Zoledronic Acid as a Novel Dual Blocker of KIR6.1/2-SUR2 Subunits of ATP-Sensitive K+ Channels: Role in the Adverse Drug Reactions
Abstract
:1. Introduction
Significant Statement
2. Materials and Methods
2.1. Tissues and Primary Cell Culture
2.2. Drugs and Solutions
2.3. Patch-Clamp Experiments
2.4. Polymerase Chain Reaction
2.5. Molecular Modeling Analysis
2.6. Pharmacovigilance Analysis
2.7. Statistical Analysis
3. Results
3.1. Zoledronic Acid Inhibits KATP Channel Currents in Native Murine Skeletal Muscle, Bone Cells, and Recombinant Subunits Expressed in Cell Lines
3.1.1. Docking Analysis
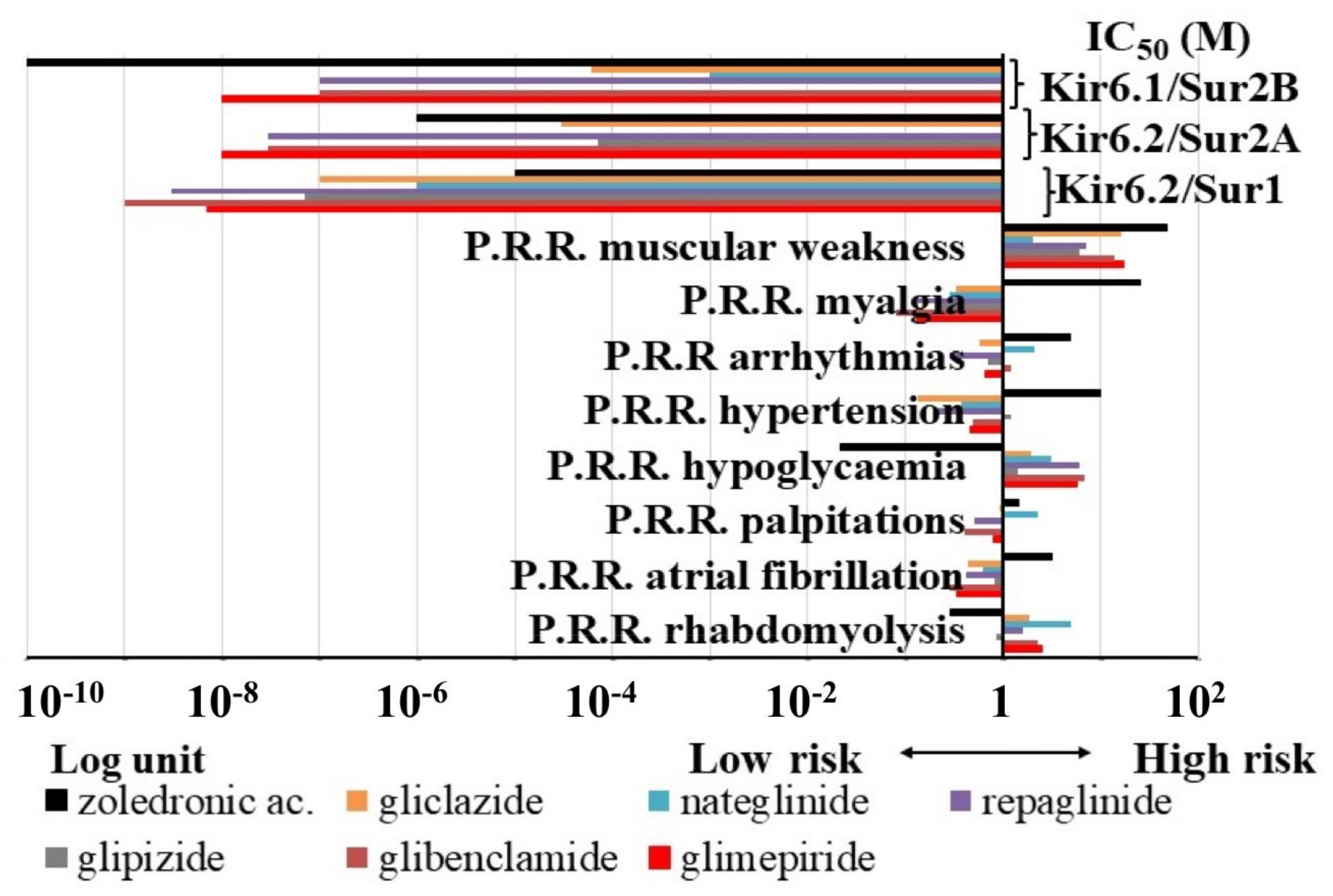
3.1.2. Pharmacovigilance Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogers, M.J.; Gordon, S.; Benford, H.L.; Coxon, F.P.; Luckman, S.P.; Monkkonen, J.; Frith, J.C. Cellular and molecular mechanisms of action of bisphosphonates. Cancer 2000, 88 (Suppl. S12), 2961–2978. [Google Scholar] [CrossRef]
- Green, J.R. Bisphosphonates: Preclinical Review. Oncologist 2004, 9 (Suppl. 4), 3–13. [Google Scholar] [CrossRef]
- Russell, R.G.G. Bisphosphonates: Mode of Action and Pharmacology. Pediatrics 2007, 119, S150–S162. [Google Scholar] [CrossRef] [Green Version]
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of Action and Role in Clinical Practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dozier, J.K.; Distefano, M.D. An enzyme-coupled continuous fluorescence assay for farnesyl diphosphate synthases. Anal. Biochem. 2012, 421, 158–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savino, S.; Toscano, A.; Purgatorio, R.; Profilo, E.; Laghezza, A.; Tortorella, P.; Angelelli, M.; Cellamare, S.; Scala, R.; Tricarico, D.; et al. Novel bisphosphonates with antiresorptive effect in bone mineralization and osteoclastogenesis. Eur. J. Med. Chem. 2018, 158, 184–200. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.; Gleason, D.M.; Murray, R.; Tchekmedyian, S.; Venner, P.; Lacombe, L.; Chin, J.L.; Vinholes, J.J.; Goas, J.A.; Zheng, M. Long-Term Efficacy of Zoledronic Acid for the Prevention of Skeletal Complications in Patients With Metastatic Hormone-Refractory Prostate Cancer. J. Natl. Cancer Inst. 2004, 96, 879–882. [Google Scholar] [CrossRef] [Green Version]
- Mhaskar, R.; Kumar, A.; Miladinovic, B.; Djulbegovic, B. Bisphosphonates in multiple myeloma: An updated network meta-analysis. Cochrane Database Syst. Rev. 2017, 2017, CD003188. [Google Scholar] [CrossRef]
- Alegre, A.; Gironella, M.; Bailén, A.; Giraldo, P. Zoledronic acid in the management of bone disease as a consequence of multiple myeloma: A review. Eur. J. Haematol. 2014, 92, 181–188. [Google Scholar] [CrossRef]
- Kwek, E.B.K.; Koh, J.S.B.; Howe, T.S. More on Atypical Fractures of the Femoral Diaphysis. N. Engl. J. Med. 2008, 359, 316–318. [Google Scholar] [CrossRef]
- Kennel, K.A.; Drake, M.T. Adverse Effects of Bisphosphonates: Implications for Osteoporosis Management. Mayo Clin. Proc. 2009, 84, 632–638. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.-L.; Gou, W.-L.; Wang, A.-Y.; Wang, Y.; Guo, Q.-Y.; Lu, Q.; Lu, S.-B.; Peng, J. Basic research and clinical applications of bisphosphonates in bone disease: What have we learned over the last 40 years? J. Transl. Med. 2013, 11, 303. [Google Scholar] [CrossRef] [Green Version]
- Jara, M.A.; Varghese, J.; Hu, M.I. Adverse events associated with bone-directed therapies in patients with cancer. Bone 2021, 115901. [Google Scholar] [CrossRef] [PubMed]
- Moreland-Head, L.N.; Coons, J.C.; Seybert, A.L.; Gray, M.P.; Kane-Gill, S.L. Use of Disproportionality Analysis to Identify Previously Unknown Drug-Associated Causes of Cardiac Arrhythmias Using the Food and Drug Administration Adverse Event Reporting System (FAERS) Database. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Scala, R.; Maqoud, F.; Angelelli, M.; Latorre, R.; Perrone, M.G.; Scilimati, A.; Tricarico, D. Zoledronic Acid Modulation of TRPV1 Channel Currents in Osteoblast Cell Line and Native Rat and Mouse Bone Marrow-Derived Osteoblasts: Cell Proliferation and Mineralization Effect. Cancers 2019, 11, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Wu, J.-X.; Ding, D.; Cheng, J.; Gao, N.; Chen, L. Structure of a Pancreatic ATP-Sensitive Potassium Channel. Cell 2017, 168, 101–110.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.-X.; Ding, D.; Wang, M.; Kang, Y.; Zeng, X.; Chen, L. Ligand binding and conformational changes of SUR1 subunit in pancreatic ATP-sensitive potassium channels. Protein Cell 2018, 9, 553–567. [Google Scholar] [CrossRef] [Green Version]
- Tricarico, D.; Servidei, S.; Tonali, P.; Jurkat-Rott, K.; Camerino, D.C. Impairment of skeletal muscle adenosine triphosphate–sensitive K+ channels in patients with hypokalemic periodic paralysis. J. Clin. Investig. 1999, 103, 675–682. [Google Scholar] [CrossRef]
- Tricarico, D.; Mele, A.; Camerino, G.M.; Bottinelli, R.; Brocca, L.; Frigeri, A.; Svelto, M.R.; George, A.L., Jr.; Camerino, D.C. The KATPchannel is a molecular sensor of atrophy in skeletal muscle. J. Physiol. 2010, 588 Pt 5, 773–784. [Google Scholar] [CrossRef]
- Ashcroft, F. KATP channels and insulin secretion: A key role in health and disease. Biochem. Soc. Trans. 2006, 34, 243–246. [Google Scholar] [CrossRef]
- Olson, T.M.; Terzic, A. Human KATP channelopathies: Diseases of metabolic homeostasis. Pflügers Arch. Eur. J. Physiol. 2009, 460, 295–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masi, A.; Narducci, R.; Mannaioni, G. Harnessing ionic mechanisms to achieve disease modification in neurodegenerative disorders. Pharmacol. Res. 2019, 147, 104343. [Google Scholar] [CrossRef]
- Fisher, C.; Johnson, K.; Okerman, T.; Jurgenson, T.; Nickell, A.; Salo, E.; Moore, M.; Doucette, A.; Bjork, J.; Klein, A.H. Morphine Efficacy, Tolerance, and Hypersensitivity Are Altered After Modulation of SUR1 Subtype KATP Channel Activity in Mice. Front. Neurosci. 2019, 13, 1122. [Google Scholar] [CrossRef]
- Luu, W.; Bjork, J.; Salo, E.; Entenmann, N.; Jurgenson, T.; Fisher, C.; Klein, A.H. Modulation of SUR1 KATP Channel Subunit Activity in the Peripheral Nervous System Reduces Mechanical Hyperalgesia after Nerve Injury in Mice. Int. J. Mol. Sci. 2019, 20, 2251. [Google Scholar] [CrossRef] [Green Version]
- Maqoud, F.; Scala, R.; Hoxha, M.; Zappacosta, B.; Tricarico, D. ATP-sensitive potassium channel subunits in the neuroinflammation: Novel drug targets in neurodegenerative disorders. CNS Neurol. Disord. Drug Targets 2021, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Nichols, C.G.; Singh, G.K.; Grange, R.K. KATP Channels and Cardiovascular Disease: Suddenly a syndrome. Circ. Res. 2013, 112, 1059–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tricarico, D.; Mele, A.; Camerino, D.C. Carbonic anhydrase inhibitors ameliorate the symptoms of hypokalaemic periodic paralysis in rats by opening the muscular Ca2+-activated-K+channels. Neuromuscul. Disord. 2006, 16, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Scala, R.; Maqoud, F.; Zizzo, N.; Mele, A.; Camerino, G.M.; Zito, F.A.; Ranieri, G.; McClenaghan, C.; Harter, T.M.; Nichols, C.G.; et al. Pathophysiological Consequences of KATP Channel Overactivity and Pharmacological Response to Glibenclamide in Skeletal Muscle of a Murine Model of Cantù Syndrome. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Bakker, A.D.; Klein-Nulend, J. Osteoblast Isolation from Murine Calvaria and Long Bones. Methods Mol. Biol. 2011, 816, 19–29. [Google Scholar] [CrossRef]
- Cooper, P.E.; McClenaghan, C.; Chen, X.; Weinzinger, A.; Nichols, C.G. Conserved functional consequences of disease-associated mutations in the slide helix of Kir6.1 and Kir6.2 subunits of the ATP-sensitive potassium channel. J. Biol. Chem. 2017, 292, 17387–17398. [Google Scholar] [CrossRef] [Green Version]
- Tricarico, D.; Mele, A.; Lundquist, A.L.; Desai, R.R.; George, A.L.; Camerino, D.C. Hybrid assemblies of ATP-sensitive K+ channels determine their muscle-type-dependent biophysical and pharmacological properties. Proc. Natl. Acad. Sci. USA 2006, 103, 1118–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mele, A.; Camerino, G.M.; Calzolaro, S.; Cannone, M.; Conte, D.; Tricarico, D. Dual response of the KATP channels to staurosporine: A novel role of SUR2B, SUR1 and Kir6.2 subunits in the regulation of the atrophy in different skeletal muscle phenotypes. Biochem. Pharmacol. 2014, 91, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Eswar, N.; Webb, B.; Marti-Renom, M.A.; Madhusudhan, M.; Eramian, D.; Shen, M.; Pieper, U.; Sali, A. Comparative Protein Structure Modeling Using Modeller. Curr. Protoc. Bioinform. 2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persson, B. Bioinformatics in protein analysis. EXS 2000, 88, 215–231. [Google Scholar] [CrossRef]
- Bossis, F.; De Grassi, A.; Palese, L.L.; Pierri, C.L. Prediction of high- and low-affinity quinol-analogue-binding sites in the aa3 and bo3 terminal oxidases from Bacillus subtilis and Escherichia coli1. Biochem. J. 2014, 461, 305–314. [Google Scholar] [CrossRef]
- Pierri, C.L.; Parisi, G.; Porcelli, V. Computational approaches for protein function prediction: A combined strategy from multiple sequence alignment to molecular docking-based virtual screening. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2010, 1804, 1695–1712. [Google Scholar] [CrossRef]
- Pierri, C.L.; Bossis, F.; Punzi, G.; De Grassi, A.; Cetrone, M.; Parisi, G.; Tricarico, D. Molecular modeling of antibodies for the treatment of TNFα-related immunological diseases. Pharmacol. Res. Perspect. 2016, 4, e00197. [Google Scholar] [CrossRef]
- Trisolini, L.; Gambacorta, N.; Gorgoglione, R.; Montaruli, M.; Laera, L.; Colella, F.; Volpicella, M.; De Grassi, A.; Pierri, C.L. FAD/NADH Dependent Oxidoreductases: From Different Amino Acid Sequences to Similar Protein Shapes for Playing an Ancient Function. J. Clin. Med. 2019, 8, 2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alford, R.F.; Leaver-Fay, A.; Jeliazkov, J.R.; O’Meara, M.J.; DiMaio, F.P.; Park, H.; Shapovalov, M.V.; Renfrew, P.D.; Mulligan, V.K.; Kappel, K.; et al. The Rosetta All-Atom Energy Function for Macromolecular Modeling and Design. J. Chem. Theory Comput. 2017, 13, 3031–3048. [Google Scholar] [CrossRef] [PubMed]
- Conway, P.; Tyka, M.D.; DiMaio, F.; Konerding, D.E.; Baker, D. Relaxation of backbone bond geometry improves protein energy landscape modeling. Protein Sci. 2013, 23, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, I.; Tragni, V.; Busto, F.; De Grassi, A.; Pierri, C.L. Protein structure analysis of the interactions between SARS-CoV-2 spike protein and the human ACE2 receptor: From conformational changes to novel neutralizing antibodies. Cell. Mol. Life Sci. 2020, 78, 1501–1522. [Google Scholar] [CrossRef]
- Vriend, G. WHAT IF: A molecular modeling and drug design program. J. Mol. Graph. 1990, 8, 52–56. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- The {PyMOL} Molecular Graphics System, Version~1.8; Schrödinger, L.L.C.: New York, NY, USA, 2015.
- Guex, N.; Peitsch, M. SWISS-MODEL and the Swiss-Pdb Viewer: An environment for comparative protein modeling. Electrophoresis 1997, 18, 2714–2723. [Google Scholar] [CrossRef] [PubMed]
- Forli, S.; Huey, R.; Pique, M.E.; Sanner, M.F.; Goodsell, D.S.; Olson, A.J. Computational protein–ligand docking and virtual drug screening with the AutoDock suite. Nat. Protoc. 2016, 11, 905–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mele, A.; Calzolaro, S.; Cannone, G.; Cetrone, M.; Conte, D.; Tricarico, D. Database search of spontaneous reports and pharmacological investigations on the sulfonylureas and glinides-induced atrophy in skeletal muscle. Pharmacol. Res. Perspect. 2014, 2, e00028. [Google Scholar] [CrossRef]
- Abdelmoneim, A.S.; Hasenbank, S.E.; Seubert, J.M.; Brocks, D.R.; Light, P.E.; Simpson, S.H. Variations in tissue selectivity amongst insulin secretagogues: A systematic review. Diabetes Obes. Metab. 2011, 14, 130–138. [Google Scholar] [CrossRef]
- Russell, R.G.G.; Watts, N.B.; Ebetino, F.H.; Rogers, M. Mechanisms of action of bisphosphonates: Similarities and differences and their potential influence on clinical efficacy. Osteoporos. Int. 2008, 19, 733–759. [Google Scholar] [CrossRef]
- Clézardin, P. Therapeutic targets for bone metastases in breast cancer. Breast Cancer Res. 2011, 13, 207. [Google Scholar] [CrossRef] [Green Version]
- Malwal, S.R.; O’Dowd, B.; Feng, X.; Turhanen, P.A.; Shin, C.J.; Yao, J.; Kim, B.K.; Baig, N.; Zhou, T.; Bansal, S.; et al. Bisphosphonate-Generated ATP-Analogs Inhibit Cell Signaling Pathways. J. Am. Chem. Soc. 2018, 140, 7568–7578. [Google Scholar] [CrossRef]
- Todisco, S.; Di Noia, M.A.; Onofrio, A.; Parisi, G.; Punzi, G.; Redavid, G.; De Grassi, A.; Pierri, C.L. Identification of new highly selective inhibitors of the human ADP/ATP carriers by molecular docking and in vitro transport assays. Biochem. Pharmacol. 2015, 100, 112–132. [Google Scholar] [CrossRef] [PubMed]
- Trisolini, L.; Laera, L.; Favia, M.; Muscella, A.; Castegna, A.; Pesce, V.; Guerra, L.; De Grassi, A.; Volpicella, M.; Pierri, C.L. Differential Expression of ADP/ATP Carriers as a Biomarker of Metabolic Remodeling and Survival in Kidney Cancers. Biomolecules 2020, 11, 38. [Google Scholar] [CrossRef] [PubMed]

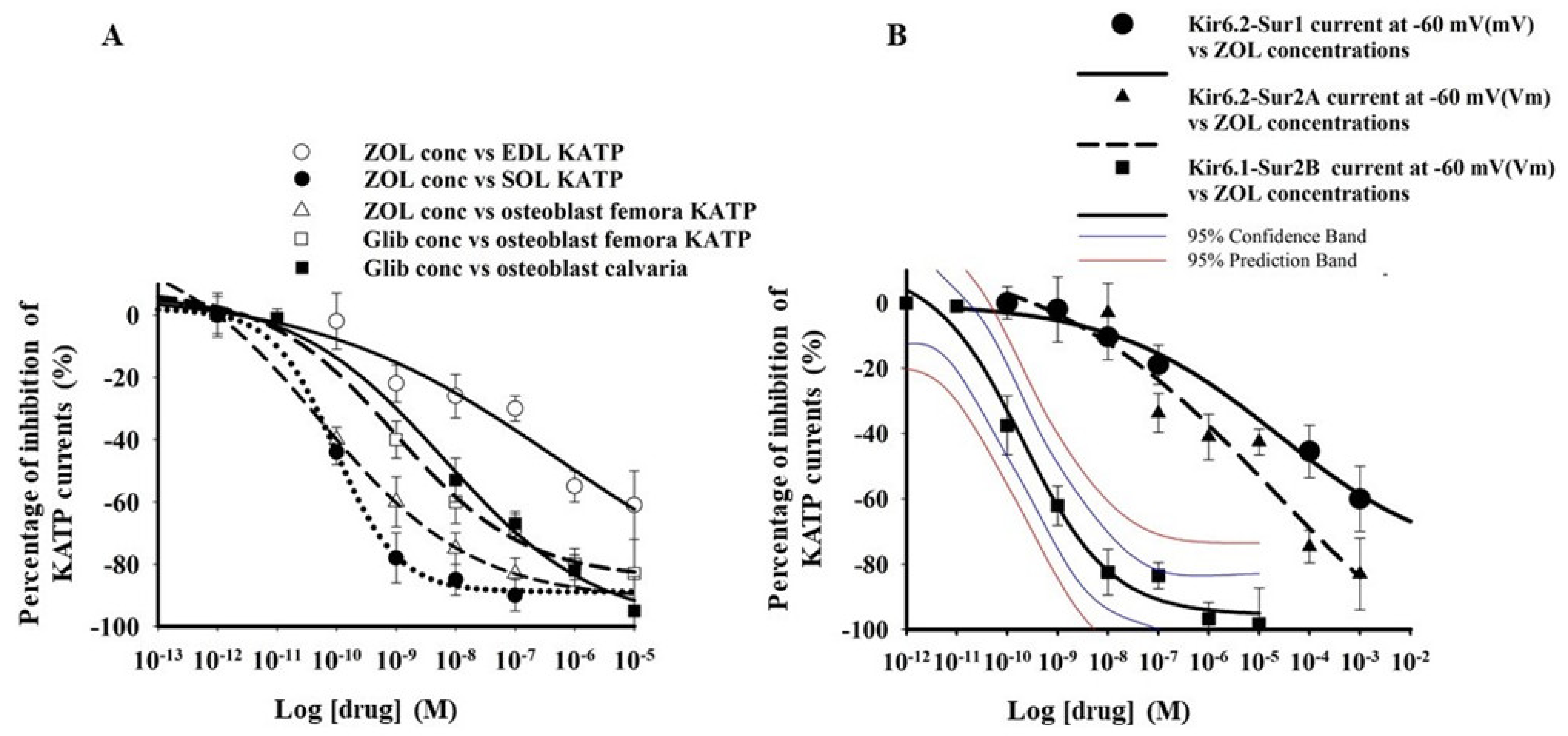

| Drugs Cell Types Patch Clamp Configuration | Imax % | IC50 (M) | Slope |
|---|---|---|---|
| Zoledronic acid EDL fibers excised macropatches | −100.1 ± 9.3 n = 17 fibers | 1.2 ± 1.4 ×10−6 | 0.38 ± 0.07 |
| Zoledronic acid SOL fibers excised macropatches | −99.4 ± 5 n = 21 fibers | * 2.1 ± 3.7 × 10−10 | 0.29 ± 0.05 |
| Zoledronic acid Femora primary bone cells Cell-attached patches | −104.8 ± 8.7 n = 21 cells | * 1.6 ± 2.8 × 10−10 | 0.31 ± 0.07 |
| Glibenclamide Femora primary bone cells cell-attached patches | −88.32 ± 21 n = 13 cells | * 5.07 ± 0.5 × 10−9 | 0.4 ± 0.08 |
| Glibenclamide Calvaria primary bone cells cell-attached patches | −93 ± 14 n = 18 cells | * 1.02 ± 0.6 × 10−8 | 0.5 ± 0.03 |
| Zoledronic acid Hek293 cells- KIR6.2-SUR2A Whole-cell | −83.7 ± 1.2 n = 15 cells | 7.1 ± 3.1 × 10−6 | * 0.12 ± 0.7 |
| Zoledronic acid Hek293 cells -KIR6.1-SUR2B Whole-cells | −94 ± 4.8 n = 15 cells | * 3.9 ± 2.7 × 10−10 | 0.59 ± 0.04 |
| Zoledronic acid Hek293 cells -KIR6.1-SUR1 Whole-cells | * −40 ± 8.8 n = 3 cells | / | / |
| Zoledronic acid Hek293 cells -KIR6.2-SUR1 Whole-cells | * −60.13 ± 4.1 n = 15 cells | 2.1 ± 4.1 × 10−5 | * 0.18 ± 0.09 |
| Ligand Binding Affinity (G (Kcal/mol)) | ||||
|---|---|---|---|---|
| KIR ATP Binding Region | SUR ATP Binding Region | SUR Glib Binding Region | ||
| HsKIR6.1SUR2B | ATP | −5.9 | −6.3 | ND |
| Zoledronic acid lowest rmsd pose | −6.3 | −5.3 | −6.5 | |
| Zoledronic lowest binding energy pose | −6.6 | −5.3 | −6.4 | |
| ATP_isopentenyl ester | −6.6 | V6.3 | ND | |
| Glib | ND | ND | −9.1 | |
| HsKIR6.1SUR1 | ATP | −5.9 | −6.3 | ND |
| Zoledronic acid lowest rmsd pose | −4.3 | −5.3 | −4.4 | |
| Zoledronic lowest binding energy pose | −4.3 | −5.3 | −4.4 | |
| ATP_isopentenyl ester | −6.6 | −6.4 | ND | |
| Glib | ND | ND | −9.1 | |
| HsKIR6.2SUR2A | ATP | −6.1 | −6.3 | ND |
| Zoledronic acid lowest RMSD pose | −5.4 | −5.3 | −6.1 | |
| Zoledronic lowest binding energy pose | −5.4 | −5.6 | −5.9 | |
| ATP_isopentenyl ester | −6.2 | −6.3 | ND | |
| Glib | ND | ND | −9.2 | |
| HsKIR6.2SUR1 | ATP | −6 | −6.3 | ND |
| Zoledronic acid lowest RMSD pose | −5.4 | −5.3 | −4.2 | |
| Zoledronic lowest binding energy pose | −4.8 | −5.4 | −4.4 | |
| ATP_isopentenyl ester | −6.2 | −6.4 | ND | |
| Glib | ND | ND | −9.9 | |
| P.R.R. of KATP Channel Blockers | IC50 (M) of KATP Channel Blockers | Equations and Coefficient of Correlation |
|---|---|---|
| hypoglycaemia | KIR6.2-SUR1 | y = aln(x) + b a = −0.708 b = −8.2542 R2 = 0.7849 |
| muscular weakness rhabdomyolysis | KIR6.2-SUR2A | y = b0 + blnx1 + b2x2 b1 = −9.114 × 10−6 b2 = 3.505 × 10−5 b0 = −3.493 × 10−5 R2 = 0.83249 |
| atrial fibrillation arrhythmias | KIR6.2-SUR2A KIR6.1-SUR2B | b1 = 4.0144 × 10−5 b2 = −2.708 × 10−5 b0 = 2.213 × 10−5 R2 = 0.602 b1 = −6.878 × 10−4 b2 = 4.342 × 10−4 b0 = 9.518 × 10−5 R2 = 0.699 |
| atrial fibrillation hypertension | KIR6.1−SUR2B | b1 = 1.6465 × 10−3 b2 = −4.862 × 10−4 b0 = −3.851 × 10−4 R2 = 0.6177 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maqoud, F.; Scala, R.; Tragni, V.; Pierri, C.L.; Perrone, M.G.; Scilimati, A.; Tricarico, D. Zoledronic Acid as a Novel Dual Blocker of KIR6.1/2-SUR2 Subunits of ATP-Sensitive K+ Channels: Role in the Adverse Drug Reactions. Pharmaceutics 2021, 13, 1350. https://doi.org/10.3390/pharmaceutics13091350
Maqoud F, Scala R, Tragni V, Pierri CL, Perrone MG, Scilimati A, Tricarico D. Zoledronic Acid as a Novel Dual Blocker of KIR6.1/2-SUR2 Subunits of ATP-Sensitive K+ Channels: Role in the Adverse Drug Reactions. Pharmaceutics. 2021; 13(9):1350. https://doi.org/10.3390/pharmaceutics13091350
Chicago/Turabian StyleMaqoud, Fatima, Rosa Scala, Vincenzo Tragni, Ciro Leonardo Pierri, Maria Grazia Perrone, Antonio Scilimati, and Domenico Tricarico. 2021. "Zoledronic Acid as a Novel Dual Blocker of KIR6.1/2-SUR2 Subunits of ATP-Sensitive K+ Channels: Role in the Adverse Drug Reactions" Pharmaceutics 13, no. 9: 1350. https://doi.org/10.3390/pharmaceutics13091350
APA StyleMaqoud, F., Scala, R., Tragni, V., Pierri, C. L., Perrone, M. G., Scilimati, A., & Tricarico, D. (2021). Zoledronic Acid as a Novel Dual Blocker of KIR6.1/2-SUR2 Subunits of ATP-Sensitive K+ Channels: Role in the Adverse Drug Reactions. Pharmaceutics, 13(9), 1350. https://doi.org/10.3390/pharmaceutics13091350









