Whey Proteins–Zinc Oxide Bionanocomposite as Antibacterial Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Nanostructured ZnO
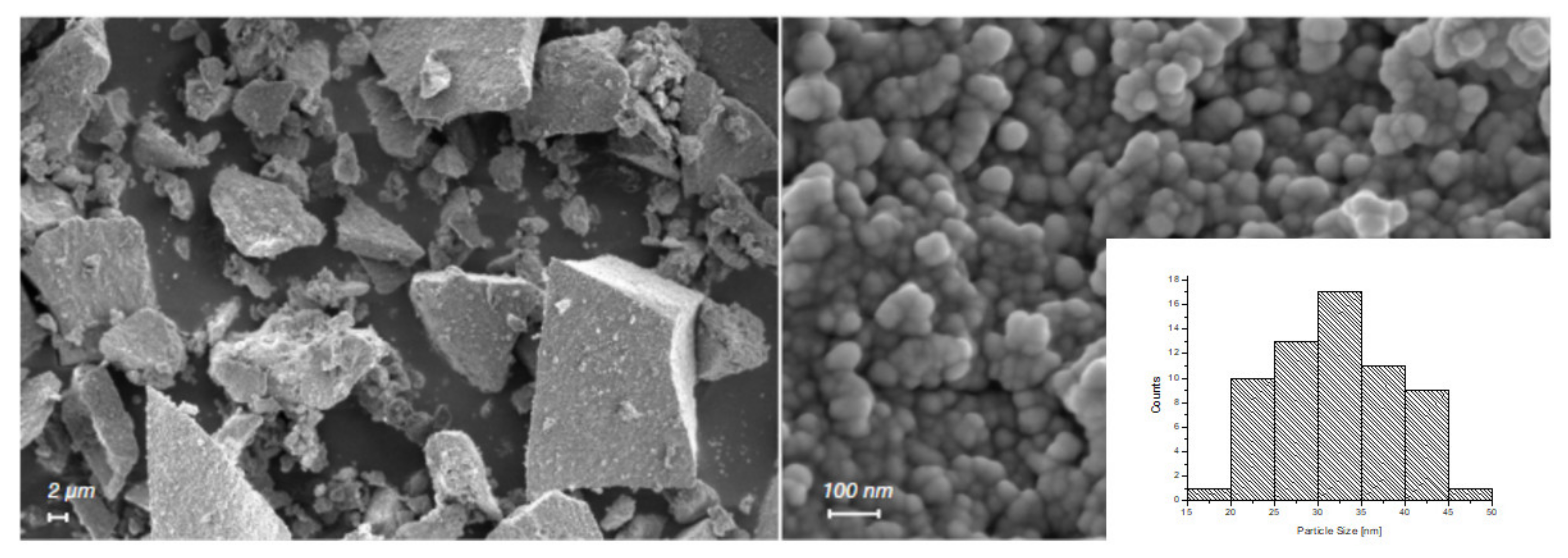
2.3. Characterization of ZnO Particles
2.4. Preparation of Whey Protein Films
2.5. Preparation of WP-ZnO Films
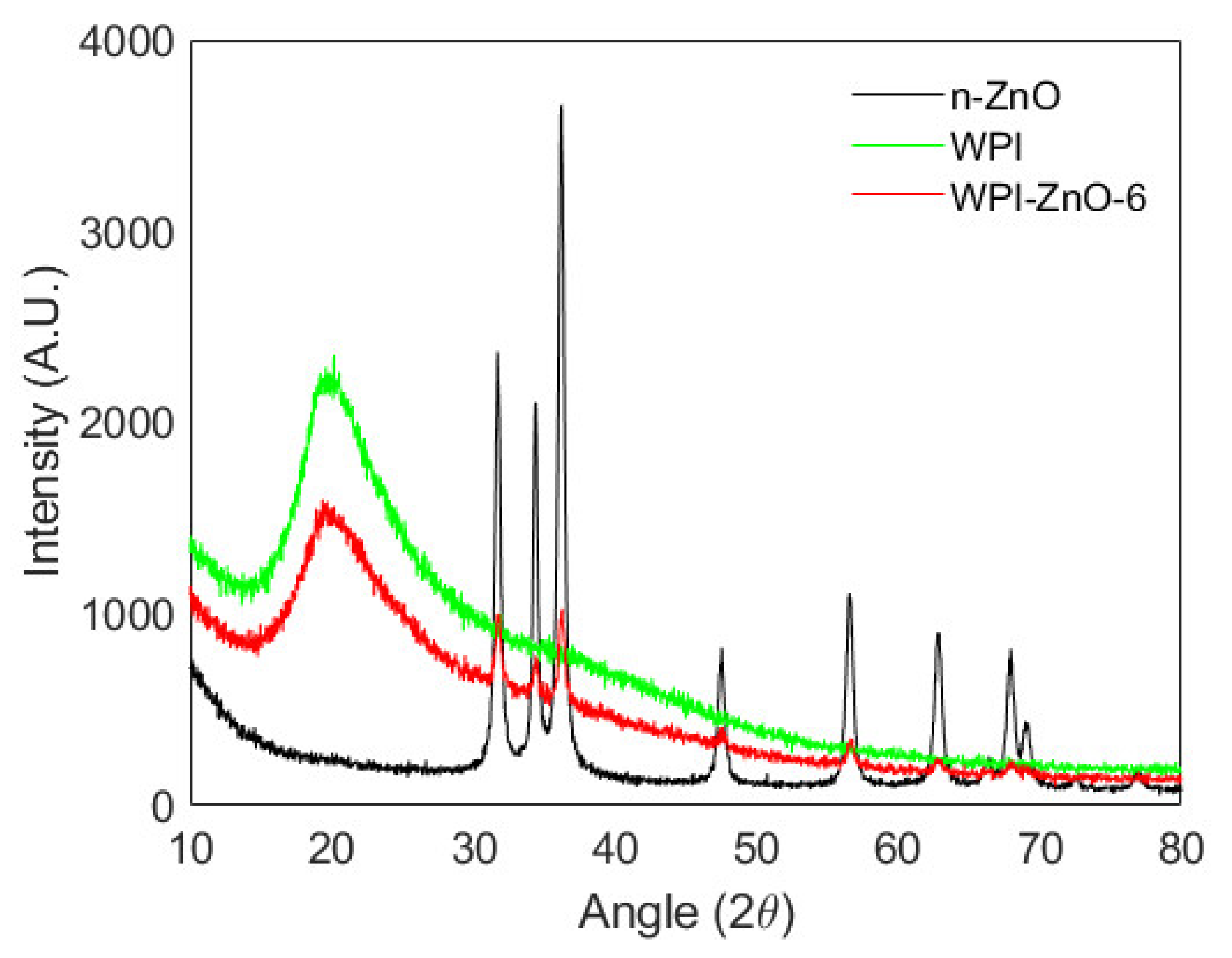
2.6. Characterization of the Films
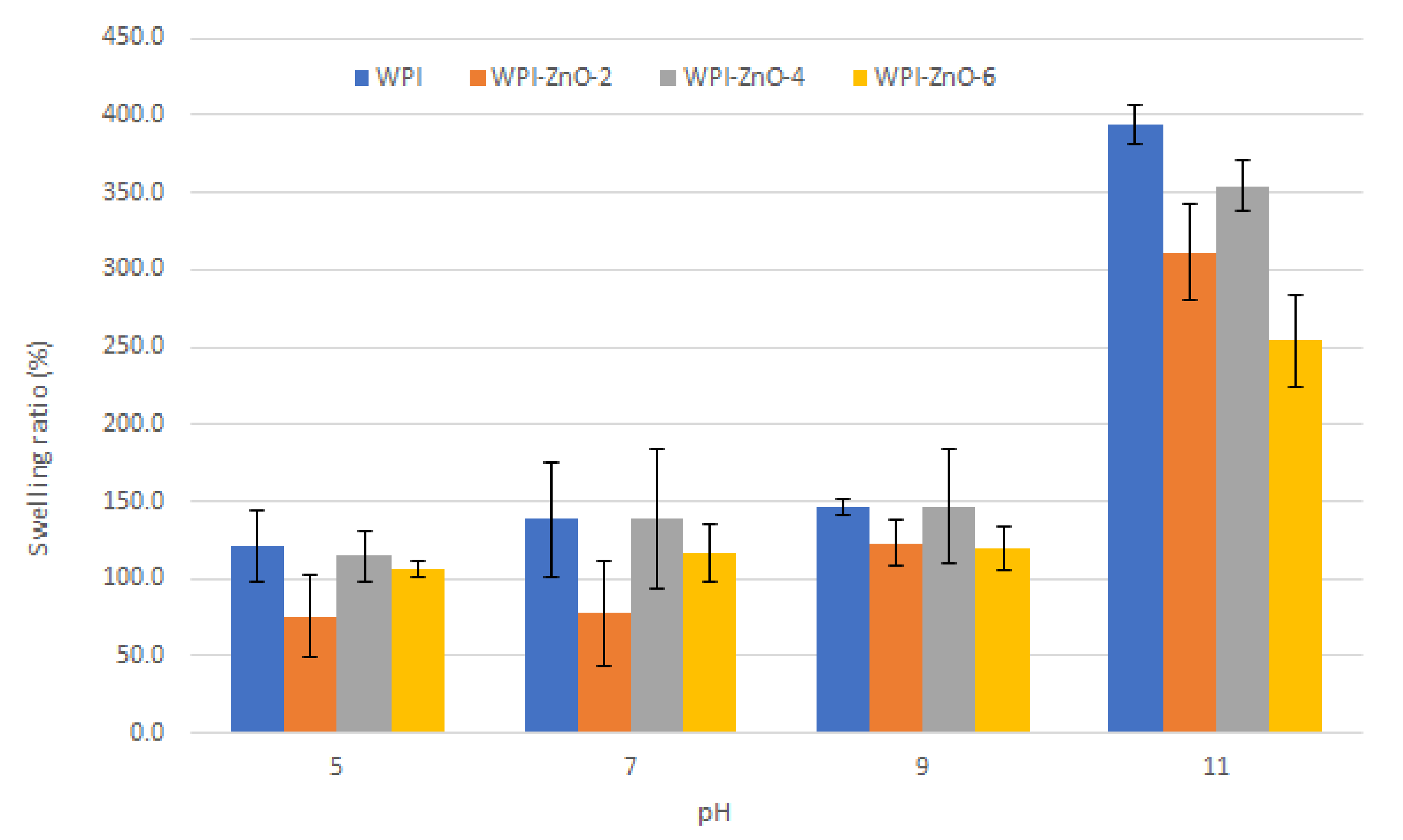
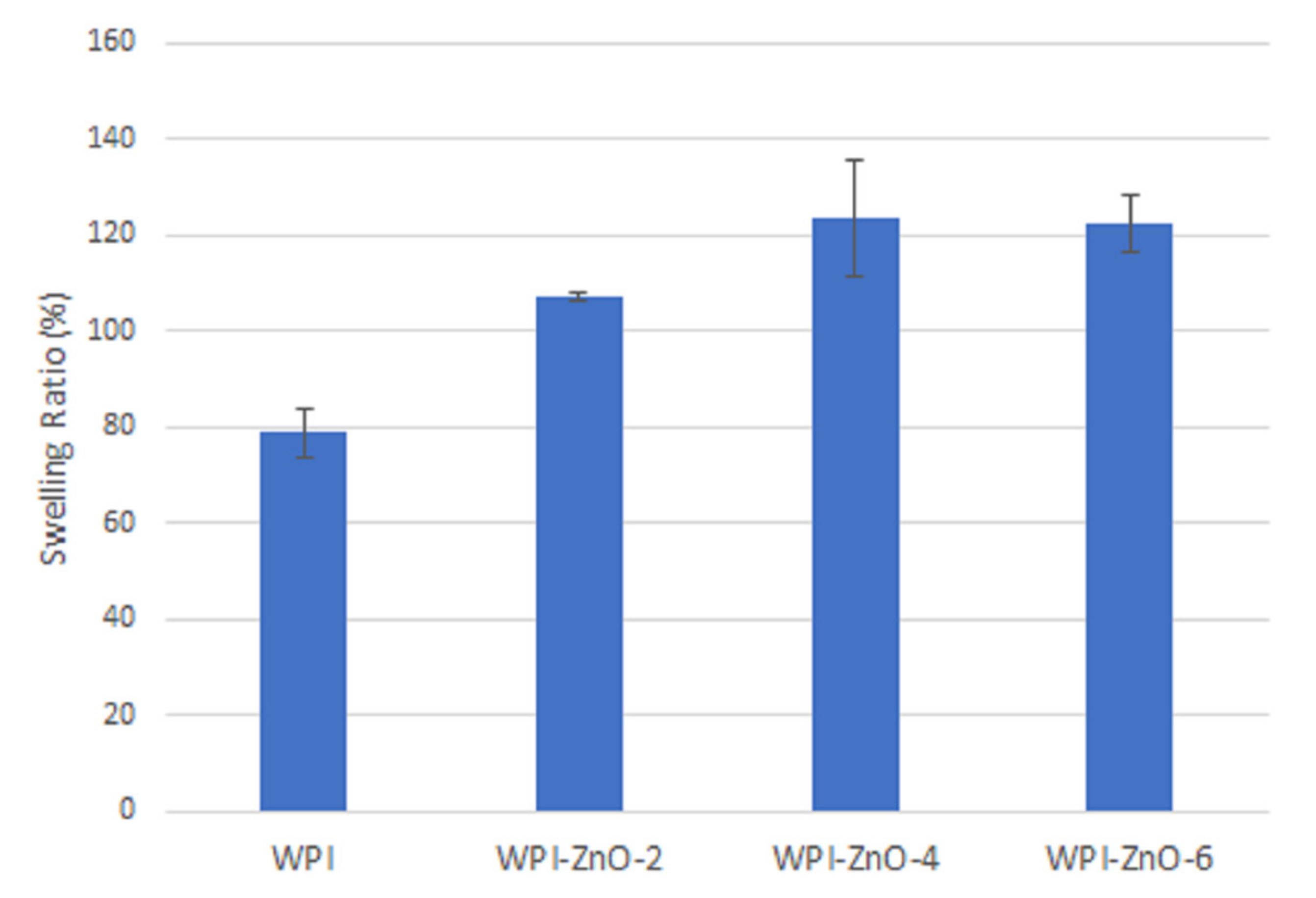
2.7. Swelling Tests
2.8. Antibacterial Activity
2.9. Antibacterial Activity of n-ZnO
2.10. Antibacterial Activity of Bionanocomposites Films
3. Results and Discussion
3.1. Characterization of n-ZnO
3.2. Characterization of the Films
3.3. Swelling Tests
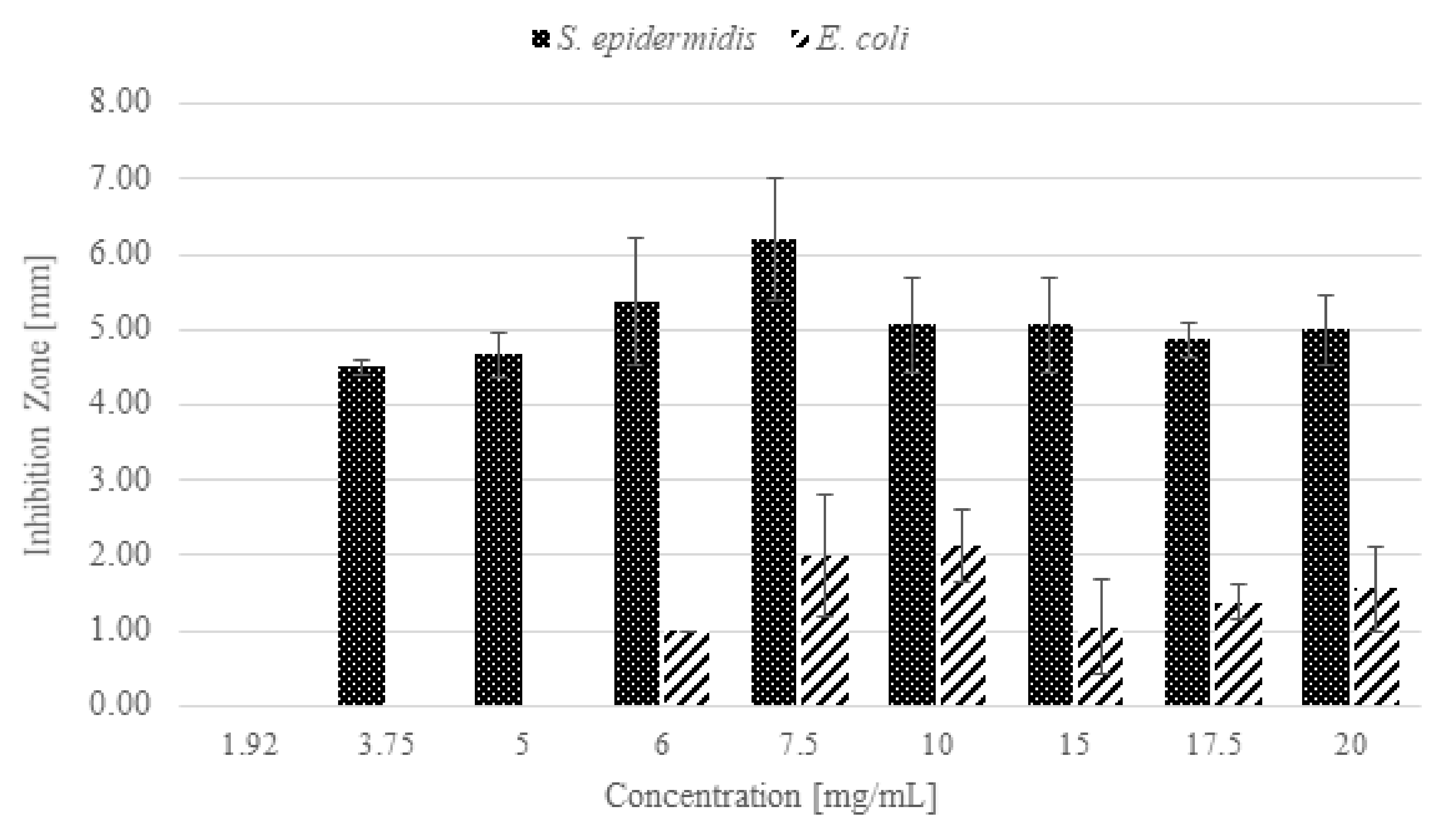
3.4. Antibacterial Activity of n-ZnO
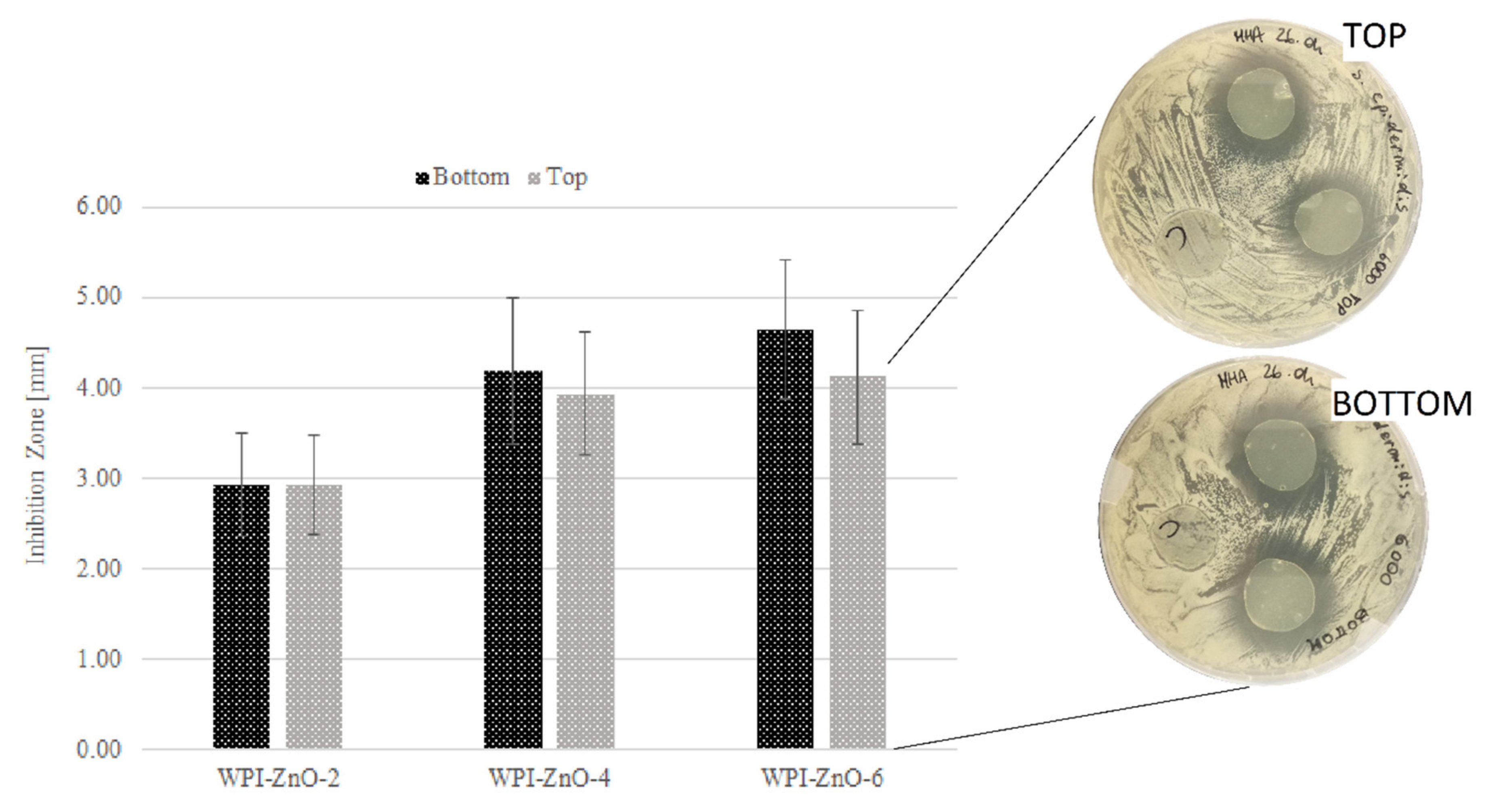
3.5. Antibacterial Activity of Bionanocomposite Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Jeong, C.H.; Kim, D.H.; Yune, J.H.; Kwon, H.C.; Shin, D.-M.; Sohn, H.; Lee, K.H.; Choi, B.; Kim, E.S.; Kang, J.H.; et al. In vitro toxicity assessment of crosslinking agents used in hyaluronic acid dermal filler. Toxicol. Vitr. 2021, 70, 105034. [Google Scholar] [CrossRef]
- Amri, M.; Firdaus, M.; Fauzi, M.; Chowdhury, S.; Fadilah, N.; Hamirul, W.W.; Reusmaazran, M.; Aminuddin, B.; Ruszymah, B. Cytotoxic evaluation of biomechanically improved crosslinked ovine collagen on human dermal fibroblasts. Bio-Med. Mater. Eng. 2014, 24, 1715–1724. [Google Scholar] [CrossRef]
- Lai, J.-Y. Relationship between structure and cytocompatibility of divinyl sulfone cross-linked hyaluronic acid. Carbohydr. Polym. 2014, 101, 203–212. [Google Scholar] [CrossRef]
- Tee, H.T.; Zipp, R.; Koynov, K.; Tremel, W.; Wurm, F.R. Poly(methyl ethylene phosphate) hydrogels: Degradable and cell-repellent alternatives to PEG-hydrogels. Eur. Polym. J. 2020, 141, 110075. [Google Scholar] [CrossRef]
- Suo, H.; Hussain, M.; Wang, H.; Zhou, N.; Tao, J.; Jiang, H.; Zhu, J. Injectable and pH-Sensitive Hyaluronic Acid-Based Hydrogels with On-Demand Release of Antimicrobial Peptides for Infected Wound Healing. Biomacromolecules 2021, 22, 3049–3059. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Guo, L.; Tao, S.; Huang, Z.; Qi, H. Freely Moldable Modified Starch as a Sustainable and Recyclable Plastic. Biomacromolecules 2021, 22, 2676–2683. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, L.; Van Hoorick, J.; Blondeel, P.; Van Vlierberghe, S. Toward Adipose Tissue Engineering Using Thiol-Norbornene Photo-Crosslinkable Gelatin Hydrogels. Biomacromolecules 2021, 22, 2408–2418. [Google Scholar] [CrossRef]
- Chen, L.; Dong, Q.; Shi, Q.; Du, Y.; Zeng, Q.; Zhao, Y.; Wang, J.J. Novel 2,3-Dialdehyde Cellulose-Based Films with Photodynamic Inactivation Potency by Incorporating the β-Cyclodextrin/Curcumin Inclusion Complex. Biomacromolecules 2021, 22, 2790–2801. [Google Scholar] [CrossRef]
- Indumathi, M.; Sarojini, K.S.; Rajarajeswari, G. Antimicrobial and biodegradable chitosan/cellulose acetate phthalate/ZnO nano composite films with optimal oxygen permeability and hydrophobicity for extending the shelf life of black grape fruits. Int. J. Biol. Macromol. 2019, 132, 1112–1120. [Google Scholar] [CrossRef]
- Shankar, S.; Teng, X.; Li, G.; Rhim, J.-W. Preparation, characterization, and antimicrobial activity of gelatin/ZnO nanocomposite films. Food Hydrocoll. 2015, 45, 264–271. [Google Scholar] [CrossRef]
- Khalid, A.; Khan, R.; Ul-Islam, M.; Khan, T.; Wahid, F. Bacterial cellulose-zinc oxide nanocomposites as a novel dressing system for burn wounds. Carbohydr. Polym. 2017, 164, 214–221. [Google Scholar] [CrossRef]
- Azevedo, V.M.; Dias, M.V.; Elias, H.H.D.S.; Fukushima, K.L.; Silva, E.K.; Carneiro, J.D.D.S.; Soares, N.D.F.F.; Borges, S. Effect of whey protein isolate films incorporated with montmorillonite and citric acid on the preservation of fresh-cut apples. Food Res. Int. 2018, 107, 306–313. [Google Scholar] [CrossRef]
- Mohammadi, M.; Mirabzadeh, S.; Shahvalizadeh, R.; Hamishehkar, H. Development of novel active packaging films based on whey protein isolate incorporated with chitosan nanofiber and nano-formulated cinnamon oil. Int. J. Biol. Macromol. 2020, 149, 11–20. [Google Scholar] [CrossRef]
- Abaee, A.; Mohammadian, M.; Jafari, S.M. Whey and soy protein-based hydrogels and nano-hydrogels as bioactive delivery systems. Trends Food Sci. Technol. 2017, 70, 69–81. [Google Scholar] [CrossRef]
- Ramos, O.; Reinas, I.; Silva, S.I.; Fernandes, J.; Cerqueira, M.; Pereira, R.N.; Vicente, A.; Poças, M.D.F.; Pintado, M.E.; Malcata, F.X. Effect of whey protein purity and glycerol content upon physical properties of edible films manufactured therefrom. Food Hydrocoll. 2013, 30, 110–122. [Google Scholar] [CrossRef] [Green Version]
- Shaw, N.; Monahan, F.; O’Riordan, E.; O’Sullivan, M. Physical Properties of WPI Films Plasticized with Glycerol, Xylitol, or Sorbitol. J. Food Sci. 2002, 67, 164–167. [Google Scholar] [CrossRef]
- McHugh, T.H.; Aujard, J.-F.; Krochta, J.M. Plasticized Whey Protein Edible Films: Water Vapor Permeability Properties. J. Food Sci. 1994, 59, 416–419. [Google Scholar] [CrossRef]
- Guo, X.; Liu, Y.; Bera, H.; Zhang, H.; Chen, Y.; Cun, D.; Foderà, V.; Yang, M. α-Lactalbumin-Based Nanofiber Dressings Improve Burn Wound Healing and Reduce Scarring. ACS Appl. Mater. Interfaces 2020, 12, 45702–45713. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, H.; Wang, X. Facile and mild preparation of fluorescent ZnO nanosheets and their bioimaging applications. Appl. Surf. Sci. 2011, 257, 6991–6995. [Google Scholar] [CrossRef]
- Nagajyothi, P.; Cha, S.J.; Yang, I.J.; Sreekanth, T.; Kim, K.J.; Shin, H.M. Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J. Photochem. Photobiol. B Biol. 2015, 146, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-E.; Jin, H.-E. Synthesis, Characterization, and Three-Dimensional Structure Generation of Zinc Oxide-Based Nanomedicine for Biomedical Applications. Pharmaceutics 2019, 11, 575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leone, F.; Cataldo, R.; Mohamed, S.S.Y.; Manna, L.; Banchero, M.; Ronchetti, S.; Mandras, N.; Tullio, V.; Cavalli, R.; Onida, B. Nanostructured ZnO as Multifunctional Carrier for a Green Antibacterial Drug Delivery System—A Feasibility Study. Nanomaterials 2019, 9, 407. [Google Scholar] [CrossRef] [Green Version]
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc oxide nanoparticles: A promising nanomaterial for biomedical applications. Drug Discov. Today 2017, 22, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, B.; Solanki, B.; Zaidi, A.; Khan, M.S.; Musarrat, J. Bacterial toxicity of biomimetic green zinc oxide nanoantibiotic: Insights into ZnONP uptake and nanocolloid–bacteria interface. Toxicol. Res. 2018, 8, 246–261. [Google Scholar] [CrossRef] [Green Version]
- Arakha, M.; Saleem, M.; Mallick, B.C.; Jha, S. The effects of interfacial potential on antimicrobial propensity of ZnO nanoparticle. Sci. Rep. 2015, 5, 9578. [Google Scholar] [CrossRef] [PubMed]
- Sharifalhoseini, Z.; Entezari, M.H.; Jalal, R. Direct and indirect sonication affect differently the microstructure and the morphology of ZnO nanoparticles: Optical behavior and its antibacterial activity. Ultrason. Sonochem. 2015, 27, 466–473. [Google Scholar] [CrossRef]
- Cai, Q.; Gao, Y.; Gao, T.; Lan, S.; Simalou, O.; Zhou, X.; Zhang, Y.; Harnoode, C.; Gao, G.; Dong, A. Insight into Biological Effects of Zinc Oxide Nanoflowers on Bacteria: Why Morphology Matters. ACS Appl. Mater. Interfaces 2016, 8, 10109–10120. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. ZnO-Reinforced Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Bionanocomposites with Antimicrobial Function for Food Packaging. ACS Appl. Mater. Interfaces 2014, 6, 9822–9834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, M.M.; Bouchami, O.; Tavares, A.; Cordoba, L.; Santos, C.; Miragaia, M.; Montemor, F. New Insights into Antibiofilm Effect of a Nanosized ZnO Coating against the Pathogenic Methicillin Resistant Staphylococcus aureus. ACS Appl. Mater. Interfaces 2017, 9, 28157–28167. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, M.E.; Cuestas, M.L.; Pérez, C.J.; Orto, V.C.D.; Copello, G.J. Smart release of antimicrobial ZnO nanoplates from a pH-responsive keratin hydrogel. J. Colloid Interface Sci. 2019, 536, 372–380. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Joorabloo, A.; Moghaddam, A.; Shamsi, H.; MansooriMoghadam, Z. Incorporation of ZnO nanoparticles into heparinised polyvinyl alcohol/chitosan hydrogels for wound dressing application. Int. J. Biol. Macromol. 2018, 114, 1203–1215. [Google Scholar] [CrossRef] [PubMed]
- Vasile, B.S.; Oprea, O.; Voicu, G.; Ficai, A.; Andronescu, E.; Teodorescu, A.; Holban, A. Synthesis and characterization of a novel controlled release zinc oxide/gentamicin–chitosan composite with potential applications in wounds care. Int. J. Pharm. 2014, 463, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Banchero, M.; Mohamed, S.S.Y.; Leone, F.; Lopez, F.; Ronchetti, S.; Manna, L.; Onida, B. Supercritical Solvent Impregnation of Different Drugs in Mesoporous Nanostructured ZnO. Pharmaceutics 2019, 11, 340. [Google Scholar] [CrossRef] [Green Version]
- Mitra, S.; Subia., B.; Patra, P.; Chandra, S.; Debnath, N.; Das, S.; Banerjee, R.; Kundu, S.C.; Pramanik, P.; Goswami, A. Porous ZnO nanorod for targeted delivery of doxorubicin: In vitro and in vivo response for therapeutic applications. J. Mater. Chem. 2012, 22, 24145–24154. [Google Scholar] [CrossRef]
- EUCAST Disk Diffusion Method for Antimicrobial Susceptibility Testing (January 2021). Available online: https://www.eucast.org/ast_of_bacteria/disk_diffusion_methodology/ (accessed on 7 September 2021).
- Berg, J.M.; Romoser, A.; Banerjee, N.; Zebda, R.; Sayes, C. The relationship between pH and zeta potential of ∼ 30 nm metal oxide nanoparticle suspensions relevant toin vitrotoxicological evaluations. Nanotoxicology 2009, 3, 276–283. [Google Scholar] [CrossRef]
- Gounga, M.E.; Xu, S.; Wang, Z. FILM FORMING MECHANISM AND MECHANICAL AND THERMAL PROPERTIES OF WHEY PROTEIN ISOLATE-BASED EDIBLE FILMS AS AFFECTED BY PROTEIN CONCENTRATION, GLYCEROL RATIO AND PULLULAN CONTENT. J. Food Biochem. 2010, 34, 501–519. [Google Scholar] [CrossRef]
- Lorenzen, P.C.; Schrader, K. A Comparative Study of Whey Protein Concentrate and Whey Protein Isolate. Le Lait 2006, 86, 259–271. [Google Scholar] [CrossRef] [Green Version]
- Vaezi, K.; Asadpour, G.; Sharifi, H. Effect of ZnO nanoparticles on the mechanical, barrier and optical properties of thermoplastic cationic starch/montmorillonite biodegradable films. Int. J. Biol. Macromol. 2019, 124, 519–529. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Yu, H.-Y.; Yang, L.; Abdalkarim, S.Y.H.; Chen, W.-L. Enhancing long-term biodegradability and UV-shielding performances of transparent polylactic acid nanocomposite films by adding cellulose nanocrystal-zinc oxide hybrids. Int. J. Biol. Macromol. 2019, 141, 893–905. [Google Scholar] [CrossRef]
- Jebel, F.S.; Almasi, H. Morphological, physical, antimicrobial and release properties of ZnO nanoparticles-loaded bacterial cellulose films. Carbohydr. Polym. 2016, 149, 8–19. [Google Scholar] [CrossRef]
- Hezma, A.; Rajeh, A.; Mannaa, M. An insight into the effect of zinc oxide nanoparticles on the structural, thermal, mechanical properties and antimicrobial activity of Cs/PVA composite. Colloids Surf. A Physicochem. Eng. Asp. 2019, 581, 123821. [Google Scholar] [CrossRef]
- Rahman, P.M.; Mujeeb, V.A.; Muraleedharan, K.; Thomas, S.K. Chitosan/nano ZnO composite films: Enhanced mechanical, antimicrobial and dielectric properties. Arab. J. Chem. 2018, 11, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Annaidh, A.N.; Otténio, M.; Bruyere, K.; Destrade, M.; Gilchrist, M. Mechanical Properties of Excised Human Skin. In VI Latin American Congress on Biomedical Engineering CLAIB 2014, Paraná, Argentina 29, 30 & 31 October 2014; Springer: Berlin/Heidelberg, Germany, 2010; Volume 31, pp. 1000–1003. [Google Scholar] [CrossRef] [Green Version]
- Koehler, J.; Wallmeyer, L.; Hedtrich, S.; Goepferich, A.M.; Brandl, F.P. pH-Modulating Poly(ethylene glycol)/Alginate Hydrogel Dressings for the Treatment of Chronic Wounds. Macromol. Biosci. 2017, 17, 1600369. [Google Scholar] [CrossRef]
- Andrade, J.; Pereira, C.G.; Junior, J.C.D.A.; Viana, C.C.R.; Neves, L.N.D.O.; da Silva, P.H.F.; Bell, M.J.V.; Anjos, V.D.C.D. FTIR-ATR determination of protein content to evaluate whey protein concentrate adulteration. LWT 2019, 99, 166–172. [Google Scholar] [CrossRef]
- Saedi, S.; Shokri, M.; Kim, J.T.; Shin, G.H. Semi-transparent regenerated cellulose/ZnONP nanocomposite film as a potential antimicrobial food packaging material. J. Food Eng. 2021, 307, 110665. [Google Scholar] [CrossRef]
- Jayakumar, R.; Kumar, P.S.; Mohandas, A.; Lakshmanan, V.K.; Biswas, R. Exploration of alginate hydrogel/nano zinc oxide composite bandages for infected wounds. Int. J. Nanomed. 2015, 10, 53–66. [Google Scholar] [CrossRef] [Green Version]
- Gunasekaran, S.; Ko, S.; Xiao, L. Use of whey proteins for encapsulation and controlled delivery applications. J. Food Eng. 2007, 83, 31–40. [Google Scholar] [CrossRef]
- Livney, Y.D. Milk proteins as vehicles for bioactives. Curr. Opin. Colloid Interface Sci. 2010, 15, 73–83. [Google Scholar] [CrossRef]
- Quevedo, M.; Karbstein, H.P.; Emin, M.A. Influence of thermomechanical treatment and pH on the denaturation kinetics of highly concentrated whey protein isolate. J. Food Eng. 2021, 292, 110294. [Google Scholar] [CrossRef]
- Wang, W.-Q.; Sheng, H.-B.; Zhou, J.-Y.; Yuan, P.-P.; Zhang, X.-F.; Lu, M.-L.; Gu, R.-X. The effect of a variable initial pH on the structure and rheological properties of whey protein and monosaccharide gelation via the Maillard reaction. Int. Dairy J. 2021, 113, 104896. [Google Scholar] [CrossRef]
- Namazi, H.; Hasani, M.; Yadollahi, M. Antibacterial oxidized starch/ZnO nanocomposite hydrogel: Synthesis and evaluation of its swelling behaviours in various pHs and salt solutions. Int. J. Biol. Macromol. 2019, 126, 578–584. [Google Scholar] [CrossRef]
- Wahid, F.; Yin, J.-J.; Xue, D.-D.; Xue, H.; Lu, Y.-S.; Zhong, C.; Chu, L.-Q. Synthesis and characterization of antibacterial carboxymethyl Chitosan/ZnO nanocomposite hydrogels. Int. J. Biol. Macromol. 2016, 88, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.P.T.; Lakshmanan, V.K.; Raj, M.; Biswas, R.; Hiroshi, T.; Nair, S.V.; Jayakumar, R. Evaluation of Wound Healing Potential of β-Chitin Hydrogel/Nano Zinc Oxide Composite Bandage. Pharm. Res. 2012, 30, 523–537. [Google Scholar] [CrossRef]
- Zhai, M.; Xu, Y.; Zhou, B.; Jing, W. Keratin-chitosan/n-ZnO nanocomposite hydrogel for antimicrobial treatment of burn wound healing: Characterization and biomedical application. J. Photochem. Photobiol. B Biol. 2018, 180, 253–258. [Google Scholar] [CrossRef]
- Muthuramalingam, K.; Choi, S.I.; Hyun, C.; Kim, Y.M.; Cho, M. β-Glucan-Based Wet Dressing for Cutaneous Wound Healing. Adv. Wound Care 2019, 8, 125–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, A.; Bhargava, R.; Poddar, P. Probing interaction of Gram-positive and Gram-negative bacterial cells with ZnO nanorods. Mater. Sci. Eng. C 2013, 33, 1247–1253. [Google Scholar] [CrossRef]
- Reddy, K.M.; Feris, K.; Bell, J.; Wingett, D.G.; Hanley, C.; Punnoose, A. Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl. Phys. Lett. 2007, 90, 213902. [Google Scholar] [CrossRef] [Green Version]
- Premanathan, M.; Karthikeyan, K.; Jeyasubramanian, K.; Manivannan, G. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 184–192. [Google Scholar] [CrossRef]
- Nigam, A.; Saini, S.; Rai, A.K.; Pawar, S. Structural, optical, cytotoxicity, and antimicrobial properties of MgO, ZnO and MgO/ZnO nanocomposite for biomedical applications. Ceram. Int. 2021, 47, 19515–19525. [Google Scholar] [CrossRef]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial properties of ZnO nanomaterials: A review. Ceram. Int. 2017, 43, 3940–3961. [Google Scholar] [CrossRef]
- Resmi, R.; Yoonus, J.; Beena, B. A novel greener synthesis of ZnO nanoparticles from Nilgiriantusciliantus leaf extract and evaluation of its biomedical applications. Mater. Today Proc. 2021, 46, 3062–3068. [Google Scholar] [CrossRef]
- Abdelhakim, H.K.; El-Sayed, E.R.; Rashidi, F.B. Biosynthesis of zinc oxide nanoparticles with antimicrobial, anticancer, antioxidant and photocatalytic activities by the endophytic Alternaria tenuissima. J. Appl. Microbiol. 2020, 128, 1634–1646. [Google Scholar] [CrossRef]
- Vinardell, M.P.; Llanas, H.; Marics, L.; Mitjans, M. In Vitro Comparative Skin Irritation Induced by Nano and Non-Nano Zinc Oxide. Nanomaterials 2017, 7, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sruthi, S.; Ashtami, J.; Mohanan, P. Biomedical application and hidden toxicity of Zinc oxide nanoparticles. Mater. Today Chem. 2018, 10, 175–186. [Google Scholar] [CrossRef]
- Azari, S.S.; Alizadeh, A.; Roufegarinejad, L.; Asefi, N.; Hamishehkar, H. Preparation and characterization of gelatin/β-glucan nanocomposite film incorporated with ZnO nanoparticles as an active food packaging system. J. Polym. Environ. 2021, 29, 1143–1152. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Benjakul, S.; Prodpran, T.; Sumpavapol, P.; Songtipya, P. Properties and antimicrobial activity of fish protein isolate/fish skin gelatin film containing basil leaf essential oil and zinc oxide nanoparticles. Food Hydrocoll. 2014, 41, 265–273. [Google Scholar] [CrossRef]
- Amato, A.; Migneco, L.M.; Martinelli, A.; Pietrelli, L.; Piozzi, A.; Francolini, I. Antimicrobial activity of catechol functionalized-chitosan versus Staphylococcus epidermidis. Carbohydr. Polym. 2018, 179, 273–281. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Khezerlou, A.; Ehsani, A. Fabrication and characterization of the bionanocomposite film based on whey protein biopolymer loaded with TiO2 nanoparticles, cellulose nanofibers and rosemary essential oil. Ind. Crop. Prod. 2018, 124, 300–315. [Google Scholar] [CrossRef]
- Sani, M.A.; Ehsani, A.; Hashemi, M. Whey protein isolate/cellulose nanofibre/TiO2 nanoparticle/rosemary essential oil nanocomposite film: Its effect on microbial and sensory quality of lamb meat and growth of common foodborne pathogenic bacteria during refrigeration. Int. J. Food Microbiol. 2017, 251, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Karimi, N.; Alizadeh, A.; Almasi, H.; Hanifian, S. Preparation and characterization of whey protein isolate/polydextrose-based nanocomposite film incorporated with cellulose nanofiber and L. plantarum: A new probiotic active packaging system. LWT 2020, 121, 108978. [Google Scholar] [CrossRef]
- Thanusha, A.V.; Dinda, A.K.; Koul, V. Evaluation of nano hydrogel composite based on gelatin/HA/CS suffused with Asiatic acid/ZnO and CuO nanoparticles for second degree burns. Mater. Sci. Eng. C 2018, 89, 378–386. [Google Scholar] [CrossRef]






| Sample | Young’s Modulus [MPa] | Tensile Strenght [MPa] | Elongation at Break [%] |
|---|---|---|---|
| WPI | 12.65 ± 0.99 | 0.56 ± 0.18 | 106.16 ± 15.97 |
| WPI–ZnO-2 | 32.27 ± 1.37 | 0.71 ± 0.15 | 44.19 ± 4.71 |
| WPI–ZnO-4 | 36.25 ± 1.47 | 0.69 ± 0.04 | 30.90 ± 2.73 |
| WPI–ZnO-6 | 39.34 ± 3.30 | 0.65 ± 0.10 | 14.67 ± 2.95 |
| WPI | WPI–ZnO-2 | WPI–ZnO-4 | WPI–ZnO-6 | |
|---|---|---|---|---|
| Inhibition halo [mm] | 0 | 2.94 ± 0.57 | 4.19 ± 0.81 | 4.66 ± 0.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pino, P.; Ronchetti, S.; Mollea, C.; Sangermano, M.; Onida, B.; Bosco, F. Whey Proteins–Zinc Oxide Bionanocomposite as Antibacterial Films. Pharmaceutics 2021, 13, 1426. https://doi.org/10.3390/pharmaceutics13091426
Pino P, Ronchetti S, Mollea C, Sangermano M, Onida B, Bosco F. Whey Proteins–Zinc Oxide Bionanocomposite as Antibacterial Films. Pharmaceutics. 2021; 13(9):1426. https://doi.org/10.3390/pharmaceutics13091426
Chicago/Turabian StylePino, Paolo, Silvia Ronchetti, Chiara Mollea, Marco Sangermano, Barbara Onida, and Francesca Bosco. 2021. "Whey Proteins–Zinc Oxide Bionanocomposite as Antibacterial Films" Pharmaceutics 13, no. 9: 1426. https://doi.org/10.3390/pharmaceutics13091426







