219,220] | 3–5 kPa [217] | 1–100 µm [217] |
| Films | ||||||
| 3D scaffolds | 2–4 MPa [206] | 10–900 µm [223] | ||||
| Polyglycolide (due to its inherent hydrolitic instability it is used in blends or copolymerized with PLA) | Fibers (nano and micro) | Good [204] Approved by FDA and EMA for human use in injectable drug products | Weeks–months depending on Mw [207] | Can be tuned depending on its blending or copolymerization with PLA | 1–100 µm [217] | |
| Films | 1–100 µm [217] | |||||
| 3D scaffolds | 10–900 µm [223] | |||||
| Poli(ε-caprolactone) (Mostly used as PLA-PCL copolymer or in blend with PLA) | Fibers (nano and micro) | Good Approved by FDA and EMA for human use in injectable drug products | Months–Years depending on Mw and on derivative [213,224] | 3–5 kPa [217] Can be tuned depending on its blending or copolymerization with PLA | 1–100 µm [217] | |
| Films |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonferoni, M.C.; Caramella, C.; Catenacci, L.; Conti, B.; Dorati, R.; Ferrari, F.; Genta, I.; Modena, T.; Perteghella, S.; Rossi, S.; et al. Biomaterials for Soft Tissue Repair and Regeneration: A Focus on Italian Research in the Field. Pharmaceutics 2021, 13, 1341. https://doi.org/10.3390/pharmaceutics13091341
Bonferoni MC, Caramella C, Catenacci L, Conti B, Dorati R, Ferrari F, Genta I, Modena T, Perteghella S, Rossi S, et al. Biomaterials for Soft Tissue Repair and Regeneration: A Focus on Italian Research in the Field. Pharmaceutics. 2021; 13(9):1341. https://doi.org/10.3390/pharmaceutics13091341
Chicago/Turabian StyleBonferoni, Maria Cristina, Carla Caramella, Laura Catenacci, Bice Conti, Rossella Dorati, Franca Ferrari, Ida Genta, Tiziana Modena, Sara Perteghella, Silvia Rossi, and et al. 2021. "Biomaterials for Soft Tissue Repair and Regeneration: A Focus on Italian Research in the Field" Pharmaceutics 13, no. 9: 1341. https://doi.org/10.3390/pharmaceutics13091341
APA StyleBonferoni, M. C., Caramella, C., Catenacci, L., Conti, B., Dorati, R., Ferrari, F., Genta, I., Modena, T., Perteghella, S., Rossi, S., Sandri, G., Sorrenti, M., Torre, M. L., & Tripodo, G. (2021). Biomaterials for Soft Tissue Repair and Regeneration: A Focus on Italian Research in the Field. Pharmaceutics, 13(9), 1341. https://doi.org/10.3390/pharmaceutics13091341